Abstract
Objective:
This study was considered to explore the possible impacts of drinking water quality from different sources on the bioavailability of doxycycline.
Materials and Methods:
Sixty-four tap and ground drinking water samples collected from poultry farms were scrutinized for their water quality limits (TH, pH, total dissolved solids, electrical conductivity, Cl−, Ca+2, Na+, and Mg+2) and heavy metals concentrations (Zn, Fe, Cu, and Ni). An in vitro study was conducted by adding the therapeutic concentrations of doxycycline to all tested water samples, and allowed to interact for 1 h, 3 h, 5 h, and 8 h followed by re-estimation of doxycycline concentrations after each contact time using thin layer chromatography.
Results:
The therapeutic concentration of doxycycline was decreased in tap water samples by 1.92%, 9.63%, 22.42%, and 30.83% for the aforementioned contact times, respectively, while the corresponding reduction percentages in ground water samples were 2.14%, 17.14%, 28.57%, and 40.09%. However, the control samples had never showed any recorded decrease in their doxycycline concentrations overall contact times. All measured concentrations of doxycycline were significantly lower in tap and ground water than those of control at all times of contact. Both pH, Mg+2 showed significant positive correlations with decreasing values of doxycycline in water.
Conclusion:
Different drinking water sources reduce the concentrations of doxycycline in vitro in a time dependent manner, which can be attributed to their different physico-chemical parameters, i.e., pH and Mg+2 ions. This emphasizes the role of water quality on the stability of antibiotics concentrations administrated via drinking water.
Keywords: Antibiotic stability, bio-availability, doxycycline, metals and water quality
Introduction
Antibiotics are administrated to different poultry species for controlling multiple infectious and dangerous diseases, improve food conversion rate, and to haste the bird growth [1,2]. Various classes of antibiotics, such as tetracyclines (TC) are commonly used in veterinary medicine especially in poultry farms [3] Doxycycline is a member of the important semisynthetic TC, it referred as “typical tetracycline.” It exhibits bacteriostatic action by means of interacting with bacterial ribosomes and inhibits a miscellany of discrete phases in cellular synthesis of protein [4–6].
Water is one of the superlative, common, and economic applied methods for giving antibiotics to different types of birds. It provides cost-effective solution, safe administration, and rapid distribution to all the birds in the primary junctures of disease, and facilitates dosage or drug change [7]. However, administration of antibiotics via drinking water has few disadvantages related to water quality, for examples, to be salubrious, with neutral pH level and appropriate hardness value besides the physicochemical possessions of medicament, including their degree of solubility in water, and combination with multiplicity of free ions that present in water forming new complexes [8].
Due to low water quality in the developing countries, antibiotics are used in higher doses than the label dosage, hoping to reach the optimal needed concentration in bodies of the treated animals [9,10] Frequent exposure to sub-optimal doses of antibiotics not only increases the possibility of increasing antibiotic residue in animal tissues, but also it can interfere with the action and pharmacokinetics of other antibacterial agents as well. Recently, both antibiotics’ fate and biological influences are extensively studied all over the world due to their prevalent existence in the surrounding environment [11–13].
Interestingly, the elemental composition of water was previously approved to have a great influence on the precipitation, biological availability, and adsorption of various antibiotics, including tetracycline inside the bodies of treated animals and birds, while there were only few studies concerned with their in vitro effects [14–16]. Recently, reduced efficacy of some antibiotics, including tetracycline that administrated in drinking water, was observed in the area of the study. The reduced drug efficacy was attributed to the low water quality which may influence the stability and bioavailability of tetracycline. However, it is clearly known that data documenting the in vitro effect of water parameters, such as water chemistry and heavy metals loads on doxycycline stability, are still scarce so far. Therefore, and for the first time, the purpose of this work was to explore the in vitro impact of the quality of drinking water from different water sources on the bioavailability of doxycycline, the widely used medication in veterinary medicine.
