Abstract
Urea transporters (UTs) are membrane proteins in the urea transporter protein A (UT-A) and urea transporter protein B (UT-B) families. UT-B is mainly expressed in endothelial cell membrane of the renal medulla and in other tissues, including the brain, heart, pancreas, colon, bladder, bone marrow, and cochlea. UT-B is responsible for the maintenance of urea concentration, male reproductive function, blood pressure, bone metabolism, and brain astrocyte and cardiac functions. Its deficiency and dysfunction contribute to the pathogenesis of many diseases. Actually, UT-B deficiency increases the sensitivity of bladder epithelial cells to apoptosis triggers in mice and UT-B-null mice develop II-III atrioventricular block and depression. The expression of UT-B in the rumen of cow and sheep may participate in digestive function. However, there is no systemic review to discuss the UT-B functions. Here, we update research approaches to understanding the functions of UT-B.
Keywords: Urea, Urea transporter proteins, UT-B, Kidd blood group, UT-B-null mice
Introduction
Urea is an organic compound with two NH2 groups linked by a carbonyl. Urea has high water-solubility and low fat-solubility. Urea is produced in the liver and concentrates in the kidney for nitrogen excretion in mammals. Although urea is not charged, it has a strong dipole moment, which makes it impossible to penetrate through non-polar lipid membranes. Hence, urea depends on its specific transporters to pass through the cell membranes.
At present, some genes for urea transporters (UTs) have been cloned from human, rabbit, mice, and Xenopus, and they include the SLC14a1 gene for two subtypes of UT-Bs and the SLC14a2 gene for six subtypes of UT-As due to varying splicing. Here, we review the gene, protein structure, and main functions of UT-B in different organs.
Urea transporters
UT proteins were discovered in the kidney medullary collecting ducts of rabbits and mice, and the SLC14a1 for UT-B (B1, B2) was cloned from the renal medulla of rabbits and from human bone marrow [19, 55]. Subsequently, the SLC14a2 gene for UT-A (A1–A6) was cloned from human, rabbit, mice, and Xenopus, and UT-A1, UT-A2, and UT-A3 are sensitive to vasopressin. These genes are located in human chromosome 18 q12.1-q21.2 [31, 54, 66]. The UT-B gene has 11 exons, of which exons 4–11 contain the coding sequence for the UT-B of 30 kDa. UTs are widely expressed in mammals. While the UT-A is expressed mainly in human kidney and other organs, including the testis, colon, and liver, the UT-B is highly expressed in human erythrocytes and many other organs (Table 1) [70]. Urea transporters are N-linked glycosylated proteins with a unique hydrophobic character. Human UT-B1 is a glycosylated protein of 46–60 kDa in most organs, except for a 41–54-kDa protein in the kidney, but it is deglycosylated as a 36-kDa protein in red blood cells. Similar tissue-specific variates in the degrees of glycosylation are observed in other species. For example, the rat glycosylated UT-B1 protein is 32 kDa in the brain while it is 45–55 kDa in the kidney [77]. Physiologically, UTs function to be responsible for urea transportation to maintain its concentration, cell homeostasis, and nitrogen balance. Moreover, UTs are important regulators of male reproductive function, blood pressure, bone metabolism, and brain astrocyte and cardiac functions. However, the available studies on the action of UT-B in the pathogenesis of varying diseases remain highly descriptive, and the functional mechanisms of UT-B currently remain elusive. Here, we provide a brief summary of the known functions of UT-B.
Table 1.
Mammalian urea transporter gene
| Gene | Chromosome | Isoform | RNA (kb) | Protein (kDa) | Cloned from | Tissue location | Inhibitors | References |
|---|---|---|---|---|---|---|---|---|
| Slc14a1 | 18 q12.1-q21.2 | UT-B1 | 3.8 | 43 | Human, rabbit, rat, mouse, Xenopus | Erythrocytes, brain, lung, heart, pancreas, colon, small intestine, prostate, kidney, bladder, skeletal muscle, bone marrow, cochlea | Phloretin, dimethylurea, acrylamide, methylurea, thiourea, methylformamide, PCMBS | [5, 55, 66, 92] |
| UT-B2 | 3.7 | 43–54 | Sheep, cow | Rumen | [66, 80] | |||
| Slc14a2 | 18 q12.1-q21.2 | UT-A1 | 4.0 | 97,117 | Human, rabbit, rat | Inner medullary collecting duct | [3, 68] | |
| UT-A1b | 3.5 | 55 | Medulla | [2] | ||||
| UT-A2 | 2.9 | Thin descending limb, liver | [57, 73, 94] | |||||
| UT-A2b | 2.5 | 44, 67 | Medulla, heart | [2, 15, 31] | ||||
| UT-A3 | 2.1 | Inner medullary collecting duct | [31, 32, 69] | |||||
| UT-A3b | 3.7 | 43 | Medulla | [2] | ||||
| UT-A4 | 2.5 | Rat* | Medulla | [31] | ||||
| UT-A5 | 1.4 | Mouse** | Testis | [19] | ||||
| UT-A6 | 1.8 | Human*** | Colon | [73] |
*Cloned from rat only
**Cloned from mouse only
***Cloned from human only
UT-B and the Kidd blood group
The Kidd blood group antigen Jk and UT-B are the same protein in erythrocytes [56]. In 1951, Mrs. Kidd gave birth to a newborn with hemolytic disease. The infant developed antibodies against a new blood group antigen, Jka, leading to the Kidd blood group [1] and its specific antibodies were identified later [61]. Currently, there are three antigens, Jka, Jkb, and Jk3, and four phenotypes, Jk (a+b-), Jk (a-b+), Jk (a+b+), and Jk (a-b-) in the Kidd blood group.
Although the Jk3-related JK (a-b-), an invalid phenotype, is rare, it is crucial for the safety of blood transfusion in humans because JK (a-b-) red blood cells are 30 times insensitive to urea lysis [28, 60]. The Jk-null phenotype is prevalent in Polynesian population, including Thailand (0.02%), Japan (0.002%), Taiwan (0.023%), China (0.008%), Chinese Han (0.019%), and Finland (0.03%) [39]. Genetic analysis indicates that the gene for Jk is located in chromosome 18q12-q21 [22] and encodes a 45-kDa glycoprotein UT-B with UT function [56, 71]. The JK-null phenotype is caused by the mutations in the UT-B splicing sites, and JK-null individuals have no functional UT-B protein, leading to a weaker urine concentrating ability. However, the absence of UT-B does not disrupt their homeostatic balance. Given the different sensitivities in gene mutation among different races, this steady state of homeostasis may not be applicable to all species. For example, aquaporin 1 (AQP1) knockout causes a severe defect in urinary concentrating in mice, whereas AQP1 deficiency does not lead to any pathological phenotype in humans. Thus, further studies are required to determine the roles of UT-B in homeostasis [48, 62, 67, 72].
