Abstract
Functional orthopedic treatment is effective for the correction of malformation. Studies demonstrated myoblasts undergo proliferation and apoptosis on certain stretch conditions. MicroRNAs (miRNAs) function in RNA silencing and post-transcriptional regulation of gene expression, and participate in various biological processes, including proliferation and apoptosis. One hypothesis suggested that miRNA was involved into the procedure via suppressing its target genes then triggered endoplasmic reticulum stress-induced apoptosis. Therefore, miRNAs play important roles in the regulation of the proliferation and apoptosis of myoblasts. In our study, the miR-147 has been explored. A cyclic mechanical stretch model was established to observe the features of rat L6 myoblasts. The detection of mRNA and protein levels was performed by qRT-PCR and western blot. L6 cell proliferation/apoptosis was checked by CCK-8 assay, DNA fragmentation assay, and caspase-3 activity assay. MiRNA transfections were performed as per the manufacturer’s suggestions: (1) cyclic mechanical stretch induced apoptosis of L6 myoblasts and inhibition of miR-147; (2) miR-147 attenuated cyclic mechanical stretch-induced apoptosis of L6 myoblasts; (3) miR-147 attenuated cyclic mechanical stretch-induced L6 myoblast endoplasmic reticulum stress; (4) BRMS1 was a direct target of miR-147 in L6 myoblasts; (5) miR-147/BRMS1 axis participated in the regulation of cyclic mechanical stress on L6 myoblasts. MiR-147 attenuates endoplasmic reticulum stress by targeting BRMS1 to inhibit cyclic mechanical stretch-induced apoptosis of L6 myoblasts.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-01037-4) contains supplementary material, which is available to authorized users.
Keywords: Cyclic mechanical stretch, Myoblast, MiR-147, Apoptosis, ER-Stress
Introduction
Functional orthopedic treatment is effective for children and teenagers with malocclusion before they reach the age of peak growth and development of their bones. In the industry of dental health, functional orthopedics consider the jaw alignment, the tooth relationship, the muscle relationship, and the head and neck posture as a whole system (Hicks et al. 2018). Unlike the bonded brackets, functional orthopedics use sophisticated, removable appliances that work with specific muscle forces to move the teeth. The previous study found that during the functional orthopedic treatment, the mandibular stretched forward could effectively stimulate the growth of the mandibular condyle (Barnouti et al. 2011; Owtad et al. 2011), and then obtained the morphological and functional adaptation of the perioral muscle and masticatory muscle system (Erdem et al. 2009; Yagci et al. 2010), and finally achieved the successful treatment. Researchers have noticed that mechanical stretch can alter the organization of stress fibers in myocytes, resulting in cell damage and repair or regeneration (Akimoto et al. 2005; Chen et al. 2013). The cyclic mechanical stretch appliances, which mimic the stress in biological environment and adjust the stretch with different time and frequency, provide a perfect tool to study the relationship of mechanical stretch and cultured myoblast cells (Akimoto et al. 2005; Chen et al. 2013; Song et al. 2018a).
Myoblast cells are a small population of undifferentiated cells that are capable of giving rise to muscle cells. They experience proliferation, differentiation, and apoptosis under certain stretch conditions, and play an extremely important role in the adaptive reconstruction of the facial muscles. Studies have demonstrated that appropriate stretch stimulated myoblast proliferation, while excessive stretch leads to apoptosis (Cucina et al. 2008; Song et al. 2018a; Zhang et al. 2016). However, the mechanism of how mechanical stretch influences the behaviors of myoblasts remains unknown. Recently, some genes were described to be bonded by miRNAs and achieved functional suppression under the mechanical stretch condition, triggered a series of signal pathways to regulate the proliferation and apoptosis myoblast cells (Fu et al. 2018; Hua et al. 2016). Endoplasmic reticulum stress (ER-Stress) was involved in the mechanical stretch as well (Jia et al. 2015). ER-Stress is an apoptotic pathway discovered in recent years (Strasser et al. 1997). It plays an important role in many diseases such as atherosclerosis, heart failure, diabetes, and Alzheimer’s disease (Yoshida 2007). The ER-Stress leading to the apoptosis of myoblasts has not clearly elucidated.
MiRNAs are a class of evolutionarily conserved endogenous non-coding RNAs that are 18 to 25 base pairs in length. They can regulate post-transcription by binding to the 3′UTR of the target genes. MicroRNAs are involved in a variety of biological processes including cell proliferation and apoptosis, angiogenesis, tissue invasion, metastasis, immune response, microbial infection, and myogenic apoptosis and differentiation (Iwakawa and Tomari 2015; White et al. 2011). In this study, we used a target scan software and screened a miRNA named miR-147, and found its sequence perfectly match the 3′UTR of gene breast carcinoma metastasis suppressor 1 (BRMS1). BRMS1 played a role in tumor suppression and cell proliferating inhibition (Chimonidou et al. 2013; Xing et al. 2015; Zhang et al. 2014). Thus, we hypothesize it might be a negative regulator of L6 myoblasts in the cyclic mechanical stretch model. MiR-147 has been studied more frequently in tumors and inflammation (Liu et al. 2009; Ning et al. 2019; Shen et al. 2018), but its function and mechanism of action in myoblasts have not been studied in depth. To further investigate the roles of miR-147 in the cyclic mechanical stretch to induce apoptosis of myoblasts, we designed and performed this study.
