Abstract
In this study, we investigated the function of co-enzyme Q10 (Q10) in autophagy of primary chicken myocardial cells during heat stress. Cells were treated with Q10 (1 μΜ, 10 μΜ, and 20 μM) before exposure to heat stress. Pretreatment of chicken myocardial cells with Q10 suppressed the decline in cell viability during heat stress and suppressed the increase in apoptosis during heat stress. Treatment with 20 μM Q10 upregulated autophagy-associated genes during heat stress. The expression of LC3-II was highest in cells treated with 20 μM Q10. Pretreatment with Q10 decreased reactive oxygen species (ROS) levels during heat stress. The number of autophagosomes was significantly increased by 20 μM Q10 treatment, as demonstrated by electron microscopy or monodansylcadaverine (MDC) fluorescence. SQSTM1 accumulation was diminished by Q10 treatment during heat stress, and the number of LC3II puncta was increased. Treatment with 20 μM Q10 also decreased the activation of the PI3K/Akt/mTOR pathway. Our results showed that co-enzyme Q10 can protect primary chicken myocardial cells by upregulating autophagy and suppressing the PI3K/Akt/mTOR pathway during heat stress.
Keywords: Co-enzyme Q10, Heat stress, Autophagy, Myocardial cells, Chicken
Introduction
As animal husbandry and veterinary medicine has improved, animal welfare has focused on animal feeding, transport, and disease treatment. Heat stress is a common phenomenon in the animal breeding industry and often occurs when the stocking density is high, resulting in hyperthermia. Heat stress resulted in losses of $2.4 billion to the animal breeding industry in the USA alone (St-Pierre et al. 2003). Heat stress in broiler chickens increases basal metabolic rate and abdominal fat (N’dri et al. 2007), that negatively affects reproductive performance; compared with broiler chickens maintained in normal conditions, the amount and quality of sperm was significantly reduced under heat stress conditions. We have previously shown that heat stress can induce cardiac damage in chickens, indicated by increased levels of heart damage associated enzymes (for example, CK and LDH), increased cellular apoptosis, and vacuolar and granular degeneration in myocardial cells; these lesions in heart tissue could be detrimental to the health of broiler chickens (Tang et al. 2016; Wu et al. 2016; Zhang et al. 2016).
Co-enzyme Q10 (Q10), also called ubiquinone, is an endogenously synthesized cofactor that shares a similar structure with vitamin K and E (Flower et al. 2014). Oxidative stress is a main consequence of heat stress (Mehta et al. 2007; Vanlangenakker et al. 2008; Kalmar and Greensmith 2009). Co-enzyme Q10 possesses antioxidant activity; Q10 improved antioxidant enzyme activity (SOD and CAT) in the serum of aged rats (Belviranli and Okudan 2019), and Q10 relieved heat stress-induced oxidative damage to skeletal muscle in chickens by downregulating levels of ROS (Kikusato et al. 2016). Co-enzyme Q10 has been used clinically as an ancillary drug for treatment of heart-associated diseases, including heart attack and chronic cardiac failure; in this capacity, co-enzyme Q10 increases the levels of adenosine triphosphate (ATP) in heart tissue, stabilizes calcium-dependent ion channels in the myocardium, and prevents membrane oxidation and lipid peroxidation (Rauchova et al. 1995; Tsutsui et al. 2002; Madmani et al. 2014).
Autophagy is a defensive and adaptive mechanism through which damaged organelles are digested and abnormal proteins are denatured to maintain balance within cells and to protect them from damage, apoptosis, or death (Mizushima et al. 2008; Mizushima and Komatsu 2011). There are three types of autophagy: microautophagy, chaperone-mediated autophagy, and macroautophagy. Accumulation of ROS and elevation of the AMP/ATP ratio can trigger oxidative damage; AMPK can also be activated to inhibit the expression of mTORC1, thereby stimulating the synthesis of LC3II, Beclin 1, ATG-5, and other autophagy-related proteins (de Andrade Ramos and Witkin 2016). AKT/mTOR and HSP70-NF-kB/JNK signal pathways also participate in the induction of autophagy in chicken spleen (Chen et al. 2018). As a demonstration of the protective capacity of autophagy, increased autophagy in desmin-related cardiomyopathy decreased the accumulation of toxic protein aggregates and attenuated disease progression (Tannous et al. 2008). Enhanced autophagy induced by co-enzyme Q10 increases antioxidants (SOD, superoxide dismutase; GSH, glutathione; GPx, glutathione peroxidase) and reduces oxidation (TBARS, thiobarbituric acid reactive substances) in rat heart tissue during acute myocardial ischemia-reperfusion injury (Liang et al. 2017). Furthermore, autophagy is inhibited by high-glucose concentrations in human retinal pigment epithelial cells, and levels of apoptosis and ROS were significantly upregulated in association with the inactivation of the ROS/PINK1/Parkin signaling pathway (Zhang et al. 2019).
The protective function of autophagy has been described for many different organ systems, but most studies have focused on mammals; few studies on autophagy have been performed in avian species, and the connections between heat stress and autophagy are not completely understood in chickens. Moreover, while co-enzyme Q10 is considered an antioxidant and we have demonstrated that co-enzyme Q10 can protect chicken heart tissue during heat stress, it remains unknown whether Q10 influences autophagy in chicken heart tissue during heat stress. In the present study, we evaluated the ability of different concentrations of co-enzyme Q10 to induce autophagy and protect chicken myocardial cells during heat stress.
Materials and methods
Cell culture and treatment
All experiments were performed in accordance with the guidelines of the Animal Ethics Committee of Jiangsu Province (China). The study protocol was approved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China). Chicken primary myocardial cells were collected from embryonated eggs and were cultured as we previously described (Xu et al. 2017b). Heart tissue was removed and cut into pieces, then digested with collagenase type I (1 mg/ml; Life Technologies, Carlsbad, CA, USA) at 4 °C for 14–16 h. Cells isolated from heart tissue were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Fibroblast growth was inhibited by addition of 0.1 mM 5-bromo-2′-deoxyuridine solution (Sigma-Aldrich, Saint Louis, MO, USA). Cells were divided into several treatment groups: control group, cells without any treatment; Q10 groups, cells treated for 2 h with 1 μΜ, 10 μΜ, or 20 μM Q10; Q10 + HS groups, cells treated with 1 μΜ, 10 μΜ, or 20 μM Q10 for 2 h before heat stress. HS group, cells treated with heat stress only for 1 h. Co-enzyme Q10 was dissolved in dimethyl sulfoxide (DMSO), the final concentration of DMSO in the culture medium was 0.1%. As a control, same concentration of DMSO was added to all groups. Heat stress was 1 h, at a temperature of 40 °C using incubator with CO2 control; all cells were collected immediately post treatments.
