Abstract
Objective:
To investigate the relationship between Krebs von den Lungen-6 [KL-6] and CC chemokine ligand 18 [CCL-18]) with severity and progression of systemic sclerosis-related interstitial lung disease (SSc-ILD).
Methods:
Patients enrolled in Scleroderma Lung Study (SLS) II (cyclophosphamide [CYC] versus mycophenolate mofetil [MMF]) were included. Baseline and 12-month plasma samples were analyzed by ELISA to assess CCL-18 and KL-6 levels. The forced vital capacity (FVC) and the diffusing capacity for carbon monoxide (DLCO) were measured every 3 months. Joint models were created to investigate the relationship between baseline CCL-18 and KL-6 and the course of the FVC and DLCO over 1 year by treatment arm.
Results:
Baseline KL-6 and CCL-18 levels each correlated with the extent of radiographic fibrosis. Levels of both CCL-18 and KL-6 declined significantly at one year. In both treatment arms, higher baseline KL-6 level predicted progression of ILD based on the course of the FVC (CYC/MMF: P=0.024/0.005) and DLCO (CYC/MMF: P<0.001/0.004) over 1 year. Higher baseline CCL-18 level predicted progression of ILD based on the course of the FVC (CYC/MMF: P<0.001;0.007), DLCO (CYC/MMF: P=0.001/<0.001) over 1 year, as well as mortality (CYC arm only P=0.0008).
Conclusion:
In a rigorously-conducted clinical trial for SSc-ILD, KL-6 and CCL-18 levels correlated with ILD severity and declined with immunosuppression. Patients with higher baseline KL-6 and CCL-18 levels were more likely to experience disease progression despite treatment. KL-6 and CCL-18 could be used to identify patients with a progressive ILD phenotype who may benefit from a more aggressive initial treatment approach.
Keywords: Systemic sclerosis, Interstitial lung disease, Mycophenolate mofetil, Cyclophosphamide, Biomarkers
INTRODUCTION
Interstitial lung disease (ILD) occurs in the majority of patients with systemic sclerosis (SSc) [1]. While ILD is the leading cause of disease-related mortality among patients with SSc [2, 3], ILD progression rates vary considerably. Results of randomized controlled trials (RCTs) have demonstrated that some patients experience an improvement in lung function after treatment with immunosuppression, while other patients experience progression of ILD despite early and aggressive treatment [4, 5]. Furthermore, not all patients with ILD will develop symptoms or will have progressive disease even in the absence of treatment [1, 6–8].
Evidenced-based clinical tools to predict which patients with SSc-ILD are more likely to experience ILD progression do not exist. Specific clinical and biological factors have been associated with progression of ILD in observational studies (e.g., low forced vital capacity (FVC) [9], greater extent of ILD on high-resolution computed tomography (HRCT) imaging [10, 11], low diffusing capacity for carbon monoxide (DLCO) [9, 12], and anti-topoisomerase I antibody positivity [9,12]). Moreover, several studies have identified serum/plasma protein candidate biomarkers that predict SSc-ILD progression, including interleukin (IL)-6 [13], C-reactive protein (CRP) [14], CC chemokine ligand 2 (CCL2) [15], CCL-18 [16, 17], CXCL4 [18], and KL-6 [19, 20].
Among these candidate biomarkers, KL-6 and CCL-18 have been found to predict outcomes in several different SSc-ILD populations [16, 17, 19, 20]. Because KL-6 and CCL-18 are pneumoproteins associated with lung parenchymal injury [21, 22], they may be more specific markers for monitoring and predicting the course of ILD in SSc. For example, in contrast to general inflammatory markers (e.g., IL-6 or C-reactive protein), the levels of KL-6 and CCL-18 may be less likely to be affected by extra-pulmonary fibrotic processes such as cutaneous sclerosis or infections.
Furthermore, KL-6 correlates with disease severity in different SSc-ILD populations [23–28]. Two observational studies [19, 20] have found that high KL-6 levels predict worse outcomes in SSc-ILD. A recent, small observational study demonstrated that high serum KL-6 level was associated with poor response to immunosuppression with cyclophosphamide (CYC) in SSc-ILD patients [29].
CCL-18 is a chemokine, which was previously known as pulmonary and activation-regulated chemokine (PARC), and studies have demonstrated higher levels of this chemokine in both serum and bronchoalveolar lavage fluid (BAL) samples of patients with ILD [30]. Observational studies have demonstrated that CCL-18 also predicts various ILD-related outcomes in SSc [16, 17, 31, 32].
Given the accumulating evidence that KL-6 and CCL-18 may be key markers of disease activity and progression in SSc-ILD, the present study sought to evaluate the predictive role of CCL-18 and KL-6 in the context of a RCT, in which all patients have equal access to care, uniform follow up and a standardized treatment approach. The present study aimed to determine whether KL-6 and CCL-18 are associated with the severity of ILD in a clinical trial cohort comprised of patients with well-characterized and active SSc-ILD. A secondary aim was to determine whether baseline levels of these peripherally-measured lung glycoproteins predict the progression of SSc-ILD in patients receiving immunosuppression with either mycophenolate mofetil (MMF) or CYC.