The Kidd blood group is important for the safety of blood transfusion because its incompatibility can cause typical acute and delayed hemolytic transfusion reactions in newborns. According to the edition of the American Association of Blood Banks Technical Manual, there are anti-globulin or enzyme tests for the detection of Kidd antigens. The urea dissolution test for screening Jk (a-b-) phenotype is also available in America, especially in Hawaii [39, 41].
UT-B proteins
The SLC14a1 for human UT-B was first cloned from human erythrocytes and is located at the single-gene locus of chromosome 18q12.1-q21.2 [77]. It is homologous to mouse [18]. There are two SLC14a1 mRNA transcripts due to variable polyadenylation, and they are expressed for a 45-kDa protein [44]. The UT-B-mediated urea transportation is inhibited by urea analogues and other compounds, including phloretin, dimethylurea, acrylamide, methylurea, thiourea, methylformamide, and PCMBS [52].
In humans, the SLC14a1 for UT-B is not only expressed in endothelial cell membrane of the renal medulla but also in erythrocytes and other organs, including the brain, lung, heart, pancreas, colon, small intestine, prostate, kidney, bladder, skeletal muscle, bone marrow, and cochlea [79]. The wide distribution of UT-B suggests that UT-B has broad physiological functions in humans.
UT-B in human body
The urinary system
UT-B is detected in the renal epithelial cells, the ureter, and the bladder of rats. Similarly, UT-B is expressed in renal medullary descending vasa recta of mice [74]. Interestingly, genome-wide association study (GWAS) suggests that the UT-B polymorphism may be associated with the development of bladder cancer in Northern India.
The bladder can transport and store urine in mammals. High-protein diets can increase urea concentration, which may be a carcinogen for rat bladder [42, 70]. UT-B-/- mice were generated by disrupting the UT-B gene, evidenced by PCR analysis and lack of UT-B protein expression in all organs [86]. Transmission electron microscopy (TEM) analysis reveals that medullary sheath is observed in urothelial cells of UT-B-/- mice, which may cause urothelial cell shrinking, chromatin condensation, and apoptosis [91]. Microarray assay indicates that UTB knockout alters the expression of 69 genes associated with apoptosis and DNA damage in the urothelium of mice [13]. Among them, the DDB1- and CUL4-related factors 11 (Dcaf11) and MCM2-4 expression were significantly upregulated, while the ubiquitin carboxyl terminal hydrolase L1 (Uch-L1), adenovirus E1B-19K/Bcl-2 interaction protein 3 (Bnip3), and 45S pre-rRNA were downregulated. The frequency of UT-B efficiency is significantly higher than that of other transporters. Given that urea concentrations in the bladder of UT-B-/- mice are several times higher than those of wild-type mice, they can induce cell cycle arrest and apoptosis [36, 72, 92]. In the urothelium of UT-B-/- bladder, the decreased BcL-2 expression and increased caspase-3 and Bax expression, together with downregulated ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), which promotes cell proliferation and enhances anti-apoptotic signaling pathway, may underlie the mechanisms by which high concentrations of urea cause urothelial cell apoptosis.
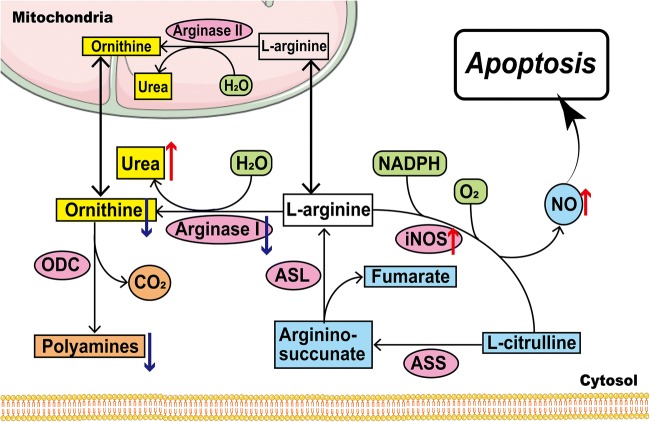
L-arginine is cleaved by arginase to produce urea and ornithine, which can be preferably converted into polyamines by ornithine decarboxylase (ODC). Interestingly, the arginase I expression is significantly downregulated in urothelial cells of UT-B-/- mice [92]. This will reduce the production of ornithine and polyamine. Previous studies have shown that polyamines, especially spermine, are responsible for regulating gene expression and maintaining the stability of DNA and chromatin [32, 33]. On the other hand, high levels of nitric oxide (NO) are detected in the bladder and urinary epithelium of UT-B-/- mice, accompanied by increased iNOS expression [91]. Actually, high concentrations of NO can cause DNA damage and cell death in NO-generating and neighboring cells [47]. Hence, UT-B deficiency can increase urea concentrations, which cause abnormal arginine metabolism and increased levels of NO, leading to DNA damage and cell apoptosis (Fig. 1).
Fig. 1.
Arginine metabolism and the urea/L-Arg/NO pathway in urothelial cell apoptosis. NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; iNOS, inducible nitric oxide synthase; ASL, argininosuccinate lyase; ASS, argininosuccinate synthase; NO, nitric oxide; NOS, nitric oxide synthase; ODC, ornithine decarboxylase
Alternatively, UT-B deficiency can enhance histone (H2AX) phosphorylation, but downregulate MCM2 expression. Given that H2AX is the indicator of DNA damage and MCM2 is an important component of MCM2-7 complex for DNA repair, their changes may also contribute to urea-induced epithelial cell apoptosis [91]. Furthermore, ataxia telangiectasia–mutated (ATM) kinase phosphorylation at SER1981 and p53 expression and phosphorylation at SER15 are significantly increased in bladder urothelium of UT-B-/- mice. These results suggest that DNA damage and apoptosis caused by the UT-B deficiency in the bladder urothelium may depend on the ATM/p53 signaling.
The reproductive system
UT-B is also expressed in the testis, mainly in Sertoli cells of the seminiferous tubules at stages II to III [20]. Sertoli cells in the seminiferous tubules function to nurture the growing sperm cells during spermatogenesis. Similarly, high arginase activity can hydrolyze arginine into urea and ornithine in Sertoli cells and high concentrations of urea appear in testicular tissue of UT-B-/- mice, particularly for aging mice. Furthermore, UT-B-/- mice had higher body weights than wild-type mice, and earlier spermatogenesis and reproductive system maturation. However, there is no abnormality in the integrity of spermatogenic epithelial cells, sperm morphology or distribution, and the tubular and lumen diameters of the spermatogenic tubules in UT-B-/- mice [92].
It is well known that follicle-stimulating hormone receptor (FSHR) and androgen-binding protein (ABP) are crucial for the development and function of Sertoli cells in the testis [37]. FSH can through its FSHR stimulates ABP expression in Sertoli cells, which is important for the maintenance of sperm number. Actually, significantly higher levels of FSHR and ABP expression are detected in the testis of UT-B-/- mice at 10 days of age, accompanied by accelerating sperm formation and reproductive system maturation [92]. These suggest that UT-B-related urea concentrations may contribute to the development of Sertoli cells in the reproductive system of mice [25, 91].
