Abstract
Based on reports in adult lung transplant recipients, we hypothesized that community acquired respiratory viral infections (CARV) would be a risk factor for poor outcome after pediatric lung transplant. We followed 61 pediatric lung transplant recipients for 2+ years or until they met a composite primary endpoint including bronchiolitis obliterans syndrome (BOS)/obliterative bronchiolitis, re-transplantation or death. Blood, bronchoalveolar lavage and nasopharyngeal specimens were obtained with standard of care visits. Nasopharyngeal specimens were obtained with respiratory viral symptoms. Respiratory specimens were interrogated for respiratory viruses using multiplex PCR. Donor-specific HLA antibodies, self-antigens, and Elispot reactivity were also evaluated. Survival was 84% (1 year) and 68% (3 years). BOS incidence was 20% (1 year) and 38% (3 years). The primary endpoint was met in 46% of patients. CARV was detected in 156 patient visits (74% enterovirus/rhinovirus). We did not find a relationship between CARV recovery from respiratory specimens and the primary endpoint (HR 0.64 (0.25, 1.59), P=0.335) or between CARV and the development of allo- or autoimmune humoral or cellular responses. These findings raise the possibility that the immunologic impact of CARV following pediatric lung transplant is different than that observed in adults.
Introduction
Advancements in recipient selection, surgical techniques, evolving immunosuppressive regimens and more effective infection prophylaxis over the past 25 years have led to improved perioperative survival in pediatric lung transplant recipients (LTRs). However, long- term graft and patient survival has not improved substantially over the same period and remains significantly worse than outcomes for recipients of heart, liver and kidney allografts. Beyond the first post-transplant year, chronic lung allograft dysfunction (CLAD) is the most common cause of graft failure. The incidence of bronchiolitis obliterans syndrome (BOS), the most common form of CLAD, is 50% at 5 years in children and roughly 80% between 5 and 10 years in adults 1-3. Between 25 and 40% of lung transplant recipients will die directly or indirectly from CLAD 3. Although re-transplantation for CLAD is feasible, its utility is limited by organ availability and poorer survival after second transplant 4-6.
Although the etiology of CLAD remains unclear, a common theme throughout the spectrum of clinical risk factors including primary graft dysfunction, recurrent and/or severe acute cellular rejection (ACR), viral infections including cytomegalovirus (CMV), bacterial infection (particularly Pseudomonas aeruginosa), fungal infection and gastroesophageal reflux is allograft injury. Acute and chronic lung allograft injury in adults were also associated with the development of autoantibodies (AutoAb) in cross sectional studies 7. Given accumulating data suggesting a role for both donor- specific anti-human leukocyte antigen (HLA) antibodies (DSA) and autoantibodies reactive to collagen type V (ColV) and k-alpha 1 tubulin (Kα1T) in the development of CLAD, it is possible that the development of DSA and AutoAb in response to allograft injury serves as a common pathway. 7,8 The mechanistic link between antibody formation/binding and chronic graft injury remains unclear, although complement mediated injury, cytokine/macrophage mediated injury, and direct effects of antibody binding on endothelial or epithelial growth/proliferation may be involved 9-13.
Community acquired respiratory viral infections (CARV) are frequently reported after lung transplantation and cause significant morbidity. Studies of viral isolation from adult LTRs have identified infection with both established respiratory viruses such as rhinovirus, respiratory syncytial virus (RSV), parainfluenza virus and influenza virus 14-18, as well as emerging respiratory viruses, including human metapneumovirus and human coronavirus 17,19,20. CARV are associated with poor long-term outcomes in adult LTRs 21-25.
Based on these findings we hypothesized that CARV infections directly activate the immune system by inducing a pro-inflammatory environment and thus could a) directly damage the lung allograft, b) augment innate immunity leading to direct lung allograft damage, c) augment innate immunity leading to enhanced adaptive cellular and humoral allo- and or auto-immunity and resultant lung allograft injury, and, d) induce anti-viral immunity that could cross react with lung allograft antigens to mediate graft injury (heterologous immunity).
In spite of accumulating evidence in adults, the role CARV play in the long-term outcome of pediatric LTRs remains uncertain. A retrospective analysis of CARV within the first year after transplantation found an association with death but not BOS in a cohort of nearly 600 pediatric LTRs 26. Moreover, there have been no reports supporting a relationship between T cell responses and the development of DSA or AutoAb in pediatric LTRs, or a relationship between DSA and/or AutoAb and CLAD in pediatric LTRs.
We conducted a prospective, longitudinal study of pediatric LTRs to test the hypothesis that CARV increase the risk of meeting a composite endpoint consisting of BOS, obliterative bronchiolitis (OB), death or re-transplantation in pediatric LTRs. A companion mechanistic study assessed the relationship between CARV and immunologic events known to be associated with CLAD development.
Methods
Study Development and Oversight
The Clinical Trials in Organ Transplant in Children study (CTOTC-03, ) enrolled patients at six pediatric institutions in the United States from 2009 to 2013. CTOTC-03 was the first prospective, longitudinal study of pediatric LTRs. This prospective multicenter observational trial had a target accrual of 80 pediatric lung or heart-lung transplant recipients (78 enrolled). The CTOTC-03 protocol development team was led by Drs. Sweet and Danziger-Isakov. Clinical and/or laboratory data were contributed by Drs. Heeger, Mohanakumar and Storch. Medical safety oversight was provided by Dr. Jonah Odim. Data analysis was the responsibility of Karen Kesler and Hyunsook Chin (with the CTOTC-03 team). Data were collected by the investigators and coordinators at each study site. All authors vouch for data accuracy and completeness. Each site participated under the auspices of its Institutional Review Board. An independent, NIAID-appointed Data Safety Monitoring Board was responsible for periodic safety review. The study was registered at clinicaltirals.gov ().
Subjects
Candidates for first single or bilateral lung or heart-lung transplant who were less than or equal to 19 years of age at enrollment were considered for inclusion pending appropriate informed consent. We excluded multi-organ transplant recipients (aside from heart-lung) and recipients with a condition or characteristic which, in the opinion of the local primary investigator, made the participant unlikely to complete the study.
Endpoints
We tested the hypothesis that CARV increase the risk of meeting a composite endpoint consisting of BOS, obliterative bronchiolitis (OB), death or re-transplantation in pediatric LTRs. We chose this composite endpoint to increase the power to detect an impact, postulating that CARV result in direct and indirect allograft injury leading to expanded graft-reactive T and B cell immunity and the development of autoimmunity through the unmasking of cryptic autoantigens. We also evaluated the impact of risk factors associated with outcomes in adults such as primary graft dysfunction (PGD) and acute cellular rejection (ACR).
Subjects were followed for at least two years or until they met one or more components of the composite endpoint which included death, re-transplantation or meeting criteria for BOS grade 0-p or 1 27 or the finding of OB on lung biopsy. The endpoint included BOS27 because this study was performed prior to CLAD becoming accepted terminology28, but all patients identified using the 2001 BOS criteria would meet current CLAD criteria. Subdivision between those with obstructive and restrictive CLAD was not performed.