Materials and Methods
This study was conducted in Assiut Governorate, Egypt, where the examined water samples were collected from different sites, including Mankabad, Abnob, Manfalout, El-Kosia and Dairout, during the period from February till August 2018.
Sampling
Water samples from different sources (taps and ground water) were gathered from multiple poultry farms that are located in Assiut Governorate, Egypt. Representative water samples were filled into dark glass bottles, each of 1 l capacity, provided with screw capped stopper. The glass bottles were carefully washed, rinsed using distilled water, and finally sterilized. Water samples were numbered, cooled at 4°C, and carried to the laboratory in an ice box till experimental investigations within 6 h [17].
Types of water samples
A total number of 64 water samples from different sources and localities in Assiut Governorate were collected and examined. Water samples (n = 32) were collected directly from taps from the inside of poultry farms, and another 32 groundwater samples (hand pump wells) were collected from multiple wells, that are located beside to some poultry farms, where their waters are used for poultry drinking. Different sites of sampling at Assiut Governorate were detailed in Figure 1.
Figure 1. Localities of the selected sampling sites on Google map.
Water estimation and analysis
The protocol of the conducted study divided into analytical and experimental works. The analytical work included estimation of some water quality parameters and heavy metals concentrations and antibiotic (doxycycline) residues. The experimental work included the studying of the possible in vitro influence of water quality, characteristics, and heavy metals concentrations on doxycycline stability in all tested drinking water samples of both tap and ground water sources.
Estimation of water quality parameters
Estimation of some water quality parameters included pH, total hardness (T.H), chloride (Cl−), total dissolved solids (TDS), calcium ions (Ca+2), magnesium ions (Mg+2), sodium ions (Na+), and electrical conductivity (EC). Values of pH were estimated by using pH meter (model JWNWAY 3505). Electrical conductivity (dc/m) was also estimated by conductivity meter (HI 9835-Italy). TDS, total water hardness were estimated by Lovibond Microprocessor Multi-direct Photometer. Free ions concentrations (mg/l), including chloride (Cl−), calcium ions (Ca+2), magnesium ions (Mg+2), and sodium ions (Na+), were estimated by Flame Photometer Model M 360 according to Costanzo and Windhager [18] in laboratories of Soil and Water Department, Faculty of Agriculture, Assiut University, Egypt.
Estimation of heavy metals concentrations
Samples preparation and digestion were done according to Chau and Lum [19]. Heavy metals analysis of iron (Fe), Zinc (Zn), nickel (Ni), and copper (Cu) was performed according to Tewari and Singh [20] by using Flame Atomic Absorption Spectrophotometer (Perkin-Elmer Atomic Absorption Spectrophotometer model A Analyst 400). Ultimately, the concentrations of Fe, Cu, Ni, and Zn in all examined samples were calculated according to Horwitz et al. [21].
Estimation of doxycycline antibiotic residues in all tested water samples
Each water sample was examined to estimate the possible residues of doxycycline by thin layer chromatography (TLC) in the Drug Researches Center, Assiut University according to Thangadurai et al. [22].
The experimental work
Doxycycline (Pfizer Egypt-Pharma) was added separately in the therapeutic doses to all tested water samples that previously had been estimated for antibiotic residues, water quality parameters, and heavy metals concentrations. These samples were allowed to interact (combine) for four specific contact times which were 1 h, 3 h, 5 h, and 8 h at room temperature and indoor light. After each contact time, the antibiotic concentration was re-evaluated using several mobile phases for the individual identification of tetracycline [23] using thin TLC. Control samples, which include the therapeutic dose of doxycycline (300 mg) dissolved in bi-distilled water, were prepared and measured in the same time and conditions. All samples were estimated in triplicates.
Statistical analysis
All data was assembled in the Microsoft Excel sheet, and then analyzed using IBM SPSS Statistics for Windows version 25 [24]. Quantitative data were presented as means ± standard deviation, while qualitative data were expressed as number and percentage. Shapiro–Wilk test and the nonparametric Mann–Whitney test were used for testing of data normality. Moreover, for data which wasn’t showing normal distribution, Friedman one-way analysis of variance test, and Spearman’s correlation were used, while independent samples T test was used for normally distributed data. The level of significance was at 5% in all applied statistical tests in the study.
