Abstract
Abdominal aortic aneurysm (AAA) is often a hidden pathological process showing no clinical symptoms. Genetic burden, smoking, male gender, age > 65 years, and white race have been identified as the main risk factors. A regular screening program has been introduced but is, as yet, unclear and is not performed in most countries. Prostate cancer is the most frequent male malignant disease in Western countries. Prostate cancer is a disease of older age with a median primary diagnosis of over 60 years. In recent years, advanced imaging methods have been established as important diagnostic tools in prostate cancer diagnostics. The incidental detection of AAA during diagnostic imaging performed due to prostate cancer diagnosis could reveal some asymptomatic aneurysms. Using our experience, the incidental detection of AAA during 18F-fluoromethylcholine PET/CT imaging, performed due to the staging, follow-up, and restaging of the prostate cancer, was reworked into a regular tool of secondary prevention within the framework of personalized medicine strategies. Experience with this type of AAA detection is demonstrated by a cohort of 500 patients who underwent 18F-fluorometylcholine PET/CT examination due to the staging or restaging of prostate cancer. A total of 28 aneurysms were detected (26 aneurysms < 50 mm, 2 aneurysms > 50 mm). In 2 cases (diameter < 50 mm), serious complications were found (penetrating aortic ulcer). The detection and monitoring of AAA in patients undergoing 18F-fluorometylcholine PET/CT due to the prostate cancer offers the possibility of a secondary prevention of AAA, patient stratification, and common follow-up for both pathologies.
Keywords: Abdominal aortic aneurysm, Prostate cancer, 18F-fluorometylcholine, Iodine contrast agent, PET/CT, Personalized follow-up, Predictive value of imaging methods, Patient stratification, Screening, Primary prevention, Secondary prevention, Prognosis, Diagnostic algorithm, Androgen deprivation, Rupture, Incidence, Diameter, Risk factors, General population, Stent graft placement, Multivariate model, Biomarker panel, American Association for Vascular Surgery, Society for Vascular Surgery
Introduction
Early detection of abdominal aortic aneurysm (AAA) has been widely discussed over a long period of time. Many efforts have been made in this field. Despite this, there are still no reliable procedures available for the primary detection of AAA. It is possible to seek out new approaches from within the framework of current knowledge which allow us to improve this unfavorable situation. With the development of advanced imaging techniques, it is possible to begin to think in new ways, approaching this problem from outside the usual boxes [1]. The classical concept of imaging examinations is to move from the original indication to the possibility of incidental detection of another defect such as AAA. Since the early detection of AAA is a crucial but as yet unresolved task, incidental detection of AAA could be reworked to become a systematic examination and patients could benefit from both prostate cancer (PCa) and AAA assessment.
AAA epidemiology
AAA is often a hidden pathological process, and can develop for several years with no clinical symptoms. AAA is defined as a dilatation of a diameter of at least one and half times the normal diameter at the level of the renal arteries, with this aortic segment usually measuring about 20 mm. A diameter greater than 30 mm is considered to be a confirmation of an AAA diagnosis. About 80% of AAAs are positioned between the renal arteries and the bifurcation of the aorta to the iliac arteries [2, 3]. Asymptomatic AAA could be detected by a physical examination or during imaging performed for a variety of reasons. Acute AAA rupture is one of the most dramatic emergencies in medicine. In western countries, ruptured AAAs are estimated to cause between 4 and 5% of all sudden deaths. Ruptured AAA presents as a terrible abdominal or back pain and a pulsatile abdominal mass. Severe hypotension occurs during rupture [4, 5]. Only about half of all patients suffering from AAA rupture reach the hospital alive and of those another half does not survive emergency surgical or endovascular repair [6–8]. The hidden growth of AAA increases the risk of a sudden rupture. Sudden rupture has a global mortality of over 90% when it is not treated and the survival of the patients who are able to undergo surgery is about 30–60% [9–11].
Multifactorial pathogenesis, individual growth rate, and the risk of sudden rupture are the main reasons preventing a standardized approach to AAA patients. Therefore, diagnostic tools, which are able to help us in the stratification of AAA patients as well as the personalization of diagnostic and therapy algorithms, are highly appreciated [12]. The main risk factors of AAA—genetic burden, smoking, male gender, age > 65 years, and white race—have been identified [13, 14]. Over the years since the risk factors were highlighted, targeted prevention of AAA has been a focus of research. For prevention to be appropriate, the ratio between the effect and costs of action, possible future costs of the disease treatment, and possible complications are always important [15]. Without these data, a general consensus, for example with regard to systematic screening, cannot be reached. The data required to make such decisions are currently lacking and so there is, as yet, no targeted primary prevention.
Prostate cancer epidemiology
PCa is the most frequent adult malignant disease occurring in men in the population of western countries. More than one million cases are diagnosed worldwide every year, and the mortality is over 300,000 deaths per year. The highest incidence was observed in Australia and North America, the lowest incidence was reported in China and Japan [16, 17]. Regarding incidence and mortality, the relation to the human development index (HDI) was proved. The age-standardized incidence rate (ASIR) was the highest in high-HDI countries led by Australia; however, the highest age-standardized mortality rate (ASMR) was observed in low-HDI countries led by Zimbabwe [18]. This unfavorable situation demands a complex solution. Predictive, preventive, and personalized medicine (PPPM) can play a useful role through its prevention strategies, patient stratification, and predicting PCa [19] using cost-effective strategies like multiomic approach and newly introduce biomarkers [20].