Detection of cell viability
Cell viability was measured using CCK-8 kits (ab228554, Abcam, USA) according to the manufacturer’s instructions. At the end of each experiment, CCK-8 reagent was added into the culture medium of chicken primary myocardial cells from all experimental groups. Cells were incubated for 2 h at 37 °C. The optical density (OD) was measured using a spectrophotometer (Tecan, USA) at a wavelength of 460 nm.
Detection of cell apoptosis
Cells were collected from each experimental group and were washed with FACS buffer. After washing, cells were digested using pancreatic enzymes without ethylenediamine tetra-acetic acid (EDTA) and resuspended in Bind Buffer, and 10 μl annexin V-fluorescein isothiocyanate and 15 μl propidium iodide were added. Cells were analyzed by flow cytometry within 1 h. FlowJo v7.6.1 was used for data analyses.
Real-time quantitative PCR
Total mRNA from chicken primary myocardial cells was isolated using RNAISO Plus (9108, Takara, Japan). The concentration of total mRNA was determined using a NanoDrop 2000 (Thermo Fisher, USA). Reverse transcription was carried out with a real-time quantitative PCR instrument (Thermo Fisher, USA). mRNA expression was analyzed between the various treatment groups. mRNA expression levels were determined using the comparative Ct (2-△△Ct) method. The target gene mRNA levels were normalized to those of GAPDH mRNA. The primers used are listed in Table 1.
Table 1.
Primers for quantitative real-time polymerase chain reaction
| Gene | PCR primers |
|---|---|
| lc3 |
Forward 5′-AGTGAAGTGTAGCAGGATGA-3′ Reverse 5′-AAGCCTTGTGAACGAGAT-3′ |
| atg5 |
Forward 5′-GGCACCGACCGATTTAGT-3′ Reverse 5′-GCTGATGGGTTTGCTTTT-3′ |
| beclin1 |
Forward 5′-CGACTGGAGCAGGAAGAAG-3′ Reverse 5′-TCTGAGCATAACGCATCTGG-3′ |
| dynein |
Forward 5′-CGGCTTGACCTATGGAATCT-3′ Reverse 5′-CATCACTGCGAGGAACTGC-3′ |
| gapdh |
Forward 5′-AGAACATCATCCCAGCGT-3′ Reverse5′-AGCCTTCACTACCCTCTTG-3′ |
Western blot analysis
Following the various cell treatments, protein was extracted from myocardial cells using RIPA lysis buffer with PMSF. After determining the protein concentration, samples were boiled for 15 min. Following separation of proteins by SDS-PAGE, proteins were transferred onto PVDF membranes. Membranes were then blocked using skimmed milk powder for 2 h at room temperature prior to incubation with primary antibodies, including LC3II (ab63817, Abcam, USA); p-AKT (ab110231, Abcam, USA), p-PI3K (TA326165, Origen, USA), p-mTOR (ab45996, Abcam, USA), GAPDH (ab181602, Abcam, USA); and beta-tubulin (ab18207, Abcam, USA) at 4 °C for 14 h. After incubation with the corresponding peroxidase-conjugated secondary antibodies, chemiluminescent reagent was applied for detection of protein bands. The intensity of protein bands on developed film was quantified using Quantity One software.
Detection of ROS
Cellular reactive oxygen species levels were detected using a Cellular ROS Assay Kit (ab186027, Abcam, USA), according to the manufacturer’s instructions. After treatment (2 h) with co-enzyme Q10, cells were incubated with ROS working solution before heat stress treatment (1 h). Following treatments, cells were incubated at 37 °C for 45 min in the dark. Cells were then washed three times with PBS and were then observed using a fluorescence microscope (Carl Zeiss, Germany).
Transmission electron microscopy
Chicken myocardial cells were collected and immersed in 2.5% glutaraldehyde in 0.01 M PBS solution for 24 h. After cells were permeabilized, they were placed into a 1% osmium tetroxide solution for 1 h at 37 °C. Cells were dehydrated with ascending concentrations of ethyl alcohol and embedded with a propylene oxide–araldite mixture. A transmission electron microscope (Carl Zeiss, Germany) was used to observe autophagosomes. Three fields of view were chosen randomly and analyzed for each sample; the numbers of autophagosome were counted.
Immunofluorescence analysis
Chicken primary myocardial cells were washed with PBS before fixation in 4% paraformaldehyde. Cells were washed with PBST, then blocked with 5% bovine serum albumin (BSA). Cells were incubated with anti-chicken LC3II and anti-SQSTM1 antibodies at 4 °C for 12 h. After incubation with primary antibodies, cells were washed with PBST before incubating with the corresponding secondary antibodies. Cells were washed with PBS, and the nuclei of myocardial cells were stained with DAPI. Fluorescence microscopy was used to observe the distribution of SQSTM1 and LC3II, and the number of LC3II puncta in every cell was counted.
MDC fluorescence
Myocardial cells were washed with PBS and were then incubated with MDC at 37 °C for 30 min in the dark. Cells were washed again with PBS and immunofluorescence microscopy was used to observe the cells.
Statistical analysis
Differences between the experimental groups and the control group were analyzed by one-way analysis of variance (ANOVA) and post hoc test using SPSS software (v20.0; IBM, Armonk, NY, USA). Results are expressed as the mean ± standard error of the mean (SEM). A P value of P < 0.05 was considered statistically significant, and P < 0.01 was considered to indicate a high degree of significance. Unless indicated otherwise, experiments were performed in triplicate (n = 3).
Results
Effects of Q10 on cell viability after heat stress
Cell viability in the Q10 groups did not differ significantly from the control group. However, as seen in the HS group, exposure to heat stress significantly reduced cell viability (P < 0.01). This reduction in cell viability was attenuated in a dose-dependent manner by treatment with 1 μΜ (P < 0.05), 10 μΜ (P < 0.01), and 20 μM (P < 0.01) Q10 before heat stress (Q10 + HS groups, Fig. 1).
Fig. 1.