Study Design
Clinical data (including pulmonary function testing and imaging studies) was collected at scheduled visits including: pre-transplant, at transplant (relevant donor demographics were also collected at transplant) and at post-transplant weeks 2, 4, 6, 8 and months 3, 6, 9, 12, 18 and 24. Blood, NP and BAL specimens were obtained at these visits for viral testing and assessment of immunity. Subjects who developed clinical symptoms consistent with CARV had a nasopharyngeal (NP) swab obtained as well as blood and BAL samples if bronchoscopy was performed as part of clinical care. Standard of care included sending BAL specimens (obtained during bronchoscopies performed for routine surveillance or with symptomatic episodes) to the local laboratory to assess for routine bacterial, fungal, mycobacterial, viral and opportunistic organisms. When BAL was not performed with a symptomatic episode, standard of care generally included sputum culture.
Subjects underwent routine pre-transplant evaluation. Post-transplant management followed International Pediatric Lung Transplant Collaborative (IPLTC) guidelines (see supplementary materials). Use of an induction agent was at the discretion of the transplant center: two centers utilized an IL-2 antagonist (42 subjects), two used rabbit anti-thymocyte globulin (10 subjects), one used both an IL-2 antagonist and rabbit anti-thymocyte globulin (6 subjects), and one administered no induction therapy (3 subjects). All subjects received triple drug maintenance immunosuppressive therapy with tacrolimus, mycophenolate and steroids, with standardized levels for tacrolimus. Pneumocystis, fungal and CMV prophylaxis were determined by the local center. Treatment of viral infection detected locally was at the discretion of the treating physician. Viral testing of specimens sent to the central laboratories was performed in batches. Therefore, the clinical sites were not informed of these results.
Clinical data was collected from each site using standardized forms. Primary graft dysfunction was determined based on published criteria 29. Bacterial, fungal, viral and mycobacterial systemic and respiratory tract Infections meeting International Society for Heart and Lung Transplantation (ISHLT) working formulation criteria 30 were captured based on local clinical data interpretation and intervention as determined by the clinical site primary investigator (PI). After verifying adequate correlation of local biopsy readings with readings made by two core pathologists, local pathology readings were used for histologic determination of acute, humoral and chronic rejection. Centers submitted all pulmonary function data to the data coordinating center (including those obtained during scheduled visits and with acute events). Based on all PFT data, a tentative BOS grade was assigned using an algorithm based on published criteria 27. The local site PI was asked to verify that no concomitant conditions excluding BOS were present before finalizing the BOS grade.
Laboratory Studies
Virology PCR
BAL and NP swab samples obtained at the time points specified above were analyzed in the CTOTC-03 virology core laboratory using the xTAG Respiratory Virus Panel manufactured by Luminex Corp. (Austin, TX) following the manufacturer’s instructions. A research use only panel was used, which included assays for the following viruses: influenza A, influenza A H1, influenza A H3, influenza B, respiratory syncytial virus A and B, parainfluenza 1–4, enterovirus/rhinovirus, coronaviruses OK43, 229E, NL63, and 229E, metapneumovirus, and adenovirus. Samples with mean fluorescence index (MFI) ≥ 1000 were considered positive. Samples with MFI 300–999 were retested and considered positive if the repeat MFI was ≥300. If the MFI of the repeat test was <300, the test was repeated a second time, and the sample was considered positive if the MFI of the second repeat test was ≥300. CMV and Epstein-Barr virus in whole blood and BAL were also assessed using PCR. 31 for inclusion in the assessment of the relationship between immunosuppression and viral infection.
Assessment of Humoral Immunity
Anti-HLA alloantibody testing was performed using Luminex assays. 32 DSA were determined by comparing HLA antibody specificities to the donor HLA. Detection of autoantibodies to type V collagen (colV) and K-alpha 1 tubulin (Kα1T) were performed by ELISA methodology using vimentin and myosin as controls. 8
Assessment of Cellular Immunity
Cellular alloreactivity was assessed by cytokine (interferon gamma, IFNγ) enzyme linked immunosorbent spot assays (ELISPOT) using donor B cell lines expanded using CD40L transfected fibroblasts and IL-4 as described 33. Specimens with > 25 positive cells per 300,000 PBL were considered positive. For T cell autoreactivity, peripheral blood mononuclear cells (PBMCs) were tested against recombinant colV (Chemicon/ Millipore, Billerica, MA) and Kα1T (recombinant protein expressed in the Mohanakumar laboratory as published) 34 using cytokine ELISPOT as well 35.
Methods – Statistical Analyses
Continuous variables were summarized with the means and standard deviations and categorical variables with counts and percentages. Subject characteristics were compared using chi-square, Fisher’s Exact, or Cochran-Mantel-Haenszel tests for categorical variables and t-tests for continuous variables. Time-dependent Cox proportional hazard models were used to examine the associations between viral infections and the composite endpoint as well as between viral infections and the selected mechanistic endpoints. Hazard ratios and corresponding 95% confidence intervals were reported. Survival curves were estimated using the Kaplan-Meier method. Because subjects’ infection status could transition between positive and negative, and due to the presence of the endogenous time-varying aspect of viral infection, a sensitivity analysis was performed using multi-state and joint survival models 36. Log10 transformations were applied as necessary to satisfy normal distribution assumptions. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Subject Population
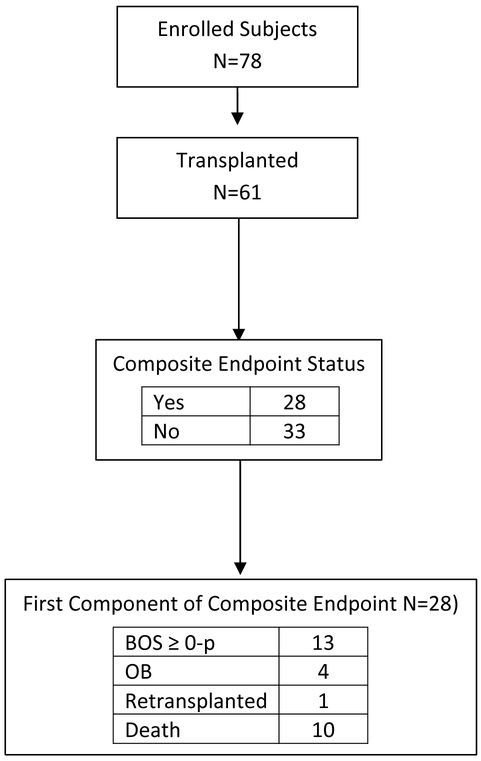
Seventy-eight subjects were enrolled in the study from which 61 underwent transplant (Figure 1). Twenty-nine (48%) subjects had Cystic Fibrosis, 11 (18%) had pulmonary hypertension, 9 (15%) had obliterative bronchiolitis, 6 (10%) had interstitial lung disease, 4 (7%) had congenital disorders of surfactant metabolism and 2 (3%) had other diseases leading to transplant. Mean age at transplant was 11.3 years, ranging from 9 months to 18 years. (Table 1). Patients were followed for an average of 20 (+/− 8) months following transplant.
Figure 1.
Consort diagram. Consort diagram illustrating the outcome of subjects throughout the course of the study including.