The circulatory system
UT-B is expressed in the heart. Electrocardiogram (ECG) indicated that P-R interval of adult UT-B-/- mice was significantly prolonged, and II-III grades of atrioventricular block appeared in elder UT-B-/- mice [53]. Further proteomic analysis reveals that nine protein expression is upregulated and one is downregulated in the heart of UT-B-/- mice and those upregulated proteins include troponin C (TNNC), troponin T2 (TNNT2) and troponin I (TNNI), desmin (DESM), acyl-CoA dehydrogenase short-chain (ACAD) precursor K7, K14, enolase (ENOA), malate dehydrogenase (MDH), and atrial natriuretic peptide (ANP). All of these proteins are associated with cardiac function, cellular energy metabolism, ion channel function, and oxidative stress [58, 84, 92, 95]. Interestingly, the levels of ANP expression in aged UT-B-/- mice are significantly higher than those in aged wild-type mice. ANP is expressed in the atrium and can regulate blood pressure, cardiac function and cardiovascular diseases, such as myocardial hypertrophy [65].
Du et al. [14] have shown that UT-B-/- mice are prone to cardiac oxidative stress and myocardial hypertrophy, accompanied by increased concentrations of urea in cardiac myocytes, and abnormal sugar and lipid metabolism. In addition, UT-B-/- mice display impaired ATP production, increased ROS production, and DNA mutations, which may result in mitochondrial dysfunction. These findings suggest that UT-B deficiency may impair myocardial energy metabolism and induce abnormal intracellular homeostasis. It is possible that UT-B-related urea concentration may affect the arginine-eNOS-NO pathway and blood pressure, leading to vascular relaxation [81].
Erythrocytes
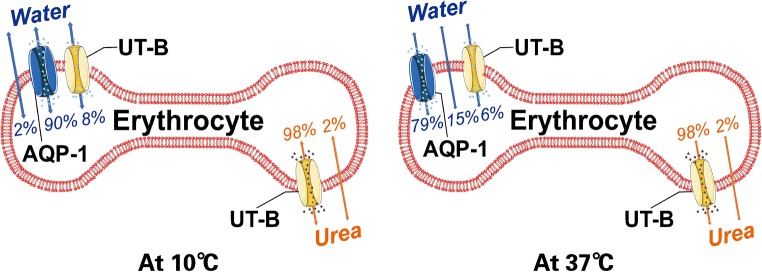
UT-B protein is highly expressed in the plasma membrane of erythrocytes and is a water channel responsible for 8% of water transportation (at 10 °C). Although urea is not charged it has a strong dipole moment, which makes it impossible to penetrate through non-polar lipid membranes. Hence, urea depends on its specific transporters to pass through the cell membranes. The transfer function of UT-B is regulated by temperature (Fig. 2) [89, 90]. These, together with high permeability to urea in human erythrocyte, reduce urea concentrations in erythrocytes. Actually, UT-B deficiency decreases the urea permeability of erythrocytes by 45 times [91].
Fig. 2.
Contribution of AQP1, UT-B, and lipid bilayers to water and urea transport in erythrocytes. The transfer function of UT-B is regulated by temperature
Physiologically, UT-B functions to protect erythrocytes from repeated osmotic pressure-related injury to maintain the stability of erythrocyte permeation and morphology. Because of higher concentrations of urea in the vasa recta higher permeability of AQP1 to water, it is crucial for the balance of transporting water and urea to avoid the high urea concentrations that damage erythrocytes in the renal medulla [21, 49]. Furthermore, rapid urea transport mediated by UT-B in erythrocyte may help establish urea concentration gradient. Erythrocytes can provide urea through UT-B for countercurrent exchange between ascending and descending rectal vessels.
The bone marrow
The gene for UT-B was cloned from human bone marrow [55] and UT-B may influence bone metabolism [63]. Osteoporosis and senile osteopenia are characterized by reduced bone tissues and increased adipose tissues in the bone marrow. Given that adipocytes and osteoblasts are from mesenchymal stem cells, they can transdifferentiate in some conditions, such as aging, post-menopause, emergencies, and others to regulate the balance of hematopoiesis and osteogenesis in the bone marrow.
A previous study has shown that UT-B expression is downregulated during the adipogenesis in the bone marrow and nuclear hormone receptor peroxisome proliferator–activated receptor gamma 2 (PPARg2) expression is upregulated. Downregulation of UT-B occurred early before the increase of PPARg2 expression. The PPARg2 expression in human trabecular bone (hOBs) is upregulated during fat formation in hOBs. And there is a reciprocal relationship between the expression of UT-B and PPARg2; the time-dependent regulation of these two is opposite [63]. These findings suggest that UT-B-related urea concentrations in the bone marrow may regulate the differentiation of mesenchymal stem cells. During the urea cycle, arginine can be converted into urea or NO and polyamines. NO is an important mediator of osteoblast activity and a stimulator for bone formation. NO can regulate the biochemical processes in chondrocytes, osteoblasts, and adipocytes [9, 24, 50]. Polyamines are polycations and can interact with negatively charged DNA, RNA, and proteins to regulate the proliferation and differentiation of many types of cells. Therefore, UT-B expression in osteoblasts may be a marker of fat formation in the bone marrow during the process of osteoporosis and also provide theoretical basis for understanding the mechanism underlying osteoblasts/adipocytes differentiation [63].
The digestive system
UT-B2 mRNA transcripts can be detected in the rumen of sheep and cow [11] and its expression is regulated by gastric acidity and alkalinity, rumen ammonia concentration, and the type of food consumed in the digestive system of sheep and cow. For example, consumed solid food can increase UT-B expression in cows [4] and feeding with a large amount of nitrogen and non-fiber carbohydrates also upregulates UT-B expression in the rumen of sheep [43]. Furthermore, short-chain fatty acids and low pH can increase the UT-B protein expression in rumen epithelial cells of sheep. Similarly, several factors can regulate UT-B2 expression in ruminants of rodents.
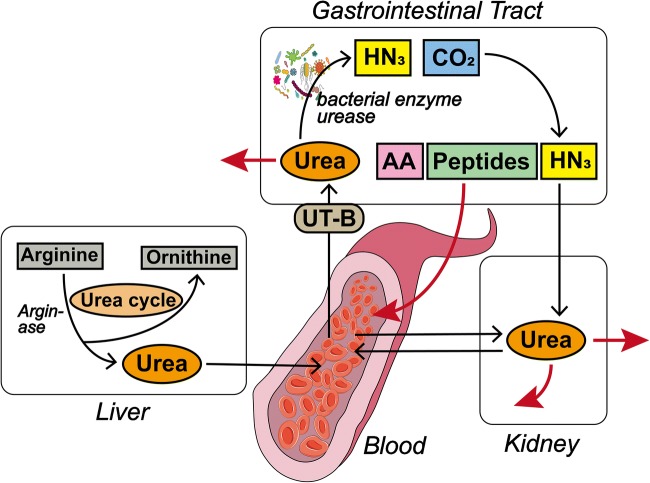
More importantly, UT-B is also expressed in human digestive system. Firstly, UT-B protein is detected in human small intestine, colon, and colonic recesses [64]. Furthermore, UT-B protein is detected in human intestinal Caco-2 cells and epithelial cells, particularly higher expression in ascending colon than in descending colon [10, 86]. UT-B has shown to promote the transportation of urea from blood to the gastrointestinal tract in the ruminants and other mammals [45, 51, 83] and the gastrointestinal UT-B in humans may participate in the process of urea nitrogen salvaging (UNS). During the UNS process, urea supplied by animals is decomposed by bacterial urease. Amino acids and peptides released by bacteria are absorbed through the transport system of epithelial cells to help the body growth and also to maintain a healthy microflora in the intestine (Fig. 3) [78]. The microbial population in human gastrointestinal tract varies greatly and is associated with the development of many diseases, such as diabetes, inflammatory bowel disease, and obesity [12, 16, 17, 34].