PATIENTS AND METHODS
Study participants
Data and plasma samples from participants enrolled in SLS II [5] were analyzed for this study. Eligibility criteria included the following key inclusion criteria: (1) adults, aged 18–75 years, (2) limited or diffuse cutaneous SSc [33], (3) active ILD as demonstrated by restrictive to borderline restrictive ventilatory impairment (FVC<80–85% but ≥ 45% predicted) AND the presence of any ground glass opacity (GGO; hazy opacity through which normal lung markings can be discerned) on high-resolution computed tomography (HRCT), (4) exertional dyspnea (Grade ≥2 on the Magnitude of Task component of the Mahler Baseline Dyspnea Index [BDI] [34]. Key exclusion criteria included pulmonary hypertension; clinically significant abnormalities on HRCT not attributable to SSc; smoking within the past 6 months; and evidence of significant airflow obstruction. Complete details of the SLS II study design have been previously reported [5].
Unaffected control participants were independently recruited at the University of Texas, Houston, and age-, ethnicity- and gender-matched to SLS II participants in an approximately 1 (control) to 3 (SLS II) ratio. The same healthy, unaffected controls were used for both the KL-6 and CCL-18 analyses. The Institutional Review Board of each site approved the studies; and only participants who provided informed consent were included in the present analyses.
Patient and Public Involvement
Patients and the public were not involved in the design or reporting of the results of this research study. Patients were involved in the conduct of the study because they served as participants.
SLS II Study Design
In SLS II, enrolled patients were randomized in a similar manner to either oral CYC for one year followed by one year of placebo or MMF for 2 years. For complete details of the SLS II protocol, please see the supplementary web appendix accompanying the main SLS II [5] manuscript. The FVC (primary SLS II endpoint) and DLCO (secondary SLS II endpoint) were measured every 3 months, and the TLC was measured every 6 months during the trial. HRCT thoracic imaging was obtained at baseline in SLS II, and a Computer Aided Design (CAD) scoring system was employed to provide quantitative measures of different patterns of ILD as previously described [35]. Quantitative ILD (QILD) score was the sum of all abnormally classified scores, including scores for quantitative lung fibrosis (QLF, linear reticular markings with architectural distortion), GGO and honeycomb changes (clustered air-filled cysts with dense walls). Scores were calculated as percentage of total counted voxels for both the whole lung (WL), including both lungs, and for the zone (area-equivalent upper, middle or lower lung zone) of maximal involvement (ZM).
KL-6 and CCL-18 Assays
SLS II plasma samples were collected at the baseline and 12-month study visits in EDTA tubes and were immediately processed on-site on the day of collection, stored at −700C, and shipped on dry-ice to the central repository at the University of Texas - Houston. All SLS II patients with an available baseline plasma sample were included in the present study. Plasma samples from healthy controls collected at the University of Texas - Houston, were handled in the same manner except that no shipping was required. CCL-18 was assayed by commercially available ELISA kits (MIP-4/CCL-18 kit, Cell Sciences), while KL-6 was measured using latex-fixed anti-KL-6 monoclonal antibody with an automated analyzer (Nanopia KL-6; Sekisui Medical Co. Ltd.). All plasma assays were performed in duplicates and the coefficient of variance was <20%. Technicians performing the assays were blinded to the clinical diagnosis and outcome data.
Statistical Analysis
Baseline characteristics
Summary statistics were generated for baseline characteristics. A two-sample t-test or Wilcoxon rank-sum test was used to compare continuous variables and a chi-square test was used to compare categorical variables. Kendall’s tau correlations were performed to examine the relationship between KL-6/CCL-18 levels and baseline measures of extent of ILD, as measured by the FVC, DLCO, QILD, and QLF.
Change of KL-6 and CCL-18 from baseline to 12 months
Summary statistics of KL-6/CCL-18 were calculated for baseline and 12 months. Wilcoxon signed-rank test was used to compare the data collected at the two time points.
Relationship between baseline KL-6 and CCL-18 with progression of SSc-ILD
A joint model analysis was used to determine whether baseline levels of KL-6 or CCL-18 predict progression of SSc-ILD. The joint model (used also in the main SLS II analysis [5]) adjusts for non-ignorable missing data due to treatment failure, death, and drop-outs [36]. The outcome for the primary outcome model was the course of FVC %-predicted measured in 3-month increments from 3 to 12 months. The longitudinal model of the joint analysis included the following covariates: baseline KL-6 or CCL-18, baseline FVC %-predicted, and a linear time trend. The outcome for the secondary outcome model was the course of DLCO %-predicted measured in 3-month increments from 3 to 12 months. The longitudinal model of the joint analysis included the following covariates: baseline KL-6 or CCL-18, baseline DLCO %-predicted, and a linear time trend. KL-6 and CCL-18 were log-transformed (with a base of 2) in these analyses to correct data skewness. We generated models for examining baseline KL-6 and CCL-18 as a continuous variable and also as a dichotomous variable (using the median as the cut point). The median was selected since there are no valid thresholds for defining high versus low KL-6 and CCL-18. In an exploratory analysis, we generated receiver operator curves (ROC) and logistic regression analysis to determine whether we could identify a threshold for KL-6 and CCL-18 that predicted disease progression. Since there is no universally accepted definition of disease progression in SSc-ILD we used the following two definitions: (1) FVC decline >= −5%; and (2) FVC decline >= −10% OR FVC decline between −5 and −9% accompanied by a DLCO decline >= −15%. The time course of 3–12 months was selected as this was the time period in which patients in both study arms (CYC and MMF) were receiving active treatment.