Notes: 5 patients were lost to follow-up: 3 of them met the composite endpoint before being lost to follow-up; 2 were lost to follow-up at the 24 month visit. All 5 patients were included in the analysis. One patient met the composite endpoint without having any viral specimens obtained.
BOS: Bronchiolitis Obliterans Syndrome
Table 1.
Clinical Characteristics and Outcomes in Relation to the Composite Endpoint
| All Subjects (N=61) n (%) |
Composite Endpoint Yes (N=28) n (%) |
Composite Endpoint No (N=33) n (%) |
P-value | |
|---|---|---|---|---|
| Recipient Characteristics | ||||
| Lung Allocation Score Diagnosis Group | ||||
| Group A | 1( 1.6) | 1( 3.6) | 0 | 0.758 |
| Group B | 11(18.0) | 5(17.9) | 6(18.2) | |
| Group C | 29(47.5) | 13(46.4) | 16(48.5) | |
| Group D | 20(32.8) | 9(32.1) | 11(33.3) | |
| Age | ||||
| N | 61 | 28 | 33 | 0.859 |
| Mean (SD) | 11.3 (5.46) | 11.5 (5.56) | 11.2 (5.46) | |
| Median | 13 | 13 | 13 | |
| Min, Max | <1, 18 | <1, 18 | <1, 18 | |
| Gender | ||||
| Male | 25(41.0) | 14(50.0) | 11(33.3) | 0.187 |
| Female | 36(59.0) | 14(50.0) | 22(66.7) | |
| Race | ||||
| White | 50(82.0) | 21(75.0) | 29(87.9) | 0.734 |
| Black or African American | 5( 8.2) | 3(10.7) | 2( 6.1) | |
| Asian | 2( 3.3) | 1( 3.6) | 1( 3.0) | |
| More Than One Race | 1( 1.6) | 1( 3.6) | 0 | NA |
| Unknown or Not Reported | 3( 4.9) | 2( 7.1) | 1( 3.0) | NA |
| Ethnicity | ||||
| Hispanic or Latino | 5( 8.2) | 2( 7.1) | 3( 9.1) | >0.999 |
| Not Hispanic or Latino | 42(68.9) | 19(67.9) | 23(69.7) | |
| Unknown or Not Reported | 14(23.0) | 7(25.0) | 7(21.2) | NA |
| Type of Transplant | ||||
| Double Lung | 56(91.8) | 25(89.3) | 31(93.9) | 0.653 |
| Heart-Lung | 5( 8.2) | 3(10.7) | 2( 6.1) | |
| Donor and Recipient Gender | ||||
| Donor Female, Recipient Female | 18(29.5) | 3(10.7) | 15(45.5) | 0.028 |
| Donor Female, Recipient Male | 13(21.3) | 6(21.4) | 7(21.2) | |
| Donor Male, Recipient Female | 18(29.5) | 11(39.3) | 7(21.2) | |
| Donor Male, Recipient Male | 11(18.0) | 7(25.0) | 4(12.1) | |
| Induction | ||||
| Anti IL-2 antibody | 44(72.1) | 22(78.6) | 22(66.7) | 0.241 |
| Thymoglobulin | 14(23.0) | 6(21.4) | 8(24.2) | |
| None | 3( 4.9) | 0 | 3( 9.1) | |
| Donor Gender | ||||
| Male | 29(47.5) | 18(64.3) | 11(33.3) | 0.010 |
| Female | 31(50.8) | 9(32.1) | 22(66.7) | |
| Clinical Events | ||||
| Acute Cellular Rejection | ||||
| Yes | 16(26.2) | 8(28.6) | 8(24.2) | 0.513 |
| No | 42(68.9) | 17(60.7) | 25(75.8) | |
| Highest Acute Cellular Rejection Grade | ||||
| A2 - Mild | 14(23.0) | 7(25.0) | 7(21.2) | >0.999 |
| A3 - Moderate | 2( 3.3) | 1( 3.6) | 1( 3.0) | |
| Antibody Mediated Rejection | ||||
| Yes | 11(18.0) | 6(21.4) | 5(15.2) | 0.504 |
| No | 47(77.0) | 19(67.9) | 28(84.8) | |
Primary graft dysfunction was common among subjects in this cohort: 33% of the 55 subjects with PGD data available met criteria for grade 2 or 3 PGD within 72 hours.
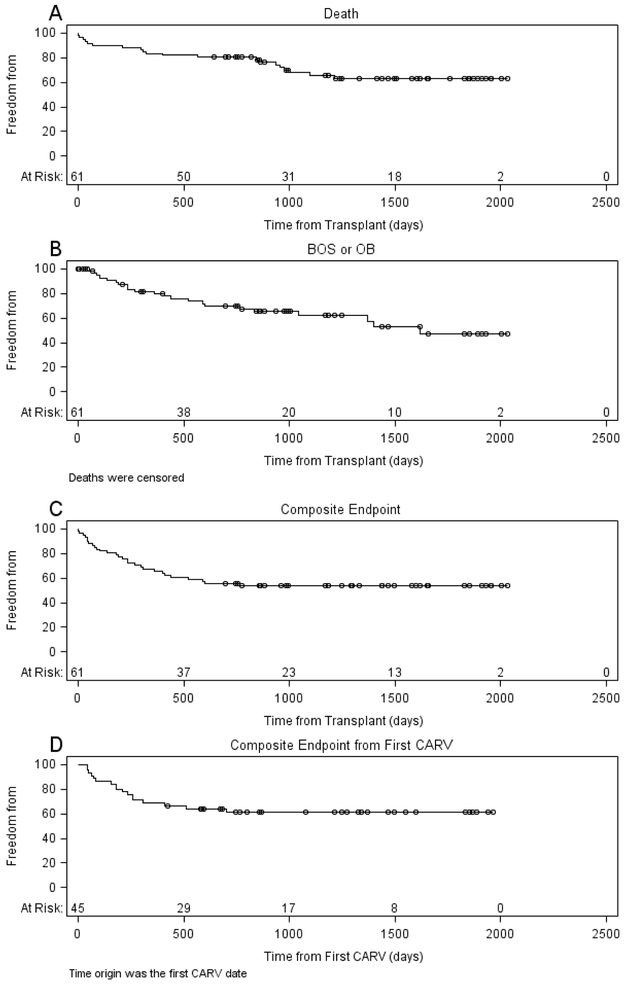
Twenty of the 61 subjects died prior to the end of the study period. Survival probability was 84% at 1 year and 68% at 3 years (Figure 2A). Chronic rejection (40%) was the most common cause of death followed by infection (20%). Death from primary graft dysfunction (10%) or post-transplant lymphoproliferative disease (5%) was uncommon in this population. Other causes of death (1 each) included renal failure, liver failure, heart failure, drug overdose and multisystem organ failure of unknown etiology.
Figure 2.
Probability of freedom from death (A) and Bronchiolitis Obliterans Syndrome (BOS) and Obliterative Bronchiolitis (OB) (B), the composite endpoint (C) and the composite endpoint as a function of time from the first community acquired respiratory virus (CARV) (D). The number of subjects at risk is presented at select time points along the x axis. Death was censored on Figure B.
