Abstract
5-Fluorouracil (5-FU) is known as a first-line chemotherapeutic agent against colorectal cancer (CRC), but drug resistance occurs frequently and significantly limits its clinical success. Our previous study showed that the protocadherin 17 (PCDH17) gene was frequently methylated and functioned as a tumor suppressor in CRC. However, the relationship between PCDH17 and 5-FU resistance in CRC remains unclear. Here, we revealed that PCDH17 was more highly expressed in 5-FU-sensitive CRC tissues than in 5-FU-resistant CRC tissues, and high expression of PCDH17 was correlated with high BECN1 expression. Moreover, this expression profile contributed to superior prognosis and increased survival in CRC patients. Restoring PCDH17 expression augmented the 5-FU sensitivity of CRC in vitro and in vivo by promoting apoptosis and autophagic cell death. Furthermore, autophagy played a dominant role in PCDH17-induced cell death, as an autophagy inhibitor blocked cell death to a greater extent than the pancaspase inhibitor Z-VAD-FMK. PCDH17 inhibition by siRNA decreased the autophagy response and 5-FU sensitivity. Mechanistically, we showed that c-Jun NH2-terminal kinase (JNK) activation was a key determinant in PCDH17-induced autophagy. The compound SP600125, an inhibitor of JNK, suppressed autophagy and 5-FU-induced cell death in PCDH17-reexpressing CRC cells. Taken together, our findings suggest for the first time that PCDH17 increases the sensitivity of CRC to 5-FU treatment by inducing apoptosis and JNK-dependent autophagic cell death. PCDH17 may be a potential prognostic marker for predicting 5-FU sensitivity in CRC patients.
Subject terms: Oncology, Gastrointestinal cancer
Introduction
Colorectal cancer (CRC) ranks third in morbidity and second in mortality among various malignancies, although much progress has been achieved in CRC therapy in past years.1 Currently, the antimetabolite 5-fluorouracil (5-FU) is widely used as chemotherapeutic drug therapy in various solid tumors such as colorectal cancer and gastric cancer. Although 5-FU in combination with other chemotherapeutic agents improves the prognosis of CRC patients, 5-FU resistance occurs frequently and significantly limits its clinical success.2,3 Epigenetic and genetic disruptions of tumor suppressor genes (TSGs) are considered to be partly attributed to 5-FU resistance.4,5 Therefore, the identification of novel genes that have therapeutic potential as predictive biomarkers of 5-FU chemosensitivity is urgently needed.
The protocadherin 17 (PCDH17) gene, a member of the protocadherin family, is frequently methylated and associated with poor prognosis in various cancer types, including esophageal squamous cell carcinoma (ESCC),6 urological cancer,7 gastric cancer,8 and nasopharyngeal cancers.9 In a previous study, we demonstrated that PCDH17 was silenced in most CRC cell lines and that restoring PCDH17 expression reduced tumor cell growth.10 Therefore, PCDH17 is a potential tumor suppressor in CRC. However, the relationship between PCDH17 and 5-FU resistance in CRC remains unclear.
Autophagy, an important homeostatic cell recycling system, plays an important role in cellular component degradation and recycling.11,12 Chemotherapy agents such as 5-FU may give rise to autophagic responses. This autophagic response can have a prodeath or a prosurvival role and thus contribute to anticancer efficacy or drug resistance, respectively.13,14 Therefore, targeting autophagy will provide a potential therapeutic strategy to overcome drug resistance and augment the clinical outcomes of anticancer therapies for patients with cancer. However, to our knowledge, there are still no reports about the role of PCDH17 in regulating autophagy and 5-FU sensitivity in CRC.
In this study, we first demonstrated that PCDH17 and BECN1 (Beclin 1, which is autophagy-related) were more highly expressed in 5-FU-sensitive CRC tissues than in 5-FU-resistant CRC tissues and showed a significant positive relationship between high expression of these genes and superior prognosis. Next, we found that PCDH17 reexpression augmented the 5-FU sensitivity of CRC cells by promoting apoptosis and autophagic cell death. Furthermore, JNK activation was proven to confer 5-FU sensitivity in PCDH17-reexpressing cells by inducing death-promoting autophagy. Thus, our findings indicate that PCDH17 plays a critical role in augmenting 5-FU sensitivity by promoting autophagy. PCDH17 will hopefully become a potential predictive biomarker for CRC patients treated with 5-FU.
Results
Loss of the PCDH17 and BECN1 proteins is associated with 5-FU resistance and poor prognosis in CRC patients
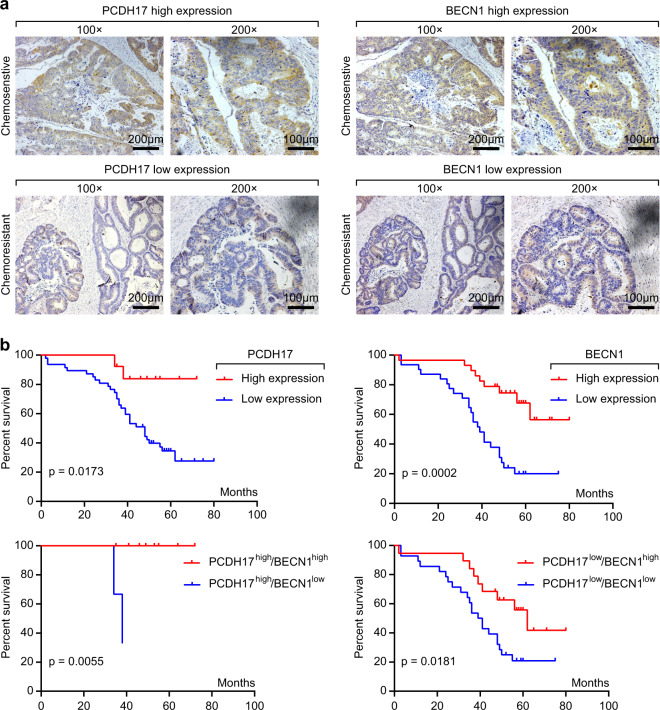
In a previous study, we demonstrated that lower protein expression of PCDH17 was significantly correlated with low T stage, decreased lymph node metastasis, and low tumor stage in CRC patients compared with paired surgical margin tissues. However, the relationship between PCDH17 expression and the 5-FU sensitivity of CRC patients is still unclear. Therefore, we examined PCDH17 expression in 21 chemosensitive and 39 chemoresistant CRC tissues by immunohistochemistry (IHC) staining. To explore the potential mechanism of PCDH17 in cell autophagy, the protein expression of BECN1 was simultaneously detected in the same paraffin-embedded block. The results showed that PCDH17 and BECN1 were highly expressed in the cytoplasm of cancer cells (Fig. 1a), and their expression was significantly correlated (Table 1, p < 0.05). In addition, high expression of PCDH17 and BECN1 was significantly correlated with low levels of lymph node metastasis in both chemosensitive and chemoresistant CRC tissues (Tables 1 and 2, P < 0.05). Furthermore, the percentages of cases with high expression of PCDH17 and BECN1 were 52.4% (11/21 cases) and 81% (17/21 cases), respectively, in chemosensitive CRC tissues but only 7.7% (2/39 cases) and 30.8% (12/39 cases), respectively, in chemoresistant tissues. Kaplan–Meier survival curve analysis indicated that CRC patients with high PCDH17 expression had significantly better overall survival (OS, p = 0.0173) than patients with low PCDH17 expression. The relationship between BECN1 protein expression and OS was consistent with that of PCDH17 (Fig. 1b). Moreover, patients with PCDH17high/BECN1high and PCDH17low/BECN1high disease had better OS than those with PCDH17high/BECN1low and PCDH17low/BECN1low disease, respectively (Fig. 1b). All these data showed that PCDH17 expression was correlated with sensitivity to 5-FU and autophagy in colorectal cancer patients.