Fig. 3.
The urea nitrogen salvaging (UNS) process. Urea is produced in the liver via the urea cycle and enters into the blood. There are two main destinations for urea produced in the liver: (a) Pass through the kidney and (b) pass through the gastrointestinal tract via UT-B. Urea entering the kidney can be freely filtered and reabsorbed or excreted directly. The gastrointestinal tract contains a large number of bacteria, and the gastrointestinal urea is decomposed into ammonia and carbon dioxide by bacterial urease. The ammonia can be directly absorbed by the blood or be used by bacteria to produce amino acids (AA) and peptides that are reabsorbed
The central nervous system
Urea can regulate the contents of ammonia and nitrogen. These polar molecules are mainly formed in the liver through the urea cycle. However, the urea cycle in the CNS is incomplete although the levels of urea are similar to that in the liver [5].
UT-B is important for the maintenance of urea concentrations in the CNS. Firstly, UT-B is expressed in the olfactory bulb, cortex, caudate nucleus, hippocampus, and hypothalamus of mice [40]. Furthermore, UT-B is also expressed by astrocytes as UT-B is co-localized with GFAP, a marker of astrocyte, in the brain sections. However, there is no evidence of UT-B expression in oligodendrocytes, microglia, and vascular endothelial cells. In addition, UT-B-/- mice display depressive-like behaviors [5]. This outcome may stem from UT-B deficiency-increased urea contents in the cerebral cortex and hypothalamus and lower NO in the hippocampus of mice. In the chronic mild stress animal model that mimics human depression, antidepressant fluoxetine inhibits NO production in the hippocampus [46]. Actually, urea loading can reduce NO production in the hippocampus of UT-B-/- and UT-B-/+ mice. UT-B deficiency does not alter NO concentrations in blood, the cortex, and hypothalamus although it increases nNOS, but not eNOS and iNOS expression in the hippocampus of mice. In addition, nNOS reduces 5-HT transporter activity, which feedback decreases the 5-HT uptake–stimulated nNOS activity and NO production [8, 23]. In a uremic condition, urea can competitively inhibit the transport of L-arginine (L-arg) to endothelial cells through the UT-B, which can be eliminated by urea transport inhibitors [92]. These observations support the notion that high urea concentrations can reduce NO level, but enhance nNOS expression [85, 88, 96]. The decrease in NO content may be related to the competition of arginine with nNOS for the NO substrate L-arginine, which needs to be further investigated. Furthermore, the reduced NO by UT-B deficiency subsequently decreases cerebral blood flow (rCBF) [82] and increases neuronal degeneration, vacuolar, and myelinated and unmyelinated fiber formation, which may contribute to the development of depression in mice.
UT-B may be crucial for the balance of sodium and water in the cerebrospinal fluid (CSF). Actually, long-term feeding with high salt diet not only significantly elevated [Na+] in the CSF but also significantly reduced UT-B, but not UT-A, expression in epithelial cells of the choroid plexus (CP) in salt-sensitive Dahl S rats, indicating that increased [Na+] in the CSF was associated with the high urea concentration caused by the downregulated UT-B expression in the CP. It is possible that the high Na+ concentrations in the CSF induced by high salt diet may increase arginine vasopressin levels to decrease UT-B expression in the Dahl S rats [26].
The cochlea
Western blot and immunohistochemistry reveal that UT-B is expressed in pillar, hair, and Boettcher’s cells of both the inner and outer ears [38]. The inner ear is responsible for sound and maintaining balance. It is well known that urea is a marker for diagnosis of too much endolymphatic effusion. The contents of urea and glycerol are commonly used for diagnosis of Meniere’s disease. Urea can increase the permeability gradient between blood and inner ear fluid and reduce the volume of endolymph hydrops [7, 30, 87]. The steady state of the volume, pressure, and chemical composition in the endolymph is crucial for the electromechanical conduction of sound in the ear. However, urea administration can increase serum osmotic pressure and osmotic gradients between blood and inner ear fluids [17, 75, 93]. In the cochlea, the penetration of small hydrophilic solutes through the blood labyrinth barrier depends on the molecular weight of solutes. Because urea transport rate is much faster than some smaller hydrophilic molecular solutes, such as L-glucose, mannitol, and sucrose, and glycine [26, 76], the urea transport system is important for the homeostasis of the volume, pressure, and chemical composition in the endolymph of the cochlea. Coincidentally, UT-B is expressed in the supporting cell system of the cochlea, such as Boettcher’s cells, which can contact with endolymph, reticular layer, and perilymph. UT-B expression in these cells may be important for the urea transport between endolymph and perilymph.
This review provides a systematic overview of the physiological function of UT-B. Currently, the available studies centered on UT-B deficiency-related phenotypic manifestations, but little is known on the precise mechanisms underlying the action of UT-B. Currently, there is no information on the exact factors that influence UT-B expression although some studies have shown that low protein diet and solid foods upregulate UT-B expression in the digestive system of rats and in the rumen of cow and sheep. UT-B is an N-linked glycosylated urea transport protein and is widely distributed in human body. Functionally, UT-B is responsible for intracellular and extracellular urea transport, maintaining urea concentrations.
Urea is the product of urea cycle (ornithine cycle), which is essential for life activities. UT-B knockout significantly increases urea concentrations in the bladder of mice and high concentrations of urea can feedback inhibit urea cycle and damage the structure and function of tissues and organs. Actually, UT-B deficiency caused DNA damage and apoptosis in bladder epithelial cells and the precocious reproductive system in mice. UTB deficiency can also lead to prolonged P-R interval and atrioventricular block in mice, accompanied by abnormal mitochondrial function and myocardial energy metabolism. Therefore, UT-B deficiency may be associated with the development of bladder cancer [29] and myocardial hypertrophy as well as other diseases.
