Abstract
The purpose of the current study was to demonstrate the neuroprotective effect of protodioscin (Prot) in an in vitro model of ischemia/reperfusion (I/R) and investigate the underlying molecular mechanism. After PC12 cells were exposed to oxygen and glucose deprivation (OGD) reperfusion, PI staining by flow cytometry was used to quantify the rate of apoptosis. The levels of hypoxia-inducible factor 1-alpha (HIF-1α), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were determined using commercially available kits. Intracellular reactive oxygen species (ROS) level was detected using the 20,70-dichlorodihy-drofluorescein diacetate (DCFH-DA) fluorescence assay. The expression levels of heat-shock proteins (HSP), PI3K, AKT, Nrf2, and miR-124 were tested by western blot or quantitative PCR. Prot significantly attenuated oxygen–glucose deprivation/reperfusion (OGD/R)-induced apoptotic death. Prot also reduced the oxidative stress as revealed by increasing the activities of SOD and GSH-Px, decreasing the levels of ROS and MDA. Moreover, mechanism investigations suggested that Prot prevented the decrease of HSP70, HSP32 (hemeoxygenase-1, HO-1), and PI3K protein expression, phosphorylation of AKT, and the accumulation of nuclear Nrf2. The level of miR-124 was decreased in PC12 cells, which was also effectively reversed by Prot treatment. Prot protected PC12 cells against OGD/R-induced injury through inhibiting oxidative stress and apoptosis, which could be associated with increasing HSP proteins expression via activating PI3K/AKT/Nrf2 pathway and miR-124 modulation.
Keywords: Protodioscin, Antioxidant, OGD/R, Heat-shock protein, miR-124, PI3K/AKT, Nrf2
Introduction
Brain stroke endangers human health and has a high risk of death and disability. (Diener et al. 2008). Clinically, it is mainly caused by cerebral artery thrombosis. After stroke, neural cells suffer from various cellular events which lead cells to apoptosis (Liu et al. 2009). Oxidants play important roles in the ischemic brain damage, and apoptosis is the main pathway that oxidative stress leads cells to death (Chan 1996). However, there is still a lack of effective strategies to protect brain cells from these ischemic injury-associated reactive cellular cascades; the progress in therapy of stroke is proceeding very slowly. So far, recombinant tissue-type plasminogen activator (rt-PA) is the only established clinical therapy for stroke, but there is only a 4.5-h time window to initiate rt-PA thrombolytic therapy after the onset of ischemic stroke symptoms, which results in a small fraction of patients for its limitations (Leker et al. 2003).
Above all, protection of neural cells against oxidative stress and apoptosis induced by ischemia–reperfusion (I/R) may be potentially beneficial for the prognosis of thrombolytic stroke therapy. The neural protection effect of heat-shock protein (HSP) 70 and 32 (hemeoxygenase-1, HO-1) have been extensively documented in association with a variety of insults, including ischemia, and may play a role in cell survival and recovery after injury (Rajdev et al. 2000; Xanthoudakis and Nicholson 2000). Several studies (Chen et al. 2018a, b; Xiao et al. 2018; Hu et al. 2018a, b; Fei et al. 2019) showed that phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT/PKB) signaling pathway positively affected nuclear factor (erythroid-derived 2)-like 2 (Nrf2) expression, alleviated oxidative damage and cell apoptosis. And PI3K/AKT signaling pathway is also demonstrated to be involved in multiple biological processes of neural cells, such as oxidative damage (Xi et al. 2018) and apoptosis (Zhong et al. 2019).
MicroRNAs (miRNAs) are noncoding RNAs that bind to the 3′-UTR of targeted messenger RNAs (mRNAs) and usually change mRNAs stability and inhibit their translation. Accumulative evidence revealed that the level of miR-124 played important roles in ischemic brain injury (Wang et al. 2017; Hamzei et al. 2016; Sun et al. 2013). Moreover, miR-124 is involved in regulating cell differentiation, survival, apoptosis, oxidative stress, and ROS production in neural cells (Yao et al. 2018; Haleh et al. 2018). This study aimed to further demonstrate the roles of miR-124 in cerebral VECs and neural cells against ischemic stroke in vitro.
Over many centuries, traditional Chinese medicine has been a reliable source of medicine with weak toxicity and multiple target spots. Steroid saponins consisting of spirostanol and furostanol forms are bioactive compounds abundantly present in various plants (Qiu et al. 2011). Protodioscin (Prot), a furostan saponin obtained from the rhizome of Dioscorea zingiberensis C.H. Wright, has a wide array of biological activity, such as anti-inflammatory (Santana et al. 2009), antioxidation (Liu et al. 2017), and anticancer (Wang et al. 2007). Though Sun reported (Zhang et al. 2016) that Prot had potential neuroprotection functions against cerebral ischemia-reperfusion injury in rats through intervening inflammation and apoptosis, its molecular mechanism of action remains unknown.
In the present study, we hypothesis that Prot could inhibit the oxidative stress and apoptosis of neural cells against ischemic stroke for neuroprotection, which were investigated by using oxygen–glucose deprivation/reperfusion (OGD/R) model in vitro (Elnaz et al. 2018). Underlying interactions associated with miR-124/AKT/Nrf2 were then explored.