Fig. 1.
Immunohistochemistry of PCDH17 and BECN1 expression and patient prognosis and survival analysis. a Two serial sections from the same paraffin-embedded block from 60 colorectal cancer patients were used for detection using anti-PCDH17 and anti-BECN1 antibodies. Representative PCDH17 and BECN1 staining from a chemosensitive and a chemoresistant sample is shown at ×100 and ×200 magnifications. b Kaplan–Meier survival curves for OS in CRC patients with different PCDH17 and BECN1 protein levels.
Table 1.
The association between PCDH17 expression with clinicopathological background and BECN1 experssion.
| PCDH17 immunoreactivity | |||||
|---|---|---|---|---|---|
| N | High (%) | Low (%) | p value | ||
| Total | 60 | 13 (21.7) | 47 (78.3) | ||
| Gender | Male | 34 | 8 (23.5) | 26 (76.5) | 0.7603 |
| Female | 26 | 5 (19.2) | 21 (80.8) | ||
| Age | |||||
| Median | 61 | ||||
| ≥61 | 28 | 5 (15.2) | 23 (84.8) | 0.9999 | |
| <61 | 32 | 8 (29.6) | 24 (70.4) | ||
| Histopathological grading | |||||
| Well/moderately | 48 | 10 (20.8) | 38 (79.2) | 0.7114 | |
| Poorly | 12 | 3 (27.2) | 9 (81.8) | ||
| pT categories | |||||
| pT2 | 9 | 0 (0) | 9 (100) | 0.1839 | |
| pT3 | 51 | 13 (25.5) | 38 (74.5) | ||
| pN categories | |||||
| pN0/1 | 31 | 11 (35.5) | 20 (64.5) | 0.0109 | |
| pN2 | 29 | 2 (6.9) | 27 (93.1) | ||
| Stage | |||||
| II | 19 | 6 (31.6) | 13 (68.4) | 0.3119 | |
| III | 41 | 7 (17.1) | 34 (82.9) | ||
| Chemosensitivity | |||||
| Sensitive | 21 | 11 (52.4) | 10 (47.6) | <0.0001 | |
| Resistant | 39 | 2 (7.7) | 37 (92.3) | ||
| BECN1 expression | |||||
| High | 29 | 10 (34.5) | 19 (65.5) | 0.0283 | |
| Low | 31 | 3 (9.7) | 28 (93.5) | ||
Table 2.
The association between BECN1 expression with clinicopathological background.
| BECN1 immunoreactivity | |||||
|---|---|---|---|---|---|
| N | High (%) | Low (%) | p value | ||
| Total | 60 | 29 (48.3) | 31 (51.7) | ||
| Gender | Male | 34 | 17 (50.0) | 17 (50.0) | 0.7997 |
| Female | 26 | 12 (46.2) | 14 (53.8) | ||
| Age | |||||
| Median | 61 | ||||
| ≥61 | 28 | 9 (15.2) | 19 (84.8) | 0.0227 | |
| <61 | 32 | 20 (29.6) | 12 (70.4) | ||
| Histopathological grading | |||||
| Well/moderately | 48 | 23 (20.8) | 25 (79.2) | >0.05 | |
| Poorly | 12 | 6 (27.2) | 6 (81.8) | ||
| pT categories | |||||
| pT2 | 9 | 3 (33.3) | 6 (66.7) | 0.4743 | |
| pT3 | 51 | 26 (51) | 25 (49) | ||
| pN categories | |||||
| pN0/1 | 31 | 25 (80.6) | 6 (19.4) | <0.001 | |
| pN2 | 29 | 4 (13.8) | 25 (86.2) | ||
| Stage | |||||
| II | 19 | 8 (42.1) | 11 (57.9) | 0.5853 | |
| III | 41 | 21 (51.2) | 20 (48.8) | ||
| Chemosensitivity | |||||
| Sensitive | 21 | 17 (81) | 4 (19) | 0.0003 | |
| Resistant | 39 | 12 (30.8) | 27 (69.2) | ||
Ectopic expression of PCDH17 induces caspase-dependent apoptosis and autophagy in CRC cells after 5-FU treatment
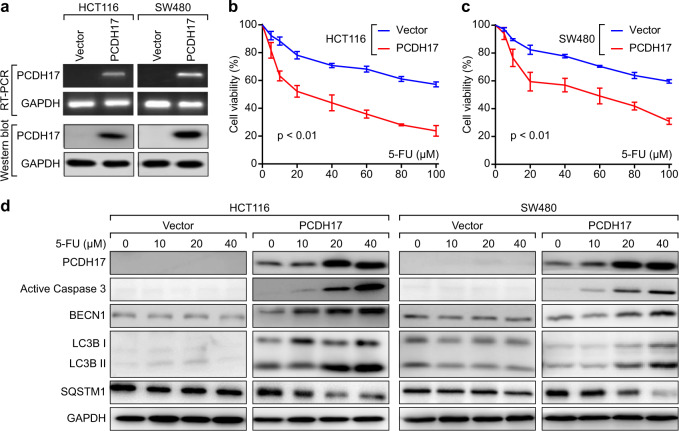
Our previous study showed that methylation mediated the transcriptional silencing of PCDH17 in CRC cell lines, including HCT116 and SW480 cells. We also established 5-FU-resistant HCT116 cells. However, in agreement with the results in 5-FU-sensitive HCT116 cells, we did not observe PCDH17 expression in HCT116/5-FU-resistant cells (Supplemental Fig. 1). In this study, we detected the effects of ectopic PCDH17 expression on CRC cell growth. The forced expression of PCDH17 in HCT116 and SW480 cells was confirmed by RT-PCR and western blot (Fig. 2a). We next investigated whether tumor cell growth was inhibited by ectopic PCDH17 expression. The CCK-8 assay results showed that