Infection
Lower respiratory tract infections (LRTI) were reported in nearly all subjects (Table 2). For bacterial LRTI, 22 of the 38 subjects had CF and accounted for 68% of the infections. None of the bacterial LRTI were the primary cause of mortality. Thirteen episodes of bacteremia were identified in 10 subjects (16%); 5 of the subjects had bacteremia within the first 90 days after transplant. Bacteremia contributed to 2 of the 4 deaths attributable to infection.
Table 2.
Summary of Respiratory Tract Infections
| Infection Type | # of Episode | # of Subject |
|---|---|---|
| Any infection including CARV, Bacterial, Viral, Fungal, Mycobacterial, and Protozoan | 416 | 57 |
| At Least one infection of CARV, Bacterial, or Fungal LRTI and Respiratory (Unspecified) | 268 | 56 |
| No infection at all | -- | 4 * |
| CARV | 156 | 50 |
| Bacterial (LRTI and Respiratory (Unspecified) | 117 (103 with LRTI only) | 41 (38 with LRTI only) |
| Fungal (LRTI and Respiratory (Unspecified) | 39 | 25 |
| Bacterial or Fungal (LRTI and Respiratory (Unspecified) | 140 | 46 |
| CARV and either Bacterial or Fungal (LRTI and Respiratory (Unspecified) at the same time | 37 | 25 ** |
| CARV and Bacterial but no Fungal (LRTI and Respiratory (Unspecified) at the same time | 27 | 21 ** |
| CARV and Fungal but no Bacterial (LRTI and Respiratory (Unspecified) at the same time | 8 | 8 ** |
| 3 infections at the same time | 2 | 2 ** |
| CARV and Neither Bacterial or Fungal (LRTI and Respiratory (Unspecified) at the same time | 119 | 46 *** |
| Bacterial *or* Fungal (LRTI and Respiratory (Unspecified) but No CARV at the same time | 112 | 42 *** |
| Bacterial and Fungal (LRTI and Respiratory (Unspecified) but No CARV at the same time | 8 | 5 *** |
3 subjects who did not have any infection died early between 1-27 days post-transplant due to the primary graft failures and cardiac failure. 1 subject completed the study without any infection and not meeting composite endpoint.
The count reflects the number of unique subjects with CARV, LRT bacterial or fungal infections, regardless of type.
Subjects may be counted in more than one row if the subject experienced multiple types of infection.
Of the locally identified viral LRTI, adenovirus (7) was the most common virus reported followed by parainfluenza (5). CMV pneumonitis was reported in four patients. Adenovirus infection contributed to 2 of the 4 deaths attributable to infection. In 20 of 22 (91%) of the locally identified viral infections for which a matching specimen was sent to the CTOTC-03 virology core laboratory the same virus was identified. Approximately 40% of the locally identified viral LRTI did not have a central specimen submitted to the core lab.
Consistent with what was previously reported, 37none of the fungal LRTI were contributors to mortality.
Rejection
A Kaplan-Meier analysis showed the probability of having at least one acute cellular rejection (ACR) grade A2 or greater was 21% at 1 year and 30% at 2 years. There were 19 ACR grade A2 or greater in 12 subjects within 1 year and 6 ACR episodes in 6 subjects between 1 year and 2 years. Thirteen episodes of antibody- mediated rejection (AMR) were identified in eleven subjects (18%) during the 2-year follow-up period.
The probability of developing either BOS grade greater than 0-p or a histologic diagnosis of OB was 20% at 1 year and 38% at 3 years (Figure 2B)
Composite Endpoint
Twenty-eight subjects (46%) met one or more of the components of the composite endpoint: death, re-transplantation or development of BOS or bronchiolitis obliterans (Figure 1). Recipient diagnosis, age, sex and race/ethnicity were not associated with the composite endpoint (Table 1). Neither was type of transplant, transplant time, ischemic time, the use of induction immunosuppressant medications, nor the development of PGD, ACR or AMR. Univariate analyses showed that recipients receiving organs from a female donor were less likely to meet the composite endpoint, particularly when the recipient was female as well (P=0.028). No other donor factors were associated with the composite endpoint. No relationship was found among demographic factors and the separate components of the composite outcome (supplementary tables S1 and S2).
Mechanistic markers and the composite endpoint
We analyzed serum DSA and autoantibodies in this cohort because the presence of these antibodies has been associated with poor lung transplant outcomes in adults 8,38-40. Twenty-eight subjects in the cohort developed de novo DSA. Twenty-one subjects without detectable serum autoantibodies pre-transplant developed post-transplant autoantibodies to ColV and 26 subjects developed de novo post-transplant autoantibodies to Kα1T. We did not detect relationships among any of the antibodies and the composite endpoint. We also quantified pre- and post-transplant, donor-reactive and Kα1T-reactive immunity in PBMCs by IFNγ ELISPOT. While ten subjects developed de novo post-transplant donor reactive cellular responses and 20 subjects developed de novo Kα1T-reactive cellular immunity, we did not observe relationships between either of these parameters and the composite endpoint (Table 3). Nor did we observe significant correlations between the composite endpoint (or its components) and the presence of post-transplant humoral or cellular reactivity to either allo- or autoantigens whether de novo or not. (Table 3)
Table 3:
Relationship between mechanistic endpoints and composite endpoint
| Mechanistic Assays | All Subjects (N=61) n (%) |
Composite Endpoint Yes (N=28) n (%) |
Composite Endpoint No (N=33) n (%) |
P-value |
|---|---|---|---|---|
| Pre to Post-Transplant Antibody Status | ||||
| Collagen V | ||||
| Neg to Neg | 10(16.4) | 7(25.0) | 3( 9.1) | 0.135 |
| Neg to Pos | 21(34.4) | 8(28.6) | 13(39.4) | |
| Ka1Tubulin | ||||
| Neg to Neg | 3( 4.9) | 2( 7.1) | 1( 3.0) | 0.553 |
| Neg to Pos | 26(42.6) | 10(35.7) | 16(48.5) | |
| Donor Specific Antibody | ||||
| Neg to Neg | 28(45.9) | 11(39.3) | 17(51.5) | 0.420 |
