Abstract
Objective:
To analyze the presence of selective antibiotic residues (oxytetracycline, amoxicillin, and ciprofloxacin) in milk during the antibiotic treatment course, and to evaluate the thermal effect on antibiotics residual status in milk of antibiotic-treated cows.
Materials and Methods:
The raw fresh milk was collected from 18 lactating cows before antibiotics treatment, which were brought to the veterinary hospital and suffered from either mastitis, foot and mouth disease, fever, local wound, or non-specific diarrhea, and so on. Out of the 18 lactating cows, six were treated with oxytetracycline, six were treated with amoxicillin, and six were treated with ciprofloxacin parenterally. Milk samples were also collected at 2nd day during treatment and final collection was done after maintaining the withdrawal period. Since milk is heated before consumption, it was boiled at 100°C for 20 min to evaluate the thermal effect on antibiotics residual status. Thin-layer chromatography was done for screening of antibiotics residue before and after boiling of the milk.
Results:
At day 0 (before antibiotic treatment), no antibiotics (oxytetracycline, ciprofloxacin and amoxicillin) residue was detected in raw milk of antibiotic treated cows. In contrast, on day 2 (during antibiotic treatment), 100% raw milk samples showed positive for antibiotics residue. After boiling, all milk samples showed positive for such specific antibiotics residue. On the other hand, no antibiotics residues were detected on day 9, which indicates the completion of the withdrawal period of the respective antibiotic. The intensities of bands for antibiotic on thin-layer chromatography plate of antibiotic residues in milk samples (oxytetracycline, amoxicillin, and ciprofloxacin) expressed that the respective antibiotic residual status was higher in the boiled milk compare to the raw milk.
Conclusion:
Proper maintenance of withdrawal period after antibiotic treatment would minimize the risk of antibiotic residues in milk, and boiling does not change these specific antibiotics residual status in milk. Therefore, awareness regarding the proper maintenance of withdrawal period after antibiotic treatment in lactating cows is one of the best strategies that may positively reduce the risk of antimicrobial drugs residue in milk.
Keywords: Antibiotic residues, milk, thermal effect, withdrawal period
Introduction
Bangladesh is an agrarian country and livestock is an integrated part of agriculture. The country has a suitable environment for milking cattle rearing. The annual milk production is 9.406 million tons in 2017–18, which covers the 1.60% of the national gross domestic product (GDP) [1]. Milk is considered as one of the most consumed foods for human as it contains sufficient amount of several essential nutrients such as protein, saturated fat, calcium, magnesium, vitamins, and essential amino acids [2,3].
Like other developing countries, antibiotics are extensively used as growth promoters in livestock sector to control diseases, facilitated feed conversion ratio, and for treatment purposes in Bangladesh. As a result, antibiotic resistant bacteria are emerging, which causes serious health hazards [4]. Previous studies also reported that veterinary antibiotics are used vastly in livestock to control disease, enhance growth, promote health, and feed conversion efficiency [5], as well as to reduce animal susceptibility to stress-related diseases or to enhance growth [6]. For these purposes, several antibiotics are extensively used in food animals, including tetracycline, sulfonamides, fluoroquinolones, macrolides, lincosamides, aminoglycosides, beta-lactams, cephalosporin, and so on. Widespread use of antibiotics close to the slaughter could lead to the presence of residues as non-altered parent form or as metabolite and/or conjugate in edible parts such as muscle (meat), organs, egg, and milk obtained from antibiotics-treated animals [7,8]. The presence of these residues is usually attributed to non-observance of withdrawal periods before the sales of edible animal food product, and also due to undesirable practices such as unregulated and indiscriminate use of drugs and lack of awareness on the rational usage of antibiotics [9]. Antibiotic residues in milk are critical issue due to human health hazard, and therefore are of great concern for dairy farmers, milk processors, consumers, and regulatory agencies. To ensure food safety for consumers, several regulatory authorities around the world including National Agency for Food and Drug Administration and Control, European Food Safety Agency, Food and Drug Administration, and USA Codex Alimentarius, established tolerance (safe) levels of antibiotic residues in milk for consumer protection [10]. However, various public authorities and researchers still have recognized the presence of antibiotic residues in milk above the Maximum Residual Limit [11,12].