Results
Most of the estimated water quality characteristics and metals concentrations were recorded in higher values in ground water than in tap water, with only a statistical significance in sodium ions concentration. Concerning heavy metals, iron was recorded in higher values in groundwater samples, while both Cu and Zn were recorded in higher values in tap water samples with no statistical significance. Nickel was not detected in any of examined water samples in both examined water sources. Doxycycline residue wasn’t recorded in any of the tested water samples as shown in Table 1.
Table 1. Mean values of water quality parameters, heavy metals concentration and doxycycline residues in different water sources.
| Water source | Water quality parameters & Heavy metals concentration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC (dc/m) | T.D.S (mg/l) | T.H (mg/l) | Ca+2 (mg/l) | Mg+2 (mg/l) | Na+ (mg/l) | Cl− (mg/l) | Ni (mg/l) | Fe (mg/l) | Cu (mg/l) | Zn (mg/l) | Doxycycline residues | |
| Tap water | 8.27 ± 0.24 | 0.69 ± 0.33 | 438.89 ± 209.55 | 208.75 ± 66.86 | 77.5 ± 50.36 | 131.25 ± 67.07 | 67.93 ± 52.86 | 53.25 ± 38.14 | ND | 4.41 ± 2.29 | 0.52 ± 0.06 | 0.59 ± 0.32 | 00 ± 00 |
| Ground water | 8.53 ± 0.35 | 1.05 ± 0.33 | 617.54 ± 267.67 | 264.28 ± 89.41 | 52.85 ± 11.12 | 211.42 ± 92.81 | 156.18 ± 78.76 | 49.68 ± 19.63 | ND | 6.47 ± 3.58 | 0.519 ± 0.073 | 0.48 ± 0.32 | 00 ± 00 |
| p-value | 0.120 | 0.51 | 0.120 | 0.193 | 0.229 | 0.075 | 0.023* | 0.828 | — | 0.201 | 0.889 | 0.500 | — |
ND = Not Detected;
p-value < 0.05 is statistically significant.
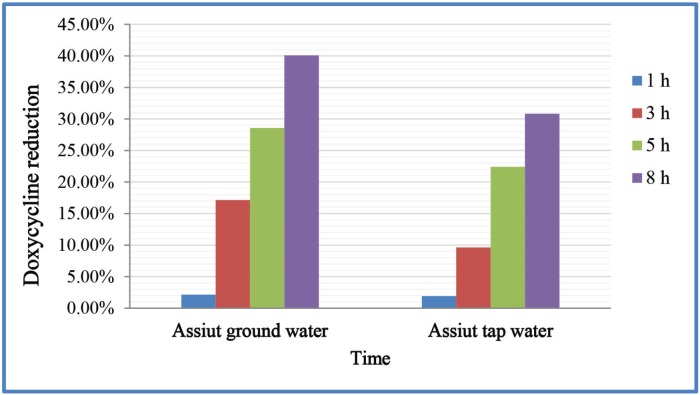
Unlike the control samples, which remained as the same initially spiked antibiotic concentration, doxycycline concentrations were significantly decreased in both tap water and ground water samples at 1 h, 3 h, 5 h, and 8 h of contact time (Table 2). The decreasing percentages was 1.92%, 9.63%, 22.42%, and 30.83%, respectively, for tap water samples compared to a reduction of 2.14%, 17.14%, 28.57%, and 40.09% for ground water, respectively (Fig. 2). Interestingly, no significant difference between the examined water sources after any of the tested contact times in decreasing doxycycline concentrations was detected. Ground water samples showed non-significant higher reduction in concentration of doxycycline than tap water samples after 8 h of contact time (Table 2).