PCDH17-transfected cells exhibited significantly lower viability than controls after treatment with various concentrations of 5-FU for 24 h (Fig. 2b, c). We further studied the effect of PCDH17 expression on apoptosis and autophagy with 5-FU treatment. As shown in Fig. 2d, an accumulation of PCDH17 protein was detected in CRC cells treated with different concentrations of 5-FU. The accumulated PCDH17 caused apoptotic cell death and triggered autophagy in a 5-FU dose-dependent manner, indicating that apoptosis and autophagy were involved in the effect of PCDH17 on CRC cell viability. Therefore, we used 20 μM 5-FU in PCDH17-transfected HCT116 and SW480 cells in subsequent experiments.
Fig. 2.
Ectopic expression of PCDH17-induced apoptosis and autophagy in HCT116 and SW480 cells. a PCDH17 expression in stably transfected cells as confirmed by RT–PCR and western blot. b, c Effect of ectopic expression of PCDH17 on the viability of CRC cells. Cell viability was measured via the CCK-8 assay after 5-FU treatment. Data are presented as the means ± standard deviations (SDs). The experiments were performed in triplicate. d Western blot analysis was performed to determine the expression of apoptosis and autophagy proteins in CRC cells transfected with empty vector or PCDH17 siRNA.
Restoration of PCDH17 expression activates autophagic cell death in CRC cells
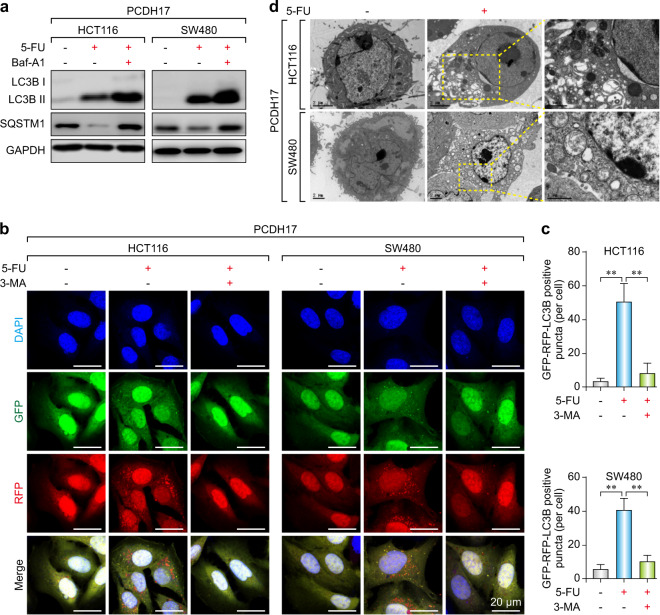
It is known that autophagy is one of the conserved protein degradation processes, and it can lead to resistance to chemotherapy treatment through a cell survival or cell death mechanism, depending on the cellular status. To determine the effect of PCDH17 on the autophagy induced by 5-FU, we investigated the level of LC3-II, the number of autophagic puncta and the formation of autophagosomes in HCT116 and SW480 cells. Western blotting results revealed that PCDH17 increased the turnover of the autophagic marker LC3B-II and SQSTM1 (sequestosome 1) degradation (Fig. 3a). Moreover, LC3B-II levels were further increased and SQSTM1 was increased after BafA1 (bafilomycin A1) treatment, indicating that 5-FU induced the autophagic flux of PCDH17-transfected CRC cells. In accordance with these results, the number of GFP-RFP-LC3-II-positive puncta in PCDH17-transfected CRC cells was significantly higher than that in the control cells after 24 h exposure to 20 μM 5-FU, whereas little signal was observed after treatment with the autophagy inhibitor 3-MA (3-methyladenine) (Fig. 3b, c). The TEM results also indicated that there were more autophagosomes and autolysosomes in the cytoplasm of PCDH17-transfected cells than in that of control cells (Fig. 3d).
Fig. 3.
PCDH17 increases autophagosome formation and autophagic flux in CRC cells. a PCDH17-transfected HCT116 and SW480 cells were treated with 20 μM 5-FU with or without 10 μM BafA1 for 24 h, and the protein levels of LC3B and SQSTM1 were assessed by western blotting. b HCT116/PCDH17 and SW480/PCDH17 cells were transfected with the GFP-RFP-LC3 plasmid overnight and transferred onto coverslips. After exposure to 20 μM 5-FU with or without 3-MA for 24 h, representative images of LC3-II-positive puncta were obtained with a confocal fluorescence microscope. c The quantitative analyses of the number of fluorescent puncta are shown. The experiments were performed in triplicate. **p < 0.01. d Electron microscopy shows the ultrastructures of autophagosome and autolysosome vesicles in these cells. The experiments were performed in triplicate. Bar = 2 μm.