After using an antibiotic in the human or animal body, it kills bacteria, and the antibiotic is broken down in the body until it becomes a non-functional agent [13], and finally, it is eliminated from the body. Withdrawal periods of different antibiotics may vary from 1 or 2 days to couple of weeks. The minimum withdrawal period for Oxytetracycline is 3 days [14], for amoxicillin is 3 days [15], and for ciprofloxacin is 6 days [16]. In addition, previous studies shown that withdrawal period may vary between different antibiotics, and the minimum withdrawal period maintained for the drugs was 7 days after the last dose of administration [17]. Antibiotics are widely used in veterinary practice, especially for dry cow therapy and mastitis treatment in lactating cows, which may cause the presence of antibiotic residues in milk [18]. There is a high chance of transmission of antibiotic-resistant organism from animal to human due to indiscriminate use of antibiotics [12].
Heat-labile antibiotic can be destroyed by cooking, pasteurization, or canning processes; however, antibiotic metabolites and/or conjugates may have remained in milk after boiling [8,19,20]. In this regard, to the best of our knowledge, no study has been carried out on the thermal effects on antibiotic residual status in boiled milk in Bangladesh. Therefore, the present research work was undertaken to determine whether proper maintenance of withdrawal period does minimize the antibiotic residue and whether thermal treatment have any effect on antibiotic residue in milk of commonly used antibiotics (oxytetracycline, amoxicillin, and ciprofloxacin) treated lactating cows brought to Veterinary Teaching Hospital, Mymensingh.
Materials and Methods
Ethical approval
The present study and all experimental procedures were approved by the Animal Welfare and Experimentation Ethics Committee, Bangladesh Agricultural University, Mymensingh [Approval number: AWEEC/BAU/2018(17-1)].
Study area and duration
The study was conducted to detect antibiotic residues in the milk of antibiotic-treated lactating cows that were brought to Veterinary Teaching Hospital, Bangladesh Agricultural University, Mymensingh. The study was performed during the period from July to December 2018. Samples preparation and laboratory analysis were performed in the Pharmacology Departmental Laboratory, BAU, Mymensingh.
Milk sampling for laboratory analysis
The raw fresh milk was collected from 18 lactating cows before antibiotics treatment which were brought to the veterinary hospital and suffered from either mastitis, foot and mouth disease, fever, local wound, or non-specific diarrhea. Milk samples were collected with the prior permission of the owners. Samples were collected directly from teat before antibiotic treatment (Day 0). Out of the 18 lactating cows, six were treated with oxytetracycline, six were treated with amoxicillin, and six were treated with ciprofloxacin parenterally. Milk samples were also collected at 2nd day during antibiotic treatment and final collection was done after maintaining the withdrawal period (Day 9). Therefore, a total of 54 (18 × 3) raw milk samples were collected for the laboratory analysis. The samples were stored at −20°C for further analysis. The minimum withdrawal period for oxytetracycline is 3 days [14], for amoxicillin is 3 days [15], and for ciprofloxacin is 6 days [16]. In addition, the minimum withdrawal period maintained for the drugs was 7 days as described by Jayalakshmi et al. [17]. Therefore, in this study, we maintained more than 7 days as the withdrawal period.
Antibiotic residue positive milk samples, during treatment (n = 18), were boiled at 100°C for 20 min to evaluate the thermal effects on antibiotic residual status. After boiling, the samples were cooled at room temperature for further analysis.
Sample screening for antibiotic residues
Screening of antibiotic residues was carried out using thin-layer chromatography (TLC) at the Department of Pharmacology, Bangladesh Agricultural University, Mymensingh. TLC apparatus includes TLC plate (MN-Germany), TLC tank, and UV detection box (UV light: F18W-Germany). TLC was performed as per the method described by sarker et al. [21] with some required adjustments. Same Rf value of standard and sample considered similar compound. All procedures are described previously by Sarker et al. [21].
Statistical analysis
The percentages of Rf values of raw and boiled milk samples were entered in MS Excel-2010 for data analysis. Then, the data were analyzed using analysis of variance (SPSS Program) to determine the level of significance. The level of significance was set at 0.05.
