Abstract
To assess if intravoxel incoherent motion (IVIM) imaging can be used to predict early response to chemoradiotherapy (CRT) in cervical cancer. IVIM imaging before and during CRT (at doses of 20 and 40 Gy) was performed in 17 patients with cervical squamous cell carcinoma. The percentage changes of IVIM parameters were significantly higher for complete remission (CR) than non-CR groups. IVIM may play a supplementary role for predicting early response to CRT in cervical cancer.
Keywords: chemoradiotherapy, intravoxel incoherent motion, treatment response, uterine cervical cancer
Introduction
Uterine cervical cancer is the second most commonly diagnosed cancer and the third leading cause of cancer death among females in less developed countries. The large geographic variation in cervical cancer rates reflects differences in the availability of screening that allows detection and removal of precancerous lesions, as well as in human papillomavirus infection prevalence. Concurrent radiotherapy and chemotherapy, in enhanced and maximal settings, is the current standard of care in patients with stage IB to IVA disease. However, local recurrence sometimes developed after chemoradiotherapy (CRT) and it is also associated with various degrees of treatment-related toxicity and morbidity. Thus, the prediction of early therapeutic response to CRT is clinically important for optimal treatment planning or modifying therapeutic strategies to avoid toxicity or adverse side effects.
Diffusion-weighted (DW) imaging is a known and useful method of assessing treatment response in advanced cervical cancers.1 Specifically, as the cellularity of tumors changes due to radiotherapy and/or chemotherapy, the apparent diffusion coefficient (ADC) may be used as a non-invasive parameter to monitor response. According to a meta-analysis by Schreuder et al.,1 DW imaging can be used for monitoring response to treatment after preoperative CRT by comparing baseline and after-treatment ADC values. The relatively recent development of intravoxel incoherent motion (IVIM) provides more detailed information on intra-tumoral diffusion than ADC alone as it uses biexponential fitting for slow and fast diffusion components. IVIM acquires multiple b values and uses a biexponential curve fit analysis to derive the true diffusion coefficient (D), the perfusion-related pseudo-diffusion coefficient (D*), and the perfusion fraction (f). Thus, because IVIM can potentially provide information on both microcirculation and tissue microstructure, the IVIM parameters are expected to be useful for the evaluation of treatment effect in comparison with other factors, such as ADC values and dynamic contrast-enhanced-MR parameters. To date, IVIM has been reported to be an early predictor of treatment response to CRT in patients with uterine cervical cancer,2,3 head and neck squamous cell carcinoma (SCC),4,5 esophageal cancer,6,7 and rectal cancer.8 Thus, the purpose of this study was to evaluate the efficacy of IVIM in predicting the early response to CRT in uterine cervical cancer.
Materials and Methods
Patients
This prospective study was approved by the human research committee of the Institutional Review Board and complied with the guidelines of the Health Insurance Portability and Accountability Act. Furthermore, written informed consent was provided by all study subjects at enrollment. Between November 2016 and January 2018, 17 consecutive patients (average age, 69.1 years; range, 28–94 years) with pathologically confirmed SCC of the uterine cervix underwent IVIM MR imaging before and during CRT (at doses of 20 and 40 Gy) in a 3T unit. Patient characteristics are summarized in Table 1. The International Federation of Gynecology and Obstetrics (FIGO) stage before CRT was IB in three patients, IIA in one, IIB in three, IIIA in three, IIIB in four, IVA in two, and IVB in one.
Table 1.
