Abstract
The advent in the last several years of effective immunotherapy for cancer has renewed interest in the role of the immune system in controlling cancer. The idea that the immune system can help control cancer has a long history. Solid organ transplant recipients (SOTRs) as well as human immunodeficiency virus (HIV)-infected people are affected by cell-mediated immune dysfunction. Epidemiologic studies of these populations reveal a pattern characterized by strongly increased incidence for virus-related cancers (e.g., Kaposi sarcoma, non-Hodgkin lymphoma, anogenital cancers). In addition, recent epidemiologic studies have evaluated cancer-specific mortality among SOTRs and HIV-infected people following a cancer diagnosis. For a wider range of cancers—not limited to those caused by viruses, and including melanoma and cancers of the colorectum, lung, and breast—these immunosuppressed cancer patients have higher cancer-specific mortality than other cancer patients. This latter group of cancers somewhat mirrors those for which immunotherapy with checkpoint inhibitors is approved. These epidemiologic observations suggest that there are two distinct immune selection processes in humans: immunosurveillance directed against premalignant cells before cancer diagnosis (most relevant for preventing virus-related cancers), and “immunocontainment” directed against established cancers. These processes thus appear relevant for different groups of malignancies and may have different mechanisms.
The advent in the last several years of effective immunotherapy for cancer has renewed interest in the role of the immune system in controlling cancer (1–3). The field of cancer immunotherapy has long drawn support from the observation that immunosuppressed individuals, including solid organ transplant recipients (SOTRs), have an elevated risk of developing cancer (2). In this Personal Viewpoint article, I review the epidemiology of cancer in SOTRs and another immunosuppressed population—individuals infected with human immunodeficiency virus (HIV)—and discuss how recent results in this area provide important insights into the role of immunity in affecting the development of cancer and outcomes following a cancer diagnosis.
Immune surveillance hypothesis
The idea that a healthy immune system can prevent development of cancer was first clearly articulated by Burnet in 1957 (4). This “immune surveillance” hypothesis has at times been controversial, but the ability of developing malignancies to avoid immune destruction is now considered an “emerging” hallmark of cancer (5).
Cancers develop over years or decades, as normal cells acquire multiple genetic changes and become progressively abnormal (5, 6). DNA mutations occur spontaneously or are caused by carcinogens (e.g., tobacco smoke, ultraviolet radiation [UVR]) (6) or, for certain cancers, genetic material is inserted by oncogenic viruses. These changes can lead to expression of novel proteins, creating “neoantigens” visible to the immune system.
The immune surveillance hypothesis, which refers specifically to the development of cancer, posits targeting of premalignant or malignant cells due to their neoantigen expression. Components of the cell-mediated immune system, including CD4-positive T-cells and cytotoxic CD8-positive T-cells and NK-cells, are relevant for this process (1, 7, 8). Based on animal experiments (7–10), Dunn et al. proposed three stages of immunosurveillance in limiting the development of cancer: elimination, equilibrium, and escape (7). Elimination corresponds most directly to the original immunosurveillance concept, in which the immune system recognizes and destroys abnormal premalignant cells. In equilibrium, a dynamic balance is established between remaining premalignant cells and the immune system. In the third stage, some cells escape immune control and proliferate to manifest as a clinically detectable tumor.
Immunosurveillance refers to the ability of the immune system to prevent cancer or eliminate it prior to formation of a clinical tumor. Immunity also contributes to control of cancer after diagnosis—most obviously demonstrated by the effectiveness of immunotherapy (reviewed below). Although mechanisms may be similar to those involved in immunosurveillance, the process involves suppression or elimination of malignant cells after cancer diagnosis. Thus, it is helpful to utilize a different term, “immunocontainment,” which will be used in this article to describe the role of the immune system after cancer diagnosis.
Characteristics of cancers arising in immunocompetent individuals
It is difficult to directly observe immunosurveillance in humans, because when it is successful, cancer does not develop. The frequent presence within solid cancers of tumor infiltrating lymphocytes (TILs), which target tumor neoantigens (7, 11–13), provides evidence that many cancers evolve in the face of immunosurveillance. This evidence is somewhat complex to interpret and suggests a dynamic process, because the presence of the cancer implies that immunosurveillance was incompletely effective. The density of infiltrating CD4-positive and CD8-positive T-cells is prognostic for many cancers including melanoma, colorectal cancer, non-small cell lung cancer (NSCLC), and urothelial carcinoma (11), indicating that these T-cells also play a role in immunocontainment after cancer diagnosis.