PCa is a characteristic disease of older age with a median of primary diagnosis over 60 years. As basic diagnostic methods, digital rectal examination (DRE), ultrasonography (USG), prostate specific antigen (PSA) levels, and biopsy have long been used. Besides these four diagnostic tools, advanced imaging methods, including computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), and hybrid imaging using PET/CT with the application of choline analog 18F-fluoromethylcholine (18F-FCH), have been established as important diagnostic tools in the staging, follow-up, and restaging of patients with a diagnosis of PCa [21].
Current therapy of PCa in comparison with other types of cancer is relatively advanced. The basic surgical treatment of prostate cancer is radical prostatectomy. Subsequently or as another option depending on the clinical stage of PCa, radiotherapy, chemotherapy, or other types of treatment could be used. In recent times, the therapy of PCa has involved androgen deprivation in most cases. Bearing in mind the proven differences in AAA development in men and women, the absence of androgen is suspected to play a role in AAA progression and increases the risk of rupture in those PCa patients [22, 23].
Coincidence of PCa and AAA
The coincidence of PCa and AAA is not frequently studied. Both diagnoses have an important impact on mortality in the older male population and their treatment should be tailored to the disease development. PCa patients are examined intensively during their treatment according to the staging, restaging, and follow-up of the disease. The treatment of patients is individually oriented according to the aggressiveness and spread of the disease as well as to the response to the treatment [24]. Given that anti-androgen therapy is one of the corner stones of the disease development control, the possible complications of the sexual hormone deprivation and its negative effect on tissues have to be taken into account. From the abovementioned facts, it is clear that the concurrently treatment of a similar age group has to be managed by dividing it into two groups according to the possible different speed and risk of AAA development. The individual development of both abovementioned diseases demands an individualized estimate of the patient’s risk of severe complications. In such cases, the advanced imaging techniques could be the right methods to serve as a tool for stratification of the patients within the framework of personalized medicine strategies [25].
Aim of the study
The aim of this study was to evaluate previously reported data regarding the detection of asymptomatic AAA in PCa patients who underwent 18F-fluoromethylcholine PET/CT imaging due to the staging, follow-up, and restaging of the disease in the University Hospital in Pilsen.
Materials and methods
Over the course of four years, a total of 586 consecutive 18F-FCH-PET/CT examinations was performed on 500 men ranging from 48 to 81 years, with a mean age of 62.5 years (Table 1). All examinations were performed after intravenous administration of 18F-FCH (IasoCholine, Iason, Graz) at 1.25 MBq/kg. Patients were prepared by fasting for 4 h, but they were allowed to receive fluids. After 45 to 120 min, imaging was performed on a hybrid scanner (Biograph 128 mCT UltraHD, Siemens Healthineers, Knoxville/Erlangen, USA/Germany) with an orthosilicate four-row PET detector subsystem and a 128-row CT subsystem. The acquisition of CT data was performed with the use of 80 ml of iodine contrast agent (Ultravist 370, Berlin, Bayer Pharma, Germany), except in those patients with iodine contrast material allergy or in those patients suffering from renal impairment. The kV dose modulation protocol was used and the kV setting was automatically selected, mostly reaching 100 kV. Submillimeter isotropic imaging was used with 128 × 0.75 mm collimation with data reconstruction at a width of 5 mm for attenuation correction and with a reconstruction of 0.75 mm-width images and 0.6 mm increment using the soft tissue and HRCT algorithms. PET dataset was obtained with a standard resolution in 5 to 7 positions ranging from the groin to the head. The data was reconstructed using the time-of-flight algorithm using a point spread function. The images were reconstructed with and without attenuation correction. Soft and bone tissues were evaluated independently using CT and PET image fusion. The retrospective analysis of the patient cohort was performed by two experienced radiologists in consensus and the main goal was detection of AAA. The diameters of abdominal aorta were assessed and measured using the Syngo.Via molecular imaging tool (Siemens Healthineers, Forchheim, Germany) also enabling vascular analysis. The aneurysms were assessed according to their maximum transverse diameter and categorized as (1) aneurysms less than 50 mm, (2) more than 50 mm and less than 70 mm, (3) more than 70 mm and less than 80, and (4) over 80 mm [2].
Table 1.
Characteristics of men in the study group
| Characteristic | Count | Detected aneurysms |
|---|---|---|
| Men | 500 | |
| Age, median of years (min–max) | 62.5 (48–81) | |
| Imaging examinations | ||
| Primary staging | 173 (30%) | 7 (30%) |
| Restaging | 327 (56%) | 21 (70%) |
| Follow-up of AAA | 86 (14%) | 2* |
| Total | 586 (100%) | 28 (100%) |
| Size of detected aneurysms | ||
| 30–50 mm | 26 (97%) | |
| 50–70 mm | 2 (3%) | |
| Total | 28 (100%) | |
| Current smoking | 195 (39%) | |
| Diabetes mellitus | 120 (24%) | |
| Hypertension | 375 (75%) | |
| Ischemic heart disease | 185 (37%) | |
*Two aneurysms primarily detected in restaging
Results
The results are summarized in Table 1. A total of 28 AAAs were detected. Twenty-six were in the range 30–50 mm. Two detected aneurysms exceeded 50 mm.