Patients’ characteristics
| Characteristics | Value |
|---|---|
| Number of patients | 17 |
| Age (years) | |
| Mean | 69.1 |
| Range | 28–94 |
| Histopathologic diagnoses Squamous cell carcinomas | 17 |
| FIGO stage | |
| IB | 3 |
| IIA | 1 |
| IIB | 3 |
| IIIA | 3 |
| IIIB | 4 |
| IVA | 2 |
| IVB | 1 |
| Method for the treatment | |
| Chemoradiotherapy | 10 |
| Radiotherapy alone | 7 |
| Total biologically effective dose (Gy) | |
| Mean | 74.9 |
| Range | 62.4–79.2 |
| Response evaluation | |
| Complete remission | 11 |
| Non-complete remission | 6 |
FIGO, The International Federation of Gynecology and Obstetrics
Concurrent chemotherapy and radiotherapy were administered in 10 patients, but the remaining seven underwent radiotherapy alone because of their advanced age, poor performance status, or chronic renal failure. In the 10 patients who underwent combination chemotherapy, the regimens of chemotherapy were cisplatin in eight patients and Z-100 in the remaining two. Mean time lapse between IVIM imaging before CRT to initiation of CRT was 4.7 days (range, 0–17 days). During MR imaging at a dose of 20 Gy, a mean radiation dose of 19.9 Gy (range, 18.0–20.0 Gy) was administered; this for 40 Gy corresponded to a mean radiation dose of 39.5 Gy (range, 36.0–40.0 Gy). All patients received intracavitary irradiation using remote afterloading system (RALS) in addition to external beam irradiation at the following doses. The mean external beam irradiation dose for the entire pelvis was 40.3 Gy (range, 20–50 Gy; five sessions per week of 1.8–2.0 Gy per session) while that of RALS was 16.6 Gy (range, 6–24 Gy; 5.5–7.0 Gy per session; point A dose). The mean total biologically effective dose was 74.9 Gy (range, 62.4–79.2 Gy). The pathological evaluation of the therapeutic effect was performed at 3 months after completion of CRT except in patients who had an obvious residual tumor on MR imaging; based on this, we divided patients into two groups, namely complete remission complete remission (CR) (n = 11) and non-CR (n = 6).
MR imaging
Three sequential MR examinations were performed at different three time points: before CRT, during CRT at the dose of 20 Gy, and during CRT at the dose of 40 Gy. MR imaging was performed using a 3T MR imaging system (Ingenia 3T CX, Philips Medical Systems, Best, The Netherlands) with a 32-channel torso phased-array receiver coil under free breathing. All images were obtained in axial planes. T2-weighted fast spin-echo (TR/TE, 5216/100 ms; imaging matrices, 512 × 512 matrix; FOV, 26 × 26 cm2; parallel imaging factor, 1.6; section thickness/gap, 5/1 mm) and IVIM single-shot spin-echo echo-planar (TR/TE, 4800/65 ms; imaging matrices, 128 × 128 matrix; FOV, 26 × 26 cm2; parallel imaging factor, 2.0; section thickness/gap, 5/1 mm; b values, 0, 50, 100, 200, 400 and 800 s/mm2) images were obtained for all patients. Motion-probing gradients of the same strength were placed in the three orthogonal directions. The total acquisition time for IVIM imaging was 186 s.
MR image review
All MR images were evaluated by an experienced radiologist with 19 years of post-training experience in interpreting genitourinary images. The reviewer was aware of the diagnosis of cervical cancer but not of the response to CRT. All IVIM images were transferred to a commercially available picture archiving and communication system (Shade-Quest; Yokogawa, Tokyo, Japan).
The ADC value was calculated using all six b values and a monoexponential model, as shown below:
where Sb represents the signal intensity in the presence of diffusion sensitization and S0 the signal intensity in the absence of diffusion sensitization.
In the biexponential IVIM model, the relationship between signal variation and b values is expressed as follows:
where f is the fractional perfusion related to microcirculation, D is the true diffusion as reflected by pure molecular diffusion, and D* is the pseudo-diffusion coefficient related to perfusion.
The following procedure was used by the reviewer to evaluate IVIM images. First, the maximum diameter (MD) of the lesion on axial T2-weighted images was measured. Next, using T2-weighted images as reference, ADC values and IVIM parameters were measured by drawing a ROI within the tumor that was as large as possible while also excluding macroscopic necroses, large vessels, and areas with obvious susceptibility artifacts caused by air–water interface. ROIs were manually drawn in one slice (the largest tumor area slice). If no residual tumor could be observed after treatment, the ROI was drawn, as best as possible, to cover the same ROI area that was used for evaluation in the ‘before-CRT’ MR image in a way similar to previous articles.2,3 Mean ADC values and IVIM parameters of the ROIs were recorded, and percentage change (Δ%) in various parameters were calculated, according to the following equations:
Statistical analysis
All statistical analyses were performed using SPSS ver. 22.0 (SPSS, Inc., IBM Company, Chicago, IL, USA). The unpaired t-test was used to compare the MD, ADC, D, D*, and f-values at three time points of MRI assessment between the CR and non-CR groups. Additionally, the unpaired t-test was also used to compare the percentage change (Δ%) in various parameters at doses of 20 and 40 Gy between the CR and non-CR groups. The null hypotheses of no difference were rejected if P-values were <0.05.