Materials and methods
PC12 cells culture
Rat pheochromocytoma PC12 cells were purchased from ATCC (American Type Culture Collection). The cells were cultured in Dulbecco’s modified eagle medium (DMEM; Invitrogen, Grand Island, NY, USA) added 10% fetal bovine serum (FBS; Wisent Biotechnology Co., Ltd., Nanjing, China), 100 mg/ml streptomycin, and 100 U/ml penicillin, grew in 95% air and 5% CO2 humidified atmosphere at 37 °C, and the medium were changed every 2–3 days.
Oxygen–glucose deprivation/reperfusion and treatment
To mimic ischemia-like conditions in vitro, PC12 cells were washed with phosphate-buffered saline to remove the residual glucose and FBS. Then, cells were cultured in glucose-free balanced salt solution at 95% N2, 5% CO2, and 37 °C for 1 h to construct hypoxia. After hypoxia, OGD was terminated, the cells were transferred to the normal conditioned DMEM (reperfusion) with or without Prot (Meilun Biotechnology Co., Ltd. Dalian, China) at different concentrations (2.5 and 5 μM) for 24 h. Cells exposed to normoxic/normoglycemic were used as control. Prot was dissolved in DMSO for in vitro experiments, and the final concentration of DMSO was below 0.1% (v/v) in culture medium so that the cell viability would not be affected (Szliszka et al. 2011; Haiying et al. 2014).
In the current study, the time selected for OGD/R was on the basis of previous studies (Elnaz et al. 2018) and our preliminary experiments.
Cell viability assay
Cell viability was determined by Cell Counting Kit-8 (CCK-8) assay. In brief, PC12 cells were seeded in 96-well plates (4 × 103 cells/well) and incubated for 24 h. The cells then underwent 1 h of OGD, followed by incubation with conditioned DMEM (reperfusion) with or without Prot at different concentrations (0, 0.5, 1, 2.5; 5; 7.5, and 10 μM) for 24 h. Afterwards 10 μl of CCK-8 solution was added to each well for another 2 h at 37̊C. The absorbance at 450 nm was read using a microplate reader.
Cell apoptosis assay
Cells were seeded in six-well plates at the density of 1 × 105 cells/well. Each well contained 2 ml of culture medium. The cells were incubated for 24 h and then were exposed to the OGD/R. Cells then were collected and rinsed with PBS and re-suspended in 300 μl of binding buffer. Next, 5 μl of PI was added to the cell suspensions and incubated for 15 min in the dark. Cell apoptosis was then detected by using a BD FACSVerse flow cytometer.
ROS determination
Intracellular ROS level was detected using the 20,70-dichlorodihy-drofluorescein diacetate (DCFH-DA) fluorescence assay (Beyotime, Beijing). Briefly, after OGD treatment, cells (8 × 103cells/well) were washed twice with PBS, and then incubated with 10 μM DCFH-DA for 30 min at 37 °C in the dark. After being washed for three times, cells were then harvested and suspended in PBS. Intracellular ROS production was detected by measuring the fluorescence intensity of DCF with a microplate reader with the excitation and emission wavelengths at 490 and 529 nm, respectively.
Enzyme-linked immunosorbent assay
After OGD/R, 100 μl per well of cell culture supernatant fluid was used for measuring hypoxia-inducible factor 1-alpha (HIF-1α) (Guangzhou Biolink Biotechnology Co., Ltd., China). PC12 cells were harvested and re-suspended in PBS. The cell suspensions were sonicated for 25 s on ice, centrifuged in 12,000g for 15 min at 4 °C, and the supernatants were collected for the determination. A BCA Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used to detect protein concentrations according to the manufacturer’s instructions. Then, the levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were measured using commercially available enzyme-linked immunosorbent assay (ELISA) Kits (Jiancheng Biotechnology Co, Nanjing, China), respectively, according to protocol instructions.
Western blotting
After OGD/R, cells were then collected and lysed with the RIPA buffer. Proteins were extracted using a protein extraction kit (KeyGen Biotech, Nanjing, China) according to the manufacturer’s instructions. The nuclear nrf2 was isolated using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific, USA) according to the manufacturer’s protocol. Protein was measured according to the bicinchoninic acid (BCA) procedure (Solarbio, Beijing, China), with bovine serum albumin as the standard. Protein samples were loaded and separated by SDS-PAGE then transferred to PVDF membranes. Membranes were sealed using 5% fat-free milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 2 h at 37 °C. β-actin was used as a loading control. Membranes were incubated overnight at 4 °C with a 1:1000 dilution of polyclonal antibody for HSP70, HO-1, Nrf2, phosphorylated and total PI3K p85, and phosphorylated and total AKT (Beijing Boisynthesis biotechnology, China), respectively, and with a 1:1500 dilution of monoclonal antibody for PCNA or β-actin (Beyotime, China). After washing with TBST, blots were then incubated using secondary antibodies. After extensive TBST washing, membranes were exposed to the enhanced chemiluminescence-plus reagents (ECL) (Beyotime Institute of Biotechnology, Haimen, China). Emitted light was documented with the BioSpectrum-410 multispectral imaging system using Chemi HR camera 410 (Bio-Rad, Hercules, CA, USA). Protein bands were photographed under transmitted ultraviolet light. The image was semi-quantitative measured based on band densitometry.