| Neg to Pos | 28(45.9) | 14(50.0) | 14(42.4) | |
| Post-Transplant Antibody Status | ||||
| ELISA: Auto Antibodies | ||||
| Collagen V | ||||
| Positive | 46(75.4) | 17(60.7) | 29(87.9) | 0.038 |
| Negative | 13(21.3) | 9(32.1) | 4(12.1) | |
| Missing | 2( 3.3) | 2( 7.1) | 0 | NA |
| Ka1Tubulin | ||||
| Positive | 56(91.8) | 24(85.7) | 32(97.0) | 0.578 |
| Negative | 3( 4.9) | 2( 7.1) | 1( 3.0) | |
| Missing | 2( 3.3) | 2( 7.1) | 0 | NA |
| Anti-HLA Antibodies | ||||
| Class I | ||||
| Positive | 33(54.1) | 12(42.9) | 21(63.6) | 0.179 |
| Negative | 26(42.6) | 14(50.0) | 12(36.4) | |
| Missing | 2( 3.3) | 2( 7.1) | 0 | NA |
| Class II | ||||
| Positive | 31(50.8) | 13(46.4) | 18(54.5) | 0.728 |
| Negative | 28(45.9) | 13(46.4) | 15(45.5) | |
| Missing | 2( 3.3) | 2( 7.1) | 0 | NA |
| Donor Specific Antibody | ||||
| Yes | 30(49.2) | 14(50.0) | 16(48.5) | 0.795 |
| No | 30(49.2) | 13(46.4) | 17(51.5) | |
| ELISPOT | ||||
| Pre to Post-Transplant ELISPOT Status | ||||
| Donor Specific ELISPOT (>=25) | ||||
| Neg to Neg | 19(31.1) | 10(35.7) | 9(27.3) | 0.433 |
| Neg to Pos | 10(16.4) | 3(10.7) | 7(21.2) | |
| Kα1T Specific ELISPOT (>=5) | ||||
| Neg to Neg | 3( 4.9) | 1( 3.6) | 2( 6.1) | >0.999 |
| Neg to Pos | 20(32.8) | 9(32.1) | 11(33.3) | |
| Post-transplant Donor Specific ELISPOT (>=25) | ||||
| Positive | 16(26.2) | 6(21.4) | 10(30.3) | 0.368 |
| Negative | 21(34.4) | 11(39.3) | 10(30.3) | |
| Post-transplant Kα1T Specific ELISPOT (>=5) | ||||
| Positive | 52(85.2) | 22(78.6) | 30(90.9) | >0.999 |
| Negative | 6( 9.8) | 3(10.7) | 3( 9.1) | |
Viral Infection and the composite endpoint
The virology core laboratory evaluated 397 BAL and 480 NP specimens for viral pathogens. Of these, CARV were detected in 156 subject visits. In 40 of these, both BAL and NP specimens were positive. In 26, the BAL alone was positive while 90 had only a positive NP sample (this includes symptomatic episodes where no BAL was obtained). Of the CARV identified in positive specimens, in 116 (74%) of the visits the specimen was positive for enterovirus/rhinovirus. Eight subjects had successive samples positive for enterovirus/rhinovirus, suggestive of persistent infection.
To investigate the association between CARV and composite endpoint, we considered only CARV that occurred on or before the subject’s known endpoint status. Of subjects who had at least one BAL or NP specimen positive for CARV, 38% (17/45) met the composite endpoint (11 BOS/OB; 5 Death; 1 re-transplant). In contrast, two thirds of subjects who did not test positive for CARV (10/15) met the composite endpoint (6 BOS/OB; 4 Death). When CARV with symptoms was evaluated, this difference was more pronounced. Only 20% of subjects with symptomatic CARV (4/20) met the composite endpoint. (Table 4).
Table 4.
Relationship between CARV and Composite Endpoint
| Met Composite Endpoint (death, retransplantation or Bronchiolitis Obliterans Syndome / Obliterative Bronchiolitis) within 24m |
|||
|---|---|---|---|
| CARV: (Rhino+Other) BAL or NP |
Total (N=60)* n (%) |
Yes (N=27) n (%) |
No (N=33) n (%) |
| Yes | 45(73.8) | 17(63.0 | 28(84.8) |
| No | 15(24.6) | 10(37.0) | 5(15.2) |
| Symptomatic CARV | |||
| Yes | 20(32.8) | 4(14.8) | 16(48.5) |
| No | 40(65.6) | 23(85.2) | 17(51.5) |
One subject met the composite endpoint without having any viral specimens obtained.
CARV: Community Acquired Respiratory Virus
BAL: Bronchoalveolar Lavage
NP: Nasopharyngeal Swab
However, recognizing that the subject’s CARV status could change over time, we utilized a time-dependent Cox analysis. First, we focused on the transitions from positive to negative or negative to positive. We found no relationship between the presence of CARV by PCR and the composite endpoint (Table 5). Second, when the presence of CARV was treated as an absorbent state (i.e. a subject is considered permanently positive for CARV after the initial exposure), the hazard ratio was 0.8 with a 95% CI of 0.3–2.5 and a p-value of 0.73. These findings, which were contrary to the primary hypothesis, led us to suspect competing risk bias in the simpler Chi-square and K-M analyses, so to support the Cox model we added a sensitivity analysis of multi-state and joint survival models. As with the Cox model, no relationship between the presence of CARV and the composite endpoint was found (data not shown).
Table 5.
Time Dependent Cox Analysis of relationship with viral infection and Composite Endpoint
| Variable | Hazard Ratio (95% Lower CL, Upper CL) | P-value |
|---|---|---|
| Any Virus | 0.57 (0.26, 1.25) | 0.161 |
| Any Virus with Symptoms | 0.66 (0.2, 2.2) | 0.497 |
| CARV | 0.64 (0.25, 1.59) | 0.335 |
| CARV with symptoms | 0.94 (0.22, 4.03) | 0.937 |
CARV: Community Acquired Respiratory Virus
No relationship was found when we limited the analysis to paramyxovirus, influenza and adenovirus episodes, when we looked for relationships between the timing of the viral episode (occurring within the first 3 months or the first 6 months) or the number of independent viral episodes and the composite endpoint. We also observed no relationship between presence of CARV by PCR and the individual components of death (HR=0.8, CI=0.2–3.2, p=0.80) and the development of BOS (HR=0.4, CI=0.1–1.7, p=0.20).
In addition, we did not observe statistically significant relationships when we repeated these analyses including viral infection data as reported by the local clinical laboratories (HR=0.5, CI=0.2–1.4, p=0.21).
Viral infection and mechanistic endpoints (allo- and autoimmunity)
We subsequently tested for relationships between viral presence and development of markers of humoral and cellular allo- and autoimmunity. Recovery of CARV from airway specimens was not related to the development of DSA, AutoAb (CoIV and Kα1T), and Kα1T specific ELISPOT. (Table 6). The presence of a CARV was associated with an increased risk for subsequent development of ColV AutoAb. Because there were multiple CARVs at different times, the time-dependent Cox model was used. (HR=3.1, CI=1.2–8.1, p=0.02).
Table 6.