Table 2. Statistical analysis of doxycycline profile after several times adding in different water sources.
| Watersources | Mean ± S.D. | Mean ± S.D. of doxycycline concentrations (mg/l) | |||
|---|---|---|---|---|---|
| p value | After 1 h | After 3 h | After 5 h | After 8 h | |
| Tap water | Mean ± S.D. | 294.3 ± 0.81 | 271.1 ± 1.41 | 232.8 ± 1.66 | 207.5 ± 1.78 |
| p-value | 0.047* | 0.035* | 0.034* | 0.033* | |
| Ground water | Mean ± S.D. | 293.5 ± 0.74 | 248.5 ± 1.36 | 214.2 ± 1.76 | 179.7 ± 1.98 |
| p-value | 0.005* | 0.000* | 0.001* | 0.001* | |
| Control (therapeutic dose) | Mean ± S.D. | 300 ± 0 | 300 ± 0 | 300 ± 0 | 300 ± 0 |
p-value < 0.05 is statistically significant.
Figure 2. Reduction (%) of doxycycline therapeutic doses in different water sources after several times.
Statistical correlation between water quality parameters, heavy metals concentrations, and decreasing values of doxycycline’s concentration showed a significant correlation between water pH and the decreasing value of doxycycline in both tap and ground water samples. Meanwhile, magnesium ions concentration was only significantly correlated with doxycycline reduction percent in tap water samples. However, other estimated parameters showed non-significant correlations with the decreased doxycycline’s concentration (Table 3).
Table 3. Statistical correlations between the decrease in doxycycline therapeutic concentration and different water parameters in different water sources.
| Water parameters | Tap water | Ground water | ||
|---|---|---|---|---|
| R | p-value | R | p-value | |
| pH | 0.772 | 0.025* | 0.685 | 0.05* |
| Electrical conductivity (EC) (dc/m) | 0.312 | 0.451 | 0.180 | 0.699 |
| Total dissolved solids (TDS) (μg/l) | 0.309 | 0.457 | 0.523 | 0.229 |
| Total Hardness (TH) (μg/l) | 0.495 | 0.213 | −0.064 | 0.892 |
| Calcium ions (Ca+2) (μg/l) | −0.234 | 0.576 | −0.389 | 0.388 |
| Magnesium ions (Mg+2) (μg/l) | 0.828 | 0.011* | −0.110 | 0.814 |
| Sodium ions (Na+) (μg/l) | 0.309 | 0.457 | 0.227 | 0.624 |
| Chloride (Cl−) (μg/l) | 0.207 | 0.623 | 0.064 | 0.891 |
| Nickle (Ni) (μg/l) | – | – | – | – |
| Iron (Fe) (μg/l) | −0.206 | 0.625 | −0.378 | 0.403 |
| Copper (Cu) (μg/l) | −0.051 | 0.904 | −0.018 | 0.969 |
| Zinc (μg/l) | 0.077 | 0.856 | 0.234 | 0.613 |
p-value < 0.05 is statistically significant.
Discussion
It was scientifically approved that the drinking water quality affects both bioavailability and absorption of antibiotics inside birds and animals’ bodies; however, there is a lack of data that focused on the in vitro effects of drinking water quality on the bioavailability and stability of different antibiotics. Water that is used in poultry farms as a medium for antibiotic administration should be carefully considered, especially in terms of their physical and chemical properties and their correlation with the stability of antibiotics.
As shown in Table 1, water pH in tap and ground water samples was alkaline, with values slightly around the maximum safe limits approved by APHA [17]. The current estimated pH values were more or less in agreement with Ramakrishnaiah et al. [25], Vasanthavigar et al. [26], and Mohebbi et al. [27].
Furthermore, in a comparison of the obtained results with the listed standers of WHO [28] it was found that water hardness and magnesium ions concentration exceeded the permissible limits in both tap and ground water samples. However, total dissolved solids in tap water were within the safe limits. Additionally, EC, Na+, Cl−, and Ca+2 ions concentrations were within the listed permissible limits in both sources of water.