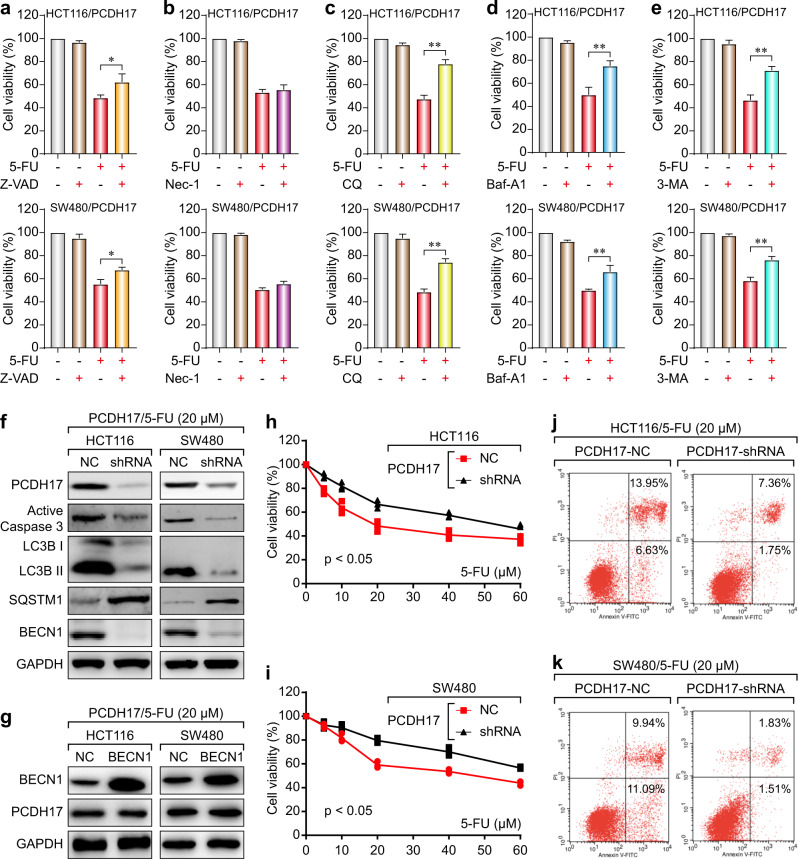
To investigate whether PCDH17-triggered autophagy plays a prosurvival or prodeath role, PCDH17-transfected CRC cells were exposed to 5-FU with or without different inhibitors. The 5-FU treatment combined with Z-VAD-FMK (a pancaspase inhibitor) or the autophagy inhibitor chloroquine (CQ), BafA1 and 3-MA, but not with the necroptosis inhibitor necrostatin-1, prevented the PCDH17-induced cell growth inhibition (Fig. 4a–e). Furthermore, autophagy played a dominant role in PCDH17-induced cell death, since the autophagy inhibitors blocked cell death to a greater extent than the pancaspase inhibitor Z-VAD-FMK (Fig. 4a, c–e). These results strongly indicate that PCDH17-induced apoptosis and autophagy collectively trigger cell death. Additionally, autophagy serves as the predominant method by which PCDH17 induces cell death.
Fig. 4.
Autophagy contributes to PCDH17-induced growth inhibition in CRC cells, and PCDH17 regulates autophagy and 5-FU sensitivity. a HCT116/PCDH17 and SW480/PCDH17 cells were treated with 5-FU with or without the pancaspase inhibitor Z-VAD-FMK for 24 h, and cell viability was assayed. The experiments were performed in triplicate. *p < 0.05. b HCT116/PCDH17 and SW480/PCDH17 cells were treated with 5-FU with or without the necroptosis inhibitor necrostatin-1 for 24 h, and cell viability was assayed. The experiments were performed in triplicate. c HCT116/PCDH17 and SW480/PCDH17 cells were treated with 5-FU with or without the autophagy inhibitor CQ (20 μM) for 24 h, and cell viability was assayed. The experiments were performed in triplicate. **p < 0.01. d HCT116/PCDH17 and SW480/PCDH17 cells were treated with 5-FU with or without the autophagy inhibitor BafA1 (0.05 nM) for 24 h, and cell viability was assayed. The experiments were performed in triplicate. **p < 0.01. e HCT116/PCDH17 and SW480/PCDH17 cells were treated with 5-FU with or without the autophagy inhibitor 3-MA (5 μM) for 24 h, and cell viability was assayed. The experiments were performed in triplicate. **p < 0.01. f HCT116/PCDH17 and SW480/PCDH17 cells were transfected with PCDH17-specific shRNA, and then PCDH17, caspase-3, BECN1 and LC3B levels were assessed by western blotting. g HCT116/PCDH17 and SW480/PCDH17 cells were transfected with BECN1 plasmid, and then BECN1 and PCDH17 levels were assessed by western blotting. h, i HCT116/PCDH17 and SW480/PCDH17 cells were transfected with PCDH17-specific shRNA, and then the cells were exposed to 5-FU at various final concentrations for 24 h. Cell viability was measured by CCK-8 assay after 48 h. Data are presented as the means ± standard deviations (SDs). The experiments were performed in triplicate. j, k HCT116/PCDH17 and SW480/PCDH17 cells were transfected with PCDH17-specific shRNA, and then the cells were exposed to 5-FU at various final concentrations for 24 h. Cell viability was measured by an Annexin V–FITC dual staining assay followed by flow cytometry after 48 h. Data are presented as the means ± standard deviations (SDs). The experiments were performed in triplicate.
PCDH17 regulates autophagy and apoptosis and augments 5-FU sensitivity
Next, we tested the effect of PCDH17 knockdown on autophagy and 5-FU sensitivity by using short hairpin RNA (shRNA) to knockdown PCDH17 expression in PCDH17-transfected CRC cells. Both HCT116/PCDH17 and SW480/PCDH17 cells were transfected with PCDH17-specific shRNA. Decreased expression of PCDH17 was confirmed by western blotting (Fig. 4f). We found that PCDH17 knockdown by specific shRNA significantly inhibited LC3B-II turnover and the expression of BECN1 and active caspase-3 (Fig. 4f); however, BECN1 overexpression did not affect PCDH17 expression (Fig. 4g), indicating that PCDH17 can modulate BECN1 and apoptosis.