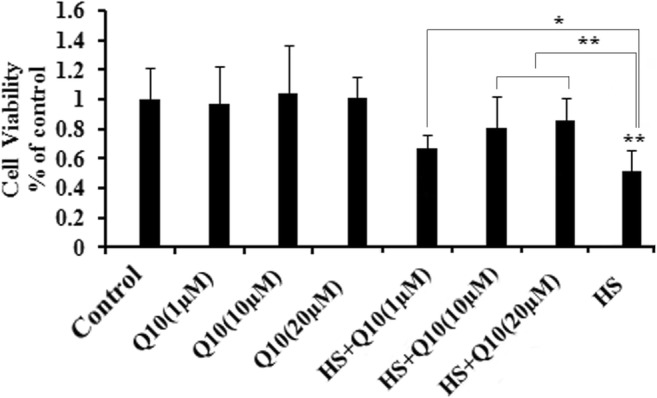
Cell viability of heat stress and Q10 treatment groups. Exposure to heat stress significantly reduced cell viability. Treatment with 1 μΜ, 10 μΜ, and 20 μM Q10 suppressed the decline in cell viability seen during heat stress. Three well plates were set for each group and each concentration. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
Effects of Q10 on cell apoptosis during heat stress
Heat stress (HS group) induced cell apoptosis compared with the control group (P < 0.01; Fig. 2). While treatment with Q10 did not completely prevent cell apoptosis during heat stress, the addition of 10 μΜ (P < 0.05) and 20 μM (P < 0.01) Q10 significantly reduces the percentage of apoptosis during heat stress compared with the HS group (Fig. 2). No obvious cell apoptosis was detected in the Q10-treated groups in the absence of heat stress (data was not shown). Q10 suppressed the HS-induced apoptosis in a dose-dependent manner.
Fig. 2.
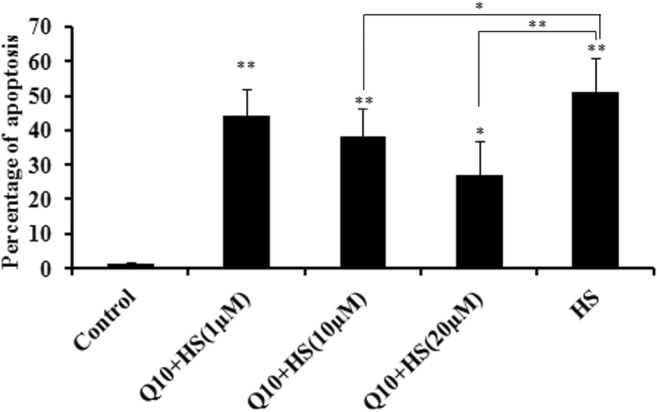
Cell apoptosis in different experimental groups after heat stress. Addition of 10 μΜ (P < 0.05) and 20 μM (P < 0.01) Q10 significantly suppressed the percentage of apoptosis during heat stress compared with the heat stress group. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
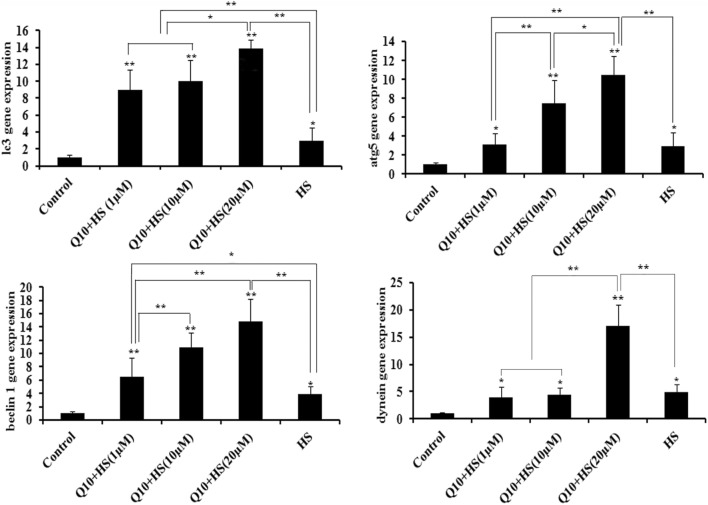
Effects of Q10 on gene expression of LC3, ATG5, Beclin-1, and dynein
Heat stress induced significant increases in expression of LC3, ATG5, Beclin-1, and dynein compared to the control group (P < 0.05; Fig. 3). Compared to the HS group, the addition of 1 μΜ Q10 increased the gene expression of LC3 (P < 0.01) and Beclin-1 (P < 0.05) in Q10 + HS groups; addition of 10 μΜ Q10 increased the gene expression of LC3 (P < 0.01), ATG5 (P < 0.01), and Beclin 1 (P < 0.01) in the Q10 + HS group; and addition of 20 μM Q10 significantly increased expression of LC3, ATG5, Beclin-1, and dynein (P < 0.01) in the Q10 + HS group. Of the different Q10 treatment groups, 20 μM Q10 induced the maximum increase in gene expression of LC3 (compared with 1 μM and 10 μM Q10, P < 0.05), ATG5 (compared with 1 μM Q10, P < 0.01 and 10 μM Q10, P < 0.05), Beclin 1 (compared with 1 μM Q10, P < 0.01), and dynein (compared with 1 μM and 10 μM Q10, P < 0.01) during heat stress when compared with HS group (Fig. 3).
Fig. 3.
Gene expression of LC3, ATG5, Beclin-1, and dynein. Heat stress induced significant increases in expression of LC3, ATG5, Beclin-1, and dynein compared to the control group. Compared to the heat stress group, the addition of 1 μΜ Q10 increased the gene expression of LC3 and Beclin-1 during heat stress; addition of 10 μΜ Q10 increased the gene expression of LC3, ATG5, and Beclin 1 during heat stress; addition of 20 μM Q10 significantly increased expression of LC3, ATG5, Beclin-1, and dynein during heat stress. Of the different Q10 treatment groups, 20 μM Q10 induced the maximum increase in gene expression of LC3 (compared with 1 μM and 10 μM Q10,), ATG5 (compared with 1 μM Q10 and 10 μM Q10), Beclin 1 (compared with 1 μM Q10), and dynein (compared with 1 μM and 10 μM Q10) during heat stress when compared with HS group. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
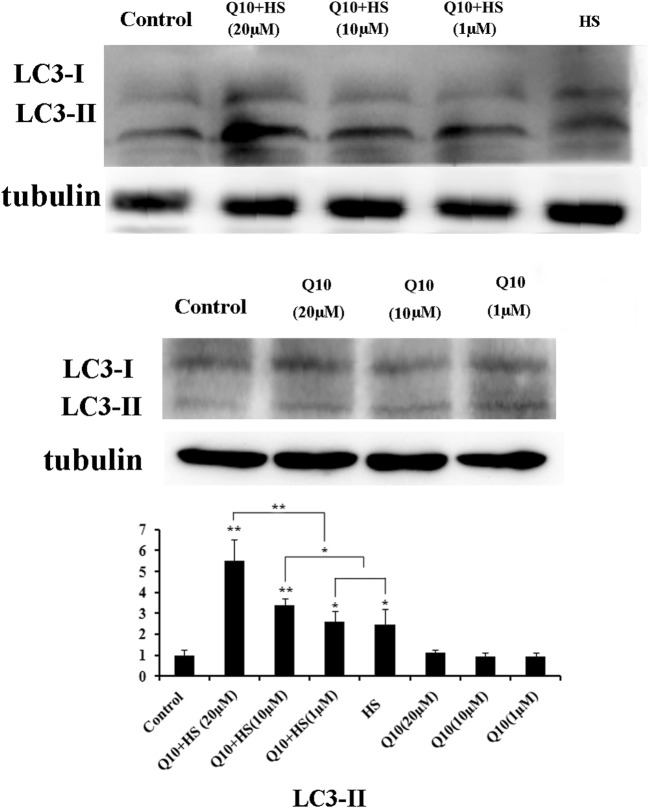
Effects of Q10 on expression of LC3-I and LC3-II
Treatment with Q10 alone did not induce expression of LC3 expression; however, heat stress increased expression of both LC3-II and LC3-I in chicken myocardial cells (Fig. 4). Treatment with 10 μM or 20 μM Q10 during heat stress significantly increased the expression of LC3-II compared with the HS only group (P < 0.05 and P < 0.01, respectively). Moreover, treatment with 20 μM Q10 significantly increased LC3-II expression over the 1 μΜ and 10 μΜ Q10 treatments (P < 0.01; Fig. 4).