Relationship between mechanistic endpoints and CARV
| Mechanistic Assays | All Subjects (N=60)* n (%) |
CARV (N=45) n (%) |
No CARV (N=15) n (%) |
P-value |
|---|---|---|---|---|
| Antibody Status Post-Transplant | ||||
| ELISA: Auto Antibodies | ||||
| Collagen V | ||||
| Positive | 46(76.7) | 37(82.2) | 9(60.0) | 0.157 |
| Negative | 13(21.7) | 8(17.8) | 5(33.3) | |
| Missing | 1( 1.7) | 0 | 1( 6.7) | NA |
| Ka1Tubulin | ||||
| Positive | 56(93.3) | 42(93.3) | 14(93.3) | >0.999 |
| Negative | 3( 5.0) | 3( 6.7) | 0 | |
| Missing | 1( 1.7) | 0 | 1( 6.7) | NA |
| Anti-HLA Antibodies | ||||
| Class I | ||||
| Positive | 33(55.0) | 28(62.2) | 5(33.3) | 0.081 |
| Negative | 26(43.3) | 17(37.8) | 9(60.0) | |
| Missing | 1( 1.7) | 0 | 1( 6.7) | NA |
| Class II | ||||
| Positive | 31(51.7) | 25(55.6) | 6(40.0) | 0.406 |
| Negative | 28(46.7) | 20(44.4) | 8(53.3) | |
| Missing | 1( 1.7) | 0 | 1( 6.7) | NA |
| Donor Specific Antibodies | ||||
| Yes | 30(50.0) | 24(53.3) | 6(40.0) | 0.493 |
| No | 29(48.3) | 21(46.7) | 8(53.3) | |
| ELISPOT | ||||
| Post-transplant Donor Specific ELISPOT (>=25) | ||||
| Positive | 16(26.2) | 11(24.4) | 5(33.3) | 0.614 |
| Negative | 21(34.4) | 16(35.6) | 5(33.3) | |
| Post-transplant Kα1T Specific ELISPOT (>=5) | ||||
| Positive | 52(85.2) | 41(91.1) | 11(73.3) | 0.149 |
| Negative | 6( 9.8) | 3( 6.7) | 3(20.0) | |
One subject met the composite endpoint without having any viral specimens obtained.
CARV: Community Acquired Respiratory Virus
Viral infection as a potential surrogate for degree of immunosuppression
Finally, we considered the hypothesis that viral infection might be a surrogate marker for the overall level of immunosuppression. We found no relationship between mean or coefficient of variation of tacrolimus trough levels and the composite endpoint (supplementary table S3). Although decreased frequencies of IFNγ ELISPOTs to polyclonal stimulus with phytohemagglutinin (PHA) were associated with viral infection (Table 7), we did not detect a relationship between IFNγ-producers and the composite endpoint (Table 8).
Table 7:
Relationship between ELISPOT Measures of immunosuppression and any viral Infection
| Mechanistic Assays | All Subjects (N=60)* n (%) |
Any Viral Infection (N=52) n (%) |
No Viral Infection (N=8) n (%) |
P-value |
|---|---|---|---|---|
| High immunosuppression per ELISPOT (at least one positive** sample) | ||||
| Yes | 43(71.7) | 41(78.8) | 2(25.0) | 0.003 |
| No | 16(26.7) | 10(19.2) | 6(75.0) | |
| Number of High immunosuppression samples** per Subject | ||||
| 0 | 16(26.7) | 10(19.2) | 6(75.0) | 0.055 |
| 1 | 9(15.0) | 9(17.3) | 0 | |
| 2 | 7(11.7) | 6(11.5) | 1(12.5) | |
| 3 | 7(11.7) | 7(13.5) | 0 | |
| 4 | 11(18.3) | 11(21.2) | 0 | |
| 5 | 5( 8.3) | 5( 9.6) | 0 | |
| 6 | 3( 5.0) | 2( 3.8) | 1(12.5) | |
| 10 | 1( 1.7) | 1( 1.9) | 0 | |
One subject met the composite endpoint without having any viral specimens obtained.
Samples were considered positive for high immunosuppression if the response to positive control (phytohemagglutinin, PHA) was less than 100 spots and cell viability was > 50%.
Table 8:
Relationship between ELISPOT Measures of immunosuppression and Composite Endpoint
| All Subjects (N=61) n (%) |
Composite Endpoint Yes (N=28) n (%) |
Composite Endpoint No (N=33) n (%) |
P-value | |
|---|---|---|---|---|
| High immunosuppression per ELISPOT (at least one positive* sample) | ||||
| Yes | 44(72.1) | 18(64.3) | 26(78.8) | 0.291 |
| No | 16(26.2) | 9(32.1) | 7(21.2) | |
| Number of high immunosuppression samples* per Subject | ||||
| 0 | 16(26.2) | 9(32.1) | 7(21.2) | 0.473 |
| 1 | 10(16.4) | 3(10.7) | 7(21.2) | |
| 2 | 7(11.5) | 2( 7.1) | 5(15.2) | |
| 3 | 7(11.5) | 3(10.7) | 4(12.1) | |
| 4 | 11(18.0) | 7(25.0) | 4(12.1) | |
| 5 | 5( 8.2) | 1( 3.6) | 4(12.1) | |
| 6 | 3( 4.9) | 2( 7.1) | 1( 3.0) | |
| 10 | 1( 1.6) | 0 | 1( 3.0) | |
Samples were considered positive for high immunosuppression if the response to positive control (phytohemagglutinin, PHA) was less than 100 spots and cell viability was > 50%.
Discussion
In this first prospective, observational, multicenter study of pediatric LTRs, we obtained clinical and mechanistic data on 61 subjects from 6 pediatric lung transplant centers followed for a minimum of 2 years and found comparable outcomes to the most recent ISHLT registry report 3. However, in contrast to studies using adult subjects, we did not find relationships among primary graft dysfunction, ACR, or CARV exposure and BOS / OB or graft loss. Based on the hypothesis that the common link between these different forms of graft injury and chronic allograft dysfunction is stimulation of a cellular and humoral responses directed at the allograft, these observations raise the possibility that the immunologic environment in pediatric lung transplant recipients is different than that of adults.
The indications for lung transplant, incidence of PGD, acute rejection, BOS and overall survival in this population are comparable to those reported in the most recent ISHLT registry report. 3 In contrast to adult LTRs ACR and PGD were not related to the development of BOS/OB or the composite endpoint 41-43.
We found that identification of common respiratory viruses in NP or BAL specimens was not predictive of poor outcome as measured by a composite endpoint of death, re-transplant or development of CLAD based on OB and BOS criteria. Although subjects with a CARV identified in NP or BAL were less likely to meet the composite endpoint, particularly when the CARV was associated with symptoms, this could be due to a competing risk bias in that subjects with good outcomes had more follow up time to develop more viral infections. The time-dependent Cox analysis partially controls for this bias and when we did not find a statistically significant difference between the groups, we concluded that the competing risk bias was driving these non-intuitive results.
These findings contrast with previous retrospective studies performed in adult populations. Both prospective 21,44,45 and retrospective studies 46 support a link between CARV and chronic rejection, particularly when the infection involves the lower respiratory tract and/or is associated with clinical symptoms 46,47. Specifically, community acquired respiratory viruses including paramyxoviruses, influenza, and adenovirus have been implicated in OB/BOS. 44,45. Most viral infections in our cohort were asymptomatic, perhaps a reflection of the fact that 75% of the CARV identified in our population were rhinovirus/enterovirus (which was not a risk factor in adult populations). No relationship was found when we limited the analysis to paramyxovirus, influenza and adenovirus episodes but the small number of these episodes limits our power to detect an effect.
Also, in contrast to adult populations, the development of donor specific humoral allo- and autoimmunity to self-antigens was not correlated to meeting the composite endpoint or any of its components. Single- center analyses of adult LTRs have identified anti-donor HLA antibodies, 39,40 as well as ColV and Kα1T autoantibodies as risk factors for CLAD 8,48. Similarly, we did not find a correlation between donor-specific alloreactive T cells responsiveness (as assessed by cytokine ELISPOT) in contrast to studies in kidney transplant recipients where ELISPOT reactivity strongly correlates with long term renal graft function 49-55.