Materials and methods
Rat L6 myoblast cell culture
Rat L6 myoblast cell line was purchased from ATCC (Manassas, VA). Early passages of L6 myoblast cells were placed in 75 cm flask and then cultured in Dulbecco’s Mod of Eagle’s (EMDM) medium (Corning, Corning, NY) with 10% of fetal calf serum (FCS, Invitrogen, Waltham, MA) plus 1% of penicillin/streptomycin (Gibco, Grand Island). Media were changed every 2–3 days for maintenance. HEK-293 cells were obtained Cell Bank in CAS. Cells were maintained in EMDM medium with 10% FCS plus 1% penicillin/streptomycin. Cells growing in log phase and about 70% confluence were used to perform following experiments.
Cyclic mechanical stretch equipment
We established a cyclic mechanical L6 myoblast stretch model (Flexcell FX-5000TM Tension System, FlexCell International Corporation, Burlington, NC, USA). The method was set up as the guideline of the manufacturer and other studies (Fu et al. 2018). Briefly, 1 × 105 L6 cells growing were placed onto a flexible-bottom six-well plates coated with type-I collagen (CellAdhere), and maintained in completed medium for overnight. When the cells were attached down to the plate bottom, the plate was subjected to cyclic strain (15% or 20% deformation) at 0.5 Hz (1 s of stretch, 1 s relaxation) for 6, 12, or 24 h as experiment required. Cells cultured under the same conditions without any cyclic strain were considered as control.
The checking of viability and apoptosis status of L6 myoblasts
For elucidating the viability of L6 myoblasts before and after treatment, we selected cell counting Kit-8 reagents (Dojindo Laboratories, Kumamoto, Japan) (Fu et al. 2018). L6 cells were replaced with fresh medium and were added Kit-8 reagents with 10 μL per 100 μL medium, then incubated at 37 °C with 5% CO2 for 2 h, read the optical density (OD) values in a microplate reader (Biotek, Winooski, VT, USA). Cell viability was calculated as the manufacturer’s suggestion formula.
For clarifying the downstream apoptosis status of L6 myoblasts, Caspase-3 Activity Assay Kit II Fluorometric (BioVision) and DNA fragmentation assay (Cell Death Detection ELISAPLUS Kit, Roche Diagnostics) were used (Dam et al. 2013; Song et al. 2018a; Zhang et al. 2016). The procedures were performed as per the manufacturer’s guidance; values were read in a plate fluorescence reader at Ex/Em = 400/505 nm.
MicroRNA transfection
SignalSilence®SirT1 siRNA I (#12241) and control siRNA (#6568) were purchased from Cell Signaling Technology (Danvers, MA). SiRNA transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. In brief, L6 cells were seeded in 60 mm plates when they are 80–90% confluent at time of transfection, 85 pmol siRNA, and 8.5 μL Lipofectamine 2000 diluted by serum-free medium, respectively, and were mixed together after 5 min, then incubated for another 20 min and added complex to the cells. Cells were incubated continuously for 48 h and RNA and protein were subsequently collected. To confirm the transfection efficiencies of miRNAs or genes, western blots were performed.
Quantitative real-time RT-PCR
Total RNA was extracted from L6 cell lines using AllPrep RNA/Protein kit (QIAGEN, Valencia, CA). cDNA synthesis was performed using iScript reverse transcription supermix (Bio-Rad; Hercules, CA). Primers were designed by references (see below). Quantitative RT-PCR for associated genes was performed using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, cat no. 172-5124) and run on 95 °C, 2 min; 95 °C, 5 s and 60 °C, 30 s, 40 cycles; and every 5 s increase 0.5 °C from 65 to 95 °C. All above assays were followed the manufacturer’s protocol. The relative expression levels of miRNA and mRNA transcripts were calculated by 2−ΔΔCt method.
Primers were referenced as (Song et al. 2018b; Wei et al. 2015; Zhang et al. 2016, 2017):
miR-147 for RT: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAGCAGAA;
miR-147 for qRT-PCR: F: GTGTGCGGAAATGCTT, R: TCAACTGGTGTCGTGG.
U6B (internal control gene) for RT: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAAATATG.
U6B for qRT-PCR: F: CAAATTCGTGAAGCGTT, R: GGAGTCGGCAATTCAG.
GRP78 for qRT-PCR: F: CATCAATGAGCCAACAGC, R: ATGGTAGAGCGGAACAGG; CHOP for qRT-PCR: F: ACCTTCACTACTCTTGACCCTG, R: CTCATTCTCCTGCTCCTTCTC;
ATF4 for qRT-PCR: F: GCCATCTCCCAGAAAGT, R: GAACCACGAGGAACACC;
GAPDH for qRT-PCR: F: GGTGCTGAGTATGTCGTGGAG, R: CAGTCTTCTGAGTGGCAGTGAT;
BRMS1 for qRT-PCR: F: TGGATGACGAGGACTATGAGC, R: TGCCACCTGAATACGAATCTTT;
BRMS1 CDS plasmid primers: F: ATTCGGAATTCATGCCCATCCAGCCTTC, R: ATTCGGGATCCTCAGGGTCCATCTGCTT.
Immunoblot
For western blots, L6 cells were lysed on ice in 1× lysis buffer (Cell Signaling Technology, Danvers, MA) in 20 mM Tris-HCl buffer, pH 7.5, containing 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, and 1% Triton with 1× Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO) or 1× phosphatase inhibitor cocktail set II (Calbiochem, La Jolla, CA). Ten to 30 μg of total protein from each sample were resolved on 8 to 12% SDS Bis-Tris.Cl polyacrylamide gel with running buffer and transferred onto 0.2 μm PVDF membranes (Bio-Rad, Hercules, CA). The membranes were probed with various antibodies.
Blots were incubated with anti-rabbit or anti-mouse secondary antibodies conjugated with HRP from GE Healthcare life sciences (Little Chalfont Buckinghamshire, UK). The signals were visualized using Supersignal West Pico Chemiluminescent Substrate or Pierce ECL Western Blotting Substrate (Zhang et al. 2017).