A total of 173 examinations were performed at primary staging, including patients on whom the examination was performed before a definitive diagnosis was established from the biopsy. Seven aneurysms were discovered, all of them below 50 mm in diameter, so, according to the diameter, no aneurysm was identified with a higher risk of rupture.
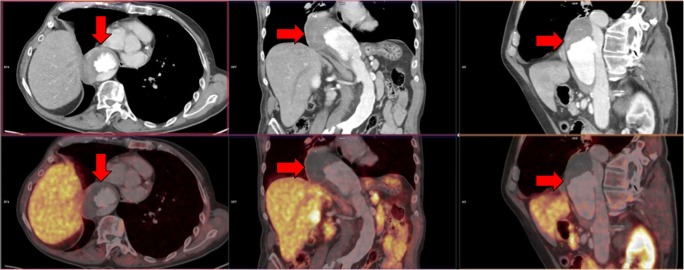
A total of 327 examinations were performed for restaging of the disease after radical prostatectomy, radical prostate irradiation, or during complete androgen blockade. In this subgroup, 21 aneurysms were detected, 2 exceeding 50 mm—1 aneurysm with a diameter of 55 mm and 1 with a diameter of 58 mm—were found. Both AAAs over 50 mm were treated using endovascular repair with stent graft implantation. Figure 1a shows the subrenal AAA with a diameter of 55 mm (red arrows) in a patient staged due to prostate cancer visualized by the fusion PET/CT image. Figure 1b shows the same patients with the subrenal AAA with a diameter of 55 mm (red arrows) in a patient staged due to prostate cancer visualized by three-dimensional CT angiography used in stent graft planning.
Fig. 1.
Staging PET/CT examination of patient with prostate cancer. a The subrenal AAA with a diameter of 55 mm (red arrows) visualized by the fusion PET/CT image. b The same patients with the subrenal AAA with a diameter of 55 mm (red arrows) visualized by three-dimensional CT angiography used in stent graft planning
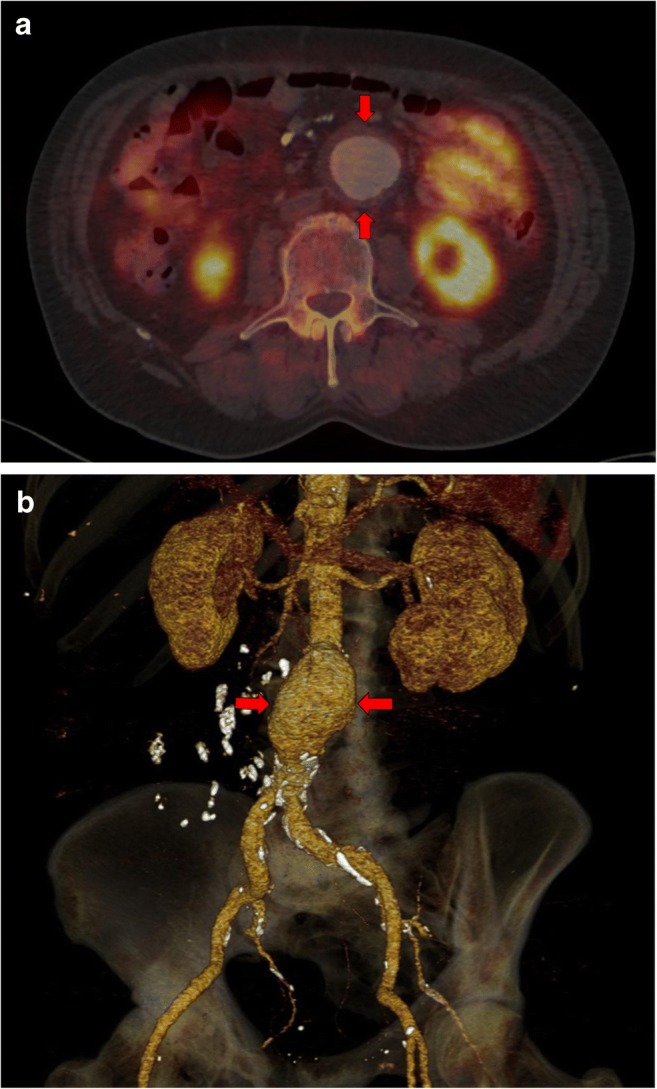
In a total of 86 cases, a follow-up examination was performed to evaluate oncological disease progression. During this follow-up also, AAA progression and severe complications were observed in two patients, namely, aortic ulcer development. Both were referred for surgical repair. Figure 2 shows the patient with 3 years of androgen deprivation therapy; aortic dissection was indicated due to the development of a deep aortic ulceration in the proximal abdominal and distal thoracic aorta (red arrows), with an originally detected subrenal aneurysm of 35 mm in size. The dissection analysis was performed using three perpendicular planes, upper row CT, and lower row PET/CT fusion. The high quality imaging enables the planning of open surgery with aortic repair using tubular graft.