Results and Discussion
The widespread and indiscriminate use of antibiotics in dairy sector may result in the presence of antibiotic residues in milk. Consumption of milk with such antibiotic residues with higher than maximum residue limit (MRL) levels by humans predisposes them to serious health effects. Commonly used antibiotics in Bangladesh are sulphonamides, penicillin, oxytetracycline, gentamycin, amoxicillin, ciprofloxacin, ceftriaxone, streptomycin, and so on. Among them, the previous studies show that antibiotics which are commonly excreted through milk are oxytetracycline, amoxicillin, and ciprofloxacin [22,23]. Therefore, in the present study, we selected oxytetracycline-, amoxicillin-, and ciprofloxacin-treated lactating cows for the present study.
Antibiotics residual status in milk during the experimental period
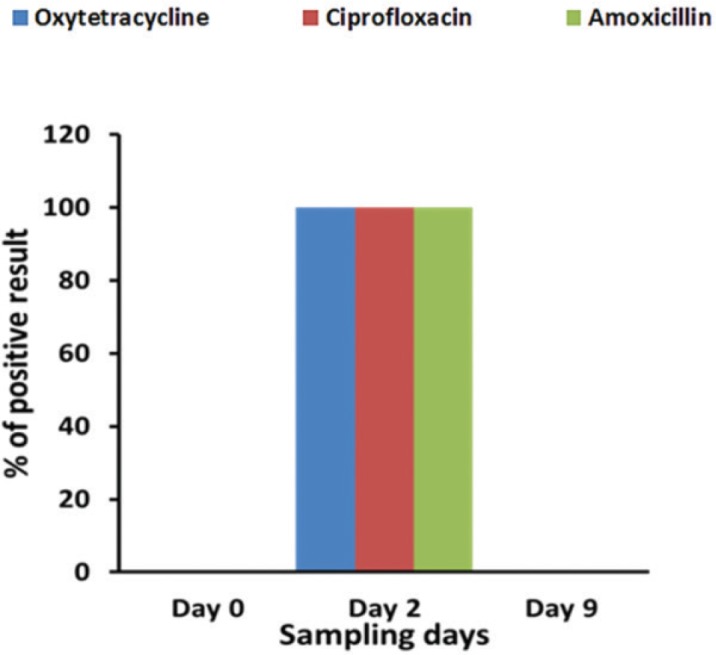
At day 0 (before antibiotic treatment), no antibiotic residues (oxytetracycline, ciprofloxacin and amoxicillin) were detected in raw milk collected from 18 lactating cows during screening by TLC. In contrast, on day 2 (during antibiotics treatment course), all milk samples were positive for antibiotic residues. These results suggested that oxytetracycline, ciprofloxacin, and amoxicillin residues were present during the antibiotic treatment course in lactating cows (Fig. 1). On the other hand, after maintaining withdrawal period of specific antibiotics (at day 9), no antibiotic residues (oxytetracycline, ciprofloxacin, and amoxicillin) were detected in raw milk obtained from 18 lactating cows. These data indicated proper maintenance of withdrawal period after antibiotic treatment would minimize the risk of antibiotic residues. In this regard, previous studies also reported that it is very much important to maintain the antibiotic withdrawal period to avoid the residual effect in milk [23,24]. The minimum withdrawal period for oxytetracycline is 3 days [4], for amoxicillin is 3 days [15], and for ciprofloxacin is six day [16]. In addition, previous studies have shown that withdrawal period may vary between different antibiotics, and the minimum withdrawal period maintained for the drugs was 7 days after the last dose of administration [17]. Therefore, the withdrawal period maintained for the present study was 7 days after the last dose of antibiotic administration.
Figure 1. Antibiotics residues (oxytetracycline, ciprofloxacin and amoxicillin) were not detected in raw milk samples collected at day 0 (before administration of antibiotics), but all milk samples were positive which were collected at day 2 (during antibiotic treatment course). On the other hand, antibiotics residues were not detected at day 9 (after maintaining withdrawal period) in TLC plate under 254 nm UV radiation.