Luciferase reporter assays
BRMS1 3′UTR wild type and mutant type were produced by PCR with L6 myoblast cDNA, primers as (Wei et al. 2016):
BRMS1 3′UTR wild type F: ATTCGCTCGAG CCCTGCATTTACACTCG, R: ATTCGGCGGCCGC TTCCAGGTCCAATTTTA.
BRMS1 3′UTR mutant type: F: TTGGGGCCGGTGGCACGGGAGCC, R: GGCTCCCGTGCCACCGGCCCCAA.
Vectors (psiCHECK-2 plasmid, Promega, Madison, WI, USA) carrying luciferase sequence were ligated with BRMS1 wild and mutant which expressed 3′UTR (Wei et al. 2016). L6 myoblast cells were seed at a 6-well plate and transfected with psiCHECK-2 plasmid, 48 h after transfection; cells were collected and the luciferase reporter analysis was performed with Firefly & Renilla Dual Assay Kit (Thermo Fisher), as per manufacturer’s instructions (Ning et al. 2019).
Biotin miRNA pulldown assay
We performed biotin miRNA pulldown assay to confirm the connection of miR-147 and BRMS1 (Yamamoto et al. 2015). Briefly, 50% confluence of L6 myoblasts were cultured on a 6-well plate, washed with cold PBS, cells were harvested and treated with 0.5 mL of 25 mM Tris-HCl (pH 7.5), 70 mM KCl, 2.5 mM EDTA, 0.05% NP-40, and 80 U/mL RNase Inhibitor (Life Technologies). After incubated on ice for 20 min and centrifuged (> 12,000g), the supernatant containing mRNAs was removed to a new tube, biotinylated double-stranded RNA of miR-147, or biotinylated control random RNA was added and incubated at 4 °C for 30 min plus 1 h with 30-rpm shaking at 30 °C. Streptavidin Mutein Matrix (Roche Applied Science) with extraction buffer (250 μg RNase-free BSA and 100 μg yeast tRNA in 500 μL of 25 mM Tris-HCl (pH 7.5), 70 mM KCl, 2.5 mM EDTA, and 0.05% NP-40) was mixed and incubated for 3 h. The biotinylated RNAs/mRNAs was washed and ready for biotinylated RNA pulldown assay. The DAPK1, whose sequence did not match with miR-147 sequences at the 3′ untranslated region (UTR) of its mRNA, was used as the negative control (Zhang et al. 2017).
Statistical analysis
The data with error bars represents the mean ± SD from three independent experiments. One-way ANOVA analysis followed by a Bonferroni post hoc test was used for data analysis. P < 0.05 is considered to be statistically different.
Results
Cyclic mechanical stretch induced apoptosis and miR-147 suppression in L6 myoblasts
For elucidating whether cyclic mechanical stretch induces L6 myoblasts apoptosis, and the miR-147 function towards L6 myoblasts proliferation, we conducted the following experiments. First, 1 × 104 rat L6 myoblasts were set up in a 6-well plate with flexible bottom, then put on cyclic mechanical stretch equipment and continually stretched for different time period. The proliferation of L6 myoblasts was detected and calculated by CCK-8 assay (Fig. 1a). Under the continuous stretching of different time period for 0, 6, 12, and 24 h, the relative viability of L6 myoblasts was gradually reduced to 75% or 50%. Compared to the 0 h, the relative cell viability was reduced to 75% (12 h) and 50% (24 h), respectively. In the time of 12 h and 24 h, the viability of L6 cells decreased significantly (P < 0.05 and P < 0.01, respectively). This result indicated that cyclic mechanical stretch inhibited the proliferation of L6 myoblasts. For examining the relationship between mechanical stretch and L6 apoptosis, caspase-3 activity and DNA fragmentation assay were performed (Fig. 1b, c). The detected caspase-3 activity of L6 cells was increased by 5–8 folds while prolonging the time, significant differences were observed in time 12 h and 24 h (Fig. 1b). Another apoptosis indicator DNA fragmentation was selected to examine the apoptosis status of L6 myoblasts, result showed as Fig. 1c; the DNA fragmentation dramatically increased along with the longer loading time, up to 30 to 40 folds compared to 0 time point; this result suggested that cyclic mechanical stretch induced L6 myoblast apoptosis. Finally, we examined the expression level of miR-147 in L6 myoblasts; the qPCR result showed the relative expression of intrinsic miR-147 was obviously going down (to 0.6 and 0.4-fold compared with time 0) (Fig. 1d), while prolonging the loading time of mechanical stretch. These experiment results suggest that cyclic mechanical stretch inhibited the proliferation of L6 myoblasts and promoted the process of the apoptosis of these cells. A microRNA miR-147 was suppressed during prolongation of the time loading of cyclic mechanical stretch.
Fig. 1.