The high urea concentrations caused by UT-B deletion can affect the NO/NOS system. NO is important for cardiovascular and nervous functions. In the CNS, NO participates in the process of sleep-wake cycles, neurosecretion, and reproduction. High levels of NO can convert into reactive nitrogen species (RNS), which can cause cell damage [6]. Interestingly, UT-B deficiency decreased the NO content in the hippocampus of mice with depressive behavior. Although the relationship between UT-B efficiency-reduced NO and depression remains to be determined this phenotype suggests that the UT-B-related NO pathway is involved in the unknown neuromechanism. In addition, NO may promote depression by regulating cerebral blood flow. Given that UT-B can regulate urea cycle by adjusting urea concentration and all enzymes related to urea cycle are expressed in the brains of AD individuals [27], it is unclear whether the altered urea cycle by UT-B deficiency contributes to the pathogenesis of AD.
The potential clinical application of UT-B is enormous. Currently, studies have shown that UT-B reduces the apparent diffusion coefficient of 13C hyperpolarized urea in tissues by mediating the uptake of urea by cells, suggesting that UT-B has the potential to become a magnetic resonance–based gene reporter in vivo [59]. In addition, UT-B inhibitors are being developed for diuretic targeting and may overcome the side effects, such as hypernatremia of conventional diuretics because UT-B inhibitors usually do not change serum sodium, chlorine or potassium levels. Therefore, UT-B inhibitors may be particularly useful in reducing circulation volume in patients with congestive heart failure [35].
In summary, future researches on UT-B should investigate whether UT-B can directly regulate organ function besides maintaining urea concentration, and which factors can modulate UT-B expression. To address these questions may provide new insights into the physiological action of UT-B and aid in design of new therapeutic strategies for treatment of relevant diseases in the clinic.
Funding information
This work was supported by the grants from the National Natural Science Foundation of China (NOs. 81370240, 81600207), Excellent Youth Fund of Jilin Provincial Science and Technology Department(NO. 182427JH010247838), and National College Students’ Innovation and Entrepreneurship Training Program (NO. 201810199034), College Project Grant (NO. 2018KJ01).
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lanying Yu and Tiantian Liu are Co-first author
Contributor Information
Yan Meng, Email: mengyan@jlu.edu.cn.
Xuejiao Lv, Email: lvxuejiao0311@163.com.
Yanwei Du, Phone: +86-0431-8617-2315, Email: duyanwei.dyw@163.com.
References
- 1.Allen FH, Diamond LK, Niedziela B. A New blood-group antigen. Nature. 1951;167:482–482. doi: 10.1038/167482b0. [DOI] [PubMed] [Google Scholar]
- 2.Bagnasco SM, Peng T, Nakayama Y, Sands JM. Differential expression of individual UT-A urea transporter isoforms in rat kidney. J Am Soc Nephrol. 2000;11:1980–1986. doi: 10.1681/ASN.V11111980. [DOI] [PubMed] [Google Scholar]
- 3.Bagnasco SM, Peng T, Janech MG, Karakashian A, Sands JM. Cloning and characterization of the human urea transporter UT-A1 and mapping of the human Slc14a2 gene. Am J Physiol Ren Physiol. 2001;281:F400–F406. doi: 10.1152/ajprenal.2001.281.3.F400. [DOI] [PubMed] [Google Scholar]
- 4.Berends H, van den Borne JJ, Rojen BA, van Baal J, Gerrits WJ. Urea recycling contributes to nitrogen retention in calves fed milk replacer and low-protein solid feed. J Nutr. 2014;144:1043–1049. doi: 10.3945/jn.114.191353. [DOI] [PubMed] [Google Scholar]
- 5.Berger UV, Tsukaguchi H, Hediger MA. Distribution of mRNA for the facilitated urea transporter UT3 in the rat nervous system. Anat Embryol. 1998;197:405–414. doi: 10.1007/s004290050152. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 7.Carlborg BI, Farmer JC., Jr Effects of hyperosmolar solutions on the labyrinthine fluid pressures. I. Effects of glycerol and urea tests. II. Effects of mannitol tests. Ann Otol Rhinol Laryngol. 1983;Supplement 104:1–16. [PubMed] [Google Scholar]
- 8.Chanrion B, la Cour CM, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, Bockaert J, Marin P. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci U S A. 2007;104:8119–8124. doi: 10.1073/pnas.0610964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolletta C, Jouzeau JY, Gegout-Pottie P, Presle N, Bordji K, Netter P, Terlain B. Modulation of IL-1-induced cartilage injury by NO synthase inhibitors: a comparative study with rat chondrocytes and cartilage entities. Br J Pharmacol. 1998;124:1719–1727. doi: 10.1038/sj.bjp.0702005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins D, Winter DC, Hogan AM, Schirmer L, Baird AW, Stewart GS. Differential protein abundance and function of UT-B urea transporters in human colon. Am J Physiol Gastrointest Liver Physiol. 2010;298:G345–G351. doi: 10.1152/ajpgi.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyle J, McDaid S, Walpole C, Stewart GS. UT-B urea transporter localization in the bovine gastrointestinal tract. J Membr Biol. 2016;249:77–85. doi: 10.1007/s00232-015-9850-5. [DOI] [PubMed] [Google Scholar]
- 12.DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460–469. doi: 10.4065/83.4.460. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, Ran J, Zhou H, Chen J, Lei T, Wang W, Sun Y, Lin G, Bankir L, Yang B. Urea transporter UT-B deletion induces DNA damage and apoptosis in mouse bladder urothelium. PLoS One. 2013;8:e76952. doi: 10.1371/journal.pone.0076952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Meng Y, Zhu J, Kang L, Jia X, Guo L, Zhang L, Ye M, Hu L, Zhao X, Gu J, Yang B, Zou H. Quantitative proteomic study of myocardial mitochondria in urea transporter B knockout mice. Proteomics. 2014;14:2072–2083. doi: 10.1002/pmic.201400123. [DOI] [PubMed] [Google Scholar]
- 15.Duchesne R, Klein JD, Velotta JB, Doran JJ, Rouillard P, Roberts BR, McDonough AA, Sands JM. UT-A urea transporter protein in heart: increased abundance during uremia, hypertension, and heart failure. Circ Res. 2001;89:139–145. doi: 10.1161/hh1401.093293. [DOI] [PubMed] [Google Scholar]