Relationship between baseline KL-6 and CCL-18 with long-term survival in SSc-ILD
Cox regression was used to assess the association between baseline KL-6/CCL-18 and long- term survival in SLS II. The model included baseline KL-6/CCL-18 (log-transformed) and baseline FVC %-predicted as covariates. The methods for obtaining long-term survival data in SLS II are described in detail in our recent publication [37].
All tests were 2-sided. The joint analyses were performed using the R package JMbayes, and all other analyses were conducted in SAS v9.4 (The SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
Baseline characteristics of SLS II participants who underwent KL-6 and CCL-18 analysis appear in Table 1. Among the 142 SLS II participants, 133 and 99 participants had KL-6 and CCL-18 measurements at baseline and 12 months, respectively. Compared with the SLS II cohort, unaffected controls (N=39) were similar in age (Mean 52.2 ± 9.5 years), sex (71.8% female), race (69.2% White, 23.1% Black, 7.7% Asian), and ethnicity (12.8% Hispanic/Latino).
Table 1.
Baseline Characteristics of SLS II participants by study group and unaffected controls.
| SLS II | Controls | ||
|---|---|---|---|
| Measure | CYC (N=71) | MMF (N=62) | (N=39) |
| Age**, years | 52.3 ± 9.5 | 52.9 ± 10.0 | 52.2 ± 9.5 |
| Female | 55 (77.5%) | 44 (71.0%) | 28 (71.8%) |
| Race*** | |||
| White | 47 (66.2%) | 46 (74.2%) | 27 (69.2%) |
| African American | 18 (25.4%) | 10 (16.1%) | 9 (23.1%) |
| Asian | 3 (4.2%) | 6 (9.7%) | 3 (7.7%) |
| Other | 3 (4.2%) | 0 (0%) | 0 (0%) |
| Diffuse cutaneous sclerosis | 39 (54.9%) | 38 (61.3%) | |
| Disease duration*, years | 2.5 ± 1.8 | 2.7 ± 1.7 | |
| FVC, % predicted | 66.2 ± 9.9 | 66.5 ± 8.3 | |
| FEV1/FVC, % | 83.5 ± 5.6 | 82.0 ± 5.7 | |
| TLC, % predicted | 65.4 ± 12.1 | 66.4 ± 10.2 | |
| DLCO***, % predicted | 53.8 ± 14.2 | 54.9 ± 11.3 | |
| BDI*** (focal score; 0–12)† | 7.0 ± 2.3 | 7.3 ± 2.2 | |
| HAQ-DI (score, 1–3)‡ | 0.7 ± 0.7 | 0.7 ± 0.6 | |
| Modified Rodnan Skin Score (MRSS) (0–51) | 14.1 ± 10.8 | 15.2 ± 10.3 | |
| Lung fibrosis (QLF) score, whole lung (WL)*, % | 9.1 ± 7.0 | 8.4 ± 7.1 | |
| Lung fibrosis (QLF) score, worst zone (ZM)*, % | 23.2 ± 19.2 | 22.8 ± 20.4 | |
| Quantitative ILD (QILD) score***, % WL | 32.1 ± 14.2 | 27.7 ± 13.8 | |
| Quantitative ILD (QILD) score***, % ZM | 53.2 ± 19.3 | 49.7 ± 21.2 | |
Data reported are mean ± SD or N (%)
p < 0.05;
p < 0.01;
p < 0.001
High score denotes worse dyspnea
High score denotes worse function
Definition of abbreviations: FVC = forced vital capacity; FEV1 = forced expired volume in 1 sec; TLC = total lung capacity; DLCO = single-breath diffusing capacity for carbon monoxide; % BDI = baseline dyspnea index; HAQ-DI = health assessment questionnaire for scleroderma-Disability Index; MRSS = Modified Rodnan Skin Score; QLF-WL, % = quantitative extent of lung fibrosis (reticulations) in whole lung on high-resolution computed tomography (HRCT); QLF-ZM, % = quantitative extent of lung fibrosis in the zone of maximal involvement on HRCT; QILD-WL, % = quantitative extent of interstitial lung disease (fibrosis + GGO + honeycombing) in whole lung on HRCT; QILD-ZM = quantitative extent of interstitial lung disease in the zone of maximal involvement on HRCT
KL-6 Levels are Associated with Disease Severity
KL-6 levels were significantly higher in SSc patients (N=133) compared with unaffected controls (N=39) (1752.05 [1274.67] versus 330.70 [125.74] u/ml, P<0.0001). KL-6 levels correlated with SSc disease severity at baseline (Table 2). Specifically, increased KL-6 levels were associated with decreased DLCO, decreased TLC, and increased radiographic extent of lung fibrosis as measured by the QILD-WL, QILD-ZM, QLF-WL, and QLF-ZM.