Cyclic mechanical stretch induced apoptosis and miR-147 suppression in L6 myoblasts. L6 myoblasts were continually stretched for 0, 6, 12, and 24 h. a The proliferation of L6 myoblasts was detected by CCK-8 assay. b, c The apoptosis of L6 myoblasts was detected by caspase-3 activity assay and DNA fragmentation assay. d The expression level of miR-147 in L6 myoblasts was analyzed by qRT-PCR. The data represent the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 compared with control group. Student’s t test
MiR-147 alleviated cyclic mechanical stretch-induced apoptosis in L6 myoblasts
Next, we investigated whether miR-147 influence the L6 myoblast apoptosis during cyclic mechanical stretch. The transfection of control microRNA and miR-147 mimic was conducted. We classified the L6 myoblasts in different groups: control group: L6 myoblasts transfected with negative control microRNA and without cyclic mechanical stretch (Control + miR-NC); stretch group: L6 myoblasts transfected with negative control microRNA plus with cyclic mechanical stretch (Stretch + miR-NC); and stretch group plus with transfection of miR-147 mimics (Stretch + miR-147). Three groups in our experiment were conducted following experiments. First, miR-147 expression levels were checked by qPCR, showed as Fig. 2a, although mechanical stretch could suppress the intrinsic miR-147 for about 50% amount of L6 myoblasts, while in Stretch + miR-147 group, the expression of miR-147 was observed 12-fold and 6-fold higher compared with Stretch + miR-NC group and Control + miR-NC group. Meanwhile, the viability and apoptosis of L6 myoblasts were also examined. As expected, cyclic mechanical stretch suppressed the L6 cell viability. However, miR-147 restored the viability for about 25% (Fig. 2b), and the restored value was significant. For the apoptosis status of L6 myoblasts, Stretch + miR-147 group alleviated 4-fold (8 to 4) of caspase-3 activity and 18% (38 to 20%) of DNA fragmentation in cyclic mechanical stretch during 24 h (Fig. 2c, d). These data indicated that, during the 24 h of continued stretch, overexpression of miR-147 in L6 myoblast cells alleviated cyclic mechanical stretch-induced apoptosis.
Fig. 2.
MiR-147 alleviated cyclic mechanical stretch-induced apoptosis in L6 myoblasts. L6 myoblasts were not subjected to continually stretch and transfected with mimic negative control (Control + miR-NC), stretched for 24 h and transfected with mimic negative control (Stretch + miR-NC) or stretched for 24 h and transfected with miR-147 mimics (Stretch + miR-147). a qRT-PCR analyzed the expression level of miR-147 in these treated L6 myoblasts. b The proliferation of these treated L6 myoblasts was detected by CCK-8 assay. c, d The apoptosis of these treated L6 myoblasts was detected by caspase-3 activity assay and DNA fragmentation assay. The data represent the mean ± SD from three independent experiments. **P < 0.01 compared with Control + miR-NC; ##P < 0.01 compared with Stretch + miR-NC. Student’s t test
MiR-147 ameliorated cyclic mechanical stretch-induced endoplasmic reticulum stress in L6 myoblasts
Many studies described that endoplasmic reticulum (ER) stress is involved in cell apoptosis. During ER-Stress, the level of ARP78 and ATF4/CHOP expressions is elevated; they performed as the reliable indicators of ER-Stress cell apoptosis. Thus, we checked the expressions of ER-Stress axis genes in GRP78 and ATF4/CHOP pathway in L6 myoblasts using qPCR and immunoblot (Fig. 3). L6 myoblasts were continuously stretched for 0 h or 24 h, then separately transfected with control microRNA or miR-147; three different treatment groups were obtained. They are Control + miR-NC, Stretch + miR-NC, and Stretch + miR-147 as previous described. The expressions of involved genes were compared with Stretch + miR-NC group. Results showed the expression of GRP78, CHOP, and ATF4 presented highest levels in Stretch + miR-NC group, which means cyclic mechanical stretch induced ER-Stress L6 myoblast apoptosis; however, in Stretch + miR-147 group, the expressions of GRP78, CHOP, and ATF4 were decreased up to 50%; significant value was obtained as well (Fig. 3a–c). Western blot showed the consistent result (Fig. 3d). Those data suggested that miR-147 ameliorated cyclic mechanical stretch-induced endoplasmic reticulum (ER) stress in L6 myoblasts.
Fig. 3.
MiR-147 ameliorated cyclic mechanical stretch-induced ER-Stress in L6 myoblasts. L6 myoblasts were continually stretched for 0 h and transfected with mimic negative control (Control + miR-NC), stretched for 24 h and transfected with mimic negative control (Stretch + miR-NC) or stretched for 24 h and transfected with miR-147 mimics (Stretch + miR-147). a–c The relative mRNA levels of ER-Stress-related genes: GRP78, CHOP, and ATF4 in these treated L6 myoblasts were determined by qRT-PCR. d The protein levels of ER-Stress-related genes: GRP78, CHOP, and ATF4 in these treated L6 myoblasts were detected by western blot. The data represent the mean ± SD from three independent experiments. **P < 0.01 compared with Control + miR-NC; ##P < 0.01 compared with Stretch + miR-NC. Student’s t test