Concerning heavy metals analysis, only iron values surpassed the listed permissible limits by WHO [29]. The iron values in both tap and ground water samples were higher than those obtained by Vasanthavigar et al. [26], while Cu concentration was lower than that estimated by Authman and Abbas [30]. In this context, high values of iron in drinking water causes rapid and shallow respiration, respiratory failure, and cardiac arrest in poultry [31].
Interestingly, the therapeutic doses of doxycycline that were added to the different water samples for several contact times were gradually decreased in an ascending manner from the 1st hour of contact time till the 8th hour thereafter, no further decrease of doxycycline was recorded in both tap and ground water samples. The reduction percent of doxycycline concentration was observed in different manner, with a higher reduction (40.09%) in ground water samples than its initial concentration. However, tap water samples showed lower decreasing percent (30.83%). This finding was lower than those of Marx et al. [32] who added doxycycline to tap water, and found a precipitate that quickly settled to the bottom of the water bottle. The authors observed high degradation value of doxycycline concentration in tap water samples where approximately 30% of doxycycline concentrations were remained stable at the 7th day of the experiment.
Comparatively, under the same condition of temperature and indoor light, the control samples showed stable concentrations over the time of experiment and even after 8 h of contact time. This emphasized that the type of water and its characteristics greatly played a critical role in decreasing the concentration of doxycycline in vitro.
In both examined water sources, the reduction of doxycycline concentration could be attributed to many factors. First, in this study, pH values of both types of water were significantly and positively correlated with the decreasing percent of doxycycline concentration. Higher pH values could greatly affect the degree of ionization and speciation of doxycycline, and hence affected its concentration [15]. The adverse impacts of pH values on the stability of doxycycline in water samples was agreed with Pioletti et al. [33], Sunaric et al. [34], Tsai et al. [16], Carlotti et al. [35], Kołodyńska et al. [17], Marx et al. [32], and Liu et al. [20]. Second, magnesium ions (Mg+2) in tap water samples was also significantly correlated with the reduction % of doxycycline, where higher magnesium ions had a great affinity to form complexes with tetracycline and extremely change the form of antibiotics [15].
Many previous studies confirmed the effect of water pH and some of free ions concentrations on the absorption of doxycycline inside the animal body, while the current results showed another similar in vitro effect of both pH and Mg+2 ions concentrations on the bioavailability of doxycycline in drinking water samples of different characteristics and from different sources.
The possible effect of heavy metals values on doxycycline concentration was still not clear; this is based on the obtained data for all estimated metals (iron, copper, and zinc) in both tap and ground water samples. In this perception, the results showed a non-significant statistical correlation with the decrease of doxycycline therapeutic concentration in vitro. This result disagreed with those of Andreu [36], who found that doxycycline had a potential interaction with heavy metals concentrations in different water samples.
Ultimately, the findings of this study prompt questions regarding the rationale of the contemporary practice of adding doxycycline to the drinking water of poultry for antibacterial treatment. Therefore, avoidance of a long-time exposure of doxycycline in drinking water in addition to using good quality water to avoid decreasing the therapeutic concentrations of doxycycline must be considered.
Future studies on water chemistry and its possible impacts on antibiotics concentrations that are administrated via drinking water are highly required. Studies should include different water sources, characteristics and other groups of antibiotics. Specific advanced both drug and water researches to overcome these in vitro effects must be evolved; this will allow setting up of general, safe guidelines for drinking water characteristics that are advisable to be used for administration of different antibiotics in poultry farming.
Conclusion
The outcomes of this work revealed that different sources of water had different chemistry that possibly affects on the stability of antibiotic. Whereby, increasing the time of contact between doxycycline and drinking water resulted in decreased its concentration up to 40.09% and 30.83% in ground and tap waters, respectively. Additionally, therapeutic doses of doxycycline in drinking water samples were reduced in a time dependent manner till 8 h of contact time thereafter, no further decrease was recorded in both tap and ground water samples. These findings were attributed to the great variances in water physico-chemical parameters, especially water pH and its Mg+2 ions contents that adversely interact with doxycycline and resulted in reduced its concentration in drinking water samples.