- 16.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton RA, Hewitt JE, Howorth A, Cottingham CA, Smith CP. The murine urea transporter genes Slc14a1 and Slc14a2 occur in tandem on chromosome 18. Cytogenet Cell Genet. 1999;87:95–96. doi: 10.1159/000015401. [DOI] [PubMed] [Google Scholar]
- 19.Fenton RA, Howorth A, Cooper GJ, Meccariello R, Morris ID, Smith CP. Molecular characterization of a novel UT-A urea transporter isoform (UT-A5) in testis. Am J Physiol Cell Physiol. 2000;279:C1425–C1431. doi: 10.1152/ajpcell.2000.279.5.C1425. [DOI] [PubMed] [Google Scholar]
- 20.Fenton RA, Cooper GJ, Morris ID, Smith CP. Coordinated expression of UT-A and UT-B urea transporters in rat testis. Am J Physiol Cell Physiol. 2002;282:C1492–C1501. doi: 10.1152/ajpcell.00567.2001. [DOI] [PubMed] [Google Scholar]
- 21.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol. 2005;16:1583–1592. doi: 10.1681/ASN.2005010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frorath B, Abney C, Abney M, Berthold H, Hunt N, Northemann W. Mapping of a linear autoantigenic epitope within the human thyroid peroxidase using recombinant DNA techniques. J Biochem. 1992;111:633–637. doi: 10.1093/oxfordjournals.jbchem.a123810. [DOI] [PubMed] [Google Scholar]
- 23.Garthwaite J. Neuronal nitric oxide synthase and the serotonin transporter get harmonious. Proc Natl Acad Sci U S A. 2007;104:7739–7740. doi: 10.1073/pnas.0702508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudiot N, Jaubert AM, Charbonnier E, Sabourault D, Lacasa D, Giudicelli Y, Ribière C. Modulation of white adipose tissue lipolysis by nitricoxide. J Biol Chem. 1998;273:13475–13481. doi: 10.1074/jbc.273.22.13475. [DOI] [PubMed] [Google Scholar]
- 25.Guo L, Zhao D, Song Y, Meng Y, Zhao H, Zhao X, Yang B. Reduced urea flux across the blood-testis barrier and early maturation in the male reproductive system in UT-B-null mice. Am J Physiol Cell Physiol. 2007;293:C305–C312. doi: 10.1152/ajpcell.00608.2006. [DOI] [PubMed] [Google Scholar]
- 26.Guo L, Meng J, Xuan C, Ge J, Sun W, O’Rourke ST, Sun C. High salt-diet reduces SLC14A1 gene expression in the choroid plexus of Dahl salt sensitive rats. Biochem Biophys Res Commun. 2015;461:254–259. doi: 10.1016/j.bbrc.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansmannel F, Sillaire A, Kamboh MI, Lendon C, Pasquier F, Hannequin D, Laumet G, Mounier A, Ayral AM, DeKosky ST, Hauw JJ, Berr C, Mann D, Amouyel P, Campion D, Lambert JC. Is the urea cycle involved in Alzheimer’s disease? J Alzheimers Dis. 2010;21:1013–1021. doi: 10.3233/jad-2010-100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaton DC, McLoughlin K. Jk(a-b-) red blood cells resist urea lysis. Transfusion. 1982;22:70–71. doi: 10.1046/j.1537-2995.1982.22182154224.x. [DOI] [PubMed] [Google Scholar]
- 29.Hou R, Alemozaffar M, Yang B, Sands JM, Kong X, Chen G. Identification of a novel UT-B urea transporter in human urothelial cancer. Front Physiol. 2017;8:245. doi: 10.3389/fphys.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juhn SK, Prado S, Rybak L. Effect of urea on osmolality of perilymph. Arch Otolaryngol. 1979;105:538–541. doi: 10.1001/archotol.1979.00790210036008. [DOI] [PubMed] [Google Scholar]
- 31.Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol. 1999;10:230–237. doi: 10.1681/ASN.V102230. [DOI] [PubMed] [Google Scholar]
- 32.Khan AU, Di Mascio P, Medeiros MH, Wilson T. Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc Natl Acad Sci U S A. 1992;89:11428–11430. doi: 10.1073/pnas.89.23.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AU, Mei YH, Wilson T. A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci U S A. 1992;89:11426–11427. doi: 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinross JM, von Roon AC, Holmes E, Darzi A, Nicholson JK. The human gut microbiome: implications for future health care. Curr Gastroenterol Rep. 2008;10:396–403. doi: 10.1007/s11894-008-0075-y. [DOI] [PubMed] [Google Scholar]
- 35.Klein JD, Sands JM. Urea transport and clinical potential of urearetics. Curr Opin Nephrol Hypertens. 2016;25:444–451. doi: 10.1097/MNH.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int. 2001;Supplement 78:S102–S107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamurthy H, Babu PS, Morales CR, Sairam MR. Delay in sexual maturity of the follicle-stimulating hormone receptor knockout male mouse. Biol Reprod. 2001;65:522–531. doi: 10.1095/biolreprod65.2.522. [DOI] [PubMed] [Google Scholar]
- 38.Kwun Y-S, Yeo SW, Ahn Y-H, Lim S-W, Jung J-Y, Kim W-Y, Sands JM, Kim J. Immunohistochemical localization of urea transporters A and B in the rat cochlea. Hear Res. 2003;183:84–96. doi: 10.1016/s0378-5955(03)00218-1. [DOI] [PubMed] [Google Scholar]
- 39.Lawicki S, Covin RB, Powers AA. The Kidd (JK) Blood group system. Transfus Med Rev. 2017;31:165–172. doi: 10.1016/j.tmrv.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Ran J, Zhou H, Lei T, Zhou L, Han J, Yang B. Mice lacking urea transporter UT-B display depression-like behavior. J Mol Neurosci. 2012;46:362–372. doi: 10.1007/s12031-011-9594-3. [DOI] [PubMed] [Google Scholar]
- 41.Linz WJ, Muenster S, Watkins J, Moore SB. Transfusion medicine illustrated. Lack of effect of 2 M urea on Jk(a-b-) cells. Transfusion. 2003;43:685. doi: 10.1046/j.1537-2995.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Li M, Liu J, Wang H, Zhong D, Zhou H, Yang B. Elevated urinary urea by high-protein diet could be one of the inducements of bladder disorders. J Transl Med. 2016;14:53. doi: 10.1186/s12967-016-0809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Z, Gui H, Yao L, Yan L, Martens H, Aschenbach JR, Shen Z. Short-chain fatty acids and acidic pH upregulate UT-B, GPR41, and GPR4 in rumen epithelial cells of goats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R283–R293. doi: 10.1152/ajpregu.00323.2014. [DOI] [PubMed] [Google Scholar]
- 44.Lucien N, Sidoux-Walter F, Olivès B, Moulds J, Le Pennec P-Y, Cartron J-P, Bailly P. Characterization of the gene encoding the human Kidd blood group/urea transporter protein: evidence for splice site mutations in Jknull individuals. J Biol Chem. 1998;273:12973–12980. doi: 10.1074/jbc.273.21.12973. [DOI] [PubMed] [Google Scholar]