Results
The quantitative measurements of MR images before CRT are summarized in Table 2; there was no significant difference between the CR and non-CR groups in the MD, ADC, D, D*, and f-values before CRT. The quantitative measurements of MR images acquired during CRT at a dose of 20 Gy are summarized in Table 3; there was no significant difference between the CR and non-CR groups in the MD, ADC, D, D*, and f-values during CRT at this dose. The quantitative measurements of MR images acquired during CRT at a dose of 40 Gy are summarized in Table 4; there was no significant difference between the CR and non-CR groups in the MD, ADC, D, D*, and f-values during CRT at this dose.
Table 2.
Quantitative measurements before CRT
| CR group (n = 11) | Non-CR group (n = 6) | P-value | |
|---|---|---|---|
| Maximum diameter (mm) | 44.6 ± 19.1 | 56.8 ± 13.1 | 0.186 |
| ADC (×10−3 mm2/s) | 0.93 ± 0.18 | 1.03 ± 0.11 | 0.226 |
| D (×10−3 mm2/s) | 0.76 ± 0.18 | 0.87 ± 0.10 | 0.186 |
| D* (×10−3 mm2/s) | 5.22 ± 0.06 | 5.26 ± 0.03 | 0.187 |
| f (%) | 14.4 ± 1.2 | 15.1 ± 0.6 | 0.174 |
Values are the mean ± 1 standard deviation. CR, complete remission; CRT, chemoradiotherapy; ADC, apparent diffusion coefficient; D, true diffusion coefficient; D*, perfusion-related pseudo-diffusion coefficient; f, perfusion fraction.
Table 3.
Quantitative measurements during CRT at a dose of 20 Gy
| CR group (n = 11) | Non-CR group (n = 6) | P-value | |
|---|---|---|---|
| Maximum diameter (mm) | 37.7 ± 19.3 | 52.3 ± 11.8 | 0.114 |
| ADC (×10−3 mm2/s) | 1.28 ± 0.25 | 1.16 ± 0.17 | 0.317 |
| D (×10−3 mm2/s) | 1.03 ± 0.18 | 0.99 ± 0.12 | 0.623 |
| D* (×10−3 mm2/s) | 5.31 ± 0.06 | 5.29 ± 0.04 | 0.617 |
| f (%) | 16.2 ± 1.2 | 15.9 ± 0.8 | 0.636 |
Values are the mean ± 1 standard deviation. CR, complete remission; CRT, chemoradiotherapy; ADC, apparent diffusion coefficient; D, true diffusion coefficient; D*, perfusion-related pseudo-diffusion coefficient; f, perfusion fraction.
Table 4.
Quantitative measurements during CRT at a dose of 40 Gy
| CR group (n = 11) | Non-CR group (n = 6) | P-value | |
|---|---|---|---|
| Maximum diameter (mm) | 27.5 ± 18.6 | 40.0 ± 11.7 | 0.158 |
| ADC (×10−3 mm2/s) | 1.58 ± 0.31 | 1.37 ± 0.16 | 0.160 |
| D (×10−3 mm2/s) | 1.16 ± 0.21 | 1.11 ± 0.12 | 0.567 |
| D* (×10−3 mm2/s) | 5.35 ± 0.07 | 5.34 ± 0.04 | 0.563 |
| f (%) | 17.1 ± 1.4 | 16.7 ± 0.8 | 0.551 |
Values are the mean ± 1 standard deviation. CR, complete remission; CRT, chemoradiotherapy; ADC, apparent diffusion coefficient; D, true diffusion coefficient; D*, perfusion-related pseudo-diffusion coefficient; f, perfusion fraction.
The percent change (Δ%) in quantitative measurements between before and during CRT at a dose of 20 Gy are summarized in Table 5; Δ%ADC20 Gy (39.0 ± 16.9% and 14.1 ± 20.5%), Δ%D20 Gy (37.4 ± 23.7% and 13.5 ± 9.7%), Δ%D*20 Gy (1.7 ± 1.0% and 0.7 ± 0.5%), and Δ%f20 Gy (12.7 ± 7.3% and 5.2 ± 3.5%) were significantly higher in the CR group than in the non-CR group (P < 0.05 for all) (Figs. 1 and 2). However, there was no significant difference in Δ%MD20 Gy values between the CR and non-CR groups.