We then assessed PCDH17 regulation of 5-FU sensitivity in HCT116 and SW480 cells. The CCK-8 assay showed that the cytotoxic effect of 5-FU was significantly inhibited in a dose-dependent manner when PCDH17 was knocked down compared with the controls (Fig. 4h, i). To quantify the cell viability, we performed an Annexin V-FITC dual staining assay and then flow cytometry. In line with the above findings, flow cytometry results also showed that PCDH17 knockdown significantly decreased 5-FU sensitivity in HCT116/PCDH17 and SW480/PCDH17 cells (Fig. 4j, k). Taken together, these data suggested that PCDH17 could modulate autophagy and augment the 5-FU sensitivity of CRC cells.
Restoration of PCDH17 expression activates autophagic cell death to augment the 5-FU sensitivity of HCT116 cells in vivo
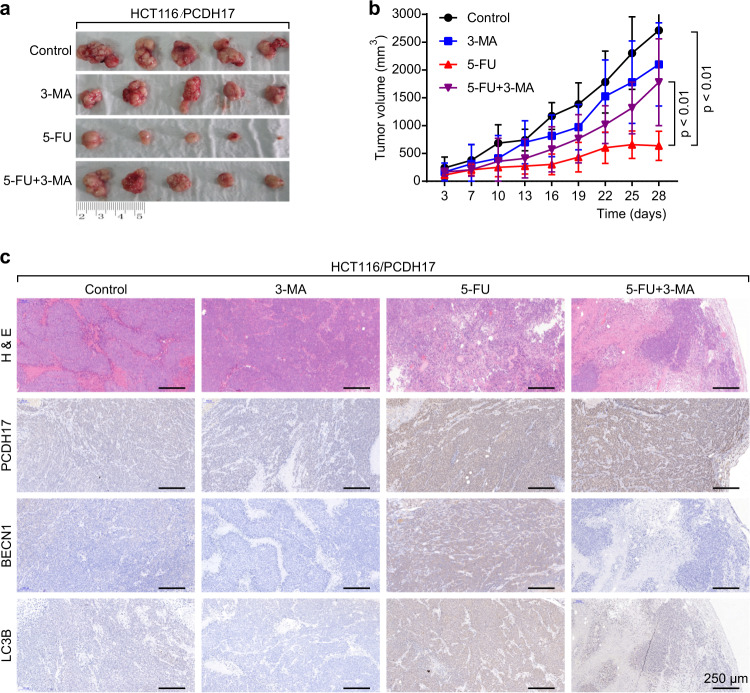
To investigate whether PCDH17 can sensitize cells to the anticancer effects of 5-FU in vivo, xenograft tumor models of HCT116/PCDH17 cells were generated. When the size of tumor xenografts reached 100 mm3, the mice were randomly divided into 4 groups: the control group, 5-FU group (40 mg/kg intraperitoneal injection every 2 days for 28 days), 3-MA group (20 mg/kg intraperitoneal injection every 2 days for 28 days), and 5-FU plus 3-MA group.
As shown in Fig. 5a, b, not 3-MA but 5-FU alone (p < 0.01) exhibited a significant effect on the growth of HCT116/PCDH17 CRC cells. In accordance with the in vitro results, the tumor size of the 5-FU plus 3-MA group was significantly larger than that of the 5-FU group (p < 0.01), indicating that PCDH17 can induce prodeath autophagy in vivo when CRC cells are treated with 5-FU. Furthermore, no significant hepatic toxicity or weight loss was observed in the 5-FU group or the 5-FU plus 3-MA group (data not shown).
Fig. 5.
PCDH17 regulates autophagy and augments 5-FU sensitivity in vivo. a Representative image of PCDH17 xenograft tumors. b Tumor volume in each group. Data are expressed as the means ± standard deviations (SDs). c H&E and immunohistochemical staining of tumor specimens. Scale bars = 250 μm.
Next, we examined the protein expression of PCDH17, BECN1 and LC3B by immunohistochemical staining. As shown in Fig. 5c, 5-FU treatment resulted in an upregulation of PCDH17 and autophagy in HCT116/PCDH17 CRC cells.
Taken together, our data indicated that PCDH17 sensitized CRC cells to the anticancer effects of 5-FU in vivo by inducing autophagic cell death. By inhibiting autophagy, 3-MA potently attenuated the antitumor activity of 5-FU in vivo.
PCDH17 activates autophagy via the c-Jun N-terminal kinase (JNK) pathway
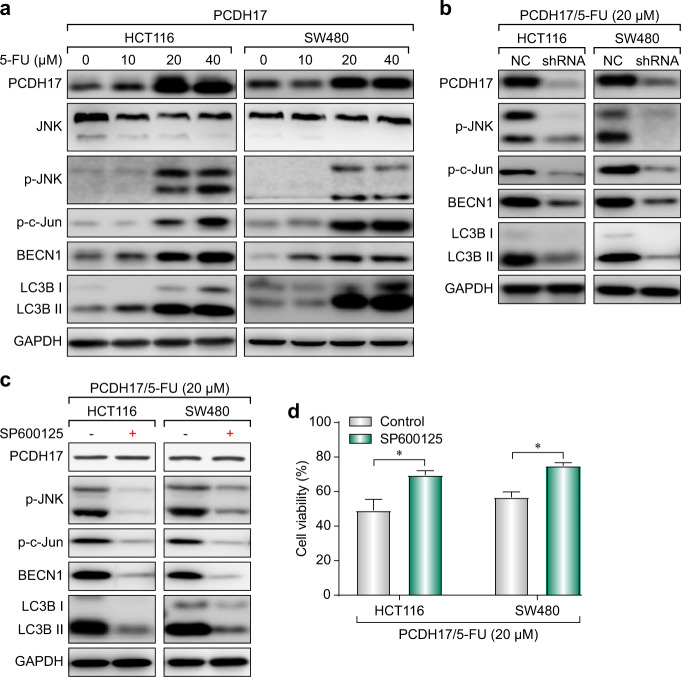
c-Jun N-terminal kinase (JNK) plays a key role in the sensitivity to and outcome of anticancer therapies. JNK activation can mediate BECN1 expression to induce autophagic cell death in response to chemotherapeutic agents.15 Because we observed that autophagic cell death was involved in the effect of PCDH17 on CRC cell viability, we next detected the protein levels of phosphorylated-JNK (p-JNK) to determine whether the JNK pathway is activated in PCDH17-transfected cells when exposed to 5-FU treatment. As shown in Fig. 6a, we found that PCDH17 was upregulated in 5-FU-treated HCT116/PCDH17 and SW480/PCDH17 cells in a dose-dependent manner, which might be attributed to endogenous PCDH17 expression (Supplemental Fig. 2). JNK activation was observed along with PCDH17 upregulation, suggesting that JNK may be activated in these cells exposed to 5-FU. For further confirmation, we preformed western blotting and found that phosphorylated c-Jun was significantly induced in HCT116/PCDH17 and SW480/PCDH17 cells in a 5-FU dose-dependent manner. Moreover, increases in BECN1 expression and the LC3-II/I ratio were observed when HCT116/PCDH17 and SW480/PCDH17 cells were treated with 5-FU (Fig. 6a).