Fig. 4.
Expression of LC3-I and LC3-II in myocardial cells after heat stress. Treatment with Q10 alone did not induce expression of LC3 expression; heat stress increased expression of both LC3-II and LC3-I in chicken myocardial cells. Treatment with 10 μM or 20 μM Q10 during heat stress significantly increased the expression of LC3-II compared with the HS only group; treatment with 20 μM Q10 significantly increased LC3-II expression over the 1 μΜ and 10 μΜ Q10 treatments. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
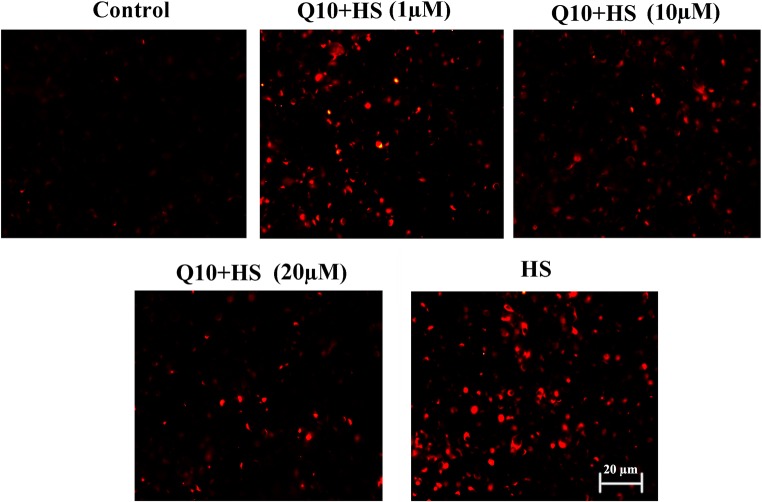
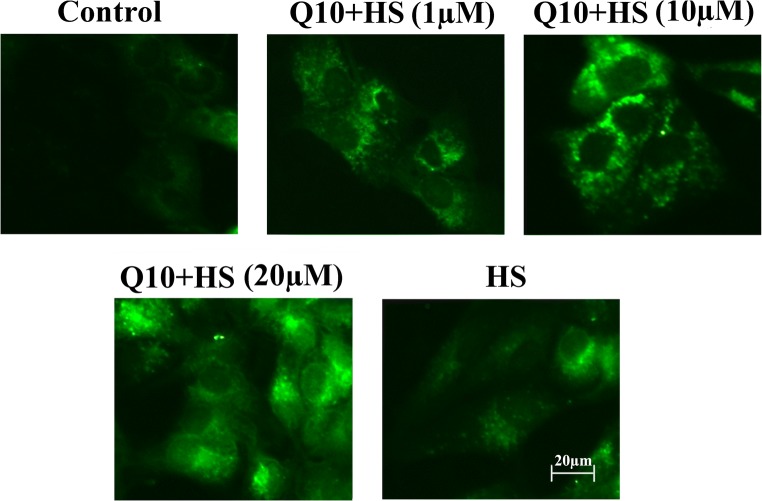
Effects of Q10 on levels of cellular ROS
Almost no ROS accumulation was detected in the control group, and heat stress induced rapid ROS production (Fig. 5). Treatment with Q10 alleviated ROS generation, and ROS levels in the 10 μΜ and 20 μM Q10 + HS groups were much lower than those in the 1 μΜ Q10 + HS group (Fig. 5).
Fig. 5.
Detection of ROS in myocardial cells after heat stress. Almost no ROS accumulation was detected in the control group, and heat stress induced rapid ROS production. Treatment with Q10 alleviated ROS generation, and ROS levels in the 10 μΜ and 20 μM Q10 treatment groups were much lower than those in the 1 μΜ Q10 treatment group. Scale bar = 20 μm
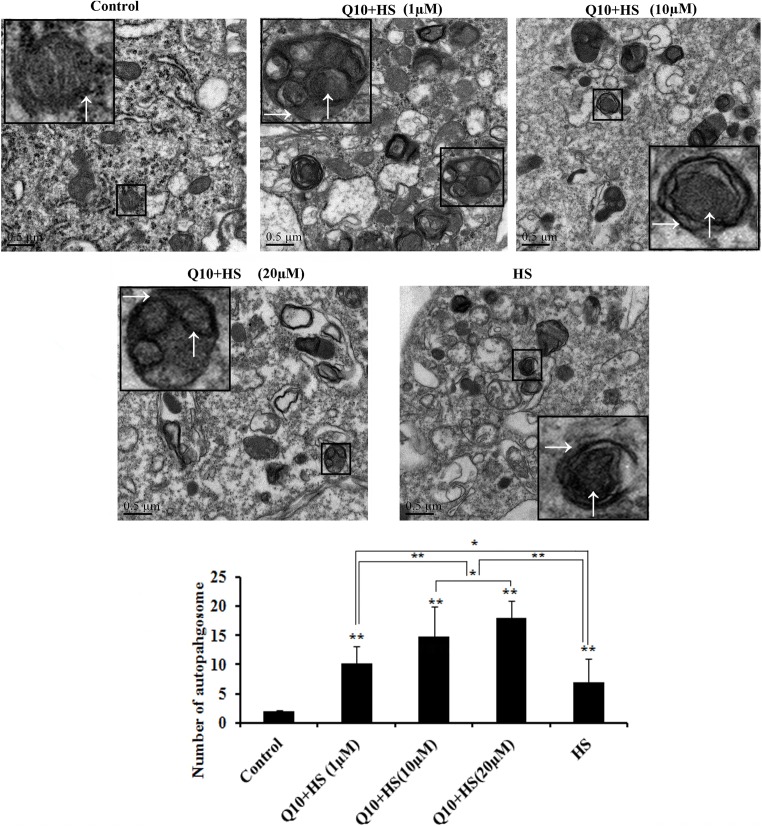
Effects of Q10 on autophagosome generation during heat stress
Almost no autophagosome bodies were observed in the control group; mitochondria were also under normal form (↑). In the heat stress group, however, after 1-h heat stress, swollen mitochondria were seen, and the number of cristae was reduced because of heat stress, and autophagosomes were present in myocardial cells (→) (magnified part, Fig. 6) by transmission electron microscopy. Treatment with co-enzyme Q10 accelerated the formation of autophagosomes, and the number of autophagosomes in the 10 μΜ and 20 μM Q10 treatment groups was significantly higher than the 1 μM Q10 treatment group or the heat stress group (P < 0.01). Moreover, the number of autophagosomes in the 20 μM Q10 treatment group was significantly higher than that in the 10μΜ Q10 + HS group (P < 0.05; Fig. 6). MDC fluorescence revealed similar autophagosome counts as those observed by electron microscopy, as treatment with 10 μΜ and 20 μM Q10 significantly increased the autophagosome formation, and 20 μM Q10 treatment resulted in the most significant increase in acceleration of autophagy during heat stress (Fig. 7).