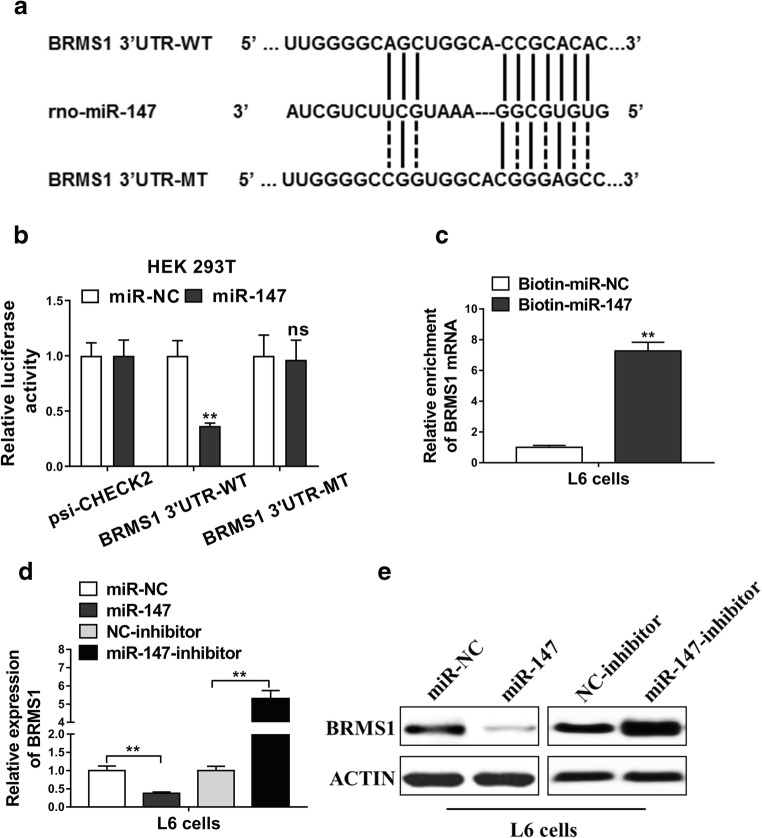
BRMS1 was a direct target of miR-147 in L6 myoblasts
For further elucidating the functions of miR-147 and its regulation genes, we searched miR-147 target genes with a Targetscan software. Among the data, we focused on a gene BRMS1. Our result indicated that BRMS1 is a direct target gene of miR-147 by binding its 3′UTR, miR-147 might act the suppression function towards BRMS1 (Fig. 4a). To prove our hypothesis, we first examined the BRMS1 expressions under the stretch period of 0, 6, 12, and 24 h in L6 cells; results showed both the mRNA and protein levels of BRMS1 were increased (Fig S1), which meant the cyclic mechanical stretch induced BRMS1 upregulation in L6 myoblasts. Then, we clarified the nucleotide sequence in this region and made BRMS1 wild (BRMS1 3′UTR-WT) and mutant type (BRMS1 3′UTR-MT) of BRMS1 in its 3′UTR region with constructed luciferase reporter vectors. psi-CHECK2 was used as control. Then, we obtained 6 types of transfectants: psi-CHECK2/miR-NC, psi-CHECK2/miR-147, BRMS1 3′UTR-WT/miR-NC, 3′UTR-WT/miR-147, BRMS1 3′UTR-MT/miR-NC, and BRMS1 3′UTR-MT/miR-147. We performed co-transfection of those transfectants to HEK 293T cells. Results showed as Fig. 4b. As expected, luciferase activity was not influenced by psi-CHECK2 vectors. When miR-147 binding 3′UTR-WT, the luciferase activity of BRMS1 was significantly suppressed (up to 60% suppression), while miR-147 failed to bind BRMS1 3′UTR-MT, resulted in no significant suppression of luciferase activity of BRMS1 3′UTR-MT. From the results above, our data clearly showed that miR-147 directly binding BRMS1 on its 3′UTR, therefore inhibited the apoptosis function of this gene. Furthermore, we used biotinylated miR-NC and miR-147 to detect BRMS1 mRNA by qPCR and found miR-147 played a role of BRMS1 mRNA enrichment from unit 1 to unit 7 (Fig. 4c). Finally, we used a miR-147 inhibitor to detect and compare the BRMS1 expressions in L6 myoblasts by qPCR; miR-147 strongly suppressed the BRMS1 expression but the suppression was dramatically removed when we add miR-147 inhibitor (Fig. 4d). Western blot clearly showed the consistent result with qPCR (Fig. 4e). From all above experiment evidences, it was clearly shown that BRMS1 is a direct target of miR-147; the apoptosis function of BRMS1 was strongly suppressed by miR-147.
Fig. 4.
BRMS1 was a direct target of miR-147 in L6 myoblasts. a A schematic diagram showed predicted binding site of miR-147 in the 3′UTR of BRMS1, with sequences of the wild type BRMS1 3′UTR (BRMS1 3′UTR-WT) and the mutant (BRMS1 3′UTR-MT). b Luciferase reporter assays of L6 cells and HEK 293T cells co-transfected with constructed luciferase reporter vectors (psi-CHECK2, BRMS1 3′UTR-WT, BRMS1 3′UTR-MT) and miR-147 or negative control mimics (miR-NC). c Detection of BRMS1 mRNAs in biotinylated miRNA/target mRNA complex by real-time RT-PCR. The relative level of BRMS1 mRNA in the complex pulled down by using biotinylated miR-147 (Bio-miR-147) was compared to that of the complex pulled down by using the biotinylated control random RNA (Bio-miR-NC). d, e The relative expression levels of BRMS1 in L6 cells transfected with miR-147 mimics and miR-147 inhibitor or their respective negative controls were detected by qRT-PCR and western blot. The data represent the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01, ns = not significant compared with the control group. Student’s t test
MiR-147/BRMS1 axis was involved in the effects of cyclic mechanical stretch on L6 myoblasts
Finally, we investigated whether the miR-147/BRMS1 axis existed and whether the axis was involved in cyclic mechanical stretch on L6 myoblasts. First, we explored whether the BRMS1 inhibition impaired cyclic mechanical stretch-induced apoptosis and ER-Stress. For this purpose, we transfected three types of siRNAs to L6 myoblasts: negative control siRNA (Control + si-NC) without stretch, negative control siRNA (Stretch + si-NC) with 24 h stretch, and siRNA against BRMS1 (Stretch + si-BRMS1) with 24 h stretch. Si-BRMS1 successfully inhibited BRMS1 expression by 70% examined by western blot (data not shown). Continuous stretch greatly improved the expression of BRMS1 in L6 cells whereas si-BRMS1 obviously reduced the expression (Fig S2A and B); the L6 cell viability was restored by the inhibition of BRMS1 (Fig S2C), and the apoptosis markers of caspase-3 and DNA fragmentation were decreased as well in L6 cells (Fig S2D and E); meanwhile, we examined the ER-Stress indicators GRP78, CHOP, and ATF4 simultaneously and data showed the ER-Stress was suppressed by si-BRMS1 (Fig S2F and G). The above results indicated that the BRMS1 inhibition attenuated the cyclic mechanical stretch-induced ER-Stress and protected the L6 cells from the stretch-induced apoptosis. Next, we used either empty vector or vector carrying BRMS1 overexpression (BRMS1-OE), to co-transfected with miR-NC or miR-147 to L6 cells. L6 myoblast cells carrying three types of transfectants were obtained: miR-NC + VEC, miR-147 + VEC, and mir-147 + BRMS1-OE. BRMS1 expression was examined by qPCR and western blot (Fig. 5a, b). Overexpression of miR-147 suppressed BRMS1 expression yet the suppression was moderated by over transfection of BRMS1. Our data clearly demonstrated the existence of miR-147/BRMS1 axis and regulation of the BRMS1 expression in L6 cells. Then, we performed continuous cyclic mechanical stretch for 24 h on L6 myoblasts. Overexpression of miR-147 promoted L6 myoblast cell viability however this function was partly removed by co-transfection of BRMS1 due to negative feedback of this axis (Fig. 5c). We examined the apoptosis status of L6 myoblasts as well with those transfectants. MiR-147 overexpression inhibited caspase-3 activity and DNA fragmentation expression, on the other hand, while co-transfected with BRMS1, the inhibition function of miR-147 was partly disarmed; obvious differences were observed in the experiments (Fig. 5d, e). The ER-Stress indicator genes were checked as well. Results are shown in Fig. 5f, g. Under continuous mechanical stretch conditions, the gene expressions of GRP78, CHOP, and ATF4 in L6 cells carrying different transfectants were detected with qPCR (Fig. 5f) and western blot (Fig. 5g). Overexpression of miR-147 decreased the expressions of those ER-Stress genes, on the other hand, BRMS1, the target gene of miR-147, partly dismissed the ER-Stress via overexpression. Overall, our data suggested that miR-147, together with its target gene BRMS1, regulate the ER-Stress response proliferation/apoptosis of L6 myoblasts under the effects of cyclic mechanical stretch.
Fig. 5.
MiR-147/BRMS1 axis was involved in the effects of cyclic mechanical stretch on L6 myoblasts. L6 myoblasts were continually stretched for 24 h and co-transfected with mimics negative control and empty vector (miR-NC + VEC), miR-147 mimics and empty vector (miR-147 + VEC), or miR-147 mimics and BRMS1 overexpressing plasmid (miR-147 + BRMS1-OE). a, b The mRNA and protein levels of BRMS1 in these treated L6 myoblasts were analyzed by qRT-PCR and western blot, respectively. c The proliferation of these treated L6 myoblasts was detected by CCK-8 assay. d, e The apoptosis of these treated L6 myoblasts was detected by caspase-3 activity assay and DNA fragmentation assay. f, g The mRNA and protein levels of ER-Stress-related genes: GRP78, CHOP, and ATF4 in these treated L6 myoblasts were detected by qRT-PCR and western blot, respectively. The data represent the mean ± SD from three independent experiments. **P < 0.01 compared with miR-NC + VEC; ##P < 0.01 compared with miR-147 + VEC. Student’s t test
Discussion
Skeletal myoblasts grow into myocytes that comprise the main composition of muscles. In dentistry, functional orthopedic treatment is effective for the correction of malformation. Appropriate force, time, and frequency of mechanical stress acting on myoblasts become significant to achieve successful orthopedic treatment. To date, several reports have described the relationship of cyclic mechanical stretch and the proliferation/apoptosis of myoblasts and the involved mechanisms as well. For example, PI3K/Akt and MARK signal pathway was related to L6 myoblasts proliferation, regulated by IGF-1 receptor under cyclic mechanic stretch model (Fu et al. 2018). Another group demonstrated cyclic stretch induces the myoblast by activation of caspase-3 protease in a magnitude-dependent manner (Liu et al. 2010). Other signal pathways including Wnt (Akimoto et al. 2005), and Toll-like receptor 3 (TLR3) (Chen et al. 2013) also regulate the features of myoblasts.
In this study, we established the cyclic mechanical stretch model under certain conditions (6–24 h, 0.5 Hz frequency) (Fu et al. 2018) and examined the cyclic mechanical stretch induced apoptosis of L6 myoblasts. The viability and apoptosis products (cleaved caspase-3 and DNA fragmentation) of the cells were all downregulated. Simultaneously, expression of the microRNA miR-147 gradually decreased while prolonging the time of cyclic mechanical stretch, indicating that miR-147 was involved in the mechanical stretch-induced cell apoptosis. This also suggests that miR-147 might act as a negative regulator in this procedure (Fig. 1).
To further elucidate the function of miR-147, overexpression of this microRNA was performed. After successful transfection of miR-147 to L6 cells (Fig. 2a), the examination of cell viability was restored and the apoptosis indicators (caspase-3 and DNA fragmentation) were significantly less (Fig. 2b–d) under the designed cyclic mechanical model. These results clearly indicate that miR-147 was a negative regulator to L6 apoptosis. Cyclic mechanical stretch induces endoplasmic reticulum (ER) stress (Jia et al. 2015) and results in cell apoptosis. To investigate whether miR-147 removes ER-Stress in L6 myoblasts, we detected the ER-Stress indicator genes (GRP78, CHOP, and ATF4) in miR-147 overexpression transfectants. As expected, overexpression of miR-147 partly ameliorated ER-Stress-induced apoptosis of L6 myoblasts (Fig. 3).