One can identify indirect effects of immunosurveillance, because premalignant cells must evolve to escape immune selection. These effects, termed immune “editing” or “sculpting,” are reflected in molecular features of a tumor once cancer is clinically diagnosed. For example, cancers often manifest alterations in antigen processing pathways, including decreased expression of HLA and related proteins (3, 14), which presumably decrease neoantigen presentation. HLA genes are frequently mutated in cervical cancer, head and neck cancer, NSCLC, and colorectal cancer (15, 16).
Moreover, tumor cells and TILs frequently express immune checkpoint proteins (17, 18). In healthy individuals, checkpoint pathways modulate cell-mediated immunity to prevent tissue damage, but tumors often exploit checkpoint signaling to evade immunological clearance. Checkpoint proteins expressed on tumor or inflammatory cells include programmed death-ligands 1 and 2 (PD-L1 and PD-L2), which interact with programmed cell death protein 1 (PD-1) on T-cells; and CD80 and CD86, which interact with cytotoxic T lymphocyte antigen 4 (CTLA4) (18). Some tumors exhibit infiltration of regulatory T-cells, myeloid-derived suppressor cells, or anti-inflammatory macrophages that may also dampen immunosurveillance or immunocontainment (11, 19).
Cancer incidence among SOTRs and HIV-infected people
As Burnet predicted in 1971 (4), “If the concept of immunological surveillance is legitimate…conditions associated with depression of the [cell-mediated] immune system whether genetic, induced by drugs, or of other origin should increase the likelihood of cancer.” Since he wrote those words, solid organ transplantation emerged as an effective treatment for end-stage organ disease but which is associated with substantial deficits in cell-mediated immune function because of the need to utilize long-term immunosuppression to prevent graft rejection (20, 21).
The first epidemiologic study of cancer risk in SOTRs was conducted in the early years of transplantation (22). Numerous studies have subsequently been conducted (23–25). Results for various cancers are summarized in Supplemental Table 1 as standardized incidence ratios (SIRs), which are relative risks comparing cancer incidence in SOTRs to that expected in the general population. These SIRs come from a meta-analysis of large epidemiologic studies (23), except when unavailable, in which case results from other studies are presented.
Notably, most malignancies for which incidence is substantially elevated in SOTRs are caused by viruses (SIRs > 5, Supplemental Table 1) (23). Incidence is especially strongly elevated for Kaposi sarcoma (KS) (caused by KS-associated herpesvirus [KSHV]) (23) and non-Hodgkin lymphoma (NHL), especially for subtypes caused by Epstein-Barr virus (EBV): diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma, and central nervous system lymphoma (26, 27). Other virus-related cancers are also increased, including anogenital cancers (caused by human papillomavirus [HPV]); liver cancer (hepatitis B and C viruses); Hodgkin lymphoma (EBV); and Merkel cell carcinoma (MCC, a rare skin cancer caused in many cases by Merkel cell polyomavirus). Cell-mediated immune function plays an important role in controlling or clearing chronic infection with these viruses (28, 29).
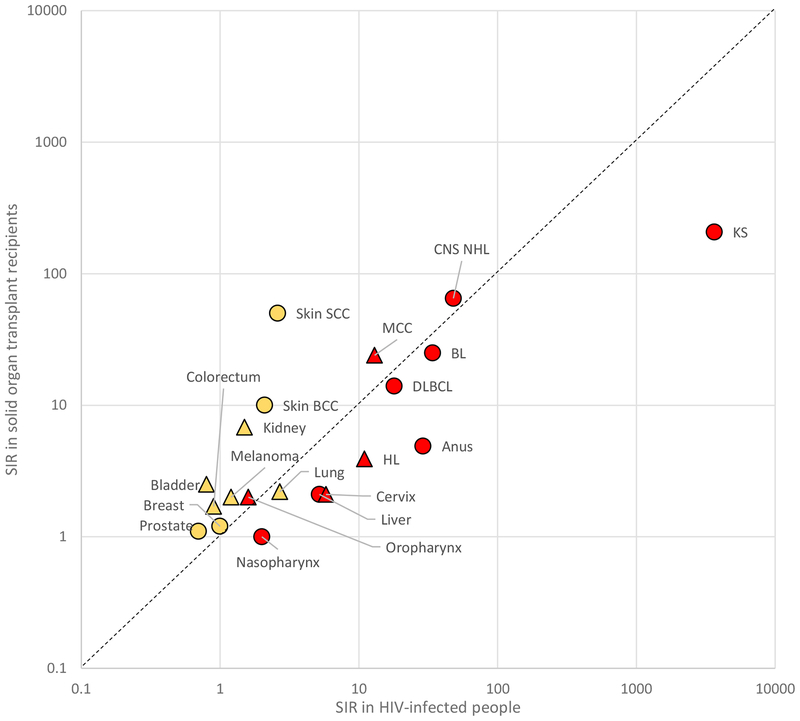
HIV infection, when untreated, typically results in progressive immunosuppression due to loss of CD4-positive T-cells (20). Both HIV-infected people and SOTRs thus suffer primarily from defective cell-mediated immune function, although both groups also manifest secondary immune abnormalities, including polyclonal B-cell activation, defective B-cell function, and chronic inflammation (30–32). Since 1996, combination antiretroviral therapy (ART) has increasingly allowed HIV suppression and immune reconstitution (33). The importance of immunosurveillance for virus-related cancers is demonstrated by somewhat parallel elevations in the incidence of these cancers in SOTR and HIV populations (Supplemental Table 1, Figure 1) (23). In addition, incidence for some cancers in HIV-infected people increases as the CD4 count declines or after onset of acquired immunodeficiency syndrome (AIDS), and these cancers declined over calendar time with increasing ART use (34).