Quantitative real-time polymerase chain reaction
The qRT-PCR analysis was performed to examine the expression of MIR-124. Total RNA was extracted via the RNAiso Plus® Reagent Kit (Takara Biotechnology), and 1 μg was used to prepare the cDNA by reversing the transcription by PrimeScript® RT Reagent Kit (Takara, Dalian, China). Amplify the cDNA using SYBR® Premix Ex Taq™ Kit (Takara, Dalian, China) and a Mx3000p instrument Agilent. PCR products were determined via the ΔΔCT method, and housekeeping gene GAPDH served as loading control. The sequences of the primers used were as follows: rat MIR-124 (F: 5′-TCG TTA AGG CAC GCG GTG -3′ and R: 5′- GTG CAG GGT CCG AGG T -3′), rat GAPDH (F: 5′- CCG TAT TCA GCA TTC TAT GCT CTC -3′ and R: 5′- TGG ATA CAC ACT CTG GGG CT -3′).
Data analysis
Statistical analysis is calculated using SPSS 13.0 software. Group data are represented as means ± S.D. The ANOVA analysis was used to compare statistically significant differences between groups. In all statistical analyses, two-sided p < 0.05 was considered to indicate a statistically significant result.
Results
Prot improved PC12 cells survival under OGD/R condition
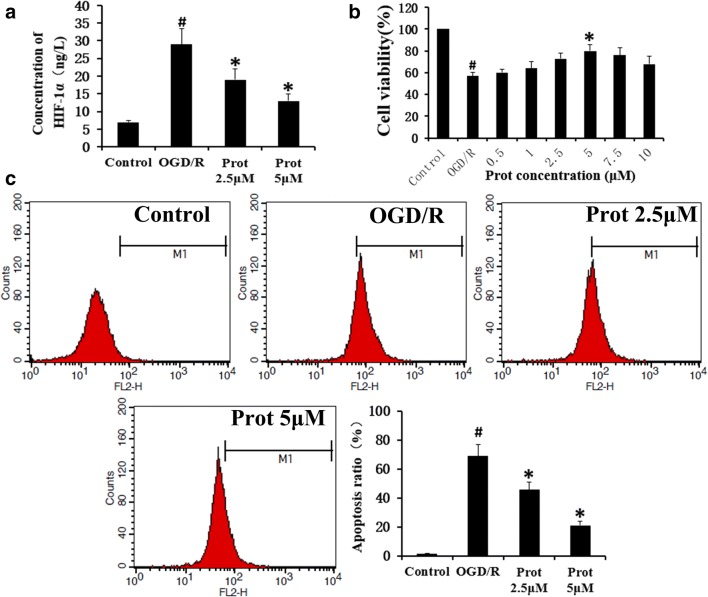
To validate our model and investigate the cellular responses to hypoxia, HIF-1a levels in cell supernatant fluid were analyzed using ELISA assay. The results showed that HIF-1α was increased significantly when the PC12 cells were under OGD/R conditions, which was improved by Prot treatment (Fig. 1a). We then examined the effect of Prot on neural cell viability. The rates of cell proliferation were evaluated using a CCK-8 assay (Fig. 1b). Prot could improve PC12 cell viability against OGD/R significantly. However, when the concentration reached 7.5 μM, the protective effect showed a decreasing tendency, which suggested that 5 μM of Prot produced a more favorable effect than any other concentrations in this study. PI staining by flow cytometry was further used to quantify the rate of apoptosis (Fig. 1c). We could observe that compared with the control group, OGD/R could markedly induce the cells apoptosis, which was significantly suppressed by Prot in a concentration-dependent manner. OGD/R stimulation increased the percentage of cells undergoing apoptosis from 1.48 to 72.24%, which was decreased by Prot to 27.3% (Fig. 1c).
Fig. 1.
Prot improved neural cells survival under OGD/R condition. a HIF-1a levels in cell supernatant fluid were analyzed using ELISA assay. b The rates of cell proliferation were evaluated using a CCK-8 assay. c Apoptosis rates were further quantified through the PI assay. Prot produced a significant protective effect against OGD/R. Histograms showing the percentage of cells undergoing apoptosis. Data are shown with mean ± SD (n = 6) (#p < 0.05 versus the control group; *p < 0.05 versus the OGD/R group)
Prot ameliorates OGD/R-induced oxidative stress in neural cells
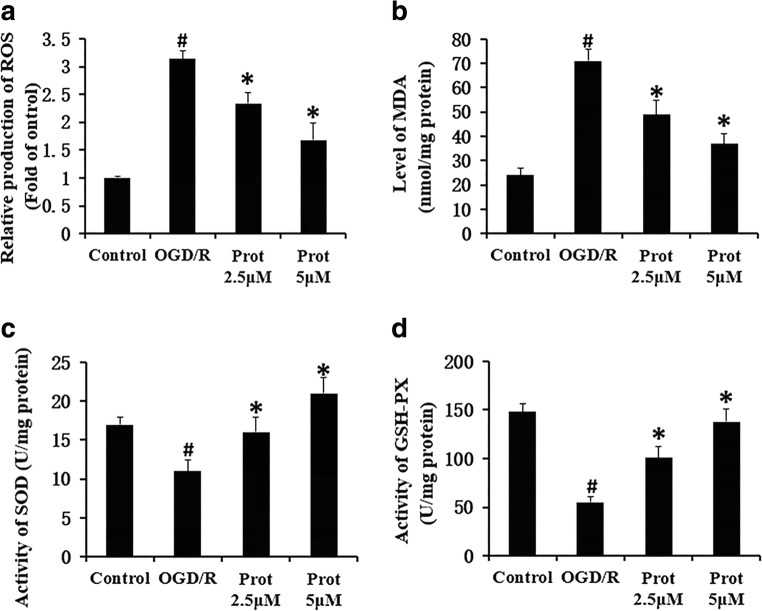
Oxidative stress played important roles in the development of cerebral I/R damage. Under physiological conditions, there is a balance between ROS generation and ROS elimination by scavengers. Several reports have revealed that cerebral ischemia significantly increased the content of ROS and reduced the activity of antioxidant enzymes in the cerebral cortex (Chen et al. 2011). The maintenance of an intact vascular network for delivering oxygen and nutrients is critical to reduce the damage. ROS, as an important oxidative stress molecule, participates in promoting the occurrence of ischemic stroke. (Nanda et al. 1996). Thus, we examined the effect of Prot on oxidative stress in OGD/R-stimulated neural cells. As shown in Fig. 2, OGD/R treatment significantly induced the level of ROS (Fig. 2a). However, treatment with Prot significantly decreased ROS production in cells after OGD/R (Fig. 2a). Additionally, treatment with Prot significantly inhibited OGD/R-increased MDA content in cells (Fig. 2b). In contrast, the activity of SOD (Fig. 2c) and GSH-Px (Fig. 2d) significantly decreased in cells exposed to OGD/R compared with that in the control group. When OGD/R injured cells were incubated with Prot, SOD and GSH-Px activity was significantly increased compared with the OGD/R group (Fig. 2c, d). Likewise, the protective effects above of Prot were all exhibited in a concentration-dependent manner. These data imply that Prot could protect from OGD-induced injury through modulating the endogenous antioxidative system.