MicroRNA usually binds its target genes in the un-translation region (UTR) as a suppressor to regulate gene functions. For clarifying our hypothesis, the Targetscan software (http://www.targetscan.org/vert_71/) was used to search the direct target genes of miR-147. Thus, breast cancer metastasis suppressor 1 (BRSM1) emerged in our study (Fig. 4a). This gene is expressed at low levels in many types of cancers. One mechanism described for BRSM1 was that it helped the binding of HDAC1 to RelA/p65, then enhanced the deacetylates of RelA/p65 and suppressed its transcriptional activity (Liu et al. 2006). Another study claimed BRMS1 regulated apoptosis in non-small lung cancer cells by modulating Stat3 activation (You et al. 2015). This gene regulated tumor metastasis in many cancers via promoting cell apoptosis and inhibiting cell proliferation (Chimonidou et al. 2013; Zhang et al. 2014). However, BRMS1 function in L6 myoblasts was poorly known. Our experiment results clearly show that BRMS1 was a direct target of miR-147; the binding site of miR-147 and BRSM1 was screened on its 3′UTR (Fig. 4), as the apoptosis function was significantly removed by miR-147 via suppressing BRMS1. We provided evidence that there existed an axis of the miR-147-BRMS1. The miR-147 bond the mRNA of BRMS1 to regulate the L6 myoblast apoptosis induced by the cyclic mechanical stretch via suppression of the ER-Stress (Fig. 5). In cyclic mechanical stretch, several microRNA were involved to join the regulation procedure, such as miR-19a function in lung cancer (Yamamoto et al. 2015), and miR-21participated proliferation and apoptosis of smooth muscle under mechanical stretch (Song et al. 2012). MiRNAs have been well demonstrated on skeletal muscle features (Lui 2017). The genetic and molecular pathways that regulate muscle development, function, and regeneration as well as muscular disease have been well established in the past decades (Diniz and Wang 2016). MiR-147 promotes macrophage activation (Croce 2009; Deng and Sui 2013), enhances PC12 cell hypoxia injury (Carthew and Sontheimer 2009), induces mesenchymal-to-epithelial transition (MET) (White et al. 2011), and suppresses cancer cell proliferation (Iwakawa and Tomari 2015; Ning et al. 2019; Shen et al. 2018; Wei et al. 2016). However, the mechanism of miR-147 regulation of the proliferation and apoptosis of L6 myoblast has not been fully elucidated. Based on the knowledge of the BRSM1 functions and the consideration of our results, we presumed that BRSM1 might trigger ER-Stress in L6 myoblasts. Our study demonstrated that miR-147 directly binds its target gene BRSM1 that in turn decreases the ER-Stress to attenuate the cyclic mechanical stretch-induced apoptosis of L6 myoblasts.
From the discussion set out above, we, for the first time, provide evidence that there exists a miR-147/BRMS1 axis that regulates the ER-Stress-induced apoptosis in L6 cells. We suppose that MiR-147 attenuates endoplasmic reticulum stress by targeting BRMS1 to inhibit cyclic mechanical stress-induced apoptosis of L6 myoblasts. Needless to say, it is unlikely that only one mechanism or pathway regulates the characteristics of L6 myoblasts. Furthermore, the studies of whether and how BRMS1 triggers ER-Stress in myoblasts are lacking; the other miR-147 target genes might also be involved with BRMS1 together to regulate ER-Stress-induced apoptosis in L6 myoblasts. Thus, further studies need to be carried out to support our hypothesis and elucidate the details of myoblast apoptosis under the condition of mechanical stretch.
Conclusion
Our study revealed one of the mechanisms of stress-induced apoptosis in myoblasts, providing an insight and a potential therapeutic strategy for malocclusion.
Electronic supplementary material
(DOCX 441 kb)
Acknowledgments
The study was supported by the Xuzhou clinical technical backbone training plan (2018).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanxiao Du and Feng Yang contributed equally to this work.
References
- Akimoto T, et al. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem Biophys Res Commun. 2005;329:381–385. doi: 10.1016/j.bbrc.2005.01.136. [DOI] [PubMed] [Google Scholar]
- Barnouti ZP, Owtad P, Shen G, Petocz P, Darendeliler MA. The biological mechanisms of PCNA and BMP in TMJ adaptive remodeling. Angle Orthod. 2011;81:91–99. doi: 10.2319/091609-522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Feng L, Ruan M, Liu X, Adriouch S, Liao H. Mechanical-stretch of C2C12 myoblasts inhibits expression of Toll-like receptor 3 (TLR3) and of autoantigens associated with inflammatory myopathies. PLoS One. 2013;8:e79930. doi: 10.1371/journal.pone.0079930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimonidou M, Kallergi G, Georgoulias V, Welch DR, Lianidou ES. Breast cancer metastasis suppressor-1 promoter methylation in primary breast tumors and corresponding circulating tumor cells. Mol Cancer Res. 2013;11:1248–1257. doi: 10.1158/1541-7786.MCR-13-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucina A, Fuso A, Coluccia P, Cavallaro A. Nicotine inhibits apoptosis and stimulates proliferation in aortic smooth muscle cells through a functional nicotinic acetylcholine receptor. J Surg Res. 2008;150:227–235. doi: 10.1016/j.jss.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Dam AD, Mitchell AS, Quadrilatero J. Induction of mitochondrial biogenesis protects against caspase-dependent and caspase-independent apoptosis in L6 myoblasts. Biochim Biophys Acta. 2013;1833:3426–3435. doi: 10.1016/j.bbamcr.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Deng G, Sui G. Noncoding RNA in oncogenesis: a new era of identifying key players. Int J Mol Sci. 2013;14:18319–18349. doi: 10.3390/ijms140918319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz GP, Wang DZ. Regulation of skeletal muscle by microRNAs. Compr Physiol. 2016;6:1279–1294. doi: 10.1002/cphy.c150041. [DOI] [PubMed] [Google Scholar]