This study was the first of its kind to examine the in vitro impacts of water quality of poultry farms on doxycycline concentration. The study showed that increasing the time of contact between doxycycline and drinking water definitely decreases its concentration. This work will aid the researches to expose the critical portions of the chemical interactions between drinking water chemistry and antibiotic stability. This will help in using the antibiotic in an accurate concentration and avoid getting suboptimal antibiotic dosages inside the bird’s body otherwise; medication failure or antibiotic resistance could be the final obtained consequence.
Acknowledgment
This study did not accept any precise funding from any agencies in the public or commercial sectors.
Conflict of interest
All authors of the study declare that there is no conflict of interest with regards to the publication of this manuscript.
Authors’ contribution
This study was conducted in collaboration between all the authors. Saber Kotb, Mostafa Ahmed got the concept of the study, critically revised the manuscript for important intellectual content, Dalia Hassan, designed the study, interpreted the data, wrote the manuscript and participated in its revision. Esraa Soltan collected the data and contributed to manuscript preparation. A final approval for publishing of the last revised copy was given by all authors.
References
- [1].Castanon JIR. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86:2466–71. doi: 10.3382/ps.2007-00249. https://doi.org/10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- [2].Fasina YO, Newman MM, Stough JM, Liles MR. Effect of clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult Sci. 2015;95:247–60. doi: 10.3382/ps/pev329. https://doi.org/10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- [3].Lindberg R, Jarnheimer PA, Olsen B, Johansson M, Tysklind M. Determination of antibiotic substances in hospital sewage water using solid phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere. 2004;57(10):1479–88. doi: 10.1016/j.chemosphere.2004.09.015. https://doi.org/10.1016/j.chemosphere.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [4].Sohmen D, Harms JM, Schlunzen F, Wilson DN. Enhanced SnapShot: antibiotic inhibition of protein synthesis II. Cell. 2009;139(1):212–12. doi: 10.1016/j.cell.2009.08.009. https://doi.org/10.1016/j.cell.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [5].Sohmen D, Harms J, Schlunzen F, Wilson DN. Cell. 2009. SnapShot: antibiotic inhibition of protein synthesis I; p. 138. https://doi.org/10.1016/j.cell.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [6].Wilson D. The A-Z of bacterial translation inhibitors. Crit Rev Biochem Mol Biol. 2009;44:393–433. doi: 10.3109/10409230903307311. https://doi.org/10.3109/10409230903307311. [DOI] [PubMed] [Google Scholar]
- [7].Landoni MF, Albarellos G. The use of antimicrobial agents in broiler chickens. Vet J. 2015;205(1):21–7. doi: 10.1016/j.tvjl.2015.04.016. https://doi.org/10.1016/j.tvjl.2015.04.016. [DOI] [PubMed] [Google Scholar]
- [8].Gbylik-Sikorska M, Posyniak A, Śniegocki T, Sell B, Gajda A, Tomczyk G, et al. Effect of doxycycline concentrations in chicken tissues as a consequence of permanent exposure to enrofloxacin traces in drinking water. Vet Res. 2016;60(3):293–9. https://doi.org/10.1515/jvetres-2016-0045. [Google Scholar]
- [9].Dosoky R, Kotb S, Farghali M. Efficiency of silver nanoparticles against bacterial contaminants isolated from surface and ground water in Egypt. J Adv Vet Anim Res. 2015;2(2):175–84. https://doi.org/10.5455/javar.2015.b79. [Google Scholar]