Taken together, the absence of a relationship between among CARV, ACR and outcome as well as the absence of a relationship between donor and/or self-antigen specific immunity and outcome suggests that these events occurring in the context of the developing immune system of a pediatric patient 56 impact transplant outcome differently than they do in adults.
The finding that female subjects, particularly females receiving organs from female donors, were less likely to meet the composite endpoint is difficult to reconcile with the other findings. Although one could hypothesize that females receiving male organs would have increased potential to develop immunity to Y chromosome gene products from the donor, our study population was not adequately powered to evaluate this hypothesis. The impact of gender combinations is not fully studied among LTRs. Indeed, some studies have shown that gender combinations appeared to have a significant impact 57,58, and others, no effects, on survival of LTRs. 59,60. One single- center, retrospective study of adult LTRs reported a trend toward improved survival in female recipients. 61 Our study was not sufficiently powered to dissect this further.
Although the study population comprised nearly 40% of pediatric lung transplants performed in the United States during the accrual period, it is underpowered. A post-hoc power calculation using the observed frequencies in this cohort would require enrollment of 100 subjects to provide 80% power to detect a hazard ratio of 2.5. Nonetheless, given that we saw no relationship between infection and the composite endpoint (including an absence of statistical trends), we feel this study provides strong evidence rejecting the original hypothesis. The absence of a relationship between allo- and autoimmune responses and the composite endpoint may also reflect inadequate power. The relatively short 2-year follow-up period may have also limited our ability to identify the development of BOS, which is often diagnosed beyond 2 years post-transplant. We plan to obtain 5-year clinical follow-up data to assess this possibility. Because we did not collect data about patients who met exclusion criteria, we cannot rule out a recruitment bias in that regard. A final limitation to our study is that it was performed using BOS rather than CLAD criteria. It is possible that our findings would have been different had we collected sufficient data to characterize patients using current CLAD criteria and chosen a composite endpoint based on one of the CLAD subtypes.
Nonetheless, in contrast to the adult population 21,44-46, respiratory viral infections in pediatric LTRs do not appear to be a predictor for poor outcome. Coupled with the observation that high immunosuppression based on ELISPOT reactivity was associated with viral infection, and the observation that a subset of our subjects had persistent rhinovirus infection 62 it is possible that viral infection was a surrogate for immunosuppression in this population. The finding, in a companion study using samples from this population, that low betatorquevirus levels at 6 weeks and 6 months after transplant were associated with death and the composite outcome supports this hypothesis63.
In summary, this comprehensive prospective analysis of pediatric LTRs found comparable outcomes to the most recent ISHLT registry report 3 but did not identify relationships among primary graft dysfunction, ACR, or CARV and graft loss/CLAD. These observations raise the possibility that the immunologic environment in pediatric lung transplant recipients is different than that observed in adults. Further research is needed to determine whether these observations can be correlated with specific immunologic markers and /or warrant changes in screening or immunosuppression therapy in pediatric LTRs.
Supplementary Material
Acknowledgments
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children, a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases. The work was supported by Grant U01 AI077810 “Viral Triggers of Alloimmunity and Autoimmunity in Pediatric Lung Transplantation” from the Division of Allergy, Immunology and Transplantation of the National Institutes of Health.
Abbreviations
- ACR
Acute cellular rejection
- AMR
Antibody- mediated rejection
- AutoAb
Autoantibodies
- BAL
Bronchoalveolar lavage
- BOS
Bronchiolitis Obliterans Syndrome
- CARV
Community acquired respiratory virus infections
- CF
Cystic Fibrosis
- CLAD
Chronic lung allograft dysfunction
- CMV
Cytomegalovirus
- ColV
Collagen type V
- CTOT-C
Clinical Trials in Organ Transplant in Children
- DSA
Donor-specific human leukocyte antigen antibody
- ELISPOT
Enzyme linked immunosorbent spot assay
- HLA
Human leukocyte antigen
- IFNγ
Interferon gamma
- IPLTC
International Pediatric Lung Transplant Collaborative
- ISHLT
International Society for Heart and Lung Transplantation
- Kα1T
K-alpha 1 tubulin
- LAS
Lung Allocation Score
- LTR
Lung transplant Recipient
- LRTI
Lower respiratory tract infection
- MFI
Mean fluorescence index
- NP
Nasopharyngeal
- OB
Obliterative Bronchiolitis
- PCR
Polymerase chain reaction
- PBMCs
Peripheral blood mononuclear cells
- PE
Primary Endpoint
- PGD
Primary graft dysfunction
- PHA
Phytohemagglutinin
- PI
Primary Investigator
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement
The data supporting this publication will be available at ImmPort (immport.org) under study accession SDY960-Viral Triggers of Alloimmunity and Autoimmunity in Pediatric Lung Transplantation64
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of this article.
References
- 1.Weigt SS, DerHovanessian A, Wallace WD, Lynch JP 3rd, Belperio JA. Bronchiolitis obliterans syndrome: the Achilles’ heel of lung transplantation. Seminars in respiratory and critical care medicine. 2013;34(3):336–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweet SC. Pediatric Lung Transplantation. Respir Care. 2017;62(6):776–798. [DOI] [PubMed] [Google Scholar]
- 3.Goldfarb SB, Levvey BJ, Cherikh WS, et al. Registry of the International Society for Heart and Lung Transplantation: Twentieth Pediatric Lung and Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1070–1079. [DOI] [PubMed] [Google Scholar]
- 4.Smits JM, Vanhaecke J, Haverich A, et al. Three-year survival rates for all consecutive heart-only and lung-only transplants performed in Eurotransplant, 1997–1999. ClinTranspl. 2003:89–100. [PubMed] [Google Scholar]
- 5.Benden C, Goldfarb SB, Edwards LB, et al. The registry of the International Society for Heart and Lung Transplantation: seventeenth official pediatric lung and heart-lung transplantation report−−2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):1025–1033. [DOI] [PubMed] [Google Scholar]
- 6.Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. Journal of Thoracic& CardiovascularSurgery. 2004;127(1):114–122. [DOI] [PubMed] [Google Scholar]
- 7.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. The Journal of clinical investigation. 2007;117(11):3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. Journal of Immunology. 2008;180(7):4487–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class I molecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Hum Immunol. 1997;53(1):90–97. [DOI] [PubMed] [Google Scholar]
- 10.Harris PE, Bian H, Reed EF. Induction of high affinity fibroblast growth factor receptor expression and proliferation in human endothelial cells by anti-HLA antibodies: a possible mechanism for transplant atherosclerosis. J Immunol. 1997;159(11):5697–5704. [PubMed] [Google Scholar]
- 11.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64(5):521–529. [DOI] [PubMed] [Google Scholar]
- 12.Reznik SI, Jaramillo A, Zhang L, Patterson GA, Cooper JD, Mohanakumar T. Anti-HLA antibody binding to hla class I molecules induces proliferation of airway epithelial cells: a potential mechanism for bronchiolitis obliterans syndrome. Journal of Thoracic & Cardiovascular Surgery. 2000;119(1):39–45. [DOI] [PubMed] [Google Scholar]