- Erdem A, Kilic N, Eroz B. Changes in soft tissue profile and electromyographic activity after activator treatment. Aust Orthod J. 2009;25:116–122. [PubMed] [Google Scholar]
- Fu Shaoting, Yin Lijun, Lin Xiaojing, Lu Jianqiang, Wang Xiaohui. Effects of Cyclic Mechanical Stretch on the Proliferation of L6 Myoblasts and Its Mechanisms: PI3K/Akt and MAPK Signal Pathways Regulated by IGF-1 Receptor. International Journal of Molecular Sciences. 2018;19(6):1649. doi: 10.3390/ijms19061649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MR, et al. ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat Cell Biol. 2018;20:46–57. doi: 10.1038/s41556-017-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua W, Zhang M, Wang Y, Yu L, Zhao T, Qiu X, Wang L. Mechanical stretch regulates microRNA expression profile via NF-kappaB activation in C2C12 myoblasts. Mol Med Rep. 2016;14:5084–5092. doi: 10.3892/mmr.2016.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa HO, Tomari Y. The functions of microRNAs: mRNA decay and translational repression trends. Cell Biol. 2015;25:651–665. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Jia LX, et al. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol. 2015;236:373–383. doi: 10.1002/path.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Smith PW, Jones DR. Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol Cell Biol. 2006;26:8683–8696. doi: 10.1128/MCB.00940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Y, Yuan X, Feng Y, Liu H. Cyclic-stretch induces the apoptosis of myoblast by activation of caspase-3 protease in a magnitude-dependent manner. Int J Biochem Cell Biol. 2010;42:2004–2011. doi: 10.1016/j.biocel.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Lui JC. Regulation of body growth by microRNAs. Mol Cell Endocrinol. 2017;456:2–8. doi: 10.1016/j.mce.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning X, Wang C, Zhang M, Wang K. Ectopic expression of miR-147 inhibits stem cell marker and epithelial-mesenchymal transition (EMT)-related protein expression in colon cancer cells. Oncol Res. 2019;27:399–406. doi: 10.3727/096504018X15179675206495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owtad P, Potres Z, Shen G, Petocz P, Darendeliler MA. A histochemical study on condylar cartilage and glenoid fossa during mandibular advancement. Angle Orthod. 2011;81:270–276. doi: 10.2319/021710-99.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Niu W, Zhang H, Jun M, Zhang H. Downregulation of microRNA-147 inhibits cell proliferation and increases the chemosensitivity of gastric cancer cells to 5-fluorouracil by directly targeting PTEN. Oncol Res. 2018;26:901–911. doi: 10.3727/096504017X15061902533715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Song J, Hu B, Qu H, Bi C, Huang X, Zhang M. Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS One. 2012;7:e47657. doi: 10.1371/journal.pone.0047657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, et al. Cleavage of caspase-12 at Asp94, mediated by endoplasmic reticulum stress (ERS), contributes to stretch-induced apoptosis of myoblasts. J Cell Physiol. 2018;233:9473–9487. doi: 10.1002/jcp.26840. [DOI] [PubMed] [Google Scholar]
- Song SE, et al. Tomatidine inhibits tumor necrosis factor-alpha-induced apoptosis in C2C12 myoblasts via ameliorating endoplasmic reticulum stress. Mol Cell Biochem. 2018;444:17–25. doi: 10.1007/s11010-017-3226-3. [DOI] [PubMed] [Google Scholar]
- Strasser A, Huang DC, Vaux DL. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim Biophys Acta. 1997;1333:F151–F178. doi: 10.1016/s0304-419x(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Wei K, et al. Herb-partitioned moxibustion and the miRNAs related to Crohn's disease: a study based on rat models. Evid Based Complement Alternat Med. 2015;2015:265238. doi: 10.1155/2015/265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, et al. Role of miR-181a-5p and endoplasmic reticulum stress in the regulation of myogenic differentiation. Gene. 2016;592:60–70. doi: 10.1016/j.gene.2016.07.056. [DOI] [PubMed] [Google Scholar]
- White NM, Fatoohi E, Metias M, Jung K, Stephan C, Yousef GM. Metastamirs: a stepping stone towards improved cancer management. Nat Rev Clin Oncol. 2011;8:75–84. doi: 10.1038/nrclinonc.2010.173. [DOI] [PubMed] [Google Scholar]
- Xing WJ, et al. MRTF-A and STAT3 promote MDA-MB-231 cell migration via hypermethylating BRSM1 IUBMB. Life. 2015;67:202–217. doi: 10.1002/iub.1362. [DOI] [PubMed] [Google Scholar]
- Yagci A, Uysal T, Kara S, Okkesim S. The effects of myofunctional appliance treatment on the perioral and masticatory muscles in class II, division 1 patients. World J Orthod. 2010;11:117–122. [PubMed] [Google Scholar]
- Yamamoto K, Ito S, Hanafusa H, Shimizu K, Ouchida M. Uncovering direct targets of MiR-19a involved in lung cancer progression. PLoS One. 2015;10:e0137887. doi: 10.1371/journal.pone.0137887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- You J, He X, Ding H, Zhang T. BRMS1 regulates apoptosis in non-small cell lung cancer cells. Cell Biochem Biophys. 2015;71:465–472. doi: 10.1007/s12013-014-0226-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Effect of BRMS1 on tumorigenicity and metastasis of human rectal cancer. Cell Biochem Biophys. 2014;70:505–509. doi: 10.1007/s12013-014-9948-x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, et al. Caspase-12 is involved in stretch-induced apoptosis mediated endoplasmic reticulum stress. Apoptosis. 2016;21:432–442. doi: 10.1007/s10495-016-1217-6. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Qiao QD, Xie HF, Wei JX. Breast cancer metastasis suppressor 1 (BRMS1) suppresses prostate cancer progression by inducing apoptosis and regulating invasion. Eur Rev Med Pharmacol Sci. 2017;21:68–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 441 kb)