Fig. 2.
Follow-up PET/CT of patient after 3 years of androgen deprivation therapy for prostate cancer. Aortic dissection due to the development of a deep aortic ulceration in the thoracic and abdominal aorta (red arrows), with an originally detected subrenal aneurysm of 35 mm. CT images (upper row) and fused PET/CT images (lower row) in three perpendicular views
Twenty-eight AAAs were detected in 500 men. Which means that our detection rate of AAA was 28 per 500 men. However, for comparison with the results available in the literature, amount of AAAs must be recalculated as the number of AAAs detected per 1000 men. When recalculated, the detection rate was 56 per 1000 men for our group of men with a median age of 62.5 years. This detection rate is more than 100 times higher than that found in the published data for an equivalent age group of men (0.55 AAA for men 65–74 years old) and even greatly exceeds the number of AAAs observed in older groups of men (1.12 AAA for men 75–84 years old and 2.98 for men older than 85 years). These data are summarized in Table 2.
Table 2.
Comparison in AAA incidence: PCa male population vs. general male population
| Characteristics of the group | Age (years) | Incidence (case/1000) |
|---|---|---|
| Our PCa male group | Median, 62.5 | 56/1000 |
| General male population | 65–74 | 0.55/1000 |
| 75–84 | 1.12/1000 | |
| ≥ 85 | 2.98/1000 |
Discussion
Before starting the study, the literature sources were reviewed in order to acquaint ourselves with the current relation between personalized medicine and AAA. Our findings are summarized in the paragraph below.
AAA and PPPM
The accumulation of evidence in AAA pathology shows that two lines of pathologies must be distinguished. One line is the genetic burden, gender, and age, and the other is the negative impact of a bad lifestyle like smoking. From the PPPM point of view, it should be emphasized the crucial role of multilevel diagnostics approach starting the genetic background of the disease and continuing with the medical imaging and laboratory methods. Integrating this information allows for targeted prevention and personalized treatment regimes, preventing severe complications with the high probability of death [26]. From the literature, sources are visible that personalized approaches to AAA diagnostics and treatment are not currently common and the publications related to this topic are rare. This situation might be the result of the fact that the majority of AAAs are detected randomly and so it is very difficult to create a systematic and personalized approach to this problem. However, certain interesting examples were found. The first group of articles might be described as those relating to expert system creation. There are examples available of expert systems for doctors [27, 28] or common system for doctors and patient. Such system could be used for treatment decisions and also for explanations for the patient [29]. The second group of articles is focused on the precision measurement of the size of the aneurysm and the speed of growth which is currently used as the main predictive factor for repair of AAA. Many authors use three-dimensional printing as a tool for professional surgical assessment and in certain cases also as a tool that is very realistic for explaining the next treatment procedure to the patient [30–32]. Some studies are targeted at the decoding of genomics of AAA to better understand the mechanism of development and growth [33]. Unlike the previous groups of articles that are small in number, the last group is larger. The last group of studies is focused on blood biomarkers mainly released from the aortic wall. This group of biomarkers has long been the focus of research. However, the results do not correspond to the extent of these efforts. From a high number of studies conducted, only a minor part was able to publish results with the potential to lead to further research. Among those studies with promising results, there is also the study conducted by our group from the University Hospital in Pilsen which was recently published in the EPMA Journal [34].
Clinical questions—four steps to the final AAA assessment
Based on the knowledge regarding AAA and PCa, the four following clinical questions were formulated:
What is the incidence of AAA in PCa patients investigated using 18F-FCH-PET/CT in comparison with the incidence in the general population?
Could progressive development of AAA be detected in patients under androgen deprivation therapy?
Is the imaging technique performed using 18F-FCH-PET/CT, including contrast substance enhanced CT, suitable for stratification of risky AAAs according to the severity of the disease, and is it sufficient for proper management?
Could be transformed the incidental detection of AAA using 18F-FCH-PET/CT into a systematic personalized approach with clearly defined benefits for patients?
Answers to these questions should serve as the main points for a final assessment of the clinical importance of such an approach and for an assessment of the benefits for patients.
What is the incidence of AAA in PCa patients?
The annual incidence of newly diagnosed AAA is approximately 0.4 to 0.67% in western populations. This equates to 2.5 to 6.5 aneurysms for every 1000 persons each year. Age has an important influence on incidence. Some studies have detected, in men aged 65 to 74 years, an incidence of 55 per 100,000 men (0.55/1000) per year, but that increases to 112 per 100,000 (1.12/1000) for men aged 75 to 85 years, with an even higher rate of 298 per 100,000 men annually (2.98/1000) for those older than 85 [7, 8, 35]. Our findings in the PCa male population undergoing imaging techniques either due to staging or restaging confirm that the incidence of AAA was 5.6% during the 4 years of observation which included 500 consecutive patients with a detection rate of 28 AAAs per 500 men (56/1000). Compared our numbers with the results in available literature, 100 times higher occurrence of AAA was observed in PCa men than in the general male population of a similar age. This high AAA incidence in PCa patients allows us to use imaging techniques as a systematic tool for PCa and AAA assessment at the same time.