Table 2.
Baseline correlations between KL-6 and CCL-18 and SSc disease activity measures
| Disease measure | KL-6-SLS II (N = 133) | CCL-18-SLS II (N = 133) |
|---|---|---|
| FVC%-predicted | −0.01 | 0.11 |
| DLCO%-predicted | −0.23*** | −0.04 |
| TLC%-predicted | −0.21*** | 0.01 |
| QILD-WL | 0.35*** | 0.14* |
| QILD-ZM | 0.35*** | 0.18** |
| QLF-WL | 0.36*** | 0.08 |
| QLF-ZM | 0.33*** | 0.10 |
p < 0.05;
p < 0.01;
p < 0.001
CCL-18 Levels are Associated with Disease Severity
CCL-18 levels were significantly higher in SSc patients (N=133) compared with healthy controls (N=39) (191.29 [111.08] versus 87.71 [28.28] ng/ml, P=0.0009). In addition, increased CCL-18 levels were associated with increased radiographic extent of lung fibrosis as measured by the QILD-WL and QLF-ZM (Table 2).
Relationship between KL-6 and CCL-18
CCL-18 levels correlated with KL-6 levels at baseline (r=0.18, P=0.036) and at 12 months (r=0.15, P=0.032). The change in CCL-18 levels from baseline to 12 months was not correlated with the change in KL-6 levels from baseline to 12 months in all participants (r=0.063, P=0.34), or in participants randomized to CYC (r=0.094, P=0.34) and to MMF (r=0.0068; P=0.95).
KL-6 and CCL-18 Levels Decrease after One Year of Immunosuppression
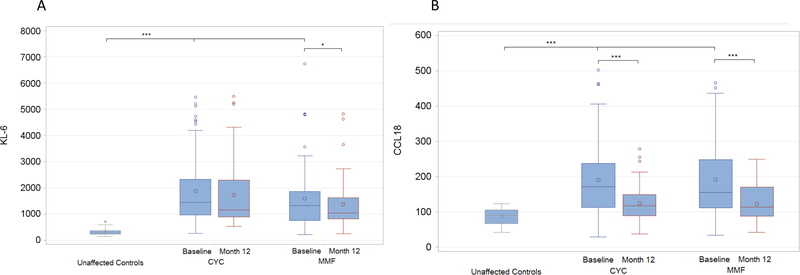
Among SLS II participants with baseline and 12-month KL-6 and CCL-18 measurements (N=99), treatment with CYC or MMF for one year led to significant reductions in these peripheral pneumoprotein levels (Figure 1). The average decline in KL-6 levels was 100.60 u/ml (N=99; p=0.045), while the average decline in CCL-18 levels was 61.24 ng/ml (N=98; P<0.0001). Among patients assigned to MMF, both KL-6 levels (N=49; P=0.016) and CCL-18 levels (N=51; P<0.0001) decreased significantly over 1 year (Supplementary Tables S1 and S2). Among patients assigned to CYC, CCL-18 levels (N=49; P=0.0008) decreased significantly over 1 year, although KL-6 levels did not (Supplementary Table S1 and S2). The average decline in KL-6 levels among patients assigned to CYC and MMF was 55.72 (819.44) and 146.40 (458.69) u/ml, respectively (Supplementary Table S2). The average decline in CCL-18 levels among patients assigned to CYC and MMF was 46.94 (87.10) and 75.55 (105.75) ng/ml, respectively (Supplementary Table S1).
Figure 1. Change in KL-6 (A) and CCL-18 (B) from baseline to 12 months in SLS II.
The units for KL-6 are u/ml. The units for CCL-18 are ng/ml.
* p< 0.05, ** p< 0.01, *** p< 0.001.
Baseline KL-6 Levels Predict SSc-ILD Progression
The predictive significance of KL-6 and CCL-18 was analyzed in each treatment arm separately. Among SLS II participants, higher baseline KL-6 levels predicted progression of ILD as measured by the course of the FVC%-predicted (CYC/MMF: Estimate −0.32/−0.72; P=0.024/0.005) and DLCO%-predicted (CYC/MMF: Estimate −1.30/−1.28; P<0.001/0.004) over 1 year in the MMF, as well as the CYC arms, even after adjusting for baseline disease severity (Table 3).
Table 3.