Fig. 2.
Prot ameliorates OGD-induced oxidative stress in neural cells. The level of intracellular ROS was also revealed by DCFH-DA staining. The levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were measured using ELISA Kits. All data are shown with mean ± SD (n = 6) (#p < 0.05 versus the control group; *p < 0.05 versus the OGD/R group)
Prot increased the activities of the HSP proteins and PI3K/AKT/Nrf2 pathway and enhanced the miR-124 expression in cells under OGD/R condition
To explore the molecular mechanism of Prot protected from OGD/R-induced cerebral damage, we measured the protein expression of HSP70, HO-1, p-PI3K, PI3K, Nrf2, phosphor-AKT, and total-AKT by Western blot. PI3K is an intracellular phosphatidylinositol kinase which consists of a catalytic subunit (p110) and a regulatory subunit (p85) (Hennessy et al. 2005). AKT is an important downstream target in the PI3K signal transduction pathway which can promote cell survival, inhibit apoptosis, and maintain normal function as a key information molecule. Previous studies have demonstrated that PI3K/Akt-mediated signaling is one of the most critical pathway regulating mammalian cell survival, proliferation, and metabolism, and that Akt phosphorylation is elevated during cerebral ischemia, especially within the penumbra, may be a part of an endogenous protection mechanism (Kilic et al. 2017). As shown in Fig. 3, the expression levels of p-PI3K/PI3K (Fig. 3d) and the p-AKT/AKT (Fig. 3e) ratio were significantly decreased in cells exposed to OGD/R, compared with the control group. However, Prot treatment efficiently reversed these in OGD/R-stimulated cells. Nrf2, one of the important downstream targets of AKT, is a key transcription factor that triggers the expression of antioxidant proteins under specific conditions. Under normal conditions, Nrf2 is retained in the cytoplasm, but production of ROS usually triggers oxidative stress in cells, resulting in the Nrf2 translocation into the nucleus, which initiates the expression of antioxidant genes and enzymes, including HSP70 (Martin et al. 2011) and HO-1 (Qiu et al. 2019). As shown in Fig. 3, the nuclear Nrf2 proteins (Fig. 3f) were downregulated in the OGD/R group compared with the control group, but was increased after the intervention of Prot incubation. Increased expression of heat-shock protein HSP70 and HO-1 has been extensively documented in relation to the cell survival and recovery after ischemia injury in the brain. The results showed that the HSP proteins were all downregulated in the OGD/R group compared with the control group, but was increased after the intervention of Prot incubation (Fig. 3b, c). There are various expression types in the nervous system, miRNAs are small noncoding, single-stranded RNAs, induce degradation or protein translation impediment via interaction with the target gene mRNA’ 3-UTR. miR-124 is reported to be closely related to the PI3K/AKT pathway in multiple pathological conditions. Interestingly, OGD/R significantly decrease the miR-124 level in PC12 cells, which were reversed by Prot treatment (Fig. 3g).
Fig. 3.
Effect of Prot on the expression of HSP70, HO-1, PI3K/AKT/Nrf2 pathway, and miR-124 in cells after OGD/R stimulation. Prot promoted the protein expression of HSP70 and HO-1, increased the activities of the PI3K/AKT/Nrf2 pathway, and enhanced the miR-124 expression in cells under OGD/R condition. The bar graph shows the relative expression ratio of each protein calculated after normalization to β-actin or PCNA. Data are shown with mean ± SD (n = 6) (#p < 0.05 versus the control group; *p < 0.05 versus the OGD/R group)
Discussion
Ischemic stroke always triggers a series of complex and interplayed signaling pathways, leading to dysfunction of the neurovascular network in the ischemic area and subsequently irreversible brain damage (Grell et al. 2014).
The neuroprotective actions of Prot have also been documented to have beneficial effects in experimental stroke therapy through intervening inflammation and apoptosis, including lowered the death rate, reduced infarct volume, improved neurological function through reducing the deficit scores, and other supporting evidence (Zhang et al. 2016). Consistent with the results of previous studies, in the current study, we observed that compared with the control group, OGD/R could markedly induce cell apoptosis. Prot produced a significant antiapoptosis effect on neural cells against OGD/R in a concentration-dependent manner. However, the underlying mechanism of action above remains unknown.