Figure 1.
Standardized incidence ratios (SIRs) for cancer in HIV-infected people and solid organ transplant recipients. SIRs are from sources indicated in the Supplemental Table 1 footnotes. Results for virus-related cancers are shown in red and for virus-unrelated cancers in yellow. Results for cancers for which checkpoint inhibitor therapy has been approved by the US Food and Drug Administration (see Supplemental Table 3) are shown as triangles and for other cancers as circles. The dashed line indicates shows equality in SIRs for the HIV and transplant population. Results are depicted on a logarithmic scale. Abbreviations: BCC basal cell carcinoma, BL Burkitt lymphoma, DLBCL diffuse large B-cell lymphoma, HL Hodgkin lymphoma, KS Kaposi sarcoma, MCC Merkel cell carcinoma, SCC squamous cell carcinoma.
SOTRs also have markedly elevated incidence of the two most common non-melanoma skin cancers, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), with SIRs of 6–10 and 65–200, respectively (35–37). Cutaneous SCC is the most common cancer in SOTRs, and some SOTRs develop multiple SCCs associated with poor treatment outcomes (36, 38, 39). A viral etiology for cutaneous SCC has been considered, with some HPV types assessed as candidates, but evidence remains inconclusive (40, 41). UVR is the major etiologic factor for skin cancers. Photosensitizing or other DNA-damaging effects of immunosuppressive medications may be the major factors contributing to development of skin cancers in SOTRs, because there are several lines of evidence suggesting direct carcinogenic mechanisms (42–45) and skin cancer incidence is much less elevated in people with AIDS (i.e., relative risk of 4.2 for individuals with CD4 counts < 200 cells/mm3 compared to HIV-uninfected people) (46). Melanoma is much less common, and incidence is only modestly increased in SOTRs and HIV-infected individuals (SIRs 1.2–2, Supplemental Table 1) (23).
Incidence of certain other major cancers is moderately elevated among SOTRs or HIV-infected people (Supplemental Table 1). Incidence is somewhat increased for lung cancer (SIRs 2–3), and while smoking plays a crucial role, other factors likely contribute (47, 48). Kidney, bladder, and colorectal cancers are increased only in SOTRs (SIRs 2–7), which probably reflects effects of end-stage renal disease, medical comorbidities, or carcinogenic effects of immunosuppressive medications, rather than immunosuppression per se (given the absence of increase in HIV-infected people). Breast and prostate cancer are not increased among SOTRs or HIV-infected people; indeed, for unclear reasons, recent studies demonstrate lower risk in these populations than in the general population (24, 49).
Associations with other immune-related conditions, although generally much weaker, parallel those seen for HIV and transplantation and likewise support a role for immunosurveillance for some cancers. For example, patients with autoimmune conditions, who are often treated with immunomodulating medications, have elevated risk for DLBCL (50). Patients with NHL or chronic lymphocytic leukemia have elevated incidence of cutaneous SCC and MCC (51–53).
Cancer outcome among SOTRs and HIV-infected people
Defective immunosurveillance in immunosuppressed SOTRs and HIV-infected people leads to an elevated incidence of cancer. Similarly, if immunocontainment is important for controlling cancers once they develop, then one would expect cancers to be more aggressive in SOTRs than other patients. Broadly speaking, however, there is no strong clinical evidence that this is the case, with the notable exception of cutaneous SCCs, which are frequently described as unusually invasive (35, 38, 39). Nonetheless, cancer aggressiveness is difficult to define, and subtle differences may readily be missed.
One way to assess the role of immunocontainment in SOTRs or other immunosuppressed individuals with cancer is to evaluate survival following the cancer diagnosis. All-cause mortality in SOTRs with cancer is generally high, partly due to underlying medical conditions and transplant-associated complications. Therefore, it is most informative to analyze the subset of deaths due to the cancer itself (i.e., cancer-specific mortality).