High baseline KL-6 predicts progression of ILD based on the course of the FVC and DLCO over 1 year in patients randomized to CYC and MMF.
| Variable | Estimate | 95% CI | P-Value | |
|---|---|---|---|---|
| Outcome: Course of FVC over 12 months in CYC arm | ||||
| Intercept | 9.99 | 7.02 | 12.26 | 0.001 |
| KL-6 | −0.32 | −0.50 | −0.11 | 0.024 |
| Baseline FVC | 0.88 | 0.87 | 0.90 | <0.001 |
| Time | 0.10 | 0.028 | 0.17 | 0.004 |
| Outcome: Course of FVC over 12 months in MMF arm | ||||
| Intercept | 16.92 | 12.12 | 21.99 | <0.001 |
| KL-6 | −0.72 | −1.03 | −0.32 | 0.005 |
| Baseline FVC | 0.85 | 0.80 | 0.89 | <0.001 |
| Time | 0.054 | −0.015 | 0.12 | 0.128 |
| Outcome: Course of DLCO over 12 months in CYC arm | ||||
| Intercept | 18.11 | 14.63 | 20.60 | <0.001 |
| KL-6 | −1.30 | −1.51 | −1.00 | <0.001 |
| Baseline DLCO | 0.85 | 0.84 | 0.87 | <0.001 |
| Time | −0.024 | −0.12 | 0.076 | 0.634 |
| Outcome: Course of DLCO over 12 months in MMF arm | ||||
| Intercept | 23.80 | 17.42 | 26.21 | 0.001 |
| KL-6 | −1.28 | −1.46 | −0.84 | 0.004 |
| Baseline DLCO | 0.80 | 0.78 | 0.84 | <0.001 |
| Time | 0.030 | −0.051 | 0.11 | 0.428 |
After dichotomizing the KL-6 variable based on the median level in baseline SLS II samples (1448.2 u/ml), a high baseline KL-6 level was associated with increased progression of ILD as measured by the course of the FVC in the MMF arm (Estimate −1.19; P=0.018), but not in the CYC arm (Estimate −0.19; P=0.44) (Supplementary Table S3). High baseline KL-6 level was associated with increased progression of ILD as measured by the course of the DLCO in the MMF arm (Estimate −0.46; P=0.030), but not in the CYC arm (Estimate −0.034; P=0.720) (Supplementary Table S4).
The results of the ROC analysis demonstrated that a KL-6 level greater than 1549 u/ml in the MMF arm was associated with an increased risk of progression using both definitions of ILD worsening. The sensitivity and specificity was 100% and 74%, respectively, when we used the definition of FVC decline >= −5%. The sensitivity and specificity was 100% and 71%, respectively, when we used the definition of FVC decline >= −10% OR FVC decline between −5 and −9% accompanied by a DLCO decline >= −15%). We were unable to identify a threshold for KL-6 with an adequate sensitivity and specificity in the CYC arm. Please see Supplementary Figure S1.
Baseline CCL-18 Levels Predict SSc-ILD Progression
Higher baseline CCL-18 levels predicted progression of ILD as measured by the course of the FVC (CYC/MMF: Estimate −1.24/−0.35; P<0.001;0.007) and DLCO (CYC/MMF: Estimate −1.87/−1.26; P=0.001/<0.001) over 1 year for both treatment arms, even after adjusting for baseline disease severity (Table 4).
Table 4.
High baseline CCL-18 predicts progression of ILD based on the course of the FVC and DLCO over 1 year in patients randomized to CYC and MMF.
| Variable | Estimate | 95% CI | P-Value | |
|---|---|---|---|---|
| Outcome: Course of FVC over 12 months in CYC arm | ||||
| Intercept | 13.80 | 11.92 | 15.65 | <0.001 |
| CCL-18 | −1.24 | −1.46 | −1.03 | <0.001 |
| Baseline FVC | 0.91 | 0.90 | 0.93 | <0.001 |
| Time | 0.10 | 0.025 | 0.17 | 0.012 |
| Outcome: Course of FVC over 12 months in MMF arm | ||||
| Intercept | 10.21 | 8.34 | 11.89 | <0.001 |
| CCL-18 | −0.35 | −0.52 | −0.16 | 0.007 |
| Baseline FVC | 0.88 | 0.86 | 0.90 | <0.001 |
| Time | 0.057 | −0.014 | 0.13 | 0.114 |
| Outcome: Course of DLCO over 12 months in CYC arm | ||||
| Intercept | 16.91 | 12.16 | 19.61 | <0.001 |
| CCL-18 | −1.87 | −2.17 | −1.19 | 0.001 |
| Baseline DLCO | 0.87 | 0.84 | 0.89 | <0.001 |
| Time | −0.020 | −0.11 | 0.065 | 0.642 |
| Outcome: Course of DLCO over 12 months in MMF arm | ||||
| Intercept | 17.36 | 14.39 | 19.45 | <0.001 |
| CCL-18 | −1.25 | −1.49 | −0.92 | <0.001 |
| Baseline DLCO | 0.85 | 0.82 | 0.87 | <0.001 |
| Time | 0.040 | −0.040 | 0.12 | 0.327 |
After dichotomizing the CCL-18 variable based on the median level in baseline SLS II samples (163.1 ng/ml), a high baseline CCL-18 level was associated with increased progression of ILD as measured by the course of the FVC both in the MMF arm (Estimate −0.61; P=0.039) and in the CYC arm (Estimate −0.01; P=0.010) (Supplementary Table S3). High baseline CCL-18 level was associated with increased progression of ILD as measured by the course of the DLCO both in the MMF arm (Estimate −0.94; P<0.001) and in the CYC arm (Estimate −2.13; P<0.001) (Supplementary Table S4).