Table 5.
Percentage change of quantitative measurements between before CRT and during CRT at a dose of 20 Gy
| CR group (n = 11) | Non-CR group (n = 6) | P-value | |
|---|---|---|---|
| Maximum diameter (%) | 15.3 ± 18.8 | 7.6 ± 9.6 | 0.365 |
| ADC (%) | 39.0 ± 16.9 | 14.1 ± 20.5 | 0.016* |
| D (%) | 37.4 ± 23.7 | 13.5 ± 9.7 | 0.039* |
| D* (%) | 1.7 ± 1.0 | 0.7 ± 0.5 | 0.039* |
| f (%) | 12.7 ± 7.3 | 5.2 ± 2.5 | 0.036* |
Significant difference was found between CR and non-CR group. Values are the mean ± 1 standard deviation. CR, complete remission; CRT, chemoradiotherapy; ADC, apparent diffusion coefficient; D, true diffusion coefficient; D*, perfusion-related pseudo-diffusion coefficient; f, perfusion fraction.
Fig. 1.
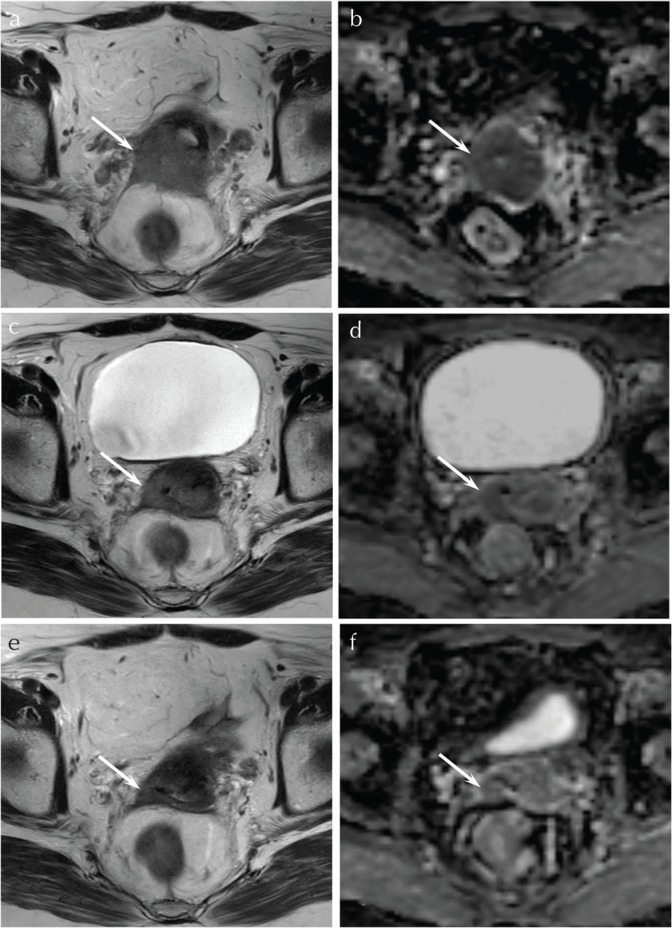
A 62-year-old woman with squamous cell carcinoma of the uterine cervix (CR group). (a) T2-weighted fast spin-echo image (TR/TE, 5216/100 ms) before CRT shows an ill-demarcated lesion with parametrial invasion (arrow). (b) ADC map before CRT shows low ADC value (0.85 × 10−3 mm2/s) (arrow). (c) T2-weighted fast spin-echo image (TR/TE, 5216/100 ms) during CRT at the dose of 20 Gy shows a decrease in size of the tumor (arrow). (d) ADC map during CRT at the dose of 20 Gy shows an increase in ADC value (1.31 × 10−3 mm2/s) with high Δ%ADC20 Gy (55.1%) (arrow). (e) T2-weighted fast spin-echo image (TR/TE, 5216/100 ms) during CRT at the dose of 40 Gy shows a further decrease in size of the tumor (arrow). (f) ADC map during CRT at the dose of 40 Gy shows a further increase in ADC value (1.53 × 10−3 mm2/s) with high Δ%ADC40 Gy (80.6%) (arrow). ADC, apparent diffusion coefficient; CR, complete remission; CRT, chemoradiotherapy
Fig. 2.