Could progressive development of AAA be detected in patients undergoing androgen deprivation therapy?
The information in literature is contradictory and therefore it is currently a very complicated task to answer the above question with any clarity. PCa requires the selection of one of several approaches, starting with active surveillance, androgen deprivation, and then radical prostatectomy or radical irradiation. Some of the therapy options carry an increased risk of the progressive development of an aneurysm: prostatectomy indicated in locally operable tumors, pelvic lymphadenectomy, but probably also the use of anti-androgen active drugs [36–38]. Even male gender is a risk factor; the aneurysms in females tend to be more progressive [39, 40]. The faster growth in females and the more rapid development of the aneurysm towards rupture implies that the sufficient levels of male sex hormones could have an important influence on the slower progression in males [41]. Although smoking has been found to be the most strongly associated risk factor for aneurysm development in males [42], some studies in patients treated for PCa with androgen deprivation have also found some relation to the missing androgen receptors. Missed receptors are being found in one subpopulation of patients with PCa—those with castration-resistant prostate cancer [43]. In the available literature, there is also information from a study conducted on animal models that blockade and genetic deletion of the androgen receptor attenuates AAA progression [23]. The severe complication of AAA was observed in two patients from our group. The first patient was treated by surgical therapy using thoracoabdominal aortic replacement. The second one only received conservative therapy due to the massive metastatic disease progression of PCa with a poor prognosis. In the case of this patient, follow-up PET/CT was performed after 9 months and AAA status was stable. The patient died 4 months later, with no signs of aortic rupture due to the cachexia and anemia related to the development of terminal disease progression. Not even the causal influence of androgen deprivation can be confirmed in either of those cases. Both cases documented the important role of follow-up in the detection of asymptomatic severe aortic disease complications [44, 45].
Is the imaging technique performed using 18F-FCH-PET/CT, including contrast substance enhanced CT, suitable for stratification of risky AAAs?
AAA size was repeatedly proved to be the strongest predictor of the risk of rupture; the risk increases seriously for aneurysms with diameters greater than 50 mm. The 5-year overall cumulative rupture rate of incidentally diagnosed aneurysms is 25–40% for aneurysms larger than 50 mm compared with 1–7% for aneurysms of 40 to 50 mm in diameter. A statement of the Joint Council of the American Association for Vascular Surgery and the Society for Vascular Surgery estimates the annual rupture risk according to AAA diameter. Although the risk of rupture in diameters of less than 49 mm can be up to 5%, it increases from 15% in aneurysms with diameters 50–59 mm, to more than 50% in those of 80 mm in diameter [3, 35, 43]. While the growth rate might be an important factor in increasing rupture risk, follow-up 18F-FCH-PET/CT may play a role, not only for follow-up of the PCa response to the therapy but also for the progress of the AAA diameter, along with its shape and the conformation of the aneurysm [23]. CT, as the anatomical component of hybrid imaging methods, is certainly the underlying approach for the assessment of the aorta and other arteries. Moreover, the high concentration of the iodine contrast agent in the arterial system is also important. The optimal situation is when the level of contrast agent concentration is comparable with CT angiography as the targeted imaging methods of arterial circulation. In this case, PET/CT could serve well for therapy decisions and the planning of aortic repair. Even the risk of expansion of more than 5 mm in 6 months was estimated as a high risk factor for rupture. The calculation of growth velocity could be made from the data obtained from the two 18F-FCH-PET/CT. The abovementioned facts confirmed that a full-diagnostic CT with intravenous iodine contrast agent application is the correct way to perform the scan, not only in oncological diagnostics but also in AAA assessment.
Could be transformed the incidental detection of AAA using 18F-FCH-PET/CT into a systematic personalized approach with clearly defined benefits for patients?
Since confirmed diagnosis of PCa is rising in the male population, with a shift in incidence towards lower age [44], the frequent use of imaging methods means serious issues in diagnostics and treatment of other diseases that are randomly detected. This problem is more significant due to the relatively high life expectancy in most patients enabled by current therapy approaches [45, 46]. Therefore the more frequent use of 18F-FCH-PET/CT in the detection, staging, and restaging of PCa has brought with it the possibility of assessing those patients in tandem for other potentially dangerous diagnoses. When AAA is incidentally detected with 18F-FCH-PET/CT, this occurrence could be important for patient survival because most AAAs are asymptomatic until the rupture, although some are identified during evaluation for any of the mild abdominal symptoms [47]. Common follow-up of PCa and AAA offers a precise and systematic tool which can detect asymptomatic but severe aortic disease complications.