- [10].Okocha R, Olatoye I, Adedeji O. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018;39:21. doi: 10.1186/s40985-018-0099-2. https://doi.org/10.1186/s40985-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cizmas L, Sharma VK, Gray CM, McDonald TJ. Pharmaceuticals and personal care products in waters: occurrence, toxicity, and risk. Environ Chem Lett. 2015;13(4):381–94. doi: 10.1007/s10311-015-0524-4. https://doi.org/10.1007/s10311-015-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De Jesus Gaffney V, Almeida CM, Rodrigues A, Ferreira E, Benolie MJ, Cardoso VV. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015;72:199–208. doi: 10.1016/j.watres.2014.10.027. https://doi.org/10.1016/j.watres.2014.10.027. [DOI] [PubMed] [Google Scholar]
- [13].Evgenidou EN, Konstantinou IK, Lambropoulou DA. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters. A review. Sci Total Environ. 2015;505:905–26. doi: 10.1016/j.scitotenv.2014.10.021. https://doi.org/10.1016/j.scitotenv.2014.10.021. [DOI] [PubMed] [Google Scholar]
- [14].Kołodyńska D, Wnętrzak R, Leahy JJ, Hayes MHB, Kwapiński W, Hubicki Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem Eng J. 2012;197:295–305. https://doi.org/10.1016/j.cej.2012.05.025. [Google Scholar]
- [15].Liu S, Xu W, Liu Y, Tan X, Zeng GX, Cai X. Facile synthesis of Cu (II) impregnated biochar with enhanced adsorption activity for the removal of Doxycycline hydrochloride from water. Sci Total Environ. 2017;592:546–53. doi: 10.1016/j.scitotenv.2017.03.087. https://doi.org/10.1016/j. scitotenv.2017.03.087. [DOI] [PubMed] [Google Scholar]
- [16].Tsai W, Huang T, Chen H, Huang J, Hsue M, Chuang H, et al. Determination of tetracycline’s in surface water and milk by the magnesium hydroxide coprecipitation method. J Chromatogr. 2010;1217(3):415–8. doi: 10.1016/j.chroma.2009.12.006. https://doi.org/10.1016/j.chroma.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [17].APHA (American Public health Association) 21th. APHA Inc; Washington, DC: 2005. Standard methods for the examination of water and waste water. Available via https://www.worldcat.org/title/standard-methods-for-the-examination-of-water-and-wastewater/oclc/156744115. [Google Scholar]
- [18].Costanzo LS, Windhager EE. Effects of PTH, ADH, and cyclic AMP on distal tubular Ca and Na reabsorption. Am J Physiol Renal Physiol. 1980;239(5):478–85. doi: 10.1152/ajprenal.1980.239.5.F478. https://doi.org/10.1152/ajprenal.1980.239.5.F478. [DOI] [PubMed] [Google Scholar]
- [19].Chau YK, Lum SCK. Determination of label and strongly bound metals in lake. Water Res. 1974;8:383–8. https://doi.org/10.1016/0043-1354(74)90052-9. [Google Scholar]
- [20].Tewari P, Singh A. Thiosalicylic acid-immobilized Amberlite XAD-2: metal sorption behaviour and applications in estimation of metal ions by flame atomic absorption spectrometry. Anal J. 2000;125(12):2350–5. doi: 10.1039/b006788l. https://doi.org/10.1039/b006788l. [DOI] [PubMed] [Google Scholar]
- [21].Horwitz W, Albert R, Deutsch MJ. Guidelines for collaborative study of procedure to validate characteristics of a method of analysis. AOAC official methods of analysis. (17th) 2000:1–11. appendix D. Available via www.aoac.org/aoac_prod_imis/.../3.5SMPRGuidelinev12.1.pdf. [Google Scholar]
- [22].Thangadurai S, Shukla S, Anjaneyulu Y. Separation and detection of certain β-lactam and fluoroquinolone antibiotic drugs by thin layer chromatography. Anal Sci J. 2002;18(1):97–100. doi: 10.2116/analsci.18.97. https://doi.org/10.2116/analsci.18.97. [DOI] [PubMed] [Google Scholar]
- [23].British Pharmacopoeia. III. British Pharmacopoeia Commission; London, UK: 2012. Formulated preparations: specific monographs, Tetracycline capsules. Available via https://www.pharmacopoeia.com/Catalogue/ProductDetails?productid=1000018792&page=1&pageSize=20&searchText=&startsWith=T. [Google Scholar]