Fig. 6.
PCDH17 meditates JNK activation to augment the cytotoxic effect of 5-FU in CRC cells by inducing prodeath autophagy. a HCT116/PCDH17 and SW480/PCDH17 cells were treated with varying concentrations of 5-FU for 24 h, and then PCDH17, JNK signaling, and LC3B were assessed by western blotting. b HCT116/PCDH17 and SW480/PCDH17 cells were transfected with PCDH17-specific shRNA, and then PCDH17, JNK signaling, BECN1 and LC3B were assessed by western blotting. c HCT116/PCDH17 and SW480/PCDH17 cells were treated with the combination of 5-FU and SP600125, and then PCDH17, JNK signaling, BECN1 and LC3B were assessed by western blotting. d HCT116/PCDH17 and SW480/PCDH17 cells were treated with the combination of 5-FU and SP600125. Cell viability was measured by CCK-8 assay. Data are presented as the means ± standard deviations (SDs). The experiments were performed in triplicate. *p < 0.05.
Next, we used shRNA to knockdown PCDH17 expression in PCDH17-transfected CRC cells and studied the effect of such PCDH17 inhibition on JNK activation and autophagy. Western blotting was used to confirm the reduced expression of PCDH17 (Fig. 6b). PCDH17 knockdown by specific shRNA significantly inhibited JNK activation and LC3B-II turnover (Fig. 6b), indicating that PCDH17 can modulate JNK activation and the induction of autophagy.
To determine whether JNK activation may be correlated with autophagy induction in HCT116/PCDH17 and SW480/PCDH17 cells in response to 5-FU, we specifically inhibited JNK activation with the JNK inhibitor SP600125 (10 µM). As shown in Fig. 6c, inhibiting JNK in PCDH17-transfected CRC cells significantly decreased the level of phosphorylated c-Jun and the ratio of LC3-II/I but had no effect on PCDH17 expression. Moreover, inhibition of autophagy by JNK knockdown attenuated the cytotoxicity activity of 5-FU (Fig. 6d). These results demonstrated that PCDH17-induced autophagic cell death by activating the JNK signaling pathway.
Discussion
CRC is the third most common malignancy worldwide and associated with high-mortality. To date, 5-FU-based chemotherapy remains widely used in the clinical treatment of CRC patients, but resistance to 5-FU is a major drawback in its clinical use.16 Thus, overcoming 5-FU resistance or increasing 5-FU treatment efficacy is urgently needed.
PCDH17, a member of the cadherin superfamily, functions as a potential tumor suppressor and is found to be transcriptionally silenced in various human cancer types due to molecular mechanisms such as deletion, mutation, and promoter methylation.17,18 Our previous study reported that PCDH17 is frequently methylated and inactivated in gastric and colorectal cancers, in which PCDH17 functions as a tumor suppresser by inducing apoptosis and autophagy.10 However, no further detailed analysis of the biological roles of PCDH17 in autophagy and the sensitivity of CRC cells to 5-FU has been conducted to date.
In this study, the protein expression of PCDH17 and BECN1 was detected in CRC tissues by IHC. We first revealed that the expression of PCDH17 and BECN1 was significantly upregulated in chemosensitive tissues compared with chemoresistant tissues. Moreover, the clinical analysis results indicated that high expression of PCDH17 and BECN1 was significantly correlated with better overall survival and higher 5-FU sensitivity than were seen with low expression of PCDH17 and BECN1. Next, we further investigated the effect of 5-FU on PCDH17 expression and found an accumulation of PCDH17 protein in CRC cells treated with different concentrations of 5-FU. The accumulated PCDH17 caused apoptosis and autophagy in a 5-FU dose-dependent manner. Moreover, autophagy serves as the predominant method by which PCDH17 induces cell death. PCDH17 knockdown by shRNA or autophagy inhibition significantly decreased the sensitivity to 5-FU in PCDH17-transfected CRC cells. Taken together, these data suggest that PCDH17 can augment the 5-FU sensitivity of CRC cells by modulating autophagy.
The JNK pathway plays a key role in cancer genesis and development, including processes such as DNA repair, cell proliferation, apoptosis, metabolism and motility. When cells encounter genotoxic stress, JNK is also involved in autophagy induction. Thus, we postulated that PCDH17 could activate the JNK pathway and mediate autophagic cell death in CRC cells. To clarify this hypothesis, we focused on the correlation between JNK pathway activation and PCDH17-induced autophagy. Here, we demonstrated that PCDH17 was upregulated in a dose-dependent manner, which was observed along with JNK activation, suggesting that JNK could be activated in these cells after 5-FU treatment. On the other hand, PCDH17 knockdown and the JNK-specific inhibitor SP600125 inhibited autophagy induction and attenuated the cytotoxicity activity of 5-FU, but JNK knockdown had no effect on PCDH17 expression.