Perspectives
The predictive value of imaging methods for AAA detection has been satisfactorily proved and is generally accepted. However, the prognostic value for AAA development is limited. One possibility of improving prognostic ability is the combination of imaging methods with a panel of suitable biomarkers and our own research in this regard is promising. Based on this research, a multivariate model using selected biomarkers was suggested [32]. As a further task, our experience in the fields of biomarkers and advanced imaging techniques is going to be merged. In accordance with the examples in literature [25–27] and in concordance with the PPPM strategies [47, 48], a diagnostic algorithm is preparing based on the imaging methods and our multivariate model of blood biomarker levels. This model could be helpful in the prognosis of AAA development, especially those with a smaller diameter (under 50 mm) which are mostly fully asymptomatic and, from the point of view of imaging techniques, these AAAs are less dangerous. Reports about the rupture of these small AAAs motivate us to further our efforts in researching this field [49].
Conclusion and expert recommendations
All facts mentioned in the answers to our clinical questions can be summarized as follows:
100 times higher incidence of AAA was shown in our group of PCa men in comparison with the similar age group of men in the general population.
A total of 28 AAAs were detected and in 2 cases progression and aortic ulcer development were observed. Given this number of cases of progression, it is not possible to draw any conclusions as to whether androgen deprivation therapy plays any role in this progression or not. In near future, the patient cohort must be broaden as well as our observations.
The imaging technique performed using 18F-FCH-PET/CT, including contrast substance enhanced CT, is a suitable method for the stratification of risky AAAs. This is clearly demonstrated by 2 cases of observed progression during follow-up for AAA.
Our experience clearly shows not only an option, but rather the necessity to transform incidental detection of AAA into a systematic tool within the framework of a personalized approach to the patients. 18F-FCH-PET/CT can serve as an effective method of secondary prevention of AAA and afterwards stratification when AAA is detected.
The main challenge for the near future, in our opinion, is the creation of a multivariate model for possible prediction of AAA development and the risk of rupture. The model should be based on stratification using imaging methods and combined with the panel of selected biomarkers. This model could be beneficial in cases of AAA with a smaller diameter (under 50 mm), and can help in the treatment decisions which would be made based solely on AAA diameter size.
As for a longer term vision and in concordance with the PPPM strategies, it is under consideration developing a larger multivariate model using the outputs of imaging techniques, biomarker levels, and proteomic and genomic data.
Abbreviations
- AAA
Abdominal aortic aneurysm
- ASIR
Age-standardized incidence rate
- ASMR
The age-standardized mortality rate
- CT
Computed tomography
- DRE
Digital rectal examination
- F
Fluor
- 18F-FCH
18F-fluoromethylcholine
- HDI
Human development index
- kV
Kilo volt
- MBq
Mega Becquerel
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- PCa
Prostate cancer
- PSA
Prostate specific antigen
Funding information
This study was supported by a Grant from the Czech Health Research Council No.15-32727A and by the Ministry of Health, Czech Republic-Conceptual Development of Research Organization (University Hospital in Pilsen-FNPl, 00669806).
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Ethical approval
All investigations conformed to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all the participants. The study was approved by the responsible Ethical Committee of the University Hospital in Pilsen on 12th of August 2014.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Golubnitschaja O, Lemke HU, Kapalla M, Kent A. Design in predictive, preventive and personalised medicine. In: Kuksa I, Fisher T, editors. Design for Personalisation. London: Gower Publishing; 2017. pp. 150–169. [Google Scholar]
- 2.Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452. doi: 10.1067/mva.1991.26737. [DOI] [PubMed] [Google Scholar]
- 3.Chaikof Elliot L., Dalman Ronald L., Eskandari Mark K., Jackson Benjamin M., Lee W. Anthony, Mansour M. Ashraf, Mastracci Tara M., Mell Matthew, Murad M. Hassan, Nguyen Louis L., Oderich Gustavo S., Patel Madhukar S., Schermerhorn Marc L., Starnes Benjamin W. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery. 2018;67(1):2-77.e2. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Forsdahl Signe Helene, Singh Kulbir, Solberg Steinar, Jacobsen Bjarne K. Risk Factors for Abdominal Aortic Aneurysms. Circulation. 2009;119(16):2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 5.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, Thompson SG, Walker NM, Multicentre Aneurysm Screening Study Group The Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/S0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 6.Svensjö Sverker, Björck Martin, Gürtelschmid Mikael, Djavani Gidlund Khatereh, Hellberg Anders, Wanhainen Anders. Low Prevalence of Abdominal Aortic Aneurysm Among 65-Year-Old Swedish Men Indicates a Change in the Epidemiology of the Disease. Circulation. 2011;124(10):1118–1123. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 7.Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, Quick CR, Ashton HA, Scott RA. Incidence among men of asymptomatic abdominal aortic aneurysms: estimates from 500 screen detected cases. J Med Screen. 1999;6:50–54. doi: 10.1136/jms.6.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Oliver-Williams C., Sweeting M. J., Turton G., Parkin D., Cooper D., Rodd C., Thompson S. G., Earnshaw J. J. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. British Journal of Surgery. 2017;105(1):68–74. doi: 10.1002/bjs.10715. [DOI] [PubMed] [Google Scholar]