- 45.Ludden PA, Stohrer RM, Austin KJ, Atkinson RL, Belden EL, Harlow HJ. Effect of protein supplementation on expression and distribution of urea transporter-B in lambs fed low-quality forage. J Anim Sci. 2009;87:1354–1365. doi: 10.2527/jas.2008-1399. [DOI] [PubMed] [Google Scholar]
- 46.Luo L, Tan RX. Fluoxetine inhibits dendrite atrophy of hippocampal neurons by decreasing nitric oxide synthase expression in rat depression model. Acta Pharmacol Sin. 2001;22:865–870. [PubMed] [Google Scholar]
- 47.Luperchio S, Tamir S, Tannenbaum SR. NO-induced oxidative stress and glutathione metabolism in rodent and human cells. Free Radic Biol Med. 1996;21:513–519. doi: 10.1016/0891-5849(96)00219-5. [DOI] [PubMed] [Google Scholar]
- 48.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 49.Macey RI, Yousef LW. Osmotic stability of red cells in renal circulation requires rapid urea transport. Am J Phys. 1988;254:C669–C674. doi: 10.1152/ajpcell.1988.254.5.C669. [DOI] [PubMed] [Google Scholar]
- 50.MacPherson H, Noble BS, Ralston SH. Expression and functional role of nitric oxide synthase isoforms in human osteoblast-like cells. Bone. 1999;24:179–185. doi: 10.1016/S8756-3282(98)00173-2. [DOI] [PubMed] [Google Scholar]
- 51.Marini JC, Klein JD, Sands JM, Van Amburgh ME. Effect of nitrogen intake on nitrogen recycling and urea transporter abundance in lambs. J Anim Sci. 2004;82:1157–1164. doi: 10.2527/2004.8241157x. [DOI] [PubMed] [Google Scholar]
- 52.Martial S, Olives B, Abrami L, Couriaud C, Bailly P, You G, Hediger MA, Cartron JP, Ripoche P, Rousselet G. Functional differentiation of the human red blood cell and kidney urea transporters. Am J Phys. 1996;271:F1264–F1268. doi: 10.1152/ajprenal.1996.271.6.F1264. [DOI] [PubMed] [Google Scholar]
- 53.Meng Y, Zhao C, Zhang X, Zhao H, Guo L, Lu B, Zhao X, Yang B. Surface electrocardiogram and action potential in mice lacking urea transporter UT-B. Sci China C Life Sci. 2009;52:474–478. doi: 10.1007/s11427-009-0047-y. [DOI] [PubMed] [Google Scholar]
- 54.Nakayama Y, Naruse M, Karakashian A, Peng T, Sands JM, Bagnasco SM. Cloning of the rat Slc14a2 gene and genomic organization of the UT-A urea transporter. Biochim Biophys Acta. 2001;1518:19–26. doi: 10.1016/s0167-4781(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 55.Olives B, Neau P, Bailly P, Hediger MA, Rousselet G, Cartron JP, Ripoche P. Cloning and functional expression of a urea transporter from human bone marrow cells. J Biol Chem. 1994;269:31649–31652. [PubMed] [Google Scholar]
- 56.Olives B, Mattei MG, Huet M, Neau P, Martial S, Cartron JP, Bailly P. Kidd blood group and urea transport function of human erythrocytes are carried by the same protein. J Biol Chem. 1995;270:15607–15610. doi: 10.1074/jbc.270.26.15607. [DOI] [PubMed] [Google Scholar]
- 57.Olives B, Martial S, Mattei MG, Matassi G, Rousselet G, Ripoche P, Cartron JP, Bailly P. Molecular characterization of a new urea transporter in the human kidney. FEBS Lett. 1996;386:156–160. doi: 10.1016/0014-5793(96)00425-5. [DOI] [PubMed] [Google Scholar]
- 58.Otten E, Asimaki A, Maass A, van Langen IM, van der Wal A, de Jonge N, van den Berg MP, Saffitz JE, Wilde AA, Jongbloed JD, van Tintelen JP. Desmin mutations as a cause of right ventricular heart failure affect the intercalated disks. Heart Rhythm. 2010;7:1058–1064. doi: 10.1016/j.hrthm.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 59.Patrick PS, Kettunen MI, Tee SS, Rodrigues TB, Serrao E, Timm KN, McGuire S, Brindle KM. Detection of transgene expression using hyperpolarized 13C urea and diffusion-weighted magnetic resonance spectroscopy. Magn Reson Med. 2015;73:1401–1406. doi: 10.1002/mrm.25254. [DOI] [PubMed] [Google Scholar]
- 60.Pinkerton FJ, Mermod LE, Liles BA, Jack JA, Jr, Noades J. The phenotype Jk(a-b-) in the Kidd blood group system. Vox Sang. 1959;4:155–160. doi: 10.1159/000478464. [DOI] [PubMed] [Google Scholar]
- 61.Plaut G, Ikin EW, Mourant AE, Sanger R, Race RR. A new blood-group antibody, anti Jkb. Nature. 1953;171:431. doi: 10.1038/171431a0. [DOI] [PubMed] [Google Scholar]
- 62.Preston G, Smith B, Zeidel M, Moulds J, Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- 63.Prichett WP, Patton AJ, Field JA, Brun KA, Emery JG, Tan KB, Rieman DJ, McClung HA, Nadeau DP, Mooney JL, Suva LJ, Gowen M, Nuttall ME. Identification and cloning of a human urea transporter HUT11, which is downregulated during adipogenesis of explant cultures of human bone. J Cell Biochem. 2000;76:639–650. doi: 10.1002/(SICI)1097-4644(20000315)76:4<639::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 64.Ritzhaupt A, Wood IS, Jackson AA, Moran BJ, Shirazi-Beechey SP. Isolation of a RT-PCR fragment from human colon and sheep rumen RNA with nucleotide sequence similarity to human and rat urea transporter isoforms. Biochem Soc Trans. 1998;26:S122. doi: 10.1042/bst026s122. [DOI] [PubMed] [Google Scholar]
- 65.Rubattu S, Sciarretta S, Valenti V, Stanzione R, Pel MI. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733–741. doi: 10.1038/ajh.2008.174. [DOI] [PubMed] [Google Scholar]
- 66.Sands JM, Blount MA. Genes and proteins of urea transporters. Subcell Biochem. 2014;73:45–63. doi: 10.1007/978-94-017-9343-8_4. [DOI] [PubMed] [Google Scholar]
- 67.Sands JM, Gargus JJ, Fröhlich O, Gunn RB, Kokko JP. Urinary concentrating ability in patients with Jk(a-b-) blood type who lack carrier-mediated urea transport. J Am Soc Nephrol. 1992;2:1689–1696. doi: 10.1681/ASN.V2121689. [DOI] [PubMed] [Google Scholar]
- 68.Shayakul C, Steel A, Hediger MA. Molecular cloning and characterization of the vasopressin-regulated urea transporter of rat kidney collecting ducts. J Clin Invest. 1996;98:2580–2587. doi: 10.1172/JCI119077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shayakul C, Tsukaguchi H, Berger UV, Hediger MA. Molecular characterization of a novel urea transporter from kidney inner medullary collecting ducts. Am J Physiol Ren Physiol. 2001;280:F487–F494. doi: 10.1152/ajprenal.2001.280.3.F487. [DOI] [PubMed] [Google Scholar]