- 13.Gunasekaran M, Xu Z, Nayak DK, et al. Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(2):474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendt CH, Fox JM, Hertz MI. Paramyxovirus infection in lung transplant recipients. Journal of Heart & Lung Transplantation. 1995;14(3):479–485. [PubMed] [Google Scholar]
- 15.Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174(12):1392–1399. [DOI] [PubMed] [Google Scholar]
- 16.Milstone AP, Brumble LM, Barnes J, et al. A single-season prospective study of respiratory viral infections in lung transplant recipients. The European respiratory journal. 2006;28(1):131–137. [DOI] [PubMed] [Google Scholar]
- 17.Gerna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78(3):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doud JR, Hinkamp T, Garrity ER Jr., Respiratory syncytial virus pneumonia in a lung transplant recipient: case report. J Heart Lung Transplant. 1992;11(1 Pt 1):77–79. [PubMed] [Google Scholar]
- 19.Garbino J, Crespo S, Aubert JD, et al. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (Non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43(8):1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larcher C, Geltner C, Fischer H, Nachbaur D, Muller LC, Huemer HP. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24(11):1891–1901. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. [DOI] [PubMed] [Google Scholar]
- 23.Garbino J, Gerbase MW, Wunderli W, et al. Respiratory viruses and severe lower respiratory tract complications in hospitalized patients. Chest. 2004;125(3):1033–1039. [DOI] [PubMed] [Google Scholar]
- 24.Bridges ND, Spray TL, Collins MH, Bowles NE, Towbin JA. Adenovirus Infection in the Lung Results in Graft Failure After Lung Transplantation. JThoracCardiovascSurg. 1998;116(4):617–623. [DOI] [PubMed] [Google Scholar]
- 25.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. Journal of Heart & Lung Transplantation. 2002;21(5):559–566. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis. 2009;11(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. Journal of Heart & Lung Transplantation. 2002;21(3):297–310. [DOI] [PubMed] [Google Scholar]
- 28.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. The Journal of Heart and Lung Transplantation. 2014;33(2):127–133. [DOI] [PubMed] [Google Scholar]
- 29.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. [24 refs]. Journal of Heart & Lung Transplantation. 2005;24(10):1454–1459. [DOI] [PubMed] [Google Scholar]
- 30.Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant. 2011;30(4):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rychert J, Danziger-Isakov L, Yen-Lieberman B, et al. Multicenter comparison of laboratory performance in cytomegalovirus and Epstein-Barr virus viral load testing using international standards. Clin Transplant. 2014;28(12):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golocheikine A, Nath DS, Basha HI, et al. Increased erythrocyte C4D is associated with known alloantibody and autoantibody markers of antibody-mediated rejection in human lung transplant recipients. Journal of Heart & Lung Transplantation. 2010;29(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poggio ED, Augustine JJ, Clemente M, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83(7):847–852. [DOI] [PubMed] [Google Scholar]
- 34.Tiriveedhi V, Gautam B, Sarma NJ, et al. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32(8):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. [DOI] [PubMed] [Google Scholar]
- 37.Ammerman E, Sweet SC, Fenchel M, et al. Risk and outcomes of pulmonary fungal infection after pediatric lung transplantation. Clin Transplant. 2017;31(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaramillo A, Smith MA, Phelan D, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation Proceedings. 1999;31(1–2):185–186. [DOI] [PubMed] [Google Scholar]
- 39.Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67(8):1155–1161. [DOI] [PubMed] [Google Scholar]
- 40.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74(6):799–804. [DOI] [PubMed] [Google Scholar]
- 41.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. American Journal of Respiratory & Critical Care Medicine. 2007;175(5):507–513. [DOI] [PubMed] [Google Scholar]
- 42.DerHovanessian A, Weigt SS, Palchevskiy V, et al. The Role of TGF-beta in the Association Between Primary Graft Dysfunction and Bronchiolitis Obliterans Syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(2):640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097–1103. [DOI] [PubMed] [Google Scholar]
- 44.Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89(8):1028–1033. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87(10):1530–1537. [DOI] [PubMed] [Google Scholar]
- 46.Fisher CE, Preiksaitis CM, Lease ED, et al. Symptomatic Respiratory Virus Infection and Chronic Lung Allograft Dysfunction. Clin Infect Dis. 2016;62(3):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peghin M, Los-Arcos I, Hirsch HH, et al. Community-acquired respiratory viruses are a risk factor for chronic lung allograft dysfunction. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. [Review] [121 refs]. AmericanJournal of Physiology - Lung Cellular& MolecularPhysiology. 2004;286(6):L1129–L1139. [DOI] [PubMed] [Google Scholar]
- 49.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17(2):564–572. [DOI] [PubMed] [Google Scholar]
- 50.Poggio ED, Roddy M, Riley J, et al. Analysis of immune markers in human cardiac allograft recipients and association with coronary artery vasculopathy. J Heart Lung Transplant. 2005;24(10):1606–1613. [DOI] [PubMed] [Google Scholar]
- 51.Poggio ED, Clemente M, Riley J, et al. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. 2004;15(7):1952–1960. [DOI] [PubMed] [Google Scholar]
- 52.Hricik DE, Rodriguez V, Riley J, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(7):878–884. [DOI] [PubMed] [Google Scholar]
- 53.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1971–1975. [DOI] [PubMed] [Google Scholar]
- 54.Gebauer BS, Hricik DE, Atallah A, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(9):857–866. [DOI] [PubMed] [Google Scholar]
- 55.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 56.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transplant. 2004;23(11):1252–1259. [DOI] [PubMed] [Google Scholar]
- 58.Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. Journal of Heart & Lung Transplantation. 2006;25(6):634–637. [DOI] [PubMed] [Google Scholar]
- 59.Sato M, Gutierrez C, Waddell TK, Liu M, Keshavjee S. Donor-recipient gender mismatch in lung transplantation: Impact on obliterative bronchiolitis and survival. J Heart Lung Transplant. 2005;24(11):2000–2001. [DOI] [PubMed] [Google Scholar]
- 60.Minambres E, Llorca J, Subrviola B, Ballesteros MA, Ortiz-Melon F, Gonzalez-Castro A. Influence of donor-recipient gender mismatch in early outcome after lung transplantation. Transplant Proc. 2008;40(9):3076–3078. [DOI] [PubMed] [Google Scholar]
- 61.Alvarez A, Moreno P, Illana J, et al. Influence of donor-recipient gender mismatch on graft function and survival following lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16(4):426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danziger-Isakov L, Buller R, Williams N, et al. Respiratory Viral Infections Are Common in the First Year After Pediatric Lung Transplantation: A Multi-Center Prospective Study. The Journal of Heart and Lung Transplantation. 2016;35(4):S35. [Google Scholar]
- 63.Blatter JA, Sweet SC, Conrad C, et al. Anellovirus loads are associated with outcomes in pediatric lung transplantation. Pediatr Transplant. 2018;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.SDY960 “Viral Triggers of Alloimmunity and Autoimmunity in Pediatric Lung Transplantation”. In. NIAID, trans: ImmPort (immport.org); 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.