Fig. 6.
Detection of autophagosomes using transmission electron microscopy. Almost no autophagosome bodies were observed in the control group; mitochondria were also under normal form (↑). In the heat stress group, however, after 1-h heat stress, autophagosomes were discovered in myocardial cells (→) (magnified part). The number of autophagosomes in the 10 μΜ and 20 μM Q10 treatment groups was significantly higher than that in the 1 μM Q10 treatment group or the heat stress control group; the number of autophagosomes in the 20 μM Q10 treatment group was significantly higher than that in the 10 μΜ treatment Q10 group. Scale bar = 0.5 μm. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
Fig. 7.
Detection of autophagosomes using monodansylcadaverine (MDC) staining. Treatment with 10 μΜ and 20 μM Q10 significantly increased the autophagosome formation, and 20 μM Q10 treatment resulted in the most significant increase in acceleration of autophagy during heat stress. Scale bar = 20 μm
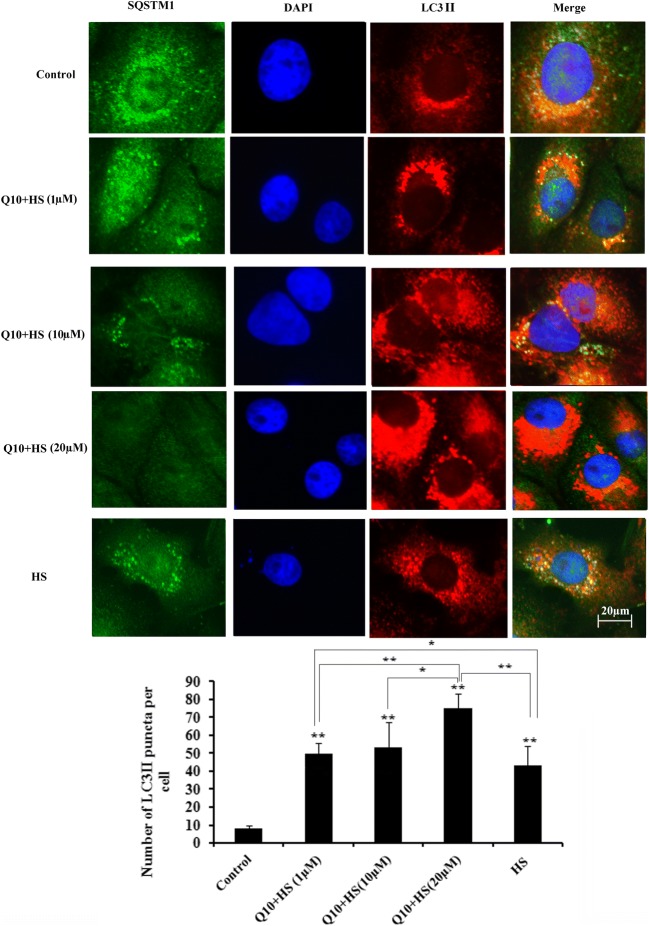
Effects of Q10 on distribution of SQSTM1 and LC3II
SQSTM1 and LC3II were mainly distributed in the cytoplasm under control conditions, but during heat stress, SQSTM1 decreased rapidly, while LC3II increased and LC3II puncta accumulated in the cytoplasm. The number of LC3II puncta was quantified, and 20 μM Q10 treatment resulted in significantly more LC3II puncta accumulation compared with the heat stress group (P < 0.01), the 1 μM Q10 + HS group (P < 0.01), and the 10 μM Q10 + HS group (P < 0.05; Fig. 8).
Fig. 8.
Distribution of SQSTM1, LC3II, and the number of LC3II puncta in myocardial cells during heat stress. The number of LC3II puncta was quantified, and 20 μM Q10 treatment resulted in significantly more LC3II puncta accumulation compared with the heat stress control group. Nuclei (DAPI, blue), SQSTM1 (FITC, green), LC3II (Rhodamine, red). Scale bar = 20 μm. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
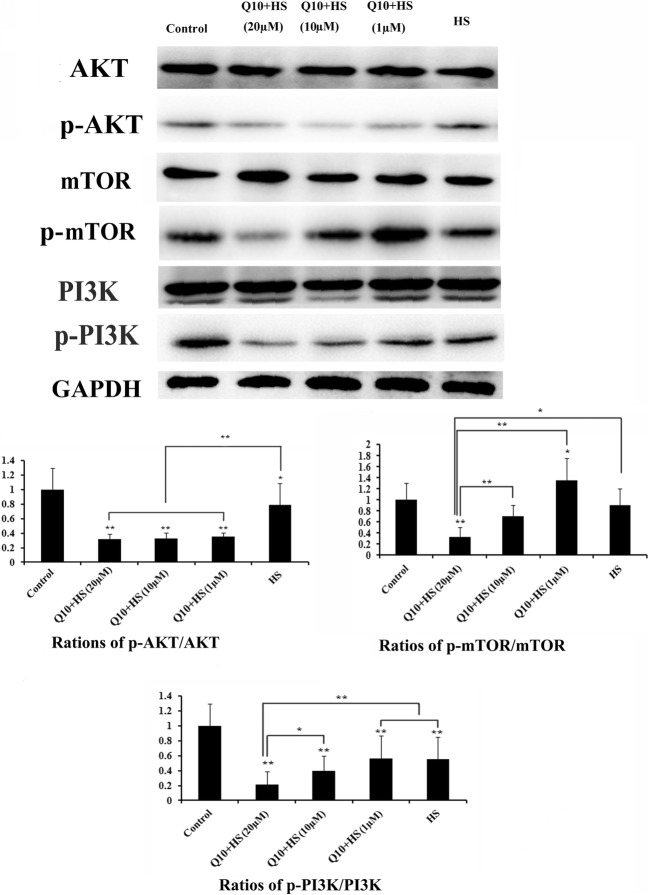
Effects of Q10 on expression of AKT, p-AKT, m-TOR, p-mTOR, PI3K, and p-PI3K
Expression of AKT, PI3K, and mTOR was not influenced by Q10 and/or heat stress. Expression of p-AKT, p-mTOR, and p-PI3K was normalized to the intensity of the relevant total protein. HS only decreased the normalized levels of p-AKT (P < 0.05), p-mTOR (P > 0.05), and p-PI3K (P < 0.01). Treatment with Q10 (at any concentration) significantly reduced the expression of p-AKT compared with HS group (P < 0.01; Fig. 9). Only treatment with 20 μM Q10 significantly downregulated the expression of both p-mTOR (P < 0.05) and p-PI3K (P < 0.01) during heat stress.