The ROC analysis failed to reveal a significant CCL-18 threshold for predicting ILD progression in either treatment with an adequate sensitivity and specificity (Supplementary Figure S2).
Baseline CCL-18, but Not KL-6, Predicts Long-term Survival in SSc-ILD
Data from the SLS II long-term follow up study [37] were used to explore whether baseline KL-6 or CCL-18 predicted long-term survival in patients with SSc-ILD. At the time of this analysis, 30 of 142 (21%) SLS II participants had died within 8 years after the first patient was randomized (CYC: 16; MMF: 14). The median follow-up time for all patients was 4 years. The majority of deaths in both cohorts were due to respiratory failure from underlying SSc (N=16) [37].
The Cox-proportional hazards model analysis demonstrated that SLS II participants with increased CCL-18 at baseline had an increased risk of mortality due to respiratory failure even after controlling for baseline disease severity in the CYC arm (HR: 3.09; P=0.018; Supplementary Table S5), but not in the MMF arm (Supplementary Table S6). Baseline KL-6 level was not associated with mortality due to respiratory failure in either treatment arm (Supplementary Tables S5, S6).
Similarly, baseline CCL-18 level was associated with mortality due to all causes (HR: 3.31; P=0.0008) in the CYC arm, but not the MMF arm. Patients with high CCL-18 based on the median had an increased risk of mortality in the CYC arm (P=0.006 by log-rank test; Supplementary Figure S3), but not in the MMF arm (Supplementary Figure S4). Baseline KL-6 level was not associated with all-cause mortality in either treatment arm (Supplementary Tables S7, S8). High KL-6 based on the median was not associated with an increased risk of mortality in the CYC arm or in the MMF arm (Supplementary Figures S5, S6).
DISCUSSION
To our knowledge, this is the first study to evaluate the relationship between plasma levels of KL-6 and CCL-18 with progression of ILD in the context of a relatively large RCT for SSc-ILD. Elevated levels of both KL-6 and CCL-18 at baseline predicted poor response to immunosuppressive therapy with either CYC or MMF.
At baseline, both KL-6 and CCL-18 levels each correlated with surrogate measures of ILD severity, including extent of radiographic fibrosis (KL-6, CCL-18), and % predicted TLC (KL-6) and DLCO (KL-6). These findings are consistent with the findings of our previous publication of patients who participated in SLS I (CYC vs. placebo), in which baseline KL-6 levels correlated with the extent of radiographic fibrosis and with the DLCO [23]. In contrast, neither KL-6 (SLS I and II) nor CCL-18 (SLS II) was associated with the baseline FVC % predicted. While the severity of SSc-ILD is often defined by the degree of ventilatory restriction (i.e. FVC % predicted), all pulmonary function test parameters are indirect and often variable measures of the extent of structural lung disease. This may explain why these peripheral pneumoproteins correlate more strongly with the extent of radiographic fibrosis as measured by quantitative computer-aided diagnostic techniques.
KL-6 and CCL-18 levels decreased in response to treatment with CYC and MMF for one year, although the magnitude of the decline was greater for CCL-18 than for KL-6. This may be due to the fact that CCL-18 is secreted by type 2 macrophages, whereas KL-6 is excreted by type II pneumocytes. Macrophages as inflammatory cells would be more likely to decrease their activity in response to immunosuppressive treatment than type II pneumocytes, which are epithelial in origin. For both pneumoproteins, patients assigned to MMF experienced the greatest decline in CCL-18 and KL-6 levels. This discrepancy could have been due to several factors. As reported previously [5], MMF was better tolerated than CYC in SLS II; thus, patients may have had better adherence to therapy with MMF than CYC and were more likely to achieve and maintain the target treatment dosage. Another possibility is that MMF targets pathways involving KL-6 and CCL-18 with greater potency than CYC. In SLS II, no difference was noted in the course of the FVC over two years between patients assigned to MMF versus CYC; however, there was a difference in the course of the DLCO, favoring MMF [5]. More research is needed to further explore why KL-6 and CCL-18 levels declined to a greater degree in response to MMF than CYC treatment.
Even after adjusting for baseline disease severity, higher levels of KL-6 and CCL-18 predicted progression (worsening) of ILD in each of the two SLS II treatment arms. We opted to examine treatment arms separately since MMF and CYC have markedly different mechanisms of action; however, even in the combined cohort, both baseline KL-6 and CCL-18 predicted progression of ILD, as measured by the course of the DLCO and FVC over one year (results available upon request).
The finding that high baseline KL-6 and CCL-18 predicted progression of ILD even after adjusting for baseline disease severity suggests that these two pneumoproteins could be used to identify patients with a more aggressive ILD phenotype. Despite treatment with MMF, patients with high baseline KL-6 and CCL-18 levels experienced a decline in their FVC and DLCO over 12 months. Among patients assigned to CYC, those who had high baseline CCL-18 (but not KL-6) levels also experienced a decline in their FVC and DLCO over 12 months, as well as increased risk of long-term mortality. In addition to helping to identify patients who may benefit from closer monitoring, KL-6 and CCL-18 measurements could also be used to select patients for combination ILD therapy (two immunosuppressants or an immunosuppressant plus and anti-fibrotic) or for cohort enrichment to identify patients who may be eligible for clinical trials investigating other novel therapies for progressive SSc-ILD.