Antibiotics residual status after heat treatment
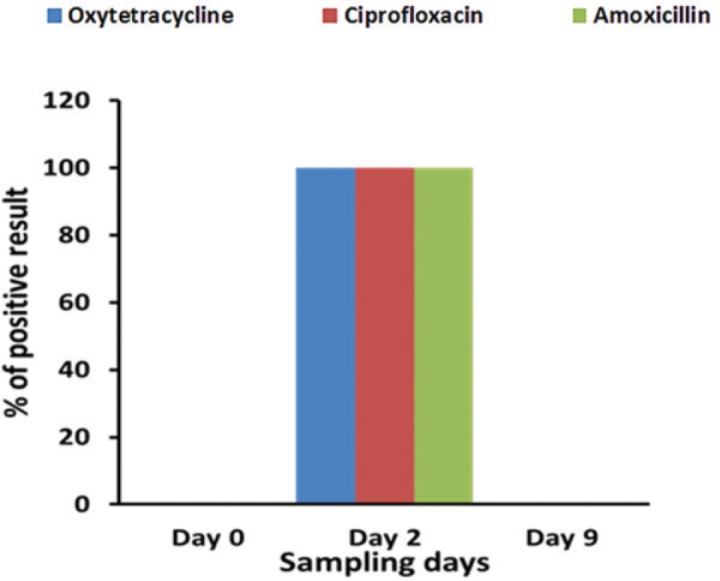
Antibiotic residues positive milk samples (collected during antibiotics treatment course) were boiled at 100°C for 20 min to evaluate the thermal effects on the antibiotic residual status. A previous study reported that boiling milk at normal pasteurization temperature (63°C for 30 min) did not eliminate oxytetracycline residue [25]. Therefore, in our present study, we used long-time boiling (100°C for 20 min) to eliminate the antibiotics residue. After boiling, the samples were cooled at room temperature and were screening for specific antibiotic residues and were found positive for antibiotic residues. These results suggested that heat treatment by boiling does not destroy the residual status in milk (Fig. 2) or their metabolites and/or conjugates may have remained in milk after boiling. In addition, the intensity of bands for antibiotic on TLC plate of boiled milk samples were more prominent than that of raw milk indicating that antibiotic residues are more concentrated in boiled milk. After boiling, the watery portion of milk is evaporated so that the milk becomes, and thereby the antibiotic residue, becomes concentrated in the boiled milk. These results indicate that antibiotic residues or its metabolites and/or its conjugates are more concentrated in boiled milk compared to raw milk. In agreement with previous study findings, the results of the present study suggest that oxytetracycline, amoxicillin, and ciprofloxacin are not destroyed by heat treatment, which also merged with the published literatures [23,25,26]. On the other hand, in this regard, a previous report showed that heat-labile antibiotic residues can be destroyed by cooking procedures, pasteurization, or canning processes; however, antibiotic metabolites and/or its conjugates may have remained in milk after boiling [8,19,20]. Since milk is usually heated or pasteurized before consumption, a few reports have been published on the effect of heating on the stability of antibiotic residues in milk [27]. However, earlier studies with drug residues in heated milk were analyzed using microbiological assays. A little or no information is available concerning the stability of various antibiotic residues in milk as affected by heat treatments [25]. Several studies have already been done on pharmacokinetic and pharmacodynamics of oxytetracycline, amoxicillin, and ciprofloxacin; however, this present study was done to increase the awareness regarding the important of withdrawal period after antibiotic treatment. Furthermore, detailed studies are needed to find out whether antibiotic residues in boiled milk are in active form or it metabolites or its conjugates.
Figure 2. Antibiotics residues (oxytetracycline, ciprofloxacin and amoxicillin) were not detected in boiled milk samples collected at day 0 (before administration of antibiotics), but all boiled milk samples were positive which were collected at day 2 (during antibiotic treatment course). On the other hand, antibiotics residues were not detected at day 9 (after maintaining withdrawal period) in TLC plate under 254 nm UV radiation.
Conclusion
Antibiotic residues are present in raw milk during the antibiotic treatment course. Even the heat treatment does not destroy the oxytetracycline, ciprofloxacin, and amoxicillin antibiotic and/or their metabolites residues in milk. In addition, proper maintaining of withdrawal period after antibiotic treatment in lactating cows no antibiotic residue is detected in raw milk samples. Therefore, withdrawal period must be followed after antibiotic treatment and only then milk should be allowed for human consumption. Government authorities should play vital role in controlling the indiscriminate use of antibiotics at field level. Farmers should be trained properly about the uses of antibiotics, antimicrobial resistance, residues, and its effect on human health, so that they can be aware about selling of milk from antibiotic-treated cows for human consumption. Finally, awareness, honesty, and responsibilities of all stakeholders of livestock industry can help produce drug-residue-free milk for our future generation.
Acknowledgment
The authors acknowledge to all teachers and staffs of the Veterinary Teaching Hospital, Bangladesh Agricultural University (BAU), and of the Department of Pharmacology, BAU, Mymensingh for their technical supports.