- [24].Field A. SAGE Publications Ltd; Thousand Oaks, CA: 2013. Discovering statistics using IBM SPSS statistics. Available via https://uk.sagepub.com/en-gb/eur/discovering-statistics-using-ibm-spss-statistics/book257672. [Google Scholar]
- [25].Ramakrishnaiah C, Sadashivaiah C, Ranganna G. Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. J Chem. 2009;6(2):523–30. https://doi.org/10.1155/2009/757424. [Google Scholar]
- [26].Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi R, Chidambaram S, Anandhan P, et al. Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess. 2010;171(1–4):595–609. doi: 10.1007/s10661-009-1302-1. https://doi.org/10.1007/s10661-009-1302-1. [DOI] [PubMed] [Google Scholar]
- [27].Mohebbi M, Saeedi R, Montazeri A, Vaghefi K, Labbafi S, Oktaie S, et al. Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI) Ecol Indic. 2013;30:28–34. https://doi.org/10.1016/j.ecolind.2013.02.008. [Google Scholar]
- [28].WHO (World Health Organization) Iron in drinking-water: Background document for development of WHO guidelines for drinking-water quality. 2003. Available via http://www.who.int/water_sanitation_health/dwq/chemicals/iron.pdf.
- [29].WHO (World Health Organization) Prepared and Published by the United Nations Environment Programme Global Environmental Monitoring System/Water Program; 2007. Water Quality for Ecosystem and Human Health & Global Drinking Water Index Development and Sensitivity Analysis Report. Available via http://wwd.unwater.org/2010/downloads/water_quality_human_ health.pdf. [Google Scholar]
- [30].Authman HM, Abbas HH. Accumulation and distribution of copper and zinc in both water and some vital tissues of two fish species(Tilapia zillii and Mugil cephalus) of Lake Qarun, Fayoum Province, Egypt. Pak J Biol Sci. 2007;10(13):2106–22. doi: 10.3923/pjbs.2007.2106.2122. https://doi.org/10.3923/pjbs.2007.2106.2122. [DOI] [PubMed] [Google Scholar]
- [31].WHO. 4th. World Health Organization; Geneva, Switzerland: 2011. Guideline for drinking water quality recommendations. ISBN Available via https://apublica.org/wp-content/uploads/2014/03/Guidelines-OMS-2011. [Google Scholar]
- [32].Marx J, Vudathala D, Murphy L, Rankin S, Hankenson F. Antibiotic administration in the drinking water of mice. J Am Assoc Lab Anim Sci. 2014;53(3):301–6. [PMC free article] [PubMed] [Google Scholar]
- [33].Pioletti M, Schlünzen F, Harms J, Zarivach R, Glühmann M, Avila H, et al. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO. 2001;20(8):1829–39. doi: 10.1093/emboj/20.8.1829. https://doi.org/10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sunaric S, Mitic S, Miletic G, Pavlovic A, Naskovic-Djokic D. Determination of Doxycycline in pharmaceuticals based on its degradation by Cu (II)/H 2 O 2 reagent in aqueous solution. J Anal Chem. 2009;64(3):231–7. https://doi.org/10.1134/S1061934809030046. [Google Scholar]
- [35].Carlotti B, Cesaretti A, Elisei F. Complexes of tetracyclines with divalent metal cations investigated by stationary and femtosecond-pulsed techniques. Phys Chem Chem Phys. 2012;14(2):823–34. doi: 10.1039/c1cp22703c. https://doi.org/10.1039/C1CP22703C. [DOI] [PubMed] [Google Scholar]
- [36].Andreu V, Gimeno-García E, Pascual A, Vázquez-Roig P, Picó Y. Presence of pharmaceuticals and heavy metals in the waters of a Mediterranean coastal wetland: Potential interactions and the influence of the environment. Sci Total Environ. 2016;540:278–86. doi: 10.1016/j.scitotenv.2015.08.007. https://doi.org/10.1016/j.scitotenv.2015.08.007. [DOI] [PubMed] [Google Scholar]