- 9.Johnsen Stein Harald, Forsdahl Signe Helene, Singh Kulbir, Jacobsen Bjarne Koster. Atherosclerosis in Abdominal Aortic Aneurysms: A Causal Event or a Process Running in Parallel? The Tromsø Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(6):1263–1268. doi: 10.1161/ATVBAHA.110.203588. [DOI] [PubMed] [Google Scholar]
- 10.Yazbek Guilherme, Nishinari Kenji, Krutman Mariana, Wolosker Nelson, Zottelle Bomfim Guilherme André, Pignataro Bruno Soriano, Fonseca Igor Yoshio Imagawa, Cavalcante Rafael Noronha, Teivelis Marcelo Passos. Treatment of Abdominal Aortic Aneurysms in Cancer Patients. Annals of Vascular Surgery. 2016;30:159–165. doi: 10.1016/j.avsg.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.329.7477.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation-EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-32100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, et al. Nat Rev Dis Primers. 2018;4:34. 10.1038/s41572-018-0030-7. [DOI] [PubMed]
- 15.Cortaredona S, Ventelou B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 2017;15:1–11. doi: 10.1186/s12916-017-0978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Chan JM. Epidemiology of prostate cancer. World J Urol. 2017;35:849. doi: 10.1007/s00345-017-2038-0. [DOI] [PubMed] [Google Scholar]
- 17.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 18.Sharma R. The burden of prostate cancer is associated with human development index: evidence from 87 countries, 1990-2016. EPMA J. 2019;10:137–152. doi: 10.1007/s13167-019-00169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens JP, Schuster K, Voss A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018;9:113–123. doi: 10.1007/s13167-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Miaolong, Zhan Xianquan. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA Journal. 2018;9(1):77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolejsova Olga, Kucera Radek, Fuchsova Radka, Topolcan Ondrej, Svobodova Hana, Hes Ondrej, Eret Viktor, Pecen Ladislav, Hora Milan. The Ability of Prostate Health Index (PHI) to Predict Gleason Score in Patients With Prostate Cancer and Discriminate Patients Between Gleason Score 6 and Gleason Score Higher Than 6—A Study on 320 Patients After Radical Prostatectomy. Technology in Cancer Research & Treatment. 2018;17:153303381878737. doi: 10.1177/1533033818787377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulug P., Powell J. T., Sweeting M. J., Bown M. J., Thompson S. G. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. British Journal of Surgery. 2016;103(9):1097–1104. doi: 10.1002/bjs.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis John P., Salmon Morgan, Pope Nicolas H., Lu Guanyi, Su Gang, Meher Akshaya, Ailawadi Gorav, Upchurch Gilbert R. Pharmacologic blockade and genetic deletion of androgen receptor attenuates aortic aneurysm formation. Journal of Vascular Surgery. 2016;63(6):1602-1612.e2. doi: 10.1016/j.jvs.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickey Sabrina L., Grayson Ciara J. The Quality of Life among Men Receiving Active Surveillance for Prostate Cancer: An Integrative Review. Healthcare. 2019;7(1):14. doi: 10.3390/healthcare7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3:14. 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed]
- 26.Younesi E, Hofmann-Apitius M. From integrative disease modeling to predictive, preventive, personalized and participatory (P4) medicine. EPMA J. 2013;4:23. 10.1186/1878-5085-4-23. [DOI] [PMC free article] [PubMed]
- 27.Borthwick KM, Smelser DT, Bock JA, Elmore JR, Ryer EJ, Pacheco JA, et al. ePhenotyping for abdominal aortic aneurysm in the Electronic Medical Records and Genomics (eMERGE) Network: algorithm development and Konstanz information miner workflow. Int J Biomed Data Min. 2015;4:113. doi: 10.4172/2090-4924.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee R., Jarchi D., Perera R., Jones A., Cassimjee I., Handa A., Clifton D.A., Bellamkonda K., Woodgate F., Killough N., Maistry N., Chandrashekar A., Darby C.R., Halliday A., Hands L.J., Lintott P., Magee T.R., Northeast A., Perkins J., Sideso E. Applied Machine Learning for the Prediction of Growth of Abdominal Aortic Aneurysm in Humans. EJVES Short Reports. 2018;39:24–28. doi: 10.1016/j.ejvssr.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman Loren, Curry Leslie, Goldberg Carolyn, Gusberg Richard, Fraenkel Liana. Pilot testing of a decision support tool for patients with abdominal aortic aneurysms. Journal of Vascular Surgery. 2011;53(2):285-292.e1. doi: 10.1016/j.jvs.2010.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meess KM, Izzo RL, Dryjski ML, Curl RE, Harris LM, Springer M, et al. 3D Printed abdominal aortic aneurysm phantom for image guided surgical planning with a patient specific Fenestrated Endovascular Graft System. Proc SPIE Int Soc Opt Eng. 2017. 10.1117/12.2253902. [DOI] [PMC free article] [PubMed]