Immediately after ischemic insult, many spontaneous protective mechanisms are activated to maintain cell homeostasis (Tang et al. 2015). Oxidative stress is involved in the pathogenesis and progression of ischemic diseases, including cerebral ischemia (Elnaz et al. 2018). It has been proven that Prot could protect the brain against oxidative stress in multiple pathological conditions, such as epilepsy (Song et al. 2018), Alzheimer’s disease (Lee et al. 2018), and others. However, whether the efficacy of Prot against oxidative stress is involved in its antistroke capability has not been reported until now. In this study, OGD/R significantly induced the level of ROS, increased MDA content, and decreased the activity of SOD and GSH-Px significantly, which were all reversed by Prot treatment.
To investigate the molecular mechanism by which the above response were dependent on, the roles of HSP proteins and PI3K/AKT/Nrf2 signal pathway were involved. AKT, one of the primary downstream targets of PI3K signaling, is an important information molecule that promotes cell survival, inhibits apoptosis (Chen et al. 2018a, b), and maintains oxidative stress (Xu et al. 2018). Activated AKT can transmit signals to a variety of downstream proteins, including Nrf2. Nrf2 is one of the master regulators of the expression of proteins involved in both endogenous antioxidation defense, and apoptosis (Sun et al. 2018a, b; Jiang et al. 2018) plays a pivotal role in the resistance to oxidative stress and apoptosis induced by ischemia stroke (Shaonan et al. 2018; Cui et al. 2018; Zhang et al. 2018). Consistent with previous studies above, the current study demonstrated that the expression levels of p-PI3K proteins and the p-AKT/AKT ratio were significantly decreased in cells exposed to OGD/R, compared with the control group. However, Prot treatment efficiently reversed these in OGD/R-stimulated cells. A number of studies have indicated that the expression of Nrf2 is largely increased in the acute phase of strokes (Zhang et al. 2017). Moreover, Chu et al. (2019) reported that the Nrf2 nuclear accumulation induced by OGD/R in PC12 cells, begins at 2 h of OGD, peaks at 4 h, and decreases below baseline at ~ 8 h. In our study, after cells were exposed to 1 h OGD followed by 24 h reperfusion, the nuclear Nrf2 level decreased significantly compared with that in the control group. However, Prot treatment significantly increased the Nrf2 nuclear accumulation above the baseline, which provides the basis for the continuous promotion of the expression of antioxidation genes. Taken together, these results suggested that the effects of Prot are related to the activation of Nrf2 pathway via PI3K/AKT signaling. Induction of HO-1 expression in vascular endothelial cells can prevent oxidative stress–induced apoptosis (Foresti et al. 1999) and attenuate the response of arteries to injury and ensure vascular wall remodeling (Tulis et al. 2001). Overexpressing HO-1 in neurons induce resistance to glutamate and hydrogen peroxide–mediated cell death (Chen et al. 2000) and protect the brain from focal ischemia injury in mice (Panahian et al. 1999). As reported, overexpression of HSP70 protected against a variety of different injuries in vitro (Sharp et al. 1999; Santos 2015) and protected against the ischemia and excitoxicity in vivo (Yenari et al., 1999). Overexpression of HSP70 significantly protected the cerebral tissues against injury induced by permanent middle cerebral artery occlusions in transgenic mice (Rajdev et al. 2000). In this study, Prot-induced HO-1and HSP70 in PC12 may protect these cells from OGD/R injury and provide a new molecular mechanism through which Prot protected the brain from ischemia injury.
More and more studies (Xue et al. 2016; Liu et al. 2016) demonstrated that miR-124 could positively modulate the activity of PI3K/AKT pathway. On the other hand, there were also reports that miR-124 expression is inversely correlated with the p-AKT level (Jin et al. 2017; Wu et al. 2017). In addition, miR-124 expression is the most obvious and most highly expressed miRNA in the cerebral cortex and cerebellum (Cheng et al. 2009), participates in a series of significant physiological activities including neuronal cell cycle regulation and cell differentiation (Gao 2008). Wang et al. (2017) reported that miR-124 participates in the neural cells protection against ischemic stroke by activating the PI3K/AKT signaling pathway. And Hu et al. (2018a, b) found that downregulating miR-124 could aggravate the hypoxia injury in PC12 cells. Liu et al. (2013) demonstrate that miR-124 level increased significantly in a mouse model of focal permanent cerebral ischemia, but no significant neuronal injury was observed in miR-124-overexpressing mice. Interestingly, Sun et al. (2018a) reported that the miR-124 levels in the patients with acute cerebral infarction rapidly decreased within 24 h after ischemia and then gradually increased at 48∼72 h after ischemia as well as the 7th day after ischemia. We thus speculate that the rise of miR-124 level in the later stage may be due to the compensatory protection of the body against ischemic injury. These results indicated that miR-124 could play diverse roles in neural cells during ischemia stroke. Our results in this study showed that OGD/R significantly decrease the miR-124 level in PC12 cells, which could be reversed by Prot treatment. In combination with the foregoing, Prot treatment protects neural cells against OGD/R via activating PI3K/AKT/Nrf2 pathway, which could be associated with the elevation of miR-124. However, the regulatory mechanism of miR-124 has not been fully elucidated; further dissection of the regulatory mechanisms may lead to new therapeutic opportunities for preventing neuronal death after stroke.