Conflict of interest
All authors declare that they have no other conflict of interest.
Authors’ contribution
Tasnia Tabassum Anika, Zakaria Al Noman, and Most. Rifat Ara Ferdous performed all the experiments and wrote the draft manuscript; Kazi Rafiq designed and supervised the research; Tasnia Tabassum Anika and Kazi Rafiq prepared and finalized the manuscript; Sayekul Hasan Khan, Mufsana Akter Mukta, Md. Shakil Islam, Md. Tarek Hossain did the statistical analysis and prepared the table and graph. All authors read and approved the final version of the manuscript.
References
- [1].Livestock economy at a glance, DLS. 2018. Available via https://www.dls.gov.bd. (Accessed 23 April 2018)
- [2].Samanidou V, Nisyriou S. Multi-residue methods for confirmatory determination of antibiotics in milk. J Sep Sci. 2008;31(11):2068–90. doi: 10.1002/jssc.200700647. https://doi.org/10.1002/jssc.200700647. [DOI] [PubMed] [Google Scholar]
- [3].Nguyen Bao K, Sandjaja S, Poh B, Rojroongwasinkul N, Huu C, Sumedi E, et al. The Consumption of dairy and its association with nutritional status in the south east Asian nutrition surveys (SEANUTS) Nutrients. 2018;10(6):759. doi: 10.3390/nu10060759. https://doi.org/10.3390/nu10060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hassan BAR. HPLC uses and importance in the pharmaceutical analysis and industrial field. Pharm Anal Acta. 2012;3:4172. https://doi.org/10.4172/2153-2435.1000e133. [Google Scholar]
- [5].Zhang H, Luo Y, Wu L, Huang Y, Christie P. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming. Environ Sci Pollut Res Int. 2014;22:5908–18. doi: 10.1007/s11356-014-3731-9. https://doi.org/10.1007/s11356-014-3731-9. [DOI] [PubMed] [Google Scholar]
- [6].Kumar K, Gupta SC, Chander Y, Singh AK. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv Agron. 2005;87:1–54. https://doi.org/10.1016/s0065-2113(05)87001-4. [Google Scholar]
- [7].Ibrahim AI, Junaidu AU, Garba MK. Multiple antibiotic residues in meat from slaughtered cattle in Nigeria. Inter J Vet Med. 2009;8:1. https://doi.org/10.5580/1fcd. [Google Scholar]
- [8].Peters RJB, Block YJC, Rutger SP, Stolker AAM, Nielen MWF. Multi residue screening of veterinary drugs in egg, fish and meat using high resolution liquid chromatography accurate mass time-of-flight mass spectrometry. J Chromatogr A. 20091216:8206–16. doi: 10.1016/j.chroma.2009.04.027. https://doi.org/10.1016/j.chroma.2009.04.027. [DOI] [PubMed] [Google Scholar]
- [9].Kabir J, Umoh VJ, Audu-okoh E, Umoh JU, Kwaga JKP. Veterinary drug used in poultry farms and determination of antimicrobial drug residues in commercial eggs and slaughtered chicken in Kaduna State, Nigeria. Food Control. 2004;15:99–105. https://doi.org/10.1016/s0956-7135(03)00020-3. [Google Scholar]
- [10].Adewuyi GO, Olatoye OI, Abafe AO, Otokpa MO, Nkukut MK. High performance liquid chromatographic method for evaluation of two antibiotic residues in liver and muscles of broilers in Ibadan city, Southern Nigeria. J Pharm Biomed Sci. 2011;11(11):1–4. [Google Scholar]
- [11].Kempe M, Verachtert B. Lunds University Centre for Chemistry and Chemical Engineering Getingevagen; Lund, Sweden: 2000. Cartridges with molecularly imprinted recognition elements for antibiotic residues monitoring in milk cream pure and applied biochemistry; pp. 1–10. [Google Scholar]
- [12].Sachi S, Ferdous J, Sikder MH, Hussani SMAK. Antibiotic residues in milk: past, present, and future. J Adv Vet Anim Res. 2019;6(3):315–32. doi: 10.5455/javar.2019.f350. https://doi.org/10.5455/javar.2019.f350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Feutz M. What is the withdrawal period of antibiotics, and why does it matter? 2017. [Jul 3;2019 ]. Available via https://www.myfearlesskitchen.com/antibiotics-withdrawal-period/)