- 31.Yuan D, Luo H, Yang H, Huang B, Zhu J, Zhao J. Precise treatment of aortic aneurysm by three-dimensional printing and simulation before endovascular intervention. Sci Rep. 2017. 10.1038/s41598-017-00644-4. [DOI] [PMC free article] [PubMed]
- 32.Bortman Jeffrey, Mahmood Faraz, Schermerhorn Marc, Lo Ruby, Swerdlow Nicholas, Mahmood Feroze, Matyal Robina. Use of 3-Dimensional Printing to Create Patient-Specific Abdominal Aortic Aneurysm Models for Preoperative Planning. Journal of Cardiothoracic and Vascular Anesthesia. 2019;33(5):1442–1446. doi: 10.1053/j.jvca.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Li Jingjing, Pan Cuiping, Zhang Sai, Spin Joshua M., Deng Alicia, Leung Lawrence L.K., Dalman Ronald L., Tsao Philip S., Snyder Michael. Decoding the Genomics of Abdominal Aortic Aneurysm. Cell. 2018;174(6):1361-1372.e10. doi: 10.1016/j.cell.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Molacek J, Treska V, Zeithaml J, Hollan I, Topolcan O, Pecen L, Slouka D, Karlikova M, Kucera R. Blood biomarker panel recommended for personalized prediction, prognosis, and prevention of complications associated with abdominal aortic aneurysm. EPMA Journal. 2019;10:125–135. doi: 10.1007/s13167-019-00173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, Krupski WC, Bandyk D, Barone GW, Graham LM, Hye RJ, Reinke DB. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg. 1997;26:595–601. doi: 10.1016/S0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- 36.Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am J Epidemiol. 2007;165:838–845. doi: 10.1093/aje/kwk063. [DOI] [PubMed] [Google Scholar]
- 37.Sweeting M. J., Thompson S. G., Brown L. C., Powell J. T. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. British Journal of Surgery. 2012;99(5):655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 38.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. UK Small Aneurysm Trial Participants. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 39.Moll F.L., Powell J.T., Fraedrich G., Verzini F., Haulon S., Waltham M., van Herwaarden J.A., Holt P.J.E., van Keulen J.W., Rantner B., Schlösser F.J.V., Setacci F., Ricco J.-B. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. European Journal of Vascular and Endovascular Surgery. 2011;41:S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Lo Ruby C., Lu Bing, Fokkema Margriet T.M., Conrad Mark, Patel Virendra I., Fillinger Mark, Matyal Robina, Schermerhorn Marc L. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. Journal of Vascular Surgery. 2014;59(5):1209–1216. doi: 10.1016/j.jvs.2013.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo Ruby C., Schermerhorn Marc L. Abdominal aortic aneurysms in women. Journal of Vascular Surgery. 2016;63(3):839–844. doi: 10.1016/j.jvs.2015.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jesus-Silva Seleno Glauber de, Oliveira Victor Rodrigues de, Moraes-Silva Melissa Andreia de, Krupa Arturo Eduardo, Cardoso Rodolfo Souza. Fatores de risco associados e sobrevida em curto e médio prazo de pacientes submetidos a correção aberta e endovascular de aneurisma de aorta abdominal. Jornal Vascular Brasileiro. 2018;17(3):201–207. doi: 10.1590/1677-5449.011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khurana Namrata, Sikka Suresh C. Interplay Between SOX9, Wnt/β-Catenin and Androgen Receptor Signaling in Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences. 2019;20(9):2066. doi: 10.3390/ijms20092066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cotter Anne R, Vuong Kim, Mustelin Linda L, Yang Yi, Rakhmankulova Malika, Barclay Colleen J, Harris Russell P. Do psychological harms result from being labelled with an unexpected diagnosis of abdominal aortic aneurysm or prostate cancer through screening? A systematic review. BMJ Open. 2017;7(12):e017565. doi: 10.1136/bmjopen-2017-017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horrill Tara. Acute Aortic Dissection Following Treatment for Castration-Resistant Prostate Cancer. Oncology Nursing Forum. 2016;43(4):413–416. doi: 10.1188/16.ONF.413-416. [DOI] [PubMed] [Google Scholar]
- 46.Bandini Marco, Mazzone Elio, Preisser Felix, Nazzani Sebastiano, Zaffuto Emanuele, Marchioni Michele, Tian Zhe, Pompe Raisa S., Tilki Derya, Graefen Markus, Shariat Shahrokh F., Montorsi Francesco, Saad Fred, Briganti Alberto, Karakiewicz Pierre. Increase in the Annual Rate of Newly Diagnosed Metastatic Prostate Cancer: A Contemporary Analysis of the Surveillance, Epidemiology and End Results Database. European Urology Oncology. 2018;1(4):314–320. doi: 10.1016/j.euo.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Hu R, Wang X, Zhan X. Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 2013;4:1–12. doi: 10.1186/1878-5085-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golubnitschaja O, Watson ID, Topic E, Sandberg S, Ferrari M, Costigliola V. Position paper of the EPMA and EFLM: a global vision of the consolidated promotion of an integrative medical approach to advance health care. EPMA J. 2013;4:12. 10.1186/1878-5085-4-12. [DOI] [PMC free article] [PubMed]
- 49.Grootes I., Barrett J. K., Ulug P., Rohlffs F., Laukontaus S. J., Tulamo R., Venermo M., Greenhalgh R. M., Sweeting M. J. Predicting risk of rupture and rupture-preventing reinterventions following endovascular abdominal aortic aneurysm repair. British Journal of Surgery. 2018;105(10):1294–1304. doi: 10.1002/bjs.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]