We attempted to identify a threshold for KL-6 and CCL-18 for predicting worsening of ILD. We discovered that a KL-6 level greater than 1549 u/ml in the MMF arm was associated with an increased risk of progression using both definitions of ILD worsening with an excellent sensitivity and good specificity. However, we were unable to identify a threshold with adequate sensitivity and specificity in the CYC arm for KL-6, or for either treatment arm for CCL-18. This may have been due to loss of power due to dichotomization of the outcome. Moreover, while we used two different definitions of ILD progression, there is currently no consensus on a universally accepted definition of ILD progression in SSc.
This study has some limitations. We did not include an external validation cohort. We had planned to use the SLS I cohort as an external validation cohort, but the sample size of participants who underwent KL-6 measurement in SLS I and had complete follow up data was too small (N=40) to perform the joint model analysis. However, the baseline correlations between KL-6 and surrogate measures of ILD severity were similar between both SLS cohorts, suggesting that our findings are likely reproducible. Moreover, we demonstrated predictive potential of both KL-6 and CCL-18 in both treatment arms of SLS II, with each arm analyzed separately and one arm being CYC, as a means of semi-internal validation.
This study has important strengths. First, we evaluated ILD progression by using a joint model that included repeated measures of the FVC and DLCO. Trends in the FVC and DLCO determined from measurements at several time points may more accurately reflect true progression of ILD compared with changes in the FVC and DLCO using measurements at only two time points. Indeed, our recent analysis of the long-term follow up data from SLS I and II revealed that the course of the FVC and DLCO were better predictors of long-term mortality than the baseline FVC or DLCO [37].
Using data from a rigorously-conducted clinical trial to study candidate biomarkers also limits potential confounding from variables, such as access to care and therapy and missing outcome data, that often occurs in the setting of observational studies in which patients receive varying medication regimens at baseline and subsequent visits (type, dose, duration) and varying follow up. Furthermore, in an exploratory analysis, we also found that high CCL-18 levels at baseline were associated with an increased risk of long-term mortality due to respiratory failure. These findings substantiate previously published work linking CCL-18 with progressive ILD and poor outcomes [16, 17, 31].
In summary, the present findings strongly suggest that KL-6 and CCL-18 are important peripheral markers of both disease severity and disease progression in patients with SSc-ILD. Measurement of these two pneumoproteins early in the course of SSc-ILD may help to identify those patients with a more aggressive SSc-ILD phenotype in both clinical practice and in research. Additional mechanistic studies are needed to determine precisely how KL-6 and CCL-18 contribute to the pathobiology of SSc-ILD. These additional studies may also reveal new therapeutic targets for intervention in SSc-ILD since currently available treatment options for this often fatal condition are still limited.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients, investigators and coordinators who participated in the Scleroderma Lung Study II. This study was funded by grants from the NHLBI/NIH (R01 HL089758 [DPT] and R01 HL089901 [RME], Rheumatology Research Foundation (Scientist Development Award [ERV]), Scleroderma Foundation (Young Investigator Award [ERV]), DoD (W81XWH-16-1-0296 [SA]). The study drug (mycophenolate) and matching placebo were supplied at no charge through Drug Supply Grant # CEL539 from Hoffmann-La Roche/Genentech.
Footnotes
Conflicts of Interest:
The authors report no financial conflicts of interest.
REFERENCES
- 1.Wells AU. Interstitial lung disease in systemic sclerosis. Press Med 2014;43:e329–e343. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 3.Steen VD, Conte C, Owens GR, Medsger TA Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283–9. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655–66. [DOI] [PubMed] [Google Scholar]
- 5.Tashkin DP, Roth MD, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease: Scleroderma lung study II (SLS-II), a double-blind, parallel group, randomised controlled trial. Lancet Resp Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkmann ER, Chung A, Tashkin DP. Managing systemic sclerosis-related interstitial lung disease in the modern treatment era. J Scleroderma Relat Disord 2017;2:72–83. [Google Scholar]
- 7.Roth MD, Tseng CH, Clements PJ, Furst DE, Tashkin DP, Goldin JG, et al. Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease. Arthritis Rheum 2011;63(9):2797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkmann ER, Tashkin DP, Sim M, Kim G-H, Goldin J, Clements PJ. Determining progression of scleroderma-related interstitial lung disease. JSRD 2018;I–9. [DOI] [PMC free article] [PubMed]
- 9.Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 10.Khanna D, Tseng CH, Farmani N, Steen V, Furst DE, Clements PJ, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum 2011;63(10):3078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore OA, Goh N, Corte T, Rouse H, Hennessey Ok Thakkar V, et al. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatology (Oxford) 2013;52(1):155–60. [DOI] [PubMed] [Google Scholar]