- [14].Haskell SR, Gehring R, Payne MA, Craigmill AL, Webb AI, Baynes RE, et al. Update on FARAD food animal drug withholding recommendations. J Am Vet Med Assoc. 2003;223(9):1277–8. doi: 10.2460/javma.2003.223.1277. https://doi.org/10.2460/javma.2003.223.1277. [DOI] [PubMed] [Google Scholar]
- [15].Martínez-Cortés I, Rosiles R, Gutierrez L, Díaz D, Sumano H. Amoxicillin resides in milk of holstein cows with double or triple intramammary administration of amoxicillin-potassium clavulanate. Int J Appl Res Vet Med. 2014;12(3):186–92. [Google Scholar]
- [16].Mahmood T, Abbas M, Ilyas S, Afzal N, Nawaz R. Quantification of fluoroquinolone (enrofloxacin, norfloxacin and ciprofloxacin) residues in cow milk. Int J Chem Biochem Sci. 2016;10:10–5. [Google Scholar]
- [17].Jayalakshmi K, Paramasivam M, Sasikala M, Tamilam TV, Sumithra A. Review on antibiotic residues in animal products and its impact on environments and human health. J Entomol Zool Stud. 2017;5(3):1446–51. [Google Scholar]
- [18].Rossi R, Saluti G, Moretti S, Diamanti I, Giusepponi D, Galarini R. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: a review. Food Addit Contam: Part A. 2018;35(2):241–57. doi: 10.1080/19440049.2017.1393107. https://doi.org/1 0.1080/19440049.2017.1393107. [DOI] [PubMed] [Google Scholar]
- [19].Isidori M, Lavorgna M, Nardelli A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci Total Environ. 2005;346:87–98. doi: 10.1016/j.scitotenv.2004.11.017. https://doi.org/10.1016/j.scitotenv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- [20].Hassani M, Lazaro R, Perez C, Condon S, Pagan R. Thermostability of oxytetracycline, tetracyclines, and doxycycline at ultrahigh temperatures. J Agric Food Chem. 2008;56:2676–80. doi: 10.1021/jf800008p. https://doi.org/10.1021/jf800008p. [DOI] [PubMed] [Google Scholar]
- [21].Sarker YA, Hasan MM, Paul TK, Rashid SZ, Alam MN, Sikder MH. Screening of antibiotic residues in chicken meat in Bangladesh by thin layer chromatography. J Adv Vet Anim Res. 2018;5(2):140–5. https://doi.org/10.5455/javar.2018.e257. [Google Scholar]
- [22].Hosen SZ, Kamal AM, Barua S, Anwar MS, Mazumder K, Kawsar MH, et al. Detection of residual antibiotics in fresh cow milk. Bangladesh Pharm J. 2010;13(2):64–6. [Google Scholar]
- [23].Chowdhury S, Hassan MM, Alam M, Sattar S, Bari MS, Saifuddin AKM, et al. Antibiotic residues in milk and eggs of commercial and local farms at Chittagong, Bangladesh. Vet World. 2015;8(4):467–71. doi: 10.14202/vetworld.2015.467-471. https://doi.org/10.14202/vetworld.2015.467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mehran MA, Hossein B, Masoud A, Ashraf-o-sadat N, Mahboob N. Simultaneous determination of tetracyclines residues in bovine milk samples by solid phase extraction and HPLC-FL method. Bio Pharm Bull. 2011;1:34–9. doi: 10.5681/apb.2011.005. https://doi.org/10.5681/apb.2011.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Loksuwan J. The effect of heating on multiple residues of tetracyclines in milk. Thammasat Int J Sci Tech. 2002;7:17–21. [Google Scholar]
- [26].Kellnerová E, Navrátilová P, Borkovcová I. Effect of pasteurization on the residues of Tetracyclines in milk. Acta Vet Brno. 2015;83(10):21–6. https://doi.org/10.2754/avb201483s10s21. [Google Scholar]
- [27].Hsieh MK, Shyu CL, Ligo JW, Franje CA, Huang YJ, Chang SK, et al. Correlation analysis of heat stability of veterinary antibiotics by structural degradation, changes in antimicrobial activity and genotoxicity. Vet Med. 2011;56(6):274–85. https://doi.org/10.17221/1548-vetmed. [Google Scholar]