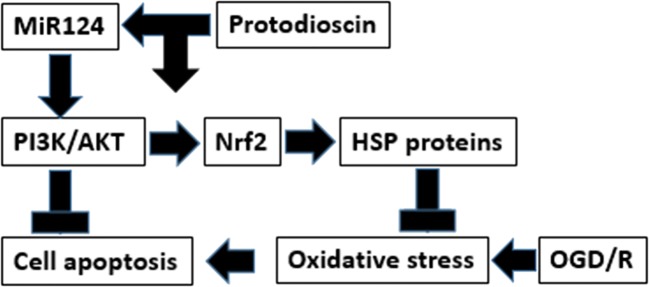
In summary, as shown in Fig. 4, we identified that the anti-OGD/R effect of Prot involves the PI3K/AKT/Nrf2 pathway and the underlying mechanism could be associated with post-transcriptional regulation by miR-124. Furthermore, we provide evidence that the enhanced PI3K/AKT/Nrf2 pathway and HSP activity could be promising drug targets for the treatment of ischemic stroke.
Fig. 4.
Prot protected PC12 cells against OGD/R-induced injury through inhibiting oxidative stress and apoptosis, which could be associated with increasing HSP proteins expression via activating PI3K/AKT/Nrf2 pathway and miR-124 modulation
Abbreviations
- Prot
Protodioscin
- OGD/R
Oxygen–glucose deprivation/reperfusion
- HIF-1α
Hypoxia-inducible factor 1-alpha
- SOD
Superoxide dismutase
- GSH-Px
Glutathione peroxidase
- MDA
Malondialdehyde
- ROS
Reactive oxygen species
- HSP
Heat shock proteins
- HO-1
Hemeoxygenase-1
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PI3K
Phosphatidylinositol-3 kinase
Funding information
This work is supported by the Xi’an Jiaotong University, Xi’an, Shanxi, China.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chan PH. Role of in ischemic brain damage. Stroke. 1996;27(6):1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yan R, Zhou K, Li X, Zhang Y, Liu C, Jiang M, Ye H, Meng X, Pang N, Zhao L, AKT-mediated platelet apoptosis and its therapeutic implications in immune thrombocytopenia. Proc Natl Acad Sci U S A 2018a Nov 06;115(45) [DOI] [PMC free article] [PubMed]
- Chen G, Chen X, Niu C, Huang X, An N, Sun J, Huang S, Ye W, Li S, Shen Y, Liang J (2018b) Baicalin alleviates hyperglycemia-induced endothelial impairment 1 via Nrf2. J Endocrinol 01 [DOI] [PubMed]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S-f, Zhang Z, Zhou X, He W-b, Chen C, Luo P, Liu D-d, Ai Q-d, Gong H-f, Wang Z-z, Sun H-s, Feng Z-p, Chen N-h. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol Sin. 2019;40:13–25. doi: 10.1038/s41401-018-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui HY, Zhang XJ, Yang Y, Zhang C, Zhu CH, Miao JY, Chen R (2018) Rosmarinic acid elicits neuroprotection in ischemic stroke Nrf2 and heme oxygenase 1 signaling. Neural Regen Res 13(12) [DOI] [PMC free article] [PubMed]
- Diener HC, Katsarava Z, Weimar C. Headache associated with ischemic cerebrovascular disease. Rev Neurol (Paris) 2008;164(10):819–824. doi: 10.1016/j.neurol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Elnaz MA, Mahdavi M, Fard FJ, Chamani S, Farajdokht F, Karimi P (2018) Metformin protects PC12 cells against oxygenglucose deprivation/reperfusion injury. Toxicol Mech Methods:28(8) [DOI] [PubMed]
- Fei L, Huang X, Luo Z, He J, Haider F, Song C, Peng L, Chen T, Buling W. Hypoxia-activated PI3K/Akt inhibits oxidative stress via the regulation of reactive oxygen species in human dental pulp cells. Oxidative Med Cell Longev. 2019;2019:6595189. doi: 10.1155/2019/6595189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R. Peroxynitrite induces haem oxygenase-1 in vascular endothelial cells: a link to apoptosis. Biochem J. 1999;339:729–736. [PMC free article] [PubMed] [Google Scholar]
- Gao FB. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008;31:20–26. doi: 10.1016/j.tins.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell AS, Thigarajah R, Edvinsson L, Samraj AK. Regulatory mechanism of endothelin receptor B in the cerebral arteries after focal cerebral ischemia. PLoS One. 2014;9:e113624. doi: 10.1371/journal.pone.0113624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiying L, Gao A, Feng D, Yang W, Zhang L, Cui Y, Li B, Wang Z, Chen G. Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood–brain barrier integrity during experimental cerebral ischemia–reperfusion injury. Transl Stroke Res. 2014;5:618–626. doi: 10.1007/s12975-014-0354-x. [DOI] [PubMed] [Google Scholar]
- Haleh M, Najafzadeh N, Vardin MM (2018) miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J Cell Physiol:1–10 [DOI] [PubMed]
- Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M (2016) MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 91 [DOI] [PubMed]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Hu Shaonan, Wu Yali, Zhao Bo, Hu Haiyan, Zhu Baochen, Sun Zongxi, Li Pengyue, Du Shouying. Panax notoginseng Saponins Protect Cerebral Microvascular Endothelial Cells against Oxygen-Glucose Deprivation/Reperfusion-Induced Barrier Dysfunction via Activation of PI3K/Akt/Nrf2 Antioxidant Signaling Pathway. Molecules. 2018;23(11):2781. doi: 10.3390/molecules23112781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Xiaoli, Liu Juan, Zhao Gang, Zheng Jiaping, Qin Xia. Long non-coding RNA GAS5 aggravates hypoxia injury in PC-12 cells via down-regulating miR-124. Journal of Cellular Biochemistry. 2018;119(8):6765–6774. doi: 10.1002/jcb.26870. [DOI] [PubMed] [Google Scholar]
- Jiang W, Meng L, Xu G, Lv C, Wang H, Tian H, Chen R, Jiao B, Wang B, Huang C (2018) Wentilactone A induces cell apoptosis by targeting AKR1C1 gene via the IGF-1R/IRS1/PI3K/AKT/Nrf2/FLIP/Caspase-3 signaling pathway in small cell lung cancer. Oncol Lett 16(5) [DOI] [PMC free article] [PubMed]
- Jin H, Li Q, Cao F, Wang SN, Wang RT, Wang Y, Tan QY, Li CR, Zou H, Wang D. Xu CX, miR-124 inhibits lung tumorigenesis induced by K-ras mutation and NNK. Mol Ther Nucleic Acids. 2017;15:9. doi: 10.1016/j.omtn.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Caglayan AB, Beker MC, Gunal MY, Caglayan B, Yalcin E, Kelestemur T, Gundogdu RZ, Yulug B, Yilmaz B, Kerman BE, Kilic E. Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol. 2017;12:657–665. doi: 10.1016/j.redox.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HA, Kim JE, Sung JE, Yun WB, Kim DS, Lee HS, Hong JT, Hwang DY. Asparagus cochinchinensis stimulates release of nerve growth factor and abrogates oxidative stress in the Tg2576 model for Alzheimer’s disease. BMC Complement Altern Med. 2018;06:18(1). doi: 10.1186/s12906-017-1775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34(8):2000–2065. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- Liu HJ, Yang JP, Wang CH, Liu RC, Li Y, Li CY. Endoplasmic reticulum in the penumbra following middle cerebral artery occlusion in the rabbit. Neurol Sci. 2009;30(3):227–232. doi: 10.1007/s10072-009-0086-y. [DOI] [PubMed] [Google Scholar]
- Liu Xiangrong, Li Fang, Zhao Shangfeng, Luo Yumin, Kang Jun, Zhao Haiping, Yan Feng, Li Sijie, Ji Xunming. MicroRNA-124–Mediated Regulation of Inhibitory Member of Apoptosis-Stimulating Protein of p53 Family in Experimental Stroke. Stroke. 2013;44(7):1973–1980. doi: 10.1161/STROKEAHA.111.000613. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao J, Liu Q, Xiong X, Zhang Z, Jiao Y, Li X, Liu B, Li Y, Lu Y. MicroRNA-124 promotes hepatic triglyceride accumulation through targeting tribbles homolog 3. Sci Rep. 2016;11(15):6. doi: 10.1038/srep37170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Jia-Yu, Hou Ya-Ling, Cao Rong, Qiu Hong Xia, Cheng Guo-Hua, Tu Ran, Wang Li, Zhang Jun-Li, Liu Dan. Protodioscin ameliorates oxidative stress, inflammation and histology outcome in Complete Freund’s adjuvant induced arthritis rats. Apoptosis. 2017;22(11):1454–1460. doi: 10.1007/s10495-017-1420-0. [DOI] [PubMed] [Google Scholar]
- Martin E, Tosi R, Bocanegra V, Manucha W, Lorenzo AG, Patricia G. VallésThe Nrf2–Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO) Cell Stress Chaperones. 2011;16:57–68. doi: 10.1007/s12192-010-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda YP, Chatterjee A, Purohit AK, Dia llo A, Innui K, Sharma RN, Lib eau G, Theva saga yam JA, Bruning A, Kitching RP, Anderson J, Barrett T, Taylor WP. The isolation of peste des petits ruminants virus from northern India. Vet Microbiol. 1996;51:207–216. doi: 10.1016/0378-1135(96)00025-9. [DOI] [PubMed] [Google Scholar]
- Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- Qiu LL, Niu H, Huang W. Ultrasonic and fermented pretreatment technology for diosgenin production from Dioscorea zingiberensis C.H. Wright. Chem Eng Res Des. 2011;89:239–247. [Google Scholar]
- Qiu L, Jin Z, Xu Z, Yang H, Liang L, Li G, Li F, Shaoli G, Zong S, Zhou J, Liang C, Wang Z, Xiao W. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 2019;24:441–452. doi: 10.1007/s12192-019-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Santana PM, Miranda M, Gutiérrez Y, García G, Orellana T, Orellana A. Antinflammatory effect and chemical composition of bursera graveolens Triana & Planch.branch oil (palo santo) from Ecuador. Rev Cuba Plantas Med. 2009;14:45–53. [Google Scholar]
- Santos TMM, Sinzato YK, Gallego FQ, Iessi IL, Volpato T, Dallaqua B, Damasceno DC. Extracellular HSP70 levels in diabetic environment in rats. Cell Stress Chaperones. 2015;20:595–603. doi: 10.1007/s12192-015-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Shaonan, Wu Yali, Zhao Bo, Hu Haiyan, Zhu Baochen, Sun Zongxi, Li Pengyue, Du Shouying. Panax notoginseng Saponins Protect Cerebral Microvascular Endothelial Cells against Oxygen-Glucose Deprivation/Reperfusion-Induced Barrier Dysfunction via Activation of PI3K/Akt/Nrf2 Antioxidant Signaling Pathway. Molecules. 2018;23(11):2781. doi: 10.3390/molecules23112781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Massa SM, Swanson RA. Heat-shock protein protection. Trends Neurosci. 1999;22:97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Song S, Fajol A, Chen Y, Ren B, Shi S. Anticonvulsive effects of protodioscin against pilocarpine-induced epilepsy. Eur J Pharmacol. 2018;15:833. doi: 10.1016/j.ejphar.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X (2013) MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther 19(10) [DOI] [PMC free article] [PubMed]
- Sun M, Hou X, Guang R, Zhang Y, Cheng H (2018a) Dynamic changes in miR-124 levels in patients with acute cerebral infarction. Int J Neurosci [DOI] [PubMed]
- Sun Pingping, Nie Xiaoke, Chen Xiaoxu, Yin Lifeng, Luo Jiashan, Sun Lingli, Wan Chunhua, Jiang Shengyang. Nrf2 Signaling Elicits a Neuroprotective Role Against PFOS-mediated Oxidative Damage and Apoptosis. Neurochemical Research. 2018;43(12):2446–2459. doi: 10.1007/s11064-018-2672-y. [DOI] [PubMed] [Google Scholar]
- Szliszka E, Czuba ZP, Bronikowska J, Mertas A, Paradysz A, Krol W. Ethanolic extract of propolis augments TRAIL-induced apoptotic death in prostate cancer cells. Evid Based Complement Alternat Med eCAM. 2011;2011:535172. doi: 10.1093/ecam/nep180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Dong W, Xie P, Cheng P, Bai S, Ren Y, Wang G, Chen X, Cui C, Zhuang Y, Huang W. The effect of pre-condition cerebella fastigial nucleus electrical stimulation within and beyond the time window of thrombolytic on ischemic stroke in the rats. PLoS One. 2015;10:e0128447. doi: 10.1371/journal.pone.0128447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 2001;155:113–122. doi: 10.1016/s0021-9150(00)00552-9. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Liu ZB, Li J, Zhong M, Li JP, et al. Determination of protodioscin in rat plasma by liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2007;848:363–368. doi: 10.1016/j.jchromb.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Wang Changming, Wei Zhijie, Jiang Guohong, Liu Haijun. Neuroprotective mechanisms of miR-124 activating PI3K/Akt signaling pathway in ischemic stroke. Experimental and Therapeutic Medicine. 2017;13(6):3315–3318. doi: 10.3892/etm.2017.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Da-Peng, Zhang Jun-Lei, Wang Jing-Yu, Cui Ming-Xing, Jia Jin-Ling, Liu Xiang-Hua, Liang Qiu-Dong. MiR-1246 Promotes LPS-Induced Inflammatory Injury in Chondrogenic Cells ATDC5 by Targeting HNF4γ. Cellular Physiology and Biochemistry. 2017;43(5):2010–2021. doi: 10.1159/000484162. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S, Nicholson DW. Heat-shock proteins as death determinants. Nat Cell Biol. 2000;2:E163–E165. doi: 10.1038/35023643. [DOI] [PubMed] [Google Scholar]
- Xi JS, Wang YF, Long XX, Ma Y. Mangiferin potentiates neuroprotection by isoflurane in neonatal hypoxic brain injury by reducing oxidative stress and activation of Phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling. Med Sci Monit. 2018;19:24. doi: 10.12659/MSM.908142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Chen B, Wang Q, Yang L, Guo H. Paeonin extracted from potatoes protects gastric epithelial cells from HO-induced oxidative damage in vitro by PI3K/AKT-mediated Nrf2 signaling pathway. Sci Rep. 2018;18:8(1). doi: 10.1038/s41598-018-28772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Xiuhua, Kim J. Julie, Li Yinuo, Xie Jia, Shao Changshun, Wei Jian-Jun. Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma. Journal of Molecular Medicine. 2018;96(10):1095–1106. doi: 10.1007/s00109-018-1682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Yu C, Wang Y, Liu L, Zhang K, Fang C, Liu F, Bian G, Song B, Yang A, Ju G, Wang J. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci Rep. 2016;05(25):6. doi: 10.1038/srep26781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Ye Y, Mao H, Lu F, He X, Lu G, Zhang S. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J Neuroinflammation. 2018;12:15(1). doi: 10.1186/s12974-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5:525–531. doi: 10.1016/s1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xue X, Xian L, Guo Z, Ito Y, Sun W. Potential neuroprotection of protodioscin against cerebral ischemia-reperfusion injury in rats through intervening inflammation and apoptosis. Steroids. 2016;09:113. doi: 10.1016/j.steroids.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. 2017;54:6006–6017. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- Zhang Wen, Song Jun-ke, Yan Rong, Li Li, Xiao Zhi-yong, Zhou Wen-xia, Wang Zhen-zhong, Xiao Wei, Du Guan-hua. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacologica Sinica. 2018;39(8):1259–1272. doi: 10.1038/aps.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhu Y, He T, Li W, Li Q, Miao Y (2019) Brain-derived neurotrophic factor inhibits hyperglycemia-induced apoptosis and downregulation of synaptic plasticity-related proteins in hippocampal neurons via the PI3K/AKT pathway. Int J Mol Med 43(1) [DOI] [PMC free article] [PubMed]