- 12.Assassi S, Sharif R, Lasky RE, McNearney TA, Estrada-Y-Martin RM, Draiger H, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010;12:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol 2013;40:435–46. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Mayes MD, Pedroza C, Draeger HT, Gonzalez EB, Harper BE, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res 2013;65:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M, Pedroza C, Salazar G, Zhou Z, Reveille JD, Mayes MD, et al. Plasma MCP-1 and IL-10 levels predict long-term progression of interstitial lung disease in patients with early systemic sclerosis [Abstract]. Arthritis Rheum 2013;65(Suppl 10):1747.23606107 [Google Scholar]
- 16.Schupp J, Becker M, Günther J, Muller-Quernheim J, Riemekasten G, Prasse A. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur Respir J 2014;43:1530–2. [DOI] [PubMed] [Google Scholar]
- 17.Tiev KP, Hua-Huy T, Kettaneh A, Gain M, Duong-Quy S, Toledano C, et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J 2011;38:1355–60. [DOI] [PubMed] [Google Scholar]
- 18.van Bon L, Affandi AJ, Broen J, Christmann RBm Marijnissen RJ, Stawski L, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med 2014;370:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwana M, Shirai Y, Takeuchi T. Elevated serum Krebs von den Lungen-6 in early disease predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. J Rheumatol 2016;43:1825–31. [DOI] [PubMed] [Google Scholar]
- 20.Salazar GA, Kuwana M, Wu M, Estrada-Y-Martin RM, Ying J, Charles J, et al. KL-6 but not CCL-18 is a predictor of early progression of systemic sclerosis-related interstitial lung disease. J Rheumatol 2018;45:1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nukiwa T The role of biomarkers in management of interstitial lung disease: implications of biomarkers derived from type II pneumocytes. European Respiratory Monograph 2009;46:47–66. [Google Scholar]
- 22.Byrne AJ, Maher TM, Lloyd CM. Pulmonary macrophages: A new therapeutic pathway of fibrosing lung disease? Trends Mol Med 2016;22:303–16. [DOI] [PubMed] [Google Scholar]
- 23.Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, et al. ; Scleroderma Lung Study Research Group. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol 2009;36:773–80. [DOI] [PubMed] [Google Scholar]
- 24.Kumanovics G, Gorbe E, Minier T, Simon D, Berki T, Czirjak L. Follow-up of serum KL-6 lung fibrosis biomarker levels in 173 patients with systemic sclerosis. Clin Exp Rheaumatol 2014;32:S-138-44. [PubMed] [Google Scholar]
- 25.Hesselstrand R, Wildt M, Bozovic G, Andersson-Sjoland A, Andreasson K, Scheja A, et al. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease. Respir Med 2013;107:1079–86. [DOI] [PubMed] [Google Scholar]
- 26.Benyamine A, Heim X, Resseguier N, Bertin D, Gomez C, Ebbo M, et al. Elevated serum Krebs von den Lungen-6 in systemic sclerosis: a marker of lung fibrosis and severity of disease. Rheumatol Int 2018;38:813–9. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Lee EY, Ha YJ, Kang EH, Lee YJ, Song YW. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 2019;21:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elhai M, Hoffmann-Vold AM, Avouac J, Pezet S, Cauvet A, Leblond A, et al. Performance of candidate serum biomarkers for systemic sclerosis-interstitial lung disease. Arthritis Rheumatol 2019. [Epub ahead of print]. [DOI] [PubMed]
- 29.Sumida H, Asano Y, Tamaki Z, Aozasa N, Taniguchi T, Toyama T, et al. Prediction of therapeutic response before and during IV cyclophosphamide pulse therapy for interstitial lung disease in systemic sclerosis: A longitudinal observational study. J Dermatol 2018;45:1425–33. [DOI] [PubMed] [Google Scholar]
- 30.Cai M, Bonella F, He X, Sixt SU, Sarria R, Guzman J, et al. CCL18 in serum, BAL fluid and alveolar macrophage culture supernatant in interstitial lung disease. Respir Med 2013;107:1444–52. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann-Vold AM, Tennoe AH, Garen T, Midtvedt O, Abraityte A, Aalokken TM, et al. High level of chemokine CCL18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest 2016;150:299–306. [DOI] [PubMed] [Google Scholar]
- 32.Elhaj M, Charles J, Pedroza C, Liu X, Zhou Z, Estrada-Y-Martin RM, et al. Can serum surfactant protein D or CC-chemokine ligand 18 predict outcome of interstitial lung disease in patients with early systemic sclerosis. J Rheumatol 2013;4:1114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masi T and Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheumatol 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 34.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest 1984;85:751–758. [DOI] [PubMed] [Google Scholar]
- 35.Kim HG, Tashkin DP, Clements PJ, Li G, Brown MS, Elashoff R, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol 2010;28:S26–35. [PMC free article] [PubMed] [Google Scholar]
- 36.Elashoff R, Li G, and Li N. Joint modeling of longitudinal and time-to-event data CRC Press; 2016. [Google Scholar]
- 37.Volkmann ER, Tashkin DP, Sim M, Li N, Goldmuntz E, Keyes-Elstein L, et al. Early progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Annals of Rheumatic Diseases 2019;78:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.