Abstract
While governments and natural resource managers grapple with how to respond to climatic changes, many marine-dependent individuals, organisations and user-groups in fast-changing regions of the world are already adjusting their behaviour to accommodate these. However, we have little information on the nature of these autonomous adaptations that are being initiated by resource user-groups. The east coast of Tasmania, Australia, is one of the world’s fastest warming marine regions with extensive climate-driven changes in biodiversity already observed. We present and compare examples of autonomous adaptations from marine users of the region to provide insights into factors that may have constrained or facilitated the available range of autonomous adaptation options and discuss potential interactions with governmental planned adaptations. We aim to support effective adaptation by identifying the suite of changes that marine users are making largely without government or management intervention, i.e. autonomous adaptations, to better understand these and their potential interactions with formal adaptation strategies.
Electronic supplementary material
The online version of this article (10.1007/s13280-019-01186-x) contains supplementary material, which is available to authorized users.
Keywords: Autonomous adaptation, Climate change, Indigenous knowledge, Local knowledge, Marine biodiversity, Species redistribution
Introduction
Climate-driven change in marine systems is already extensive and predicted to escalate (Hoegh-Guldberg et al. 2014). Many aspects of marine ecosystems, including their human components, are being profoundly impacted, and historically adequate mechanisms for coping with change are being severely challenged. Societal responses to such change have comprised both mitigation and adaptation, with the former dominating national and international agendas, and the latter prevailing at finer scales such as regional, sectoral and community levels. While both responses are needed, the demand for human system adaptation that maximizes opportunities and minimizes environmental, economic and social consequences will rapidly increase. This is, particularly under business-as-usual climate scenarios (Pecl et al. 2017), if the longevity of natural marine systems and associated livelihoods is to be ensured.
Human system adaptation to climate change involves decisions across a potentially complex multi-agent landscape comprising both private and public actors (Adger et al. 2005). Adaptation can be initiated by private individuals and be in that individual’s self-interest; however, adaptation can also be initiated by governments, and in the public interest. In the marine context, individuals, firms, peak bodies, environmental non-governmental organisations, civil society, management agencies and governments at various geographical and jurisdictional scales all make adaptation decisions. Adaptation decisions in the context of marine systems are influenced by, for instance, strategy, timeline, costs and other limitations (Miller et al. 2018). Actors differ in terms of the extent and nature of their stake in the marine resource and in the way in which they will experience change, as well as in terms of their capacity and agency to act (van Putten et al. 2015). They also differ in terms of their values, beliefs and attitudes as reflected in the diverse range of processes, behaviours, activities and actions implemented by these groups. Adaptation requires not only the willingness to change, but also the practical possibility to change in terms of agency, power and resources (Huntington et al. 2017).
Adaptation actions and processes have been categorised according to a number of different schema for a variety of purposes (see Table 1 in Grüneis et al. 2016). Beyond the private- public actor divide, the distinction is drawn on the basis of their purposefulness, timing, temporal and spatial scopes, form, function and performance at achieving intended objectives (Malik et al. 2010). A distinction is also often drawn on the basis of the extent of an actor’s intent to adapt, with some actions arising spontaneously and without conscious deliberation, in response to the manifestations of climate change in natural and human systems. At the other end of the spectrum of intent to adapt (Fankhauser et al. 1999) lies planned adaptation which is the result of a coordinated decision, based on an awareness that conditions have changed, or are about to change, and that action is required to return to, to maintain or to achieve a desired state (Smith et al. 2000).
The marine domain poses special challenges for unravelling the nature of human system adaptation (Miller et al. 2018). The fingerprint of climate change in marine systems is complex, with changes in sea temperatures and acidity, sea levels and currents acting individually and in combination to produce highly uncertain conditions that impact the operations and performance of all actors (Pecl et al. 2014a, 2017). Problems associated with potentially countervailing adaptation responses, and the conflicts they may fuel, are magnified by the large number of non-government actors who derive benefit from marine resources, and who also often hold different values. The pivotal role of government actors in marine management and governance is also notable. The increasing role of co-management processes and models of participatory governance in key marine sectors, such as fisheries, have ostensibly served to increase the agency and adaptive capacity of recognised marine users (Ogier et al. 2016; Nursey-Bray et al. 2018). However, as a number of theorists have noted (Berkes 2009; Pinkerton 2011, 2018), the power geometries between government and non-government actors remain problematic as these mechanisms (usually applied only symbolically or technically between government and non-government industry actors) have largely failed to substantively re-structure power relations. This can have the unintended consequence of further marginalisation of non-government actors in some cases (e.g. Indigenous fishers); effects which could have direct implications for the possibility of user-based autonomous adaptation.
We define autonomous adaptation as adaptation actions that were initiated by non-government actors, rather than those with direct powers over the planned management of the changing resources themselves (i.e. the government in the case of common pool marine resources). This includes responses by individuals or where a community or group of marine users works with others (collaboratively) (Huntington et al. 2017). For autonomous adaptation to be effective, the right incentive, knowledge, resources and skills are combined (Fankhauser et al. 1999).
To date, studies of marine adaptation in human systems have focused on developing the frameworks and principles of adaptation planning. Empirically based studies of implemented adaptation actions, the evaluation of the outcomes of these actions (Bradley et al. 2015; Miller et al. 2018), and how the actions of government and non-government actors might connect, are scarce. Where sector- or region-wide adaptation planning has progressed, this has been largely done without reference to an understanding of the non-government adaptation landscape (Cinner et al. 2011; Jennings et al. 2016; Miller et al. 2018), which often progresses ahead of the generally more deliberative and proactive adaptations characteristic of government agencies (Nayak 2017). In the absence of explicit mechanisms to ensure, for instance, adaptive management, individuals, organisations and communities may have greater flexibility to adjust their behaviour more readily to accommodate changes evident in the natural ecosystems they depend on (Ojea et al. 2017; Huntington et al. 2017). Moreover, although there is a burgeoning literature on climate change related adaptation, little attention has been paid to identifying the best mix of adaptation responses in the design of adaptation strategies at various scales (Tompkins and Eakin 2012). Meeting this challenge involves understanding the extent to which different adaptation actions may impede or enhance the effectiveness or possibilities of other actions, and the manner in which individual actions act to complement or substitute for one another or produce co-benefits (or costs) for other actors or groups.
Here, we aim to identify, collate and analyse the suite of changes that marine users are initiating in response to climate-driven changes in biodiversity, without direct or leading government or management interventions, i.e. user-based autonomous adaptations. We use the east coast of Tasmania, Australia, as an illustrative case as it is one of the world’s fastest warming marine areas (Hobday and Pecl 2014) with climate-driven changes already observed in the distribution, abundance, and productivity of marine resources, as well as substantial climate-driven changes in critical marine habitats (Last et al. 2011; Ramos et al. 2015). There has been considerable investment in research to underpin marine adaptation in the broader region (Creighton et al. 2016), and the east coast of Tasmania has been specifically acknowledged as a region with the potential for study of human system adaptation (Frusher et al. 2014). The east coast of Tasmania also supports a wide range of marine resource uses and interests, including production of high value seafood by commercial fishers and aquaculture sectors (ABARES 2016), Tasmanian Aboriginal peoples cultural connections to sea country (Lee 2017), and high levels of participation in recreational fishing, with nearly 30% of the population participating annually (Lyle et al. 2014).
We describe the changing resource conditions and local-level adaptation actions of seven diverse groups of marine resource users in the region. Applying Ostrom’s social-ecological systems framework (2009), we organise our analysis of autonomous adaption actions by marine resource users, rather than ethnic or social units or communities, in order to specifically examine the potential interactions of any user-based autonomous adaptation actions with planned or government-led adaptation strategies for marine resource uses. Identified adaptation actions by resource user groups are then mapped to an existing adaptation action typology, and the outcomes of actions further categorised using the conceptual framework for assessing vulnerability to climate change in climate-sensitive social-ecological systems developed by Marshall et al. (2010). The relationship of these autonomous adaptation actions to the existing governance system settings is discussed, providing an important step towards a comprehensive understanding of the potential mix of adaptation responses, and the level of agency and power relations that may need to be further explored and considered in the overall design of adaptation strategies. After describing the approach in more detail below, we provide a synthesis of these climate-driven changes in biodiversity, including how such changes have been observed by marine system users, before progressing to the adaptation actions documented.
Approach
We undertook synthesis of existing data from multiple sources which drew on varying knowledge systems and applied established analytical frameworks to examine the kinds of local-level autonomous adaptation behaviours the selected cases of marine resource users are undertaking on the east coast region of Tasmania, south-eastern Australia. Our approach was designed to identify and examine characteristics of autonomous adaptation in marine socioecological systems to inform further in-depth structured and systematic analysis.
Examples of user-based adaptation behaviours were selected on the basis of our collective domain expertise and experience in relation to changes in the actions of users of the marine systems in the case study region (see Huntington et al. 2017 for a similar approach). Cases of realised autonomous adaption actions (i.e. behaviours), rather than cases of user intent but uncompleted action, were selected as the aim of the study was to explore the characteristics of a range of user-based adaptation behaviours and the potential interactions with formal, planned adaptation on that which Nelson et al. (2009) describe as the “adaptation dynamic”. For this reason also, the sample of cases selected was not intended to be necessarily representative or complete but to explore the range of behaviours by a diversity of users with varying levels of agency in relation to the governance of the marine resources at stake (Yin 2014).
We then examined the following characteristics of the selected examples in order to generate insights into how different users are responding to climate-driven changes and the implications for governance:
What forms of adaptation actions are being undertaken by users (i.e. non-government sector actors) in response to high levels of ecological change in marine systems?
Who are the primary actors?
What level of access do users have to formal power over marine resources?
What other key assets do users have to enable adaptation actions?
What is the geographic scale of these behaviours and expected outcomes? How dependent is the adaptation action on government cooperation?
What are the expected outcomes of the adaptation behaviour for levels of ecological vulnerability of the marine socioecological system and socioeconomic vulnerability of affected marine users? What type of benefit is generated?
How may the observed adaptation behaviours potentially interact with planned government efforts and what are the implications for further adaptation planning?
The adaptation behaviours identified for each type of marine resource user were categorised using an existing typology of forms of adaption behaviours derived from a synthesis of theoretical frameworks and tested using empirical studies by Biagini et al. (2014). The typology was selected because it explicitly accounts for adaptation behaviours by non-government actors (Tompkins and Eakin 2012).