Fig. 9.
Expression of AKT, mTOR, PI3K, p-AKT, p-mTOR, and p-PI3K. Expression of AKT, PI3K, and mTOR was not influenced by heat stress and addition of Q10. Expression of p-AKT, p-mTOR, and p-PI3K was normalized to the intensity obtained for the blot of the relevant total protein. Only treatment with 20 μM Q10 significantly downregulated the expression of p-AKT, p-mTOR, and p-PI3K during heat stress. Values represent the means ± SE. *P < 0.05; **P < 0.01. The asterisks immediately above each bar indicate significance from the control. The asterisks immediately above each line indicate significance between different groups linked by line
Discussion
In this study, we found that pretreatment of primary chicken myocardial cells with 20 μM co-enzyme Q10 protected the cells from heat stress by enhancing autophagy during heat stress through suppression of the PI3K/AKT/mTOR pathway.
We found that treatment with co-enzyme Q10 alone did not influence the viability of myocardial cells, demonstrating that co-enzyme Q10 may be a safe drug to use to protect chickens from heat stress (Xu et al. 2017a). Consistent with previous studies, we found that heat stress reduced the viability of primary chicken myocardial cells (Wang et al. 2017; Hu et al. 2019; Liu et al. 2019); this reduced viability may be associated with the generation and accumulation of ROS that can result in increased oxidative damage (He et al. 2019; Tiwari et al. 2019). Pretreatment with co-enzyme Q10 significantly suppressed the decline in cell viability during heat stress, reduced cell apoptosis, and reduced ROS generation induced by heat stress. These results are consistent with previous studies on the regulation of ROS-AMPK-mTOR pathway by Q10. For example, co-enzyme Q10 ameliorates pancreatic fibrosis by suppressing the mTOR pathway triggered by ROS (Xue et al. 2019). Similarly, Q10 reduced the level of ROS to protect astrocytes from oxidative stress (Jing et al. 2015). ROS induced autophagy that was associated with the ROS-AMPK-mTOR pathway (Li et al. 2019; Yang et al. 2019).
Autophagy is an adaptive response to various stress conditions and was discovered during conditions of heat stress (Bao et al. 2017; Wang et al. 2019). Autophagy removes denatured or misfolded proteins and damaged organelles caused by heat stress through the formation of autolysosomes (Mizushima 2005; Blázquez et al. 2014; Johansen and Lamark 2014; Lee et al. 2014; Zhou et al. 2014). Autophagy-related gene 5 (ATG5) and Beclin-1 are essential for the autophagy process. During this process, beclin 1 binds to Vps34 and Vps15, two subunits of class III PI3K. When this binding is finished, vps34-beclin 1-vps15 binds to Atg14. The ATG5-ATG12 complex binds to ATG16, and then this complex with Beclin 1 and Atg14 results in the formation of LC3 (Abrahamsen et al. 2012; Mizushima et al. 1998; Liang et al. 1999; Ravikumar et al. 2008). LC3 is a commonly used indicator of autophagy and mediates the formation and elongation of the phagophore (Groulx et al. 2012; Lysenko et al. 2015). Knockout of ATG5 in mice nearly completely abrogates autophagy, and autophagy was reduced by 50% when Beclin-1 was inhibited; on the other hand, when Beclin-1 was overexpressed, the levels of autophagy in mouse heart tissue were upregulated (Nakai et al. 2007; Zhu et al. 2007). Dynein is a cytoskeletal motor protein that can transport autophagosomes to lysosomes during the process of autophagosome-lysosome fusion (Schmidt and Ferger 2001; Rubinsztein et al. 2005). In our study, the gene expression of LC3, ATG5, Beclin-1, and dynein was upregulated by 20 μM co-enzyme Q10 compared with the heat stress group, indicating that treatment with Q10 promotes autophagy during heat stress.
When autophagy is triggered, cytosolic LC3 is cleaved to form LC3-I, then LC3-I binds to phosphatidylethanolamine (PE) to become LC3-II. LC3-II targets phagophores, and the ratio of LC3-II/LC3-I is used to evaluate autophagy levels (Kabeya et al. 2000). In our study, the levels of LC3-II in myocardial cells were low under control conditions, and that heat stress significantly induced autophagy. Moreover, the levels of autophagy were highest in the 20 μM co-enzyme Q10 + HS treatment group, consistent with our evaluation of autophagy-related gene expression. Our results are consistent with other studies showing that co-enzyme Q10 can protect cells by increasing autophagy during stress conditions (Liang et al. 2017; Mohamed et al. 2019).
Transmission electron microscopy (TEM) was used to detect the double-membrane structures of autophagosomes that contain damaged or undigested organelles (Mizushima 2004). Our TEM results showed that pretreatment of co-enzyme Q10 upregulated autophagy; this increase in autophagy was positively correlated with the concentration of Q10. MDC fluorescence is another method used to monitor autophagy. When autophagy is triggered in cells, autophagosomes appear rapidly and engulf organelles (such as mitochondria). These organelles are degraded in autophagosomes that possess a highly acidic environment that is different from other parts of the cell. MDC is an eosinophilic substance that binds in a highly acidic environment. The stronger the acidic environment inside the autophagosome, the more MDC is bound. The results of the MDC assay agreed with the results of our TEM evaluation and indicated that treatment with co-enzyme Q10 increased the level of autophagy in chicken myocardial cells during heat stress.
Based on our results, we suggest that co-enzyme Q10 is a well-tolerated drug and that treatment with higher concentrations of co-enzyme Q10 could protect chicken myocardial cells from autophagy. This conclusion was similar to our previous study, where when autophagy reached a maximum in sertoli cells, cell apoptosis levels were significantly decreased (Bao et al. 2017). A paradoxical hypothesis of apoptosis/autophagy was also established based on previous studies (González-Polo et al. 2005).