- 70.Singh V, Jaiswal PK, Mittal RD. Replicative study of GWAS TP63C/T, TERTC/T, and SLC14A1C/T with susceptibility to bladder cancer in North Indians. Urol Oncol. 2014;32:1209–1214. doi: 10.1016/j.urolonc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Sinor LT, Eastwood KL, Plapp FV. Dot-blot purification of the Kidd blood group antigen. Med Lab Sci. 1987;44:294–296. [PubMed] [Google Scholar]
- 72.Smith CP, Rousselet G. Facilitative urea transporters. J Membr Biol. 2001;183:1–14. doi: 10.1007/s00232-001-0048-7. [DOI] [PubMed] [Google Scholar]
- 73.Smith CP, Potter EA, Fenton RA, Stewart GS. Characterization of a human colonic cDNA encoding a structurally novel urea transporter, hUT-A6. Am J Physiol Cell Physiol. 2004;287:C1087–C1093. doi: 10.1152/ajpcell.00363.2003. [DOI] [PubMed] [Google Scholar]
- 74.Spector DA, Yang Q, Liu J, Wade JB. Expression, localization, and regulation of urea transporter B in rat urothelia. Am J Physiol Ren Physiol. 2004;287:F102–F108. doi: 10.1152/ajprenal.00442.2003. [DOI] [PubMed] [Google Scholar]
- 75.Sterkers O, Ferrary E, Saumon G, Amiel C. Na and nonelectrolyte entry into inner ear fluids of the rat. Am J Phys. 1987;253:F50–F58. doi: 10.1152/ajprenal.1987.253.1.F50. [DOI] [PubMed] [Google Scholar]
- 76.Sterkers O, Ferrary E, Amiel C. Production of inner ear fluids. Physiol Rev. 1988;68:1083–1128. doi: 10.1152/physrev.1988.68.4.1083. [DOI] [PubMed] [Google Scholar]
- 77.Stewart G. The emerging physiological roles of the SLC14A family of urea transporters. Br J Pharmacol. 2011;164:1780–1792. doi: 10.1111/j.1476-5381.2011.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart GS, Smith CP. Urea nitrogen salvage mechanisms and their relevance to ruminants, non-ruminants and man. Nutr Res Rev. 2005;18:49–62. doi: 10.1079/Nrr200498. [DOI] [PubMed] [Google Scholar]
- 79.Stewart GS, Fenton RA, Wang W, Kwon TH, White SJ, Collins VM, Cooper G, Nielsen S, Smith CP. The basolateral expression of mUT-A3 in the mouse kidney. Am J Physiol Ren Physiol. 2004;286:F979–F987. doi: 10.1152/ajprenal.00334.2003. [DOI] [PubMed] [Google Scholar]
- 80.Stewart GS, Graham C, Cattell S, Smith TP, Simmons NL, Smith CP. UT-B is expressed in bovine rumen: potential role in ruminal urea transport. Am J Physiol Regul Integr Comp Physiol. 2005;289:R605–R612. doi: 10.1152/ajpregu.00127.2005. [DOI] [PubMed] [Google Scholar]
- 81.Sun Y, Lau CW, Jia Y, Li Y, Wang W, Ran J, Li F, Huang Y, Zhou H, Yang B. Functional inhibition of urea transporter UT-B enhances endothelial-dependent vasodilatation and lowers blood pressure via L-arginine-endothelial nitric oxide synthase-nitric oxide pathway. Sci Rep. 2016;6:18697. doi: 10.1038/srep18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas AJ, O’Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 83.Tickle P, Thistlethwaite A, Smith CP, Stewart GS. Novel bUT-B2 urea transporter isoform is constitutively activated. Am J Physiol-Reg I. 2009;297:R323–R329. doi: 10.1152/ajpregu.00199.2009. [DOI] [PubMed] [Google Scholar]
- 84.Tsuneyoshi H, Nishina T, Nomoto T, Kanemitsu H, Kawakami R, Unimonh O, Nishimura K, Komeda M. Atrial natriuretic peptide helps prevent late remodeling after left ventricular aneurysm repair. Circulation. 2004;110:II174–II179. doi: 10.1161/01.CIR.0000138348.77856.ef. [DOI] [PubMed] [Google Scholar]
- 85.Wagner L, Klein JD, Sands JM, Baylis C. Urea transporters are distributed in endothelial cells and mediate inhibition of L-arginine transport. Am J Physiol Ren Physiol. 2002;283:F578–F582. doi: 10.1152/ajprenal.00355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walpole C, McGrane A, Al-Mousawi H, Winter D, Baird A, Stewart G. Investigation of facilitative urea transporters in the human gastrointestinal tract. Phys Rep. 2018;6:e13826. doi: 10.14814/phy2.13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wright CG, Lee DH, Meyerhoff WL, Roland PS. Morphologic effects of glycerol and urea on cochlear tissues of the chinchilla. Ann Otol Rhinol Laryngol. 1988;97:67–73. doi: 10.1177/000348948809700111. [DOI] [PubMed] [Google Scholar]
- 88.Xiao S, Wagner L, Mahaney J, Baylis C. Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol-Renal. 2001;280:F989–F995. doi: 10.1152/ajprenal.2001.280.6.F989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang BX, Verkman AS. Analysis of double knockout mice lacking aquaporin-1 and urea transporter UT-B - Evidence for UT-B-facilitated water transport in erythrocytes. J Biol Chem. 2002;277:36782–36786. doi: 10.1074/jbc.M206948200. [DOI] [PubMed] [Google Scholar]
- 90.Yang Baoxue, Ma Tonghui, Verkman A. S. Erythrocyte Water Permeability and Renal Function in Double Knockout Mice Lacking Aquaporin-1 and Aquaporin-3. Journal of Biological Chemistry. 2000;276(1):624–628. doi: 10.1074/jbc.M008664200. [DOI] [PubMed] [Google Scholar]
- 91.Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective Concentrating Defect in Transgenic Mice Lacking Urea Transporter UT-B. J Biol Chem. 2002;277:10633–10637. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]
- 92.Yang B, Li X, Guo L, Meng Y, Dong Z, Zhao X. Extrarenal phenotypes of the UT-B knockout mouse. Subcell Biochem. 2014;73:153–164. doi: 10.1007/978-94-017-9343-8_10. [DOI] [PubMed] [Google Scholar]
- 93.Yazawa Y, Shea JJ. Effect of urea on endolymphatic hydrops in guinea pigs. ORL. 1985;47:281–287. doi: 10.1159/000275786. [DOI] [PubMed] [Google Scholar]
- 94.You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. Cloning and characterization of the vasopressin-regulated urea transporter. Nature. 1993;365:844–847. doi: 10.1038/365844a0. [DOI] [PubMed] [Google Scholar]
- 95.Yu H, Meng Y, Wang LS, Jin X, Gao LF, Zhou L, Ji K, Li Y, Zhao LJ, Chen GQ, Zhao XJ, Yang B. Differential protein expression in heart in UT-B null mice with cardiac conduction defects. Proteomics. 2009;9:504–511. doi: 10.1002/pmic.200701079. [DOI] [PubMed] [Google Scholar]
- 96.Zhao D, Bankir L, Qian L, Yang D, Yang B. Urea and urine concentrating ability in mice lacking AQP1 and AQP3. Am J Physiol Ren Physiol. 2006;291:F429–F438. doi: 10.1152/ajprenal.00011.2006. [DOI] [PubMed] [Google Scholar]