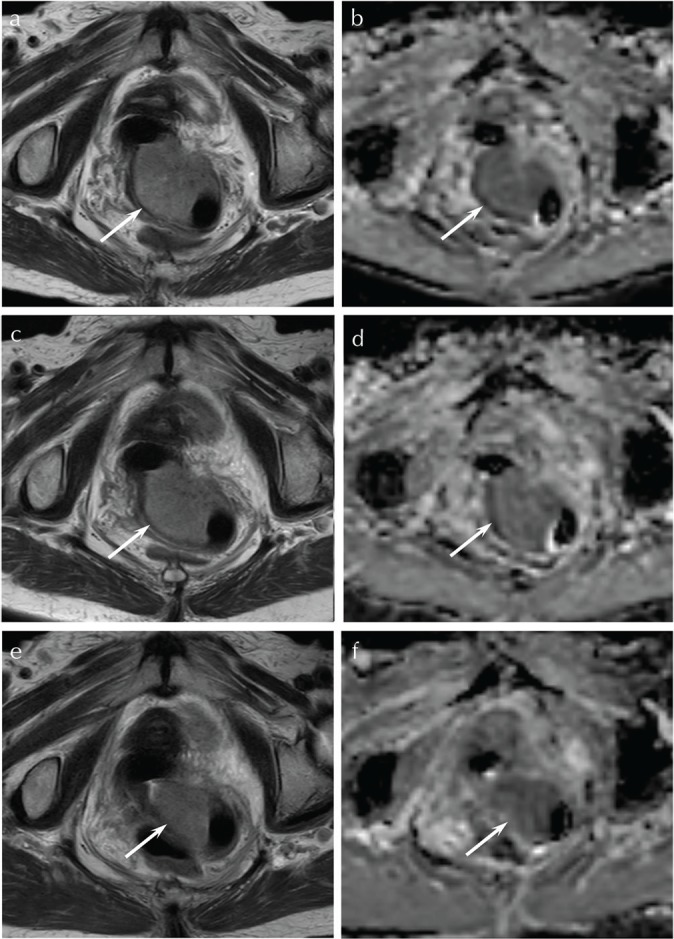
A 94-year-old woman with squamous cell carcinoma of the uterine cervix (non-CR group). (a) T2-weighted fast spin-echo image (TR/TE, 5216/100 ms) before CRT shows a partially ill-demarcated lesion with parametrial invasion (arrow). (b) ADC map before CRT shows low ADC value (1.12 × 10−3 mm2/s) (arrow). (c) T2-weighted fast spin-echo image (TR/TE, 5216/100 ms) during CRT at the dose of 20 Gy shows no significant change in size of the tumor (arrow). (d) ADC map during CRT at the dose of 20 Gy shows a slight increase in ADC value (1.21 × 10−3 mm2/s) with low Δ%ADC20 Gy (8.0%) (arrow). (e) T2-weighted fast spin-echo image (TR/TE, 5216/100 ms) during CRT at the dose of 40 Gy shows a decrease in size of the tumor (arrow). (f) ADC map during CRT at the dose of 40 Gy shows a slight increase in ADC value (1.27 × 10−3 mm2/s) with low Δ%ADC40 Gy (13.4%) (arrow). ADC, apparent diffusion coefficient; CR, complete remission; CRT, chemoradiotherapy
The percent change (Δ%) in quantitative measurements between before and during CRT at a dose of 40 Gy are summarized in Table 6; only Δ%ADC40 Gy was significantly higher in the CR group than in the non-CR group (73.3 ± 38.5% and 34.4 ± 20.7%, respectively; P < 0.05) (Figs. 1 and 2). Further, Δ%D40 Gy was marginally higher in the CR group than in the non-CR group (56.0 ± 30.4% and 28.2 ± 16.8%; P = 0.057). However, there was no significant difference in Δ%MD40 Gy, Δ%D*40 Gy, and Δ%f40 Gy between the CR and non-CR groups.
Table 6.