LC3 is an indicator of autophagy and is uniformly distributed within the cytoplasm under control conditions. However, when autophagy is initiated, LC3-I is rapidly transformed into LC3-II and LC3-II puncta accumulate on the membrane of autophagosomes (Tanida et al. 2004; Feng et al. 2013). Accordingly, the number of LC3II puncta can be used to measure autophagy (Kabeya et al. 2000). Sequestosome-1 (SQSTM1), also known as the ubiquitin-binding protein p62, is a substrate of autophagy. SQSTM1 is a protein that is encoded by the SQSTM1 gene, and is an autophagosome cargo protein that targets other proteins that bind to it for selective autophagy (Joung et al. 1996). Reduced autophagy can result in the accumulation of SQSTM1, and levels of SQSTM1 can be used as an additional indicator of autophagy, especially in combination with LC3 puncta (Mizushima et al. 2010; Klionsky et al. 2012). Our results indicated that the number of LC3II puncta were significantly induced by co-enzyme Q10 treatment before heat stress. Accumulation of SQSTM1 was also reduced by 20 μM co-enzyme Q10 treatment during heat stress, further demonstrating that Q10 treatment induces autophagy.
The PI3K/Akt/mTOR pathway plays an important role in mediating autophagy, and inhibition of this signaling pathway results in autophagic death of cardiomyocytes (Hou et al. 2014). Akt acts as a downstream mediator of PI3K signaling, that is activated by phosphorylation and can activate mTOR to inhibit autophagy (Luo et al. 2014). PI3K/Akt pathway participated cell apoptosis in chicken pancreas caused by cadmium (Jin et al. 2018). In our study, treatment with 20 μM co-enzyme Q10 suppressed the expression of p-PI3K, p-AKT, and p-mTOR, suggesting that co-enzyme Q10 can induce autophagy in primary chicken myocardial cells by suppressing the PI3K/Akt/mTOR pathway. In conclusion, we propose that co-enzyme Q10 can protect primary chicken myocardial cells by upregulating autophagy during heat stress; this function is associated with the suppression of PI3K/Akt/mTOR pathway.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 31602027, 31672520), the Jiangsu Natural Science Foundation of China (grant number BK20160732), the China Postdoctoral Science Foundation (grant number 2016M591860), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Graduate Research, and the Innovation Projects in Jiangsu Province.
Compliance with ethical standards
The study protocol was approved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China).
Conflict of interest
The authors declare that they have no competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abrahamsen H, Stenmark H, Platta HW. Ubiquitination and phosphorylation of Beclin 1 and its binding partners: tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett. 2012;586:1584–1591. doi: 10.1016/j.febslet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- Bao ZQ, Liao TT, Yang WR, Wang Y, Luo HY, Wang XZ. Heat stress-induced autophagy promotes lactate secretion in cultured immature boar Sertoli cells by inhibiting apoptosis and driving SLC2A3, LDHA and SLC16A1 expression. Theriogenology. 2017;87:339–348. doi: 10.1016/j.theriogenology.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Belviranli M, Okudan N (2019) Effect of coenzyme Q10 alone and in combination with exercise training on oxidative stress biomarkers in rats. Int J Vitam Nutr Res 88:126–136 [DOI] [PubMed]
- Blázquez AB, Escribano-Romero E, Merino-Ramos T, Saiz JC, Martín-Acebes MA. Stress responses in flavivirus-infected cells: activation of unfolded protein response and autophagy. Front Microbiol. 2014;5:266. doi: 10.3389/fmicb.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Li XJ, Fan RF, Yang J, Jin X, Hamid S, Xu SW. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere. 2018;194:396–402. doi: 10.1016/j.chemosphere.2017.12.026. [DOI] [PubMed] [Google Scholar]
- de Andrade Ramos BR, Witkin SS. The influence of oxidative stress and autophagy cross regulation on pregnancy outcome. Cell Stress Chaperones. 2016;21:755–762. doi: 10.1007/s12192-016-0715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Liu L, Zhu Y, Chen Q. Molecular signaling toward mitophagy and its physiological significance. Exp Cell Res. 2013;319:1697–1705. doi: 10.1016/j.yexcr.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Flower N, Hartley L, Todkill D, Stranges S, Rees K (2014) Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 12 [DOI] [PMC free article] [PubMed]
- González-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquère S, Eskelinen EL, Pierron G, Saftig P, Kroemer G. The apoptosis/autophagy paradox: autophagi cvacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- Groulx JF, Khalfaoui T, Benoit YD, Bernatchez G, Carrier JC, Basora N, Beaulieu JF. Autophagy is active in normal colon mucosa. Autophagy. 2012;8:893–902. doi: 10.4161/auto.19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Lu Z, Ma B, Zhang L, Li J, Jiang Y, Zhou G, Gao F. Chronic heat stress alters hypothalamus integrity, the serum indexes and attenuates expressions of hypothalamic appetite genes in broilers. J Therm Biol. 2019;81:110–117. doi: 10.1016/j.jtherbio.2019.02.025. [DOI] [PubMed] [Google Scholar]
- Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, Wang N. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol. 2014;13:78. doi: 10.1186/1475-2840-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YY, Chen X, Li X, Li Z, Diao H, Liu L, Zhang J, Ju J, Wen L, Liu X, Pan Z, Xu C, Hai X, Zhang Y. MicroRNA-1 downregulation induced by carvedilol protects cardiomyocytes against apoptosis by targeting heat shock protein 60. Mol Med Rep. 2019;19:327–3536. doi: 10.3892/mmr.2019.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Jia TT, Liu RH, Xu SW. The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-γ/PI3K/Akt pathway in chicken pancreas. J Hazard Mater. 2018;357:355–362. doi: 10.1016/j.jhazmat.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Jing L, He MT, Chang Y, Mehta SL, He QP, Zhang JZ, Li PA. Coenzyme Q10 protects astrocytes from ROS-induced damage through inhibition of mitochondria-mediated cell death pathway. Int J Biol Sci. 2015;11:59–66. doi: 10.7150/ijbs.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2014;7:279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung I, Strominger JL, Shin J. Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain. Proc Natl Acad Sci U S A. 1996;93:5991–5995. doi: 10.1073/pnas.93.12.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kikusato M, Nakamura K, Mikami Y, Mujahid A, Toyomizu M. The suppressive effect of dietary coenzyme Q10 on mitochondrial reactive oxygen species production and oxidative stress in chickens exposed to heat stress. Anim Sci J. 2016;87:1244–1251. doi: 10.1111/asj.12543. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–444. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Koo Y, Ng A, Wei Y, Luby-Phelps K, Juraszek A, Xavier RJ, Cleaver O, Levine B, Amatruda JF. Autophagy is essential for cardiac morphogenesis during vertebrate development. Autophagy. 2014;10:572–587. doi: 10.4161/auto.27649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MY, Zhu XL, Zhao BX, Shi L, Wang W, Hu W, Qin SL, Chen BH, Zhou PH, Qiu B, Gao Y, Liu BL. Adrenomedullin alleviates the pyroptosis of Leydig cells by promoting autophagy via the ROS-AMPK-mTOR axis. Cell Death Dis. 2019;10:489. doi: 10.1038/s41419-019-1728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang S, Ping Z, Ge J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxidative Med Cell Longev. 2017;2017:9863181. doi: 10.1155/2017/9863181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZF, Ji JJ, Zheng D, Su L, Peng T. Calpain-2 protects against heat stress-induced cardiomyocyte apoptosis and heart dysfunction by blocking p38 mitogen-activated protein kinase activation. J Cell Physiol. 2019;234:10761–10770. doi: 10.1002/jcp.27750. [DOI] [PubMed] [Google Scholar]
- Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu J, Ge P, Liang J. Inhibition of autophagy via activation of PI3K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults. Int J Mol Sci. 2014;15:15426–15442. doi: 10.3390/ijms150915426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko V, Fedorenko G, Fedorenko A, Kirichenko E, Logvinov A, Varduny T. Targeting of organelles into vacuoles and ultrastructure of flower petal epidermis of Petunia hybrida. Braz J Bot. 2015;39:327–336. [Google Scholar]
- Madmani ME, Yusuf Solaiman A, Tamr Agha A, Madmani K, Shahrour Y, Essali Y, Kadro W. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2014;6:Cd008684. doi: 10.1002/14651858.CD008684.pub2. [DOI] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raqhubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;2:1535–1541. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed DI, Khairy E, Tawfek SS, Habib EK, Fetouh MA. Coenzyme Q10 attenuates lung and liver fibrosis via modulation of autophagy in methotrexate treated rat. Biomed Pharmacother. 2019;109:892–901. doi: 10.1016/j.biopha.2018.10.133. [DOI] [PubMed] [Google Scholar]
- N’dri AL, Mignon-Grasteau N, Sellier C, Beaumont M, Tixier-Boichard M. Interactions between the naked neck gene, sex, and fluctuating ambient temperature on heat tolerance, growth, body composition, meat quality, and sensory analysis of slow growing meat type broilers. Livest Sci. 2007;110:33–45. [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Rauchova H, Drahota Z, Lenaz G. Function of coenzyme Q in the cell: some biochemical and physiological properties. Physiol Res. 1995;44:209–216. [PubMed] [Google Scholar]
- Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci. 2008;121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Ravikumar B, Acevedoarozena A, Imarisio S, O’Kane CJ, Brown SD. Dyneins, autophagy, aggregation and neurodegeneration. Autophagy. 2005;1:177–178. doi: 10.4161/auto.1.3.2050. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm. 2001;108:1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Tang S, Yin B, Song EB, Cheng HB, Cheng YF, Zhang XH, Bao ED, Hartung J. Aspirin upregulates αB-Crystallin to protect the myocardium against heat stress in broiler chickens. Sci Rep. 2016;6:37273. doi: 10.1038/srep37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, Benjamin IJ, Nguyen L, Gerard RD, Levine B, Rothermel BA, Hill JA. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Singh P, Riyazat Khadim S, Singh AK, Singh P, Asthana RK. Role of Ca2+ as protectant under heat stress by regulation of photosynthesis and membrane saturation in Anabaena PCC 7120. Protoplasma. 2019;256:681–691. doi: 10.1007/s00709-018-1328-8. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Ohnishi M, Kinoshita M. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;39:957–962. doi: 10.1016/s0735-1097(02)01721-7. [DOI] [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- Wang M, Tian Y, Du Y, Sun G, Xu X, Jiang H, Xu H, Meng X, Zhang J, Ding S, Zhang M, Yang M, Sun X. Protective effects of Araloside C against myocardial ischaemia/reperfusion injury: potential involvement of heat shock protein 90. J Cell Mol Med. 2017;21:1870–1880. doi: 10.1111/jcmm.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Cheng CY, Chen CJ, Chan HL, Chen HH, Tang PC, Chen CF, Lee YP, Huang SY. Acute heat stress changes protein expression in the testes of a broiler-type strain of Taiwan country chickens. Anim Biotechnol. 2019;30:129–145. doi: 10.1080/10495398.2018.1446972. [DOI] [PubMed] [Google Scholar]
- Wu D, Zhang M, Lv YJ, Tang S, Kemper N, Hartung J, Bao ED. Aspirin-induced heat stress resistance in chicken myocardial cells can be suppressed by BAPTA-AM in vitro. Cell Stress Chaperones. 2016;21:817–827. doi: 10.1007/s12192-016-0706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Tang S, Song EB, Yin B, Wu D, Bao ED. Hsp70 expression induced by co-enzyme Q10 protected chicken myocardial cells from damage and apoptosis under in vitro heat stress. Poult Sci. 2017;96:1426–1437. doi: 10.3382/ps/pew402. [DOI] [PubMed] [Google Scholar]
- Xu J, Tang S, Yin B, Sun JR, Song EB, Bao ED. Co-enzyme Q10 and acetyl salicylic acid enhance Hsp70 expression in primary chicken myocardial cells to protect the cells during heat stress. Mol Cell Biochem. 2017;435:73–86. doi: 10.1007/s11010-017-3058-1. [DOI] [PubMed] [Google Scholar]
- Xue R, Wang JX, Yang LX, Liu XJ, Gao Y, Pang YH, Wang YB, Hao JY. Coenzyme Q10 ameliorates pancreatic fibrosis via the ROS-triggered mTOR signaling pathway. Oxid Med Cell Longev. 2019;2019:8039694. doi: 10.1155/2019/8039694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qiu TM, Yao XF, Jiang LP, Wei S, Pei P, Wang ZD, Bai J, Liu XF, Yang G, Liu S, Sun XC. Taurine protects against arsenic trioxide-induced resistance via ROS-autophagy pathway in skeletal muscle. Int J Biochem Cell Biol. 2019;112:50–60. doi: 10.1016/j.biocel.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Qian Z, Zhu HS, Tang S, Wu D, Zhang M, Kemper N, Hartung J, Bao ED. HSP90 gene expression induced by aspirin is associated with damage remission in a chicken myocardial cell culture exposed to heat stress. Br Poult Sci. 2016;5:462–473. doi: 10.1080/00071668.2016.1174978. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xi X, Mei Y, Zhao X, Zhou L, Ma M, Liu S, Zha X, Yang Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed Pharmacother. 2019;111:1315–1325. doi: 10.1016/j.biopha.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Qi J, Chi Y, Fan B, Yu JQ, Chen Z. E3 ubiquitin ligase CHIP and NBR1-mediated selective autophagy protect additively against proteotoxicity in plant stress responses. PLoS. Genet. 2014;10:e1004116. doi: 10.1371/journal.pgen.1004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. Clin Investig. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]