Taken together, the present study showed that CRC patients with high expression of PCDH17 and BECN1 had a better prognosis than CRC patients with low expression of PCDH17 and BECN1. 5-FU induces PCDH17 upregulation and autophagic cell death in CRC cells, and the ectopic expression of PCDH17 augments the 5-FU sensitivity of CRC cells by promoting JNK-dependent autophagic cell death in vitro and in vivo. Our findings first suggest that PCDH17 increases the 5-FU sensitivity of CRC cells by inducing JNK-dependent autophagic cell death, which might support the clinical potential of PCDH17 for predicting 5-FU sensitivity in CRC patients.
Methods
Cell lines and reagents
The human colorectal cancer cell lines HCT116 and SW480 were obtained from the ATCC (LGC Standards SLU, Barcelona, Spain). They were cultured in McCoy’s 5 A or Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Rockville, MD, USA) containing 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 µg/mL streptomycin (Invitrogen), and 2 mmol/L L-glutamine in a humidified atmosphere of 5% carbon dioxide in air at 37 °C. The fetal bovine serum was obtained from Corille (184590, Australia). 5-FU was obtained from Jinyao Amino Acid Co., Ltd. (Tianjin, China).
BafA1 (B1793), 3-methyladenine (M9281), chloroquine (C6628), the pancaspase inhibitor Z-VAD-FMK (C2105), necrostatin-1 (N9037) and SP600125 (S5567) were purchased from Sigma Aldrich. Anti-LC-3B (#3868), anti-p62 (#8025), anti-cleaved caspase-3 (#9661), anti-SQSTM1/p62 (#5114), anti-BECN1 (#3495), anti-SAPK/JNK (#9252), anti-phospho-SAPK/JNK (Thr183/Tyr185) (81E11) (#4668), anti-phospho-c-Jun (Ser63) (54B3) (#2361), and anti-GAPDH (#5174) antibodies were purchased from Cell Signaling Technology (CST). Anti-PCDH17 (HPA026817) for IHC was obtained from Sigma Aldrich. Anti-PCDH17 (ab128815) for WB was obtained from Abcam. The PCDH17 plasmid (NM_001040429) was purchased from OriGene.
Patient-specimen selection and immunohistochemistry
IHC was performed with 60 formalin-fixed and paraffin-embedded tissue samples obtained from CRC patients diagnosed from January 2008 to October 2010. All patients volunteered to participate in the initial surgery and then received chemotherapy based on 5-FU. The follow-up deadline was June 30, 2018. Patients who developed disease during the initial treatment or relapsed within 6 months after completing the initial treatment were termed 5-FU-resistant. Patients who relapsed after 6 months or had no recurrence were termed 5-FU-sensitive.
Briefly, according to the instructions of the ChemMate EnVision test kit (Dako, Carpinteria, California, USA), the sections were incubated with the respective primary antibody overnight at 4 °C. The ChemMate EnVision/HRP, Rabbit/Mouse (ENV) reagent was applied to these sections, and then the ChemMate DAB chromogen was used. The slides were then slightly counterstained with hematoxylin.
The expression of PCDH17 and BECN1 proteins was scored according to the intensity of membrane and cytoplasmic staining using a four-point system: 0, negative; 1, weak; 2, moderate; and 3, strong. To investigate the correlation between PCDH17 and BECN1 expression and clinicopathological features, CRC patients were divided into two groups: low expression (0 and 1, –) or high expression (2 and 3, + ). Immunostaining was independently scored by two investigators who did not know the patient’s clinical information in separate cases.
PCDH17-expressing plasmids and transfection
The pCMV6 Entry-PCDH17 plasmid or empty vector (pCMV6 Entry-mock; OriGene, MD, USA) was transfected into the colorectal cell lines HCT116 and SW480 with the MegaTran 1.0 transfection reagent (OriGene), followed by selection of stable clones expressing PCDH17 or the empty vector for further study.
Cell Viability and Apoptosis Measurement
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) (LJ621, DOJINDO, JAPAN) following the product’s introduction. Cells were seeded at a density of 5,000 cells per well in 96-well flat bottom microtiter plates. After 24 h, 5-FU was added at different times at the indicated concentrations. The absorbance was measured at 450 nm on a microplate reader (Synergy HT, Bio-Tek, USA). Apoptosis was detected using a Pharmingen Annexin V-FITC Apoptosis Detection Kit I (BD, USA) following the manufacturer’s instructions. Briefly, cells were seeded at a density of 2 × 106 cells per 6 cm culture dish. After attachment overnight, cells were washed twice with PBS and cultured in medium with 20 µM 5-FU for 24 h. All cells were collected and resuspended in ice-cold 1 × binding buffer. Approximately 1 × 105 cells were then stained with 5 μL of Annexin V-FITC and 5 μL of PI for 15 min and analyzed using a FACS Calibur flow cytometer (Beckman Coulter, CytoFLEX S, USA).
Immunofluorescent confocal laser microscopy for LC3 and lysosome colocation
HCT116/PCDH17 and SW480/PCDH17 cells were transfected overnight with a GFP-RFP-LC3 plasmid and transferred to coverslips. After adhering to the coverslips, the cells were treated with 10 nM 5-FU plus 3-MA or not treated for 24 h. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, D9542), fixed with 4% paraformaldehyde (Sigma-Aldrich, 158127), and permeated with 0.5% Triton X-100 (Solarbio, T8200-100) in PBS (Corning, R21-040-CV). Images were taken with a spinning disk confocal fluorescence microscope (the system consists of the CSU-X1 rotating disk produced by Yokogawa, an IX81 microscope from Olympus and an IXON3 CCD from Andor) with a magnification of 600.
Western Blot Analysis
Cells were collected from cultured dishes and lysed in lysis buffer. The main components of the lysis buffer were 20 mM Tris-HCl pH 7.6, 1 mM EDTA, 140 mM NaCl, 1% NP-40, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM sodium vanadate. Protein concentration was determined using a BCA Protein Assay Kit (Pierce). Protein samples (40 μg of protein per cell line) were separated on a 5 to 20% Tris-Tricine Ready Gel (Bio-Rad) by SDS-PAGE for nitrocellulose membrane blotting. The blotted membrane was blocked with 5% skim milk for 1 h at room temperature and incubated overnight at 4 °C with primary antibody. The immunoreactive band was observed by enhanced chemiluminescence using horseradish peroxidase-conjugated IgG secondary antibodies. Band density was measured by densitometry, quantified using NIH image 1.62 gel map macros, and normalized to the specified sample in the same membrane.