Percentage change of quantitative measurements between before CRT and during CRT at a dose of 40 Gy
| CR group (n = 11) | Non-CR group (n = 6) | P-value | |
|---|---|---|---|
| Maximum diameter (%) | 40.2 ± 23.8 | 29.4 ± 17.2 | 0.347 |
| ADC (%) | 73.3 ± 38.5 | 34.4 ± 20.7 | 0.038* |
| D (%) | 56.0 ± 30.4 | 28.2 ± 16.8 | 0.057 |
| D* (%) | 2.6 ± 1.3 | 1.5 ± 0.8 | 0.093 |
| f (%) | 18.9 ± 9.8 | 10.3 ± 12.1 | 0.070 |
Significant difference was found between CR and non-CR group. Values are the mean ± 1 standard deviation. CR, complete remission; CRT, chemoradiotherapy; ADC, apparent diffusion coefficient; D, true diffusion coefficient; D*, perfusion-related pseudo-diffusion coefficient; f, perfusion fraction.
Discussion
The usefulness of IVIM for early prediction of response to CRT4,5 or chemotherapy9–11 for head and neck SCC has been previously reported, and significant differences in Δ%D5,9 or change (Δ) in D4,10,11 between before and during treatment are always observed between CR (responders) and non-CR (non-responders) groups. Additionally, these studies have shown that Δ%ADC or ΔADC,5,9,11 ΔD*,10,11 and Δf 4,10 are also significantly higher in CR (responders) than in non-CR (non-responders) groups. Thus, Δ% or Δ of IVIM parameters are widely known and accepted methods of early prediction of therapeutic response in patients with head and neck SCC. The usefulness of IVIM for early prediction of response to CRT for esophageal cancer,6,7 CRT for rectal cancer,8 and chemotherapy for breast cancer12 has also been reported, and the results from these studies show that both Δ%D and Δ%ADC in esophageal and rectal cancer,6–8 and both ΔD and Δf in breast cancer12 were significantly higher in CR (responders) groups than in non-CR (non-responders) groups.
In patients with uterine cervical cancer, early prediction of response to CRT using percentage change or value change of IVIM parameters has been reported in only two articles by the same investigator.2,3 Similar to our study, they also used IVIM imaging at three points (before CRT and during CRT at doses of 20 and 40 Gy) and found that the IVIM parameters during CRT at doses of 20 and 40 Gy were higher than those before CRT.2,3 However, they found no significant differences in early changes in IVIM-induced parameters such as ADC, D, D* and f that could predict CR and PR groups,3 and no change in the rate index of IVIM parameters during CRT proved useful for prognostic differentiation between good and poor prognosis groups.2 On the other hand, in this study, Δ%ADC20 Gy, Δ%D20 Gy, Δ%D*20 Gy, Δ%f20 Gy, and Δ%ADC40 Gy were significantly higher in the CR group than in the non-CR group, implying that similar to carcinoma at other sites, Δ% of IVIM parameters was useful for predicting early response to CRT in uterine cervical cancer. When comparing the differences between our study and the two previous articles, our study included stage I cancers in 3/17 (18%), whereas stage I cancers were not included in the two previous articles. Because advanced stage cancers tend to include necrosis and hemorrhage, IVIM parameters might not be able to be calculated accurately due to these tissue degeneration in the two previous articles. In addition, there are fewer differences in irradiation protocol between our study and the two previous articles, but the chemotherapy regimen was somewhat different. These factors might cause different results with the previous articles. Although further investigation is needed to determine the true utility of IVIM parameters for early prediction of response to CRT in uterine cervical cancer, this is the first study to describe the usefulness of Δ% of IVIM parameters for predicting early response to CRT in uterine cervical cancer.
Intravoxel incoherent motion during CRT was performed at doses of 20 and 40 Gy in our study, whereas other studies have used doses of 16–20,5 20,6 30,7 and patients were evaluated at 3–44 and 2/4 weeks2,3 after the initiation of CRT. Although the optimal timing for using IVIM for early prediction of response to CRT has not yet been determined, our results demonstrate that compared to those at a dose of 40 Gy, IVIM parameters at a dose of 20 Gy were more useful for distinguishing between the CR and non-CR groups.