Levels of agency of resource users as primary actors or “initiators” in these cases is a critical factor. This is attested to by the work of various theorists such as Taylor (2014) and Nasiritousi et al. (2014) who have highlighted the relational basis of vulnerability, and therefore of adaptive capacity of non-government actors, which requires an explicit focus on “how people seek to gain access to and control over changing resources” (Nightingale 2017). In order to understand the level of agency held by resource users responding to change, both the relationship to power and the capacity to act need to be examined in order to understand the context within which adaptive responses occur (Adger 2003). In their study of small communities responding to environmental change, Huntington et al. (2017) examined a range of forms of community capitals (per Emery and Flora 2006) in order to understand the conditions within which communities had sufficient agency to enable action. In our case study region, living marine resources are owned by the state (Kailis 2013) and, as such, the level of agency held by resource users is inevitably relational to the institutional power of the state. For each example, we determined the level of access of marine resource users to formal power (i.e. as typically held by government processes and actors) over resource conditions, as well as the level of dependence of the adaptation action on government cooperation, using a synthesis of approaches to analyse power relations applied by Sova et al. (2014) and Stöhr et al. (2014).
Non-government actors ranged from individual recreational users through to multinational aquaculture firms; therefore, the key capitals or assets available to these users were also examined. We drew on the approach used by both Badjeck et al. (2010) and Huntington et al. (2017) to understand what key assets actors have available to mobilize to enable adaptive behaviours.
We applied the conceptual framework for assessing vulnerability to climate change in climate-sensitive social-ecological systems developed by Marshall et al. (2010). This framework offers a modified iteration of the Exposure-Sensitivity-Adaptive Capacity vulnerability assessment framework (IPCC 2007) in response to a number of identified limitations of the IPPC’s framework in relation to socioeconomic scales of concern and determinants (Hahn et al. 2009). We use this framework to analyse and compare potential outcomes for ecological and socioeconomic vulnerability for the observed adaptation behaviours. In addition, we examined the extent to which the observed adaptation behaviours were likely to interact with (that is, were synergistic with or countervailing to) formal, planned adaptation strategies led by government actors, and whether there were implications of this interaction for future pathways for transformative adaptation (Wise et al. 2014).
Context and background
Physical environment
Waters off the east coast of Tasmania are influenced by the southward extension of the warm East Australian Current (EAC), which carries warm and nutrient poor tropical waters south along the east coast of Australia (Ridgway 2007), and by the cooler eastward-flowing Leeuwin Current extension, which can extend to the east coast of Tasmania (Ridgway and Condie 2004). The extension of the EAC over recent decades to the east coast of Tasmania has seen the region become one of fastest warming marine regions globally (Hobday and Pecl 2014) and has been associated with major southward range extensions in the distributions for almost 100 species (Last et al. 2011; Sunday et al. 2015).
While long-term change has been a focus in the physical and biological studies in the region, extreme events have attracted more attention in recent years, including marine heatwaves (Hobday et al. 2016a). For example, the 2015 Tasman Sea marine heatwave was the longest and most intense marine heatwave ever recorded, lasting 251 days and reached a maximum intensity of 2.9 °C above climatological values (Oliver et al. 2017a, b). The marine heatwave was associated with a number of nearshore ecosystem events including new disease outbreaks in farmed shellfish, mortality of wild abalone, and records of species normally associated with warmer ocean conditions. Extreme marine heatwave events are projected to increase in this region as climate change continues (Oliver et al. 2017a, b).
Biological changes observed in the system
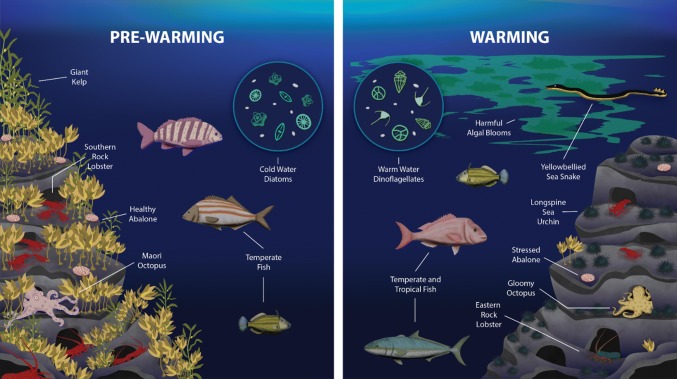
Biological changes at all trophic levels have been observed in the east coast waters of Tasmania, that have either been attributed to climate change, or are entirely consistent with expectations under climate change (Fig. 1 and see Frusher et al. 2014). These responses include changes in abundance, distribution, recruitment, and physiology of key commercial and recreational fishery (Pecl et al. 2014a) and key aquaculture species (Doubleday et al. 2013). In addition, major impacts have also resulted from increases in the timing and duration of harmful algal blooms (HABs, e.g. Noctiluca scintillans and Gymnodinium catenatum, Hallegraeff et al. 2008), and the occurrence of new strains (e.g. Alexandrium tamarense) and species (e.g. Pacific Oyster Mortality Syndrome (POMS)).
Fig. 1.
Schematic illustrating the key changes in biodiversity on the east coast of Tasmania that are either considered climate-driven changes or are consistent with changes expected under climate change. Although the figure is split into “pre-warming” and “warming” these changes have obviously occurred over time, and some changes are starker than others. All changes depicted are referenced in the main text
Changes in productivity on the east coast of Tasmania are linked to a ~ 50% decline in the biomass of the spring bloom, associated with a shift in relative abundance of cold water diatoms to warm water dinoflagellates (Thompson et al. 2009). Warming waters have also resulted in a shift in the southern range of many species (Pitt et al. 2010; Johnson et al. 2011; Last et al. 2011; Couturier et al. 2015; Robinson et al. 2015; Kelly et al. 2016; Stuart-Smith et al. 2016; Ramos et al. 2018), which is leading to the establishment and persistence of species in “new” areas of the Tasmanian coast. Reports by divers and recreational fishers to a local citizen science project of “out-of-range” observations of a wide variety of species are frequent (Table S1). Habitat changes have also resulted from warming waters, including a decline in giant kelp (Wahl et al. 2015), and an increased prevalence of urchin barrens (Ling et al. 2009). Collectively, the arrival of new species, the reduction in availability of preferred species, and changes in environmental conditions, have altered risks and opportunities for local marine industries (van Putten et al. 2015; Champion et al. 2018). Some of these changes are already affecting Tasmanian livelihoods, such as the barrens forming sea urchin Centrostephanus rodgersii (Ling 2008), and POMS (de Kantzow et al. 2017).
Combining industry observations with scientific data and analyses gives a more complete understanding of current changes in the fisheries and broader environment. In a workshop format held in 2012, we asked 40 fishery managers, industry representatives (commercial and recreational) and researchers from our region for observations, possibility related to climate, that were either specific to their operations and fishery or the ecosystem in general (for full details see Pecl et al. 2014b, Table S2). Of the 23 observations reported, 35% were observations not familiar to researchers, indicating the potential benefit of fisher’s knowledge expanding scientific knowledge. While the majority of observations (70%) could be linked to climate change or variability, fishers also reported observations potentially linked to climate change, but which are currently uncertain (15%) and would involve more detailed research to confirm.
Community understanding and perceptions of these changes
At a fundamental level, autonomous responses to change depend on people’s awareness that changes are indeed occurring. Barriers to awareness about climate change are likely to also be barriers to action (Hodgkinson et al. 2014), and communication plays a crucial role in growing awareness. Observation of change and awareness are intricately linked and are partly a function of direct exposure to climate change and the type of experiences people have with the changing marine environment (Keller and McInerney 2008). For instance, divers may observe change directly underwater, while fishers may observe change in a more indirect manner through variations in catch, bycatch, or even fish prices. Direct and indirect observation of change can influence people’s perception of risk and influence their adaptive behaviours (Weber 2010; Weber and Stern 2011). However, people’s perceptions of environmental change are socially constructed (Taylor 2014), and their direct and indirect observations are influenced by cognitive processes, including ingrained mental models, scientific complexities, and positive or negative dissonance (van Putten et al. 2015) that underpin the formation of beliefs about the cause of observed change (Mccright and Dunlap 2011; Whitmarsh 2011). These cognitive processes help explain people’s rejection or acceptance of attributing observed marine environmental change to climate change (van Putten et al. 2015) and may ultimately influence their autonomous adaptive behaviour.
Many fishers are keen observers of the marine environment and develop functionally oriented knowledge of marine ecosystems (McGoodwin 2001; Gledhill et al. 2015). However, perception and acceptance of the science of climate change by fishers are intricately linked to their ability to untangle the many interacting factors that affect fishery performance, including overfishing, management-driven change, market demands (size, season), natural fluctuations in population dynamics, inter-annual variation in biophysical environment, interactions with other species, habitat loss and finally climate change (Taylor 2014; Metcalf et al. 2015). For abalone fishers for example, our collective understanding is that two recent catastrophic heatwave events—events never seen before by multi-generation fishers—were the trigger for awareness of the potential for climate change to affect their livelihoods, and the associated need to consider operational changes in the fishery, where there is capacity to make such change. Prior to the extreme heatwaves, in the rock lobster fishery at least, acceptance of climate change was very low despite lobster fisher’s observations of changes in the marine environment being almost entirely consistent with climate change (Nursey-Bray et al. 2012). Research since then shows that there has been a significant improvement in marine user knowledge of marine processes, particularly changes in species distributions as a result of warming waters (Bannon 2016; Oliver et al. 2017a, b). The citizen science project Redmap (Range Extension Database and Mapping project, www.redmap.org.au and see Table S1) has likely had an influence on marine users in Tasmania’s understanding and attributions of change, and possibly increased their trust in the regional climate science (Bannon 2016; Nursey-Bray et al. 2018), illustrating the importance and potential value of trusted information as potential conduits for autonomous adaptation.
For Indigenous Australians, local observations are transmitted generationally through oral and artistic endeavours, in means described by Caillon et al. (2017) as vertically, horizontally and obliquely kinship-linked knowledges. The reliance on kinship as a tool to encode and share knowledges (Lee and Tran 2016) is demonstrated through Nunn and Reid’s (2016) finding that across 21 diverse Australian coastlines knowledges of sea level rises dating back 7000–13 000 years have been retained through oral histories. Climate change is endemic to Indigenous Australian communities, as much as the localised responses over time to maintain sustainability in conservation stewardship (Wali et al. 2017). For Indigenous Australians, many of the implications of climate-driven changes in biodiversity cannot be adapted to, including cultural loss of governance and connections to sea country (see Box 1).
| Box 1: Implications for indigenous peoples—not all impacts can be adapted to | |
|---|---|
Dr Aunty Patsy Cameron is worried that sea country traditional knowledges of tebrakunna country, now known as Cape Portland, north-east Tasmania, are being washed away with every new and rising tide, estimating that the coastline has receded some 15 metres. The loss of living midden sites that are thousands of years old (Lourandos 1968) are “now ancient insights lost under the oceans” (Aunty Patsy Cameron pers. comm. 22/07/17) and has massive implications on the transmission of Indigenous knowledges (Mustonen and Mustonen 2011). For example, the remains of the yolla or muttonbird (short tailed shearwater), a coastal nesting bird, found in living middens are an aid in the passing on and teaching of tebrakunna deep histories and knowledges. Yet Aunty Patsy is worried if the yolla will continue to nest, be a food source for families and nurture our cultures as their habitat is reduced. This destruction of heritage is paired with the depletion of kelp and seaweed beds that are home to the maireener shell and shell necklaces, an iconic shell and ‘potent signifier’ (Norman 2013) of tebrakunna and other women and their caring for sea country. Where the maireener rainbow kelp shells are depleted in rapid numbers, women feel the cultural loss of governance and connections to sea country (see Lee 2017). While extant rights for Tasmanian Aboriginal peoples to gather and use marine resources under the Living Marine Resources Management Act 1995 creates the spaces, for example, of autonomy and adaptation for women of tebrakunna country to shift the shell types in necklace-making, there is a distinct lack of collaboration with scientists and others to record, implement and build on traditional knowledges in research (Huntington et al. 2017). Aunty Patsy laments “where is science to help look at our health and wellbeing of the precious resources of sea country?” and highlights the restriction of Indigenous agency to care for sea country without the combination of traditional knowledge and modern science.

|
Industry and community responses to climate-driven changes in biodiversity
Recreational fishing
Extensions in the distributional range of fish and invertebrate species from mainland Australia, across the Bass Strait, and into the waters adjacent to Tasmania are well documented (Johnson et al. 2011; Last et al. 2011; Robinson et al. 2015; Stuart-Smith et al. 2016). Several of these species are particularly popular amongst recreational fishers from the southeast region of mainland Australia, including pink snapper (Chrysophrys auratus), yellowtail kingfish (Seriola lalandi), and King George whiting (Sillaginodes punctata) and as such provide new recreational fishing opportunities for fishers in Tasmania (Robinson et al. 2015). Anecdotally, there are reports of snapper and yellowtail kingfish being encountered over several decades in Tasmania, mostly along the north and northeast coasts; however, the frequency of encounters of recreational fishers with these species is increasing as is the geographic extent of the encounters (Robinson et al. 2015; Stuart-Smith et al. 2016; Champion et al. 2018).
The increasing availability of kingfish has led to several social media pages and forums dedicated to targeting the species in Tasmanian waters. The information gained from these forums, on methods to effectively target the fish, as well as real time information on where and when they can be caught, has undoubtedly increased fisher interactions. As an example, a seasonal abundance of kingfish in the vicinity of the Tasman Peninsula in 2016 led to several charter operators advertising kingfish charters for the first time. In stark contrast, the fishers who have been successful in capturing snapper in the southern waters of Tasmania tend to keep the information about where and when the fish are being caught very quiet. Discussions with the fishers revealed concerns that if the information is shared the increased effort could have a significant effect on the fish that are present, with several of the fishers noting that the people currently catching snapper generally practice catch and release. This highlights an interesting quandary for fisheries managers, given that there are significant knowledge gaps as to whether the fish are resident or migratory and whether their growth and maturity schedules are similar to fish found in the warmer waters of the mainland. This creates challenges in terms of developing effective size and individual catch limits, which are traditionally the methods used for management of recreational species.
SCUBA diving clubs
The effects of climate change on recreational SCUBA diving can be traced through shifts in dive locations and increased participation of club members in environmental activities. Fortescue Bay in south-east Tasmania was once one of Tasmania’s most popular dive sites, providing easy shore access to giant kelp (Macrocystis) forests (Johnson et al. 2011). Dive clubs would each run multiple dive events to the bay every year, often including overnight stays at the adjacent campgrounds. The climate-related loss of giant kelp from the Fortescue Bay, coupled with subsequent increases in urchin barrens (Ling 2008; Ling and Jacques 2009), has greatly reduced recreational diving in the area. For example, the state’s largest dive club, the Tasmanian University Dive Club, which once facilitated regular dives to Fortescue Bay has not dived the area in 5 years. The remaining kelp forests further south in the state are now only accessible by boat, making dives to them more infrequent and expensive. Climate change, in particular, the increasing abundance and range extension of the destructive long spined sea urchin (Ling and Jacques 2009), C. rodgersii, has also resulted in recreational dive clubs becoming increasingly involved in environmental activities. Seven Tasmanian dive clubs were involved in the “Subtidal Reef Monitoring and Community Awareness Project” that aimed to monitor and describe impacts of increasing urchin densities and associated barrens (Ling and Jacques 2009). Climate change topics (e.g. Redmap Australia project, urchin distribution, impacts and management) feature regularly in presentations at the annual Tasmanian Combined Dive Clubs Weekend.
Commercial fishing
Southern rock lobster (Jasus edwardsii) recruitment has undergone a decline in Tasmania although it is currently uncertain if it has stabilised at a lower limit, or if variability is masking a longer-term continued decline. The fishery is co-managed by government and industry, and responses to declines in stock so far have included a decrease in the overall quota from 1540 to 1050 tonnes (https://dpipwe.tas.gov.au/sea-fishing-aquaculture/commercial-fishing/rock-lobster-fishery/rock-lobster-catch). Further reductions have occurred in the most affected areas of the east coast through the introduction of a regional commercial Total Allowable Catch (TAC) cap, in addition to the statewide TAC cap. This is the first time that spatial management measures specifying catch have been introduced to the fishery. Industry have also strongly supported measures to increase testing for toxins. With the increased prevalence of HABs (Hallegraeff 2010), there has been the need for testing of a variety of invertebrates including abalone and rock lobsters to ensure public safety. Warm water associated with the Tasman Sea 2015 heatwave (Oliver et al. 2017a, b) coincided with large mortalities of lobsters held in processor tanks. As a response, many operators have changed their landing practices so that they are unloading their (live) catch in areas with cooler waters.
The Tasmanian abalone fishery remains the largest wild-harvest abalone fishery globally, a position held for more than four decades. The primary target species Haliotis rubra has a broad geographic range and populations in Tasmania represent the southern and cooler extremes of its thermal range (Mundy and McAllister 2018). Fishing effort and catch have been managed within geographic zones since 2000 (Mundy and Jones 2017), which has enabled some insight on the interaction between effects of fishing and effects of climate change. In the Eastern zone, TAC has reduced from 1120 tonnes in 2001 to 293 tonnes in 2018, but the stock has not shown signs of rebuilding, as it has in other zones (Mundy and McAllister 2018). The Eastern zone has experienced significant habitat change linked to climate shifts (loss of giant kelp and increased urchin barrens (Ling et al. 2009; Wahl et al. 2015), two significant marine heatwave events (2009/2010 and 2015/2016 Oliver et al. 2017a, b), and a 1 in 100 year storm in 2016. The timing of these events also coincides with low points in performance of the fishery over the last 17 years (Mundy and Jones 2017).
Awareness of the sensitivity of H. rubra to temperature (Moltschaniwskyj et al. 2014) has led to operational changes in the fishery, including changes to the way abalone are held and handled during transport, reluctance by many divers and processors to fish when forecast air temperatures are above 18 °C, or changes in the time of day that fishing occurs when temperatures are likely to exceed 18 °C. Processors and divers have utilized global swell forecast models for the past decade, and increasingly they are utilizing available information on sea surface temperature and seasonal climate outlooks to plan their fishing activities. From 2012, a seasonal closure was implemented in the Eastern zone for January to March inclusive, due to the recognition that abalone are in poor condition during this period, coinciding with the warmest sea temperatures on the east coast (Mundy and McAllister 2018).
Climate-driven range extension of the sea urchin C. rodgersii has led to the establishment of a wild harvest industry for this species in Tasmania. Unreported in the state before 1978, winter warming of waters above the critical temperature threshold for larval development of 12 °C has resulted in the species becoming highly abundant, even causing extensive urchin barrens in some regions (Ling 2008; Ling et al. 2009). Trial harvests for the lucrative urchin roe were first conducted in 2009, with the fishery growing to yield landings of 96 tonnes by 2014. Current production supplies the domestic market, although options to export product have also been explored. Industry subsidies are being trialled in an attempt to accelerate the development of the fishery and offset destructive urchin grazing and barren expansion on coastal reefs.
Aquaculture
Shellfish farming in Tasmania has developed since the 1980s and is based on the introduced Pacific oyster, Crassostrea gigas, with a smaller amount of blue mussels, Mytlius galloprovincialis (Crawford et al. 2003). Over the last 5 years, two events with direct links to climate change have had a major impact on the industry. Firstly, mussels, scallops, oysters, clams, abalone and rock lobsters on the east coast of Tasmania were found to have high levels of Paralytic Shellfish Toxins, originating from a bloom of the harmful alga, Alexandrium tamarense (Hallegraeff and Bolch 2016). This alga is highly toxic to humans and resulted in a global product recall and significant economic losses. The alga was identified as a new strain of A. tamarense that has developed in response to changing environmental conditions (Hallegraeff et al. 2017), and research indicates it is associated with nutrient poor waters from the EAC, with more stratified coastal waters and downwelling conditions favouring these dinoflagellates. This resulted in additional costs for farmers, who have supported development of a rapid immunological test-kit, fine-tuned to Tasmanian shellfish toxin profiles, to ensure the oysters are safe to eat. Shellfish farmers have been trained to use this simple, rapid and cost-effective test kit and are now implementing it into their routine harvesting protocols (Dorantes-Aranda et al. 2017; DPIPWE 2017a, b).
Another major challenge for Pacific oyster farmers has been the incursion of a virulent virus which causes Pacific Oyster Mortality Syndrome (POMS). This was first detected in south eastern Tasmania in January 2016 when up to 90% of all farmed oysters died (Whittington et al. 2016). At this time, water temperatures were higher than normal due to the strong southerly penetration of the EAC. Although the epidemiology of POMS is still not well understood, viral outbreaks are clearly triggered by warmer summer water temperatures (Paul-Pont et al. 2014; de Kantzow et al. 2017). Oyster farmers have responded to this disease by developing a selective breeding program for disease-resistant oysters. Many have also changed their farm management practices, such as growing only juveniles in POMS-infected areas over winter, when the temperatures are too low to trigger the virus, or placing more small oyster spat on their leases in summer, to accommodate losses and have sufficient survive to supply the market. The larger hatcheries have restructured to try to ensure POMS-free spat, including incorporating upgraded biosecurity standards, and building new hatcheries in South Australia. Ironically, one option being considered by several oyster farmers is the potential to culture the Sydney Rock Oyster, Saccrostea commercialis, which is not affected by POMS but which now occurs in Tasmania, presumably as a result of the extended southward flow of the EAC.
Salmonid aquaculture is growing rapidly in Tasmania, is a major contributor to the state economy (DPIPWE 2017a, b), but faces significant challenges from climate-driven changes (Battaglene et al. 2008). The main species grown in Tasmania is Atlantic salmon (Salmo salar) which in its endemic locale has an optimal temperature range of between 4 and 10° C (Reddin et al. 2000), and an upper thermal tolerance limit of 22–24 °C (Barton 1996). In Tasmania, summer farming temperatures can range from 14 to 22 °C (Pankhurst and King 2010). Whilst Tasmanian stocks would appear to have adapted over time to be tolerant of these warmer water conditions (Pankhurst and King 2010), increased temperatures will challenge the fish physiologically (i.e. in terms of nutrition—Miller et al. 2006; reproduction—Pankhurst and King 2010; respiration—Barnes et al. 2011), and phenologically (Fjelldal et al. 2011) and may make them more susceptible to disease (Douglas-Helders et al. 2001). These issues are confounded by the fact that warmer waters carry less oxygen, adding significant additional stress. Furthermore, warming waters have the potential to bring new disease problems; fish range extensions can result in both new species interactions and novel vectors for pathogens which can cause serious diseases. Recent outbreaks of a pilchard orthomyxovirus (POMV), potentially as a result of interactions with wild fish, highlight these risks (Galea et al. 2018).
The aquaculture industry has a limited suite of options to reduce direct effects of climate change (Hobday et al. 2016b): (i) move to cooler waters, (ii) move onshore, or (iii) breed more robust fish. All of these options are currently being investigated in Tasmania. Seasonal forecasting can provide early warming of challenging conditions (Spillman and Hobday 2014), allowing a range of responses, such as early harvesting, to be implemented where the forecasts suggest negative conditions (Hobday et al. 2016b). A cost–benefit analysis of offshore sea-pens, land-based growout and larger post-smolt production highlighted that climate change and warming waters were likely to be significant drivers of major technological change in the Tasmanian salmon industry (King et al. 2016). However, the subtler climate changes such as slightly increased temperatures and lower oxygen concentrations may already be being accommodated through changes in husbandry practices such as reducing stocking levels, or aeration/oxygenation (Hobday et al. 2016b). Selective breeding has been a major investment and longer-term development strategy for the industry over the last 20 years, with temperature tolerance amongst the key breeding improvements achieved (Taylor et al. 2007, 2009; Dominik et al. 2010; Pankhurst and King 2010; Kube et al. 2012). Whilst selective breeding and changing farming practices can help address some of the symptoms of climate change ultimately, the future of aquaculture in Tasmania will be underpinned by appropriate site selection that explicitly accounts for warming waters (Hobday et al. 2018); this will be critical to ensure the long-term environmental sustainability of salmon aquaculture.
Characteristics of adaptations by marine resource users
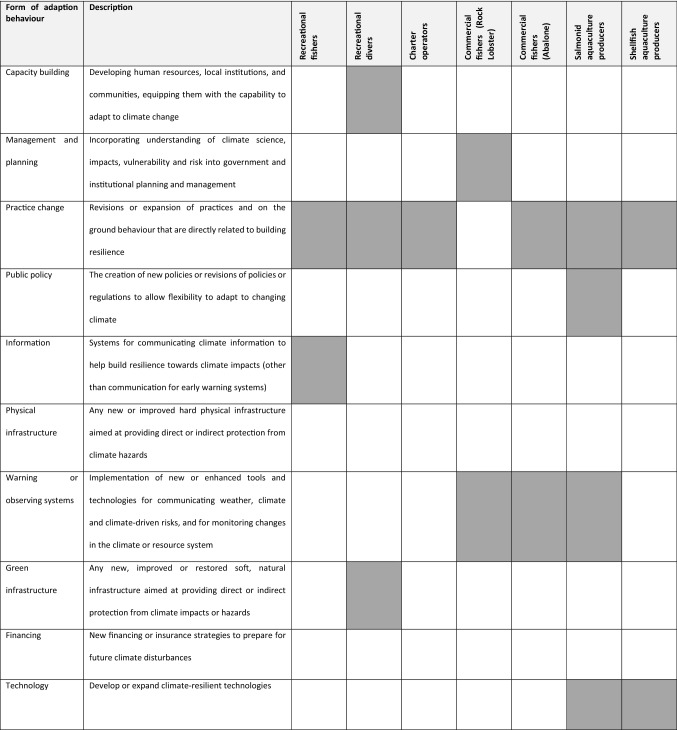
Marine users in Tasmania are undertaking a wide range of forms of autonomous adaptation behaviours (Table 1; Table S3), typical of non-government, rather than government actors (Nasiritousi et al. 2014; Gutiérrez and Morgan 2017). The most common form of adaptation behaviour is practice change, from spatial re-distribution of effort and targeting to account for less available/more available species; to changing commercial product farming, handling and landing practices to maintain supply and quality. The predominant forms of adaptation behaviours are practice change, warning or observing systems, and technology which likely reflects the extent to which marine resource users are independent of government-led action. However, observed adaptation behaviours also included examples of management and planning, and public policy, in two cases. These forms of adaptation behaviours (for example, industry support for regional management interventions to reduce ecological sensitivity) exemplify that the possibilities for user-initiated adaptation actions can be highly dependent on or constrained by government actors.
Table 1.
Forms of adaptation behaviours undertaken by Tasmanian marine resource users compared with global typology adapted from Biagini et al. (2014)
Actors initiating adaptation actions varied in the degree of agency over changing resource conditions in terms of access to formal power over changing resource conditions, the extent and type of key assets available to them, and dependence on government cooperation. A wider array of forms of adaptive actions were observed for industry actors than non-industrial (i.e. recreational and Indigenous) actors, and these actions had a wider geographic scale of effect. This may reflect that industry actors had moderate levels of access to formal power and higher levels of social, cultural and financial capital (Table S3). Furthermore, with the exception of adaptive actions to promote more conservative levels of resource extraction, the types of adaptive actions industry actors had available to them and pursued were more likely to have lower levels of dependence on government cooperation. Comparatively, recreational actors were found to have lower levels of agency and available assets and therefore fewer adaptive response possibilities. However, the lower diversity of type and more localised geographic scale of action and potential effect of adaptive responses by recreational users is also likely to reflect the significant differences in stakes and interests between marine users pursuing livelihoods from marine resource use (i.e. industry actors) and recreational hobbyists pursuing discretionary activities (Jentoft 2007). In contrast, the marginalised position of Tasmanian Aboriginal peoples and their low levels of access to formal power, institutional forms of governance (i.e. research) and assets, other than through limited recognition of some extant rights to specific marine resources, is likely to account for the absence of an observed adaption response space for Tasmanian Aboriginal peoples (see Box 1).
High dependence on resources, and responses or formal sanction by government actors was identified for three of the observed adaptation behaviours (Table S3). These included adaptation behaviours undertaken under both formal and informal co-management arrangements, such as fishing industry actor support for changes to output controls (total allowable catch settings) which ultimately requires a Ministerial decision; and aquaculture industry actor requests for both resources for new testing kits and incorporation of testing into routine, mandated public health checks. Moderate dependence was identified for seven of the observed adaptation behaviours. These included behaviours involving re-distribution of activities which are reliant on government-approved access to new marine areas or marine species, as well as activities dependent on government cooperation in adjusting monitoring and assessment regimes or co-investment in research and development. This highlights the relational basis of the adaption response space available to marine users to formal governance structures for those resources.
The range of observed adaptation behaviours are expected to reduce ecological exposure (e.g. commercial fishers and post-harvest operators maintaining fish condition by landing them in cooler waters); reduce ecological sensitivity (e.g. supporting higher levels of uncaught lobster biomass that reduces sensitivity to ecological change; selective breeding programs for farmed shellfish and salmonids; and diversification of marine resources targeted as part of livelihood strategies to reduce sensitivity arising from dependence on single fisheries sectors); reduce industry exposure to ecological vulnerability and socioeconomic resource dependence on ecologically exposed marine resources (e.g. development of new industry targeting range shifting species associated with reduced productivity of target species); and increase adaptive capacity (e.g. social media platforms which facilitate greater knowledge mobilisation; and early warning systems that enable marine farmers to predict challenging conditions and anticipate required practice changes) (Table S3). Approximately half of the behaviours were expected to both build adaptive capacity and reduce either ecological sensitivity or exposure to climate-driven impacts. Practice change, which is the dominant form of adaptation observed in these examples, is most commonly linked with potential reduction of ecological sensitivity.
Closer consideration of the potential interaction between adaptation behaviours of marine users as non-government actors and the government sector (Table S3) highlights the extent of potentially countervailing interactions. Non-government actor behaviours could lead to delays in signals received by public sector management agencies concerning the extent of negative impacts (e.g. changing farm management practices that mask significant changes in farming conditions and delay more deliberative spatial planning responses), and potentially dampen the incentive for more transformational, but higher cost solutions. They may also lead to perverse, unintended impacts, or maladaptations (e.g. ecological impacts of concentrated effort on new dive sites or newly targeted species). In contrast, non-government adaptation behaviours to build adaptive capacity of marine users through heightened knowledge mobilisation, and warning and observing systems, were likely to be synergistic with planned adaptations.
The adaptation seascape
Our domain expert synthesis of marine user adaptations and the characterization of these adaptations has revealed an active and diverse private agent adaptation landscape (Table S3). Adaptive actions undertaken in Tasmania are moderating damage of climate change to social and economic systems, particularly through actions aimed at building adaptive capacity of actors. It has also highlighted the role of autonomous adaptation in those components of marine socioecological systems (e.g. shifts in dive locations, technological uptake) which are less reliant on top-down government actions. Other identified adaptations involve private actors (both individually and collectively) exerting bottom-up influence through their engagement in co-management process for management actions aimed at reducing the sensitivity of marine resources (e.g. support for reduced quota, requests for increased HAB testing). While the approach used to collate adaptations does not assure a comprehensive representation of this private adaptation landscape, it comprises an evidence-based first step describing where and how user-based autonomous adaptation is occurring, and the manner in which such actions may interact with government-led adaptation. Some of the adaptation actions described here may have multiple drivers behind the behaviour (e.g. a change in TAC) or may be difficult to attribute directly to climate drivers. However, even if this is the case, it is in some respects less important to know the exact causes (or interaction of causes) as long as adaptation commences before it is too late. Moreover, adaptation is only possible if the adaptive capacity exists within these marine sectors and they are well organised.
High-level characterisation of adaptation actions by industry and community actors suggests that for all sectors demand for and access to information is key, and for aquaculture, technology development also appears to be particularly important, with large private “investments” already taking place (e.g. real time environmental intelligence systems and investment in selective breeding). “Practice change” adaptation actions occasionally have the potential to be countervailing to government planned adaptation. The observed adaptation behaviours include those that are anticipatory (for example, resourcing of new warning or observing systems to monitor climate-driven changes in the environmental condition of marine farm lease sites), concurrent (for example, selective breeding programs for fish and shellfish stock, support for commercial fishery quota reductions) and reactive (for example, shifting dive locations).
While we do not compare these non-government adaptation actions with government-led adaptation, our findings suggest public benefits could be arising from some of the synergistic marine user adaptations. Tompkins and Eakin (2012) found a high reliance on non-government actors to resource adaptation actions and limited government resources to drive planned adaptation. In effect, non-government actors were producing public goods although they note that such adaptation is not a substitute for government-led adaptation because of the disjuncture between temporal and spatial scales of the types of adaptations pursued by these different actors, and of the limited instruments available to ensure equitable distribution of public goods produced—the same limitations may also apply in the case explored here.
We found evidence of likely complex interactions between private and public adaptation in the region, with examples across multiple user groups of private actions having the possibility to distort both the timing and extent of potentially more beneficial government adaptation. The current adaptations documented here only avoid disruption to the existing system, and none of the observed adaptation actions are anticipated to be transformative, in part due to the inability of non-government actor-driven adaptation alone to effect transformations on the resilience of common pool resources systems (Tompkins and Eakin 2012). However, the development of an urchin harvesting sector could possibly be considered as transformational adaptation as it creates a new industry addressing the root of the invasive pest problem (albeit not addressing the ultimate root of the problem—climate change). In the context of future potential transformations, it may also be important to consider the relative permanency of current adaptation choices. Infrastructure and technology investments (such as undertaken by the aquaculture industry) can be expensive, and they might make it more difficult to be adaptive in the future as the expensive investment has to be paid off through company profits first. Costly adaptations may potentially restrict flexibility and adaption options in the future. We also note that as not all impacts of climate change are equivalent in terms of the implications for the different user groups, e.g. SCUBA divers having to shift locations to maintain a recreational pursuit versus potentially irreversible loss of species through which Indigenous people feel their governance and connections to sea country; therefore, it is in some ways difficult to compare the effectiveness and outcomes of any autonomous actions.
The east coast of Tasmania may be unique in some contexts as there is a close relationship between industry, science and government (and many managers in government have worked in their positions for many years helping the building of trust) that may have facilitated the dissemination and indeed co-production in some cases of information used to precipitate or inform adaptation actions (Frusher et al. 2014). There are many enabling pre-conditions at play—strong research networks and capacity, good conservation, remoteness, low human population and high biodiversity—however, there is still a dissatisfaction in framing a whole or complete picture at a local level. Moreover, there is clearly insufficient attention and resources allocated to potential impacts of marine climate change for Tasmanian Aboriginal peoples, and critically, not all implications of climate change can be adapted to. Additionally, the contribution here of diverse co-authors demonstrates, for example, that the linkages between Indigenous knowledges and science is still an unknown quantity; a priority should be to bridge this gap. Furthermore, constant tweaking of the governance arrangements between science, policy and broader, wider communities, together with conceiving and integrating Indigenous rights, requires a vigilance to make sense of the immediate-user experiences and observations and the interplay with top-down decisions.
Marine environments have been variously described as complex, interconnected, uncertain, dynamic and opaque. Equally so are the physical and biological manifestations of climate change in these environments, and the needs for adaptation in the human systems reliant on the ecosystem services they produce. Such adaptation needs cannot be addressed successfully by any one type of actor. Instead, individuals, firms, peak bodies, environmental non-governmental organisations, civil society, management agencies and governments will all need to play a role. Comprehensive knowledge of the way in which different actors construct and implement adaptation actions, of the costs and effectiveness of their actions and their consequences for others, will be crucial to ensure adaptation occurs in a coordinated manner that is efficient, fair, avoids waste and minimises conflict. The importance of marine resources in supporting human well-being at local and regional scales, and the local context of many climate change impacts, mean that adaptation needs will best be addressed at these same scales. The need for marine governance that supports this coordination across different types of actors, sectors and activities, and political and administrative boundaries presents a grand challenge, even in countries where single sector governance is well developed. Our study reveals a rich autonomous adaptation landscape within the user groups of Tasmanian marine resources and highlights the need for a comprehensive understanding of such changes to ensure the greatest potential for strategic and coordinated adaptation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the recreational and commercial fishers, divers, resource managers, tourism operators and seafood processors that shared their knowledge and information regarding their practices. We are particularly grateful to the Tasmanian Indigenous community that generously shared their experiences and perspectives, especially Dr Aunty Patsy Cameron. Citizen science contributors to the Redmap Australia project (www.redmap.org.au) provided the observations and associated images for Table S1. We are grateful to the resource managers, researchers and fishing industry representatives from the project “Preparing fisheries for climate change: identifying adaptation options for four key fisheries in South Eastern Australia”, FRDC Project No 2011/039 that attended the March 2012 workshop and provided the observations in Table S2. GP was supported by an Australian Research Council Future Fellowship. Animate Your Science produced Fig. 1, under our guidance.
Biographies
Gretta T. Pecl
is a Professor at the Institute for Marine and Antarctic Studies at the University of Tasmania and the Director of the Centre for Marine Socioecology. Her research interests include ecological implications of marine climate change, human adaptation to these changes, citizen science and science communication.
Emily Ogier
is a Research Fellow in social sciences at the Institute for Marine and Antarctic Studies. Her research focus is the human dimension of marine systems (fisheries and aquaculture in particular), and the way this interaction is governed through both formal institutional and social processes. Currently she is investigating the incorporation of public interest and value in marine governance systems, through both top-down public policy processes and changes in social acceptability driven by social movements and conflicts.
Sarah Jennings
is an Adjunct Senior Researcher at the Tasmanian School of Business and Economics at the University of Tasmania. As a marine economist, her research aims to support policy makers and managers design incentives and institutions that effectively align the outcome of resource users’ behaviours and actions with those that best meet the needs and aspirations of society.
Ingrid van Putten
is a Research Scientist at CSIRO. Ingrid’s research focusses on understanding what prompts resource user’s behaviour and on finding tractable ways to influence it by developing incentives, management structures, and policies that ensure the long-term viability of marine systems.
Christine Crawford
is a Senior Research Fellow at the Institute for Marine and Antarctic Studies at the University of Tasmania. Her research focuses on ecologically sustainable utilization and conservation of estuaries and inshore coastal waters, with a current emphasis on the interactions between aquaculture and the environment.
Hannah Fogarty
is a PhD student at the Institute for Marine and Antarctic Studies at the University of Tasmania and the Centre for Marine Socioecology. Her research interests include the implications of climate change on marine ecosystems and fishery resources, as well as fisheries management adaptations for climate change.
Stewart Frusher
is an adjunct Professor in the Centre for Marine Socioecology at the University of Tasmania. His most recent work focuses on bring together different disciplines to develop solutions to enhance seafood production in an environmentally acceptable manner.
Alistair J. Hobday
is a Senior Principal Research Scientist at CSIRO. Much of his current research focuses on investigating the impacts of climate change on marine biodiversity and fishery resources, and developing, prioritising and testing adaptation options to underpin sustainable use and conservation into the future.
John Keane
is a Research Fellow at the Institute for Marine and Antarctic Studies at the University of Tasmania. He has had 10 years research experience across a range of fields including commercial dive and small pelagic fisheries assessment and management. John is also an executive of the Tasmanian University Dive Club and chair of the annual Tasmanian Combined (dive) Clubs Weekend.
Emma Lee
is a trawlwulwuy woman of tebrakunna country and Research Fellow at the Centre for Social Impact at Swinburne University of Technology. Her research interests centre upon Indigenous peoples and local communities, traditional knowledges and governance practices, both local and global, to enhance Indigenous benefits of marine environments.
Catriona Macleod
is a senior research fellow and benthic ecologist at the Institute for Marine and Antarctic Studies at the University of Tasmania and the Centre for Marine Socioecology. Catriona’s research is focussed on improving our understanding of ecosystem processes, interactions and potential impacts in order to support sustainable long-term management of our coastal and marine systems.
Craig Mundy
is a Senior Research Fellow at the Institute for Marine and Antarctic Studies. Craig’s research interests spans tropical and temperate large marine ecosystems with a recent focus on fisheries ecology of wild harvest abalone fisheries. His current interests center on the interactions between abalone ecology, large scale physical processes and harvest management, particularly with respect to key drivers of fleet dynamics.
Jemina Stuart-Smith
is a Research Fellow at the Institute for Marine and Antarctic Studies. She has experience in marine ecology, marine biodiversity monitoring and conservation, the role of citizen scientists in data collection and the education of marine issues. Jemina’s research involves marine monitoring, citizen science and changes in marine biodiversity.
Sean Tracey
is a Senior Research Fellow at the Institute for Marine and Antarctic Studies. With a diverse portfolio in fisheries and marine ecosystems science, he conducts innovative research across multiple disciplines to address critical questions of ecological consequence, and that facilitates sustainable management of marine resources. His work spans the study of marine species and how they interact with each other and their environment, and assessing commercial and recreational fisheries.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gretta T. Pecl, Phone: +61 408 626 792, Email: Gretta.Pecl@utas.edu.au
Emily Ogier, Email: Emily.Ogier@utas.edu.au.
Sarah Jennings, Email: Sarah.Jennings@utas.edu.au.
Ingrid van Putten, Email: Ingrid.vanputten@csiro.au.
Christine Crawford, Email: Christine.Crawford@utas.edu.au.
Hannah Fogarty, Email: Hannah.Fogarty@utas.edu.au.
Stewart Frusher, Email: stewart.frusher@utas.edu.au.
Alistair J. Hobday, Email: Alistair.Hobday@csiro.au
John Keane, Email: jpkeane@utas.edu.au.
Emma Lee, Email: ejlee@swin.edu.au.
Catriona MacLeod, Email: Catriona.Macleod@utas.edu.au.
Craig Mundy, Email: craig.mundy@utas.edu.au.
Jemina Stuart-Smith, Email: Jemina.StuartSmith@utas.edu.au.
Sean Tracey, Email: sean.tracey@utas.edu.au.
References
- ABARES. 2016. Australian Fisheries and Aquaculture Statistics 2015. Canberra.
- Adger WN. Social capital, collective action, and adaptation to climate change. Economic Geography. 2003;79:387–404. doi: 10.1111/j.1944-8287.2003.tb00220.x. [DOI] [Google Scholar]
- Adger WN, Arnell NW, Tompkins EL. Successful adaptation to climate change across scales. Global Environmental Change. 2005;15:77–86. doi: 10.1016/j.gloenvcha.2004.12.005. [DOI] [Google Scholar]
- Badjeck M-C, Allison EH, Halls AS, Dulvy NK. Impacts of climate variability and change on fishery-based livelihoods. Marine Policy. 2010;34:375–383. doi: 10.1016/j.marpol.2009.08.007. [DOI] [Google Scholar]
- Bannon SLB. Citizen science in a marine climate change hotspot. Hobart: University of Tasmania; 2016. [Google Scholar]
- Barnes RK, King H, Carter CG. Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture. 2011;318:397–401. doi: 10.1016/j.aquaculture.2011.06.003. [DOI] [Google Scholar]
- Barton BA. General biology of salmonids. In: Pennell W, Barton BA, editors. Principles of Salmonid Aquaculture. Amsterdam: Elsevier; 1996. pp. 29–95. [Google Scholar]
- Battaglene, S., C. Carter, A. J. Hobday, V. Lyne, and B. Nowak. 2008. Scoping Study into Adaptation of the Tasmanian Salmonid Aquaculture Industry to Potential Impacts of Climate Change. National Agriculture & Climate Change Action Plan: Implementation Programme report.
- Berkes F. Evolution of co-management: Role of knowledge generation, bridging organizations and social learning. Journal of Environmental Management. 2009;90:1692–1702. doi: 10.1016/j.jenvman.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Biagini B, Bierbaum R, Stults M, Dobardzic S, McNeeley SM. A typology of adaptation actions: A global look at climate adaptation actions financed through the Global Environment Facility. Global Environmental Change. 2014;25:97–108. doi: 10.1016/j.gloenvcha.2014.01.003. [DOI] [Google Scholar]
- Bradley M, van Putten I, Sheaves M. The pace and progress of adaptation: Marine climate change preparedness in Australia’s coastal communities. Marine Policy. 2015;53:13–20. doi: 10.1016/j.marpol.2014.11.004. [DOI] [Google Scholar]
- Caillon S, Cullman G, Verschuuren B, Sterling EJ. Moving beyond the human–nature dichotomy through biocultural approaches: Including ecological well-being in resilience indicators. Ecology and Society. 2017 doi: 10.5751/es-09746-220427. [DOI] [Google Scholar]
- Champion C, Hobday A, Zhang X, Pecl G, Tracey S. Changing windows of opportunity: Past and future climate-driven shifts in temporal persistence of kingfish (Seriola lalandi) oceanographic habitat within southeast Australian bioregions. Marine & Freshwater Research. 2018;69:1–10. doi: 10.1071/MF17387. [DOI] [Google Scholar]
- Cinner JE, Folke C, Daw T, Hicks CC. Responding to change: Using scenarios to understand how socioeconomic factors may influence amplifying or dampening exploitation feedbacks among Tanzanian fishers. Global Environmental Change. 2011;21:7–12. doi: 10.1016/j.gloenvcha.2010.09.001. [DOI] [Google Scholar]
- Couturier LIE, Jaine FRA, Kashiwagi T. First photographic records of the giant manta ray Manta birostris off eastern Australia. PeerJ. 2015;3:e742. doi: 10.7717/peerj.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CM, Macleod CK, Mitchell IM. Effects of shellfish farming on the benthic environment. Aquaculture. 2003;224:117–140. doi: 10.1016/S0044-8486(03)00210-2. [DOI] [Google Scholar]
- Creighton C, Hobday AJ, Lockwood M, Pecl GT. Adapting Management of Marine Environments to a Changing Climate: A Checklist to Guide Reform and Assess Progress. Ecosystems. 2016;19:187–219. doi: 10.1007/s10021-015-9925-2. [DOI] [Google Scholar]
- de Kantzow MC, Hick PM, Dhand NK, Whittington RJ. Risk factors for mortality during the first occurrence of Pacific Oyster Mortality Syndrome due to Ostreid herpesvirus—1 in Tasmania, 2016. Aquaculture. 2017;468:328–336. doi: 10.1016/j.aquaculture.2016.10.025. [DOI] [Google Scholar]
- Dominik S, Henshall JM, Kube PD, King H, Lien S, Kent MP, Elliott NG. Evaluation of an Atlantic salmon SNP chip as a genomic tool for the application in a Tasmanian Atlantic salmon (Salmo salar) breeding population. Aquaculture. 2010;308:S56–S61. doi: 10.1016/j.aquaculture.2010.05.038. [DOI] [Google Scholar]
- Dorantes-Aranda JJ, Campbell K, Bradbury A, Elliott CT, Harwood DT, Murray SA, Hallegraeff GM. Comparative performance of four immunological test kits for the detection of Paralytic Shellfish Toxins in Tasmanian shellfish. Toxicon. 2017;125:110–119. doi: 10.1016/j.toxicon.2016.11.262. [DOI] [PubMed] [Google Scholar]
- Doubleday Z, Clarke S, Li X, Pecl G, Ward T, Battaglene S, Frusher S, Gibbs P, et al. Assessing the risk of climate change to aquaculture: A case study from south-east Australia. Aquaculture Environment Interactions. 2013;3:163–175. doi: 10.3354/aei00058. [DOI] [Google Scholar]
- Douglas-Helders M, Saksida S, Raverty S, Nowak BF. Temperature as a risk factor for outbreaks of Amoebic Gill Disease in farmed Atlantic salmon (Salmo salar) Bulletin of the European Association of Fish Pathologists. 2001;21:114–116. [Google Scholar]
- DPIPWE. 2017. Sustainable industry growth plan for the salmon industry. Hobart, Tasmania. https://dpipwe.tas.gov.au/Documents/salmonplan.pdf.
- DPIPWE. 2017. Tasmanian Shellfish Quality Assurance Program—Biotoxin Management Plan, Version 5, 4th December 2017. https://dpipwe.tas.gov.au/Documents/TSQAP_Biotoxin_Management_Plan.PDF.
- Fankhauser S, Smith JB, Tol RSJ. Weathering climate change: Some simple rules to guide adaptation decisions. Ecological Economics. 1999;30:67–78. doi: 10.1016/S0921-8009(98)00117-7. [DOI] [Google Scholar]
- Fjelldal PG, Hansen T, Huang T. Continuous light and elevated temperature can trigger maturation both during and immediately after smoltification in male Atlantic salmon (Salmo salar) Aquaculture. 2011;321:93–100. doi: 10.1016/j.aquaculture.2011.08.017. [DOI] [Google Scholar]
- Frusher SD, Hobday AJ, Jennings SM, Creighton C, D’Silva D, Haward M, Holbrook NJ, Nursey-Bray M, et al. The short history of research in a marine climate change hotspot: From anecdote to adaptation in south-east Australia. Reviews in Fish Biology and Fisheries. 2014;24:593–611. doi: 10.1007/s11160-013-9325-7. [DOI] [Google Scholar]
- Galea, S., E. Street, and J. Dunlevie. 2018. Macquarie Harbour salmon: 1.35 million fish deaths prompt call to “empty” waterway of farms. ABC News.
- Gledhill DC, Hobday AJ, Welch DJ, Sutton SG, Lansdell MJ, Koopman M, Jeloudev A, Smith A, et al. Collaborative approaches to accessing and utilising historical citizen science data: A case-study with spearfishers from eastern Australia. Marine & Freshwater Research. 2015;66:195–201. doi: 10.1071/MF14071. [DOI] [Google Scholar]
- Grüneis H, Penker M, Höferl KM. The full spectrum of climate change adaptation: Testing an analytical framework in Tyrolean mountain agriculture (Austria) SpringerPlus. 2016;5:1–14. doi: 10.1186/s40064-016-3542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez AT, Morgan S. Impediments to fisheries sustainability—Coordination between public and private fisheries governance systems. Ocean and Coastal Management. 2017;135:79–92. doi: 10.1016/j.ocecoaman.2016.10.016. [DOI] [Google Scholar]
- Hahn MB, Riederer AM, Foster SO. The Livelihood Vulnerability Index: A pragmatic approach to assessing risks from climate variability and change—A case study in Mozambique. Global Environmental Change. 2009;19:74–88. doi: 10.1016/j.gloenvcha.2008.11.002. [DOI] [Google Scholar]
- Hallegraeff GM. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. Journal of Phycology. 2010;46:220–235. doi: 10.1111/j.1529-8817.2010.00815.x. [DOI] [Google Scholar]
- Hallegraeff G, Bolch C. Unprecedented toxic algal blooms impact on Tasmanian seafood industry. Microbiology Australia. 2016;37:143–144. [Google Scholar]
- Hallegraeff, G, C. Bolch, A. Bradbury, K. Campbell, S.A. Condie, J. Dorantes, T. Harwood, S. Murray, A. Turnball, S.C. Ugalde, K. Wilson. 2017. Improved understanding of Tasmanian harmful algal blooms and biotoxin events to support seafood risk management. Fisheries Research & Development Corporation 1–132.
- Hallegraeff G, Hosja W, Knuckey R, Wilkinson C. Recent range expansion of the red-tide dinoflagellate Noctiluca scintillans in Australian coastal waters. Harmful Algae News. 2008;38:10–11. [Google Scholar]
- Hobday AJ, Alexander LV, Perkins SE, Smale DA, Straub SC, Oliver ECJ, Benthuysen JA, Burrows MT, et al. A hierarchical approach to defining marine heatwaves. Progress in Oceanography. 2016;141:227–238. doi: 10.1016/j.pocean.2015.12.014. [DOI] [Google Scholar]
- Hobday AJ, Pecl GT. Identification of global marine hotspots: Sentinels for change and vanguards for adaptation action. Reviews in Fish Biology and Fisheries. 2014;24:415–425. doi: 10.1007/s11160-013-9326-6. [DOI] [Google Scholar]
- Hobday AJ, Spillman CM, Eveson P, Hartog JR, Zhang X, Brodie S. A framework for combining seasonal forecasts and climate projections to aid risk management for fisheries and aquaculture. Frontiers in Marine Science. 2018 doi: 10.3389/fmars.2018.00137. [DOI] [Google Scholar]
- Hobday AJ, Spillman CM, Paige Eveson J, Hartog JR. Seasonal forecasting for decision support in marine fisheries and aquaculture. Fisheries Oceanography. 2016;25:45–56. doi: 10.1111/fog.12083. [DOI] [Google Scholar]
- Hodgkinson JH, Hobday AJ, Pinkard EA. Climate adaptation in Australia’s resource-extraction industries: Ready or not? Regional Environmental Change. 2014;14:1663–1678. doi: 10.1007/s10113-014-0618-8. [DOI] [Google Scholar]
- Hoegh-Guldberg, O., R. Cai, E.S. Poloczanska, P.G. Brewer, S. Sundby, K. Hilmi, V.J. Fabry, and S. Jung. 2014. The Ocean. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. V. R. Barros, C. B. Field, D. J. Dokken, M. D. Mastrandrea, K. J. Mach, T. E. Bilir, M. Chatterjee, K. L. Ebi, et al., 1655–1731. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press. 10.1017/cbo9781107415386.010.
- Huntington HP, Begossi A, Fox Gearheard S, Kersey B, Loring PA, Mustonen T, Paudel PK, Silvano RAM, Vave R. How small communities respond to environmental change: Patterns from tropical to polar ecosystems. Ecology and Society. 2017;22:9. [Google Scholar]
- Reay Dave, Sabine Christopher, Smith Pete, Hymus Graham. Spring-time for sinks. Nature. 2007;446(7137):727–728. doi: 10.1038/446727a. [DOI] [PubMed] [Google Scholar]
- Jennings S, Pascoe S, Hall-Aspland S, Le Bouhellec B, Norman-Lopez A, Sullivan A, Pecl G. Setting objectives for evaluating management adaptation actions to address climate change impacts in south-eastern Australian fisheries. Fisheries Oceanography. 2016;25:29–44. doi: 10.1111/fog.12137. [DOI] [Google Scholar]
- Jentoft Svein. In the Power of Power: The Understated Aspect of Fisheries and Coastal Management. Human Organization. 2007;66(4):426–437. doi: 10.17730/humo.66.4.a836621h2k5x46m2. [DOI] [Google Scholar]
- Johnson CR, Banks SC, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, et al. Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology. 2011;400:17–32. doi: 10.1016/j.jembe.2011.02.032. [DOI] [Google Scholar]
- Kailis G. Unintended consequences? Rights to fish and the ownership of wild fish. Macquarie Law Journal. 2013;11:99–123. [Google Scholar]
- Keller K, McInerney D. The dynamics of learning about a climate threshold. Climate Dynamics. 2008;30:321–332. doi: 10.1007/s00382-007-0290-5. [DOI] [Google Scholar]
- Kelly P, Clementson L, Davies C, Corney S, Swadling K. Zooplankton responses to increasing sea surface temperatures in the southeastern Australia global marine hotspot. Estuarine, Coastal and Shelf Science. 2016;180:242–257. doi: 10.1016/j.ecss.2016.07.019. [DOI] [Google Scholar]
- King AS, Elliott NG, James MA, MacLeod CK, Bjorndal T. Technology selection—The impact of economic risk on decision making. Aquaculture Economics & Management. 2016 doi: 10.1080/13657305.2016.1261962. [DOI] [Google Scholar]
- Kube PD, Taylor RS, Elliott NG. Genetic variation in parasite resistance of Atlantic salmon to amoebic gill disease over multiple infections. Aquaculture. 2012;364–365:165–172. doi: 10.1016/j.aquaculture.2012.08.026. [DOI] [Google Scholar]
- Last PR, White WT, Gledhill DC, Hobday AJ, Brown R, Edgar GJ, Pecl G. Long-term shifts in abundance and distribution of a temperate fish fauna: A response to climate change and fishing practices. Global Ecology and Biogeography. 2011;20:58–72. doi: 10.1111/j.1466-8238.2010.00575.x. [DOI] [Google Scholar]
- Lee E. Performing colonisation: The manufacture of Black female bodies in tourism research. Annals of Tourism Research. 2017;66:95–104. doi: 10.1016/j.annals.2017.06.001. [DOI] [Google Scholar]
- Lee E, Tran T. From boardroom to kitchen table: Shifting the power seat of Indigenous governance in protected area management. Australian Aboriginal Studies. 2016;2:81–93. [Google Scholar]
- Ling SD. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: A new and impoverished reef state. Oecologia. 2008;156:883–894. doi: 10.1007/s00442-008-1043-9. [DOI] [PubMed] [Google Scholar]
- Ling, S. D., and M. Jacques. 2009. Subtidal reef monitoring and community awareness project: data report on the long-spined sea urchin. A Tasmanian Government Fishwise Community Grant project.
- Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M. Climate-driven range extension of a sea urchin: Inferring future trends by analysis of recent population dynamics. Global Change Biology. 2009;15:719–731. doi: 10.1111/j.1365-2486.2008.01734.x. [DOI] [Google Scholar]
- Lourandos H. Dispersal of activities—the east Tasmanian aboriginal sites. Papers and Proceedings of the Royal Society of Tasmania. 1968;102:41–46. [Google Scholar]
- Lyle J, Stark KE, Tracey SR. 2012-13 survey of recreational fishing in Tasmania. Hobart: Tasmania, IMAS, University of Tasmania; 2014. [Google Scholar]
- Malik, A., X. Qin, and S. C. Smith. 2010. Autonomous adaptation to climate change: A literature review. Institute for International Economic Policy Working Paper Series 1–25.
- Marshall NA, Marshall PA, Tamelander J, Obura D, Mallaret King D, Cinner JM. A framework for social adaptation to climate change; sustaining tropical coastal communities and industries. Gland, Switzerland: IUCN; 2010. p. 36. [Google Scholar]
- Mccright AM, Dunlap RE. The politicization of climate change and polarization in the American public’s views of global warming, 2001-2010. Sociological Quarterly. 2011;52:155–194. doi: 10.1111/j.1533-8525.2011.01198.x. [DOI] [Google Scholar]
- McGoodwin JR. Understanding the culture of fishing communities: A key to fisheries management and food security. Rome: Food and Agriculture Organization of the United Nations; 2001. [Google Scholar]
- Metcalf SJ, van Putten EI, Frusher S, Marshall NA, Tull M, Caputi N, Haward M, Hobday AJ, et al. Measuring the vulnerability of marine social-ecological systems: A prerequisite for the identification of climate change adaptations. Ecology and Society. 2015;20:35. doi: 10.5751/ES-07509-200235. [DOI] [Google Scholar]
- Miller MR, Nichols PD, Barnes J, Davies NW, Peacock EJ, Carter CG. Regiospecificity profiles of storage and membrane lipids from the gill and muscle tissue of Atlantic salmon (Salmo salar L.) grown at elevated temperature. Lipids. 2006;41:865–876. doi: 10.1007/s11745-006-5042-5. [DOI] [PubMed] [Google Scholar]
- Miller DD, Ota Y, Sumaila UR, Cisneros-Montemayor AM, Cheung WWL. Adaptation strategies to climate change in marine systems. Global Change Biology. 2018;24:e1–e14. doi: 10.1111/gcb.13829. [DOI] [PubMed] [Google Scholar]
- Moltschaniwskyj, N.A., C. Mundy, and J. Harris. 2014. Maximising value by reducing stress-related mortality in wild harvested black-lip Abalone (Haliotis rubra). Fisheries Research & Development Corporation Report, Project No. 2010/704. www.frdc.com.au/project?id=692.
- Mundy C., and H. Jones. 2017. Tasmanian abalone fishery assessment 2016. Institute for Marine and Antarctic Studies Report, University of Tasmania.
- Mundy, C., and J. McAllister. 2018. Tasmanian abalone fishery assessment 2017. Institute for Marine and Antarctic Studies Report, University of Tasmania.
- Mustonen T, Mustonen K. Eastern Sámi Atlas. Kontiolahti: Snowchange Cooperative; 2011. [Google Scholar]
- Nasiritousi N, Hjerpe M, Linnér B-O. The roles of non-state actors in climate change governance: Understanding agency through governance profiles. International Environmental Agreements: Politics, Law and Economics. 2014;16:109–126. doi: 10.1007/s10784-014-9243-8. [DOI] [Google Scholar]
- Nayak PK. Fisher communities in transition: Understanding change from a livelihood perspective in Chilika Lagoon, India. Maritime Studies. 2017;1:16. doi: 10.1186/s40152-017-0067-3. [DOI] [Google Scholar]
- Nelson DR, West CT, Finan TJ. Introduction to “In focus: Global change and adaptation in local places”. American Anthropologist. 2009;111:271–274. doi: 10.1111/j.1548-1433.2009.01131.x. [DOI] [Google Scholar]
- Nightingale AJ. Power and politics in climate change adaptation efforts: Struggles over authority and recognition in the context of political instability. Geoforum. 2017;84:11–20. doi: 10.1016/j.geoforum.2017.05.011. [DOI] [Google Scholar]
- Norman R. Necklace making and placedness in Tasmania. Coolabah. 2013;11:1–16. [Google Scholar]
- Nunn PD, Reid NJ. Aboriginal memories of inundation of the Australian coast dating from more than 7000 years ago. Australian Geographer. 2016;47:11–47. doi: 10.1080/00049182.2015.1077539. [DOI] [Google Scholar]
- Nursey-Bray M, Palmer R, Pecl G. Spot, log, map: Assessing a marine virtual citizen science program against Reed’s best practice for stakeholder participation in environmental management. Ocean and Coastal Management. 2018;151:1–9. doi: 10.1016/j.ocecoaman.2017.10.031. [DOI] [Google Scholar]
- Nursey-Bray M, Pecl GT, Frusher S, Gardner C, Haward M, Hobday AJ, Jennings S, Punt AE, et al. Communicating climate change: Climate change risk perceptions and rock lobster fishers, Tasmania. Marine Policy. 2012;36:753–759. doi: 10.1016/j.marpol.2011.10.015. [DOI] [Google Scholar]
- Ogier EM, Davidson J, Fidelman P, Haward M, Hobday AJ, Holbrook NJ, Hoshino E, Pecl GT. Fisheries management approaches as platforms for climate change adaptation: Comparing theory and practice in Australian fisheries. Marine Policy. 2016;71:82–93. doi: 10.1016/j.marpol.2016.05.014. [DOI] [Google Scholar]
- Ojea E, Pearlman I, Gaines SD, Lester SE. Fisheries regulatory regimes and resilience to climate change. Ambio. 2017;46:399–412. doi: 10.1007/s13280-016-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver ECJ, Benthuysen JA, Bindoff NL, Hobday AJ, Holbrook NJ, Mundy CN, Perkins-Kirkpatrick SE. The unprecedented 2015/16 Tasman Sea marine heatwave. Nature Communications. 2017;8:16101. doi: 10.1038/ncomms16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver ECJ, Lago V, Holbrook NJ, Ling SD, Mundy CN, Hobday AJ. Eastern Tasmania marine heatwave atlas. Hobart: Institute for Marine and Antarctic Studies, University of Tasmania; 2017. [Google Scholar]
- Ostrom E. A general framework for analyzing sustainability of social-ecological systems. Science. 2009;325:419–422. doi: 10.1126/science.1172133. [DOI] [PubMed] [Google Scholar]
- Pankhurst NW, King HR. Temperature and salmonid reproduction: Implications for aquaculture. Journal of Fish Biology. 2010;76:69–85. doi: 10.1111/j.1095-8649.2009.02484.x. [DOI] [PubMed] [Google Scholar]
- Paul-Pont I, Evans O, Dhand NK, Rubio A, Coad P, Whittington RJ. Descriptive epidemiology of mass mortality due to Ostreid herpesvirus-1 (OsHV-1) in commercially farmed Pacific oysters (Crassostrea gigas) in the Hawkesbury River estuary, Australia. Aquaculture. 2014;422–423:146–159. doi: 10.1016/j.aquaculture.2013.12.009. [DOI] [Google Scholar]
- Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen I-C, Clark TD, Colwell RK, et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science. 2017;355:eaai9214. doi: 10.1126/science.aai9214. [DOI] [PubMed] [Google Scholar]
- Pecl, G.T., T. Ward, F. Briceño, A. Fowler, S. Frusher, C. Gardner, P. Hamer, K. Hartmann, et al. 2014b. Preparing fisheries for climate change: identifying adaptation options for four key fisheries in South Eastern Australia. Fisheries Research and Development Corporation, Project 2011/039.
- Pecl GT, Ward TM, Doubleday ZA, Clarke S, Day J, Dixon C, Frusher S, Gibbs P, et al. Rapid assessment of fisheries species sensitivity to climate change. Climatic Change. 2014;127:505–520. doi: 10.1007/s10584-014-1284-z. [DOI] [Google Scholar]
- Pinkerton E. Co-operative management of local fisheries: New directions for improved management and community development. Vancouver: UBC Press; 2011. [Google Scholar]
- Pinkerton E. Legitimacy and effectiveness through fisheries co-management. Brill Nijhoff: The Future of Ocean Governance and Capacity Development; 2018. pp. 333–337. [Google Scholar]
- Pitt NR, Poloczanska ES, Hobday AJ. Climate-driven range changes in Tasmanian intertidal fauna. Marine & Freshwater Research. 2010;61:963–970. doi: 10.1071/MF09225. [DOI] [Google Scholar]
- Ramos JE, Pecl GT, Moltschaniwskyj NA, Semmens JM, Souza CA, Strugnell JM. Population genetic signatures of a climate change driven marine range extension. Scientific Reports. 2018;8:9558. doi: 10.1038/s41598-018-27351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JE, Pecl GT, Semmens JM, Strugnell JM, León RI, Moltschaniwskyj NA. Reproductive capacity of a marine species (Octopus tetricus) at its range extension. Marine and Freshwater Research. 2015 doi: 10.1071/mf14126. [DOI] [Google Scholar]
- Reddin, D.G., J. Helbig, A. Thomas, B.G. Whitehouse, and K. D. Friedland. 2000. Survival of Atlantic salmon (Salmo salar L.) related to marine climate. In The ocean life of Atlantic salmon, ed. D. Mills, 88–91. Oxford: Blackwell Science.
- Ridgway KR. Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophysical Research Letters. 2007;34:1–5. doi: 10.1029/2007GL030393. [DOI] [Google Scholar]
- Ridgway KR, Condie SA. The 5500-km-long boundary flow off western and southern Australia. Journal of Geophysical Research, C: Oceans. 2004;109:1–18. doi: 10.1029/2003JC001921. [DOI] [Google Scholar]
- Robinson LM, Gledhill DC, Moltschaniwskyj NA, Hobday AJ, Frusher S, Barrett N, Stuart-Smith J, Pecl GT. Rapid assessment of an ocean warming hotspot reveals “high” confidence in potential species’ range extensions. Global Environmental Change. 2015;31:28–37. doi: 10.1016/j.gloenvcha.2014.12.003. [DOI] [Google Scholar]
- Smith B, Burton I, Klein RJT, Wang IANJ, Smit B, Wandel J. An anatomy of adaptation to climate change and variability. Climate Change. 2000 doi: 10.1023/a:1005661622966. [DOI] [Google Scholar]
- Sova CA, Helfgott A, Chaudhury AS, Matthews D, Thornton TF, Vermeulen SJ. Multi-level stakeholder influence mapping: Visualizing power relations across actor levels in Nepal’s agricultural climate change adaptation regime. Systemic Practice and Action Research. 2014;28:383–409. doi: 10.1007/s11213-014-9335-y. [DOI] [Google Scholar]
- Spillman CM, Hobday AJ. Dynamical seasonal ocean forecasts to aid salmon farm management in a climate hotspot. Climate Risk Management. 2014;1:25–38. doi: 10.1016/j.crm.2013.12.001. [DOI] [Google Scholar]
- Stöhr, C., C. Lundholm, B. Crona and I. Chabay. 2014. Stakeholder participation and sustainable fisheries: an integrative framework for assessing adaptive comanagement processes. Ecology and Society 19
- Stuart-Smith J, Pecl G, Pender A, Tracey S, Villanueva C, Smith-Vaniz WF. Southernmost records of two Seriola species in an Australian ocean-warming hotspot. Marine Biodiversity. 2016 doi: 10.1007/s12526-016-0580-4. [DOI] [Google Scholar]
- Sunday JM, Pecl GT, Frusher S, Hobday AJ, Hill N, Holbrook NJ, Edgar GJ, Stuart-Smith R, et al. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecology Letters. 2015;18:944–953. doi: 10.1111/ele.12474. [DOI] [PubMed] [Google Scholar]
- Taylor M. Political ecology of climate change adaptation: Livelihoods, agrarian change and the conflicts of development. London: Routledge/Earthscan; 2014. [Google Scholar]
- Taylor Richard S., Kube Peter D., Muller Warren J., Elliott Nicholas G. Genetic variation of gross gill pathology and survival of Atlantic salmon (Salmo salar L.) during natural amoebic gill disease challenge. Aquaculture. 2009;294(3-4):172–179. doi: 10.1016/j.aquaculture.2009.06.007. [DOI] [Google Scholar]
- Taylor RS, Wynne JW, Kube PD, Elliott NG. Genetic variation of resistance to amoebic gill disease in Atlantic salmon (Salmo salar) assessed in a challenge system. Aquaculture. 2007;272:94–99. doi: 10.1016/j.aquaculture.2007.08.007. [DOI] [Google Scholar]
- Thompson PA, Baird ME, Ingleton T, Doblin MA. Long-term changes in temperate Australian coastal waters: Implications for phytoplankton. Marine Ecology Progress Series. 2009;394:1–19. doi: 10.3354/meps08297. [DOI] [Google Scholar]
- Tompkins EL, Eakin H. Managing private and public adaptation to climate change. Global Environmental Change. 2012;22:3–11. doi: 10.1016/j.gloenvcha.2011.09.010. [DOI] [Google Scholar]
- van Putten IE, Frusher S, Fulton EA, Hobday AJ, Jennings SM, Metcalf S, Pecl GT. Empirical evidence for different cognitive effects in explaining the attribution of marine range shifts to climate change. ICES Journal of Marine Science. 2015 doi: 10.1093/icesjms/fsv192. [DOI] [Google Scholar]
- Wahl M, Molis M, Hobday AJ, Dudgeon S, Neumann R, Steinberg P, Campbell AH, Marzinelli E, et al. The responses of brown macroalgae to environmental change from local to global scales: direct versus ecologically mediated effects. Perspectives in Phycology. 2015;2:11–29. doi: 10.1127/pip/2015/0019. [DOI] [Google Scholar]
- Wali A, Alvira D, Tallman PS, Ravikumar A, Macedo MO. A new approach to conservation: Using community empowerment for sustainable well-being. Ecology and Society. 2017 doi: 10.5751/es-09598-220406. [DOI] [Google Scholar]
- Weber EU. What shapes perceptions of climate change? Wiley Interdisciplinary Reviews: Climate Change. 2010;1:332–342. doi: 10.1002/wcc.41. [DOI] [Google Scholar]
- Weber EU, Stern PC. Public understanding of climate change in the United States. American Psychologist. 2011;66:315–328. doi: 10.1037/a0023253. [DOI] [PubMed] [Google Scholar]
- Whitmarsh L. Scepticism and uncertainty about climate change: Dimensions, determinants and change over time. Global Environmental Change. 2011;21:690–700. doi: 10.1016/j.gloenvcha.2011.01.016. [DOI] [Google Scholar]
- Whittington R, Hick P, Evans O, Rubio A, Dhand N, Paul-Pont I. Pacific oyster mortality syndrome: A marine herpesvirus active in Australia. Microbiology Australia. 2016;37:126–128. [Google Scholar]
- Wise RM, Fazey I, Stafford Smith M, Park SE, Eakin HC, Archer Van Garderen ERM, Campbell B. Reconceptualising adaptation to climate change as part of pathways of change and response. Global Environmental Change. 2014;28:325–336. doi: 10.1016/j.gloenvcha.2013.12.002. [DOI] [Google Scholar]
- Yin RK. Case study research design and methods. 5. Thousand Oaks: Sage; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.