RNA interference
PCDH17 human shRNA (OriGene, TR310584) was transfected with Lipofectamine RNAi Max (Invitrogen, 13778150) following the manufacturer’s instructions.
Transmission electron microscopy
The treated cells were washed and fixed in 2.5% glutaraldehyde (Sigma-Aldrich, G5882) for 30 min. The samples were treated with 1.5% osmium tetroxide, dehydrated with acetone and embedded in Durcupan resin. Thin sections were poststained with lead citrate and examined with a 60 kV TECNAI 10 electron microscope (Philips, Holland).
In vivo subcutaneous tumor model
All in vivo experimental protocols were approved by the Animal Protection Committee of Hangzhou Normal University. Viable HCT116/PCDH17 cells (1 × 107) were subcutaneously injected into the right dorsal flank of 6-week-old female BALB/c nude mice (five mice per group). Tumor volume was measured every 2 days for 4 weeks and calculated by the following formula: (short diameter)2 × (long diameter)/2.
Statistical analyses
Data are expressed as the means ± SD of three independent experiments. Prism 7.0c GraphPad software was used for statistical analysis. Significant differences between groups were analyzed by Student’s t test, and a p value < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81672932, 81730108, 81874380, 81802371, and 81973635), Zhejiang Provincial Natural Science Foundation of China for Distinguished Young Scholars (Grant No. LR18H160001), Zhejiang Province Science and Technology Project of TCM (Grant No. 2019ZZ016), Zhejiang Province Medical Science and Technology Project (Grant No. 2017RC007), Talent Project of Zhejiang Association for Science and Technology (Grant No. 2017YCGC002), Key Project of Hangzhou Ministry of Science and Technology (Grant No. 20162013A07), Zhejiang Provincial Project for the Key Discipline of Traditional Chinese Medicine (Grant No. 2017-XK-A09), Open Project Program of Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica (No. JKLPSE201807) and Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author contributions
X.S. and T.X. designed the research; S.L., H.L. and D.W. performed the immunohistochemical staining, autophagy analyses and cell viability assays. Q.L., H.L., G.L., X.C. and Y.L. provided technical support. P.C., B.Z., W.W., R.Z., B.C., M.Z. and X.H. performed the animal experiments. Q.L., L.C. and Y.L. performed the Kaplan–Meier survival analysis. X.C., G.L., Y.X., T.D., J.F. and J.L. contributed materials and performed data analysis. X.H., Q.Z., T.P., L.Y., T.J., W.Z., L.Z. and Y.S. collected data. X.S. wrote the manuscript with contributions from the other authors.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Shuiping Liu, Haoming Lin, Da Wang
Contributor Information
Tian Xie, Email: xbs@hznu.edu.cn.
Xinbing Sui, Email: hzzju@zju.edu.cn.
Supplementary information
The online version of this article (10.1038/s41392-019-0087-0) contains supplementary material, which is available to authorized users.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman I, et al. SMAD4 loss in colorectal cancer patients correlates with recurrence, loss of immune infiltrate, and chemoresistance. Clin. Cancer Res. 2019;25:1948–1956. doi: 10.1158/1078-0432.CCR-18-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou J, et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat. Commun. 2019;10:1078. doi: 10.1038/s41467-019-08902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henricks LM, Opdam FL, Beijnen JH, Cats A, Schellens JHM. DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: call for a drug label update. Ann. Oncol. 2017;28:2915–2922. doi: 10.1093/annonc/mdx411. [DOI] [PubMed] [Google Scholar]
- 6.Haruki S, et al. Frequent silencing of protocadherin 17, a candidate tumour suppressor for esophageal squamous cell carcinoma. Carcinogenesis. 2010;31:1027–1036. doi: 10.1093/carcin/bgq053. [DOI] [PubMed] [Google Scholar]
- 7.Costa VL, et al. TCF21 and PCDH17 methylation: An innovative panel of biomarkers for a simultaneous detection of urological cancers. Epigenetics. 2011;6:1120–1130. doi: 10.4161/epi.6.9.16376. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Liu J, Li X, Li JC. PCDH17 gene promoter demethylation and cell cycle arrest by genistein in gastric cancer. Histol. Histopathol. 2012;27:217–224. doi: 10.14670/HH-27.217. [DOI] [PubMed] [Google Scholar]
- 9.He, Y. et al. Protocadherin 17 is a tumor suppressor and is frequently methylated in nasopharyngeal carcinoma. Cancer Manag. Res.11, 1601–1613 (2019). [DOI] [PMC free article] [PubMed] [Retracted]
- 10.Hu X, et al. Protocadherin 17 acts as a tumour suppressor inducing tumour cell apoptosis and autophagy, and is frequently methylated in gastric and colorectal cancers. J. Pathol. 2013;229:62–73. doi: 10.1002/path.4093. [DOI] [PubMed] [Google Scholar]
- 11.Nassour J, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–663. doi: 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sui X, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaballah HH, Gaber RA, Mohamed DA. Apigenin potentiates the antitumor activity of 5-FU on solid Ehrlich carcinoma: Crosstalk between apoptotic and JNK-mediated autophagic cell death platforms. Toxicol. Appl Pharm. 2017;316:27–35. doi: 10.1016/j.taap.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Kaehler C, Isensee J, Hucho T, Lehrach H, Krobitsch S. 5-Fluorouracil affects assembly of stress granules based on RNA incorporation. Nucleic Acids Res. 2014;42:6436–6447. doi: 10.1093/nar/gku264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranova I, et al. Aberrant methylation of PCDH17 gene in high-grade serous ovarian carcinoma. Cancer Biomark. 2018;23:125–133. doi: 10.3233/CBM-181493. [DOI] [PubMed] [Google Scholar]
- 18.Byzia E, et al. Recurrent transcriptional loss of the PCDH17 tumor suppressor in laryngeal squamous cell carcinoma is partially mediated by aberrant promoter DNA methylation. Mol. Carcinog. 2018;57:878–885. doi: 10.1002/mc.22808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.