This study has several limitations. First, the study cohort was small and the results are from a single institution. Second, the treatment protocols were not identical among all patients; i.e., while 10 patients underwent concurrent chemotherapy and radiotherapy, radiotherapy alone was administered to the remaining seven. Third, we calculated the IVIM parameters using six b values, whereas previous studies have used 14,2,10 12,4–6,8,11,12 10,9 or 93,7 b values to calculate IVIM parameters for early prediction of response to CRT. Furthermore, a large number of low b values <100 s/mm2 were used in these studies, and the use of different b values might result in bias. Although the scan time was limited, proper evaluation of IVIM needs more b values especially <200 s/mm2. Nonetheless, no study has assessed whether the number of b values affects the early prediction of response to CRT.
Conclusion
This study shows that IVIM may play a supplementary role for assessing and predicting early response to CRT in patients with uterine cervical cancer. These IVIM parameters can contribute to designing therapeutic, additional treatment, and follow-up strategies, and thus help improve patient prognosis.
Footnotes
Funding
This work was supported by JSPS KAKENHI Grant Number 16K10309.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Schreuder SM, Lensing R, Stoker J, Bipat S. Monitoring treatment response in patients undergoing chemoradiotherapy for locally advanced uterine cervical cancer by additional diffusion-weighted imaging: a systematic review. J Magn Reson Imaging 2015; 42:572–594. [DOI] [PubMed] [Google Scholar]
- 2.Zhu L, Wang H, Zhu L, et al. Predictive and prognostic value of intravoxel incoherent motion (IVIM) MR imaging in patients with advanced cervical cancers undergoing concurrent chemo-radiotherapy. Sci Rep 2017; 7:11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, Wang H, Yan J, et al. Predicting and early monitoring treatment efficiency of cervical cancer under concurrent chemoradiotherapy by intravoxel incoherent motion magnetic resonance imaging. J Comput Assist Tomogr 2017; 41:422–429. [DOI] [PubMed] [Google Scholar]
- 4.Ding Y, Hazle JD, Mohamed AS, et al. Intravoxel incoherent motion imaging kinetics during chemoradiotherapy for human papillomavirus-associated squamous cell carcinoma of the oropharynx: preliminary results from a prospective pilot study. NMR Biomed 2015; 28:1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujima N, Yoshida D, Sakashita T, et al. Prediction of the treatment outcome using intravoxel incoherent motion and diffusional kurtosis imaging in nasal or sinonasal squamous cell carcinoma patients. Eur Radiol 2017; 27:956–965. [DOI] [PubMed] [Google Scholar]
- 6.Li FP, Wang H, Hou J, et al. Utility of intravoxel incoherent motion diffusion-weighted imaging in predicting early response to concurrent chemoradiotherapy in oesophageal squamous cell carcinoma. Clin Radiol 2018; 73: 756.e717–756.e726. [DOI] [PubMed] [Google Scholar]
- 7.Zheng H, Ren W, Pan X, et al. Role of intravoxel incoherent motion MRI in early assessment of the response of esophageal squamous cell carcinoma to chemoradiotherapy: a pilot study. J Magn Reson Imaging 2018; 48:349–358. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Jing H, Ju-Mei Z, et al. Intravoxel incoherent motion diffusion-weighted imaging for discriminating the pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Sci Rep 2017; 7:8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao-ping Y, Jing H, Fei-ping L, et al. Intravoxel incoherent motion MRI for predicting early response to induction chemotherapy and chemoradiotherapy in patients with nasopharyngeal carcinoma. J Magn Reson Imaging 2016; 43:1179–1190. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Pan J, Chen Y, Chen Y, He Z, Zheng X. Intravoxel incoherent motion-magnetic resonance imaging as an early predictor of treatment response to neoadjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Medicine (Baltimore) 2015; 94:e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Luo D, Lin M, et al. Pretreatment intra-voxel incoherent motion diffusion-weighted imaging (IVIM-DWI) in predicting induction chemotherapy response in locally advanced hypopharyngeal carcinoma. Medicine (Baltimore) 2016; 95:e3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Che S, Zhao X, Ou Y, et al. Role of the intravoxel incoherent motion diffusion weighted imaging in the pre-treatment prediction and early response monitoring to neoadjuvant chemotherapy in locally advanced breast cancer. Medicine (Baltimore) 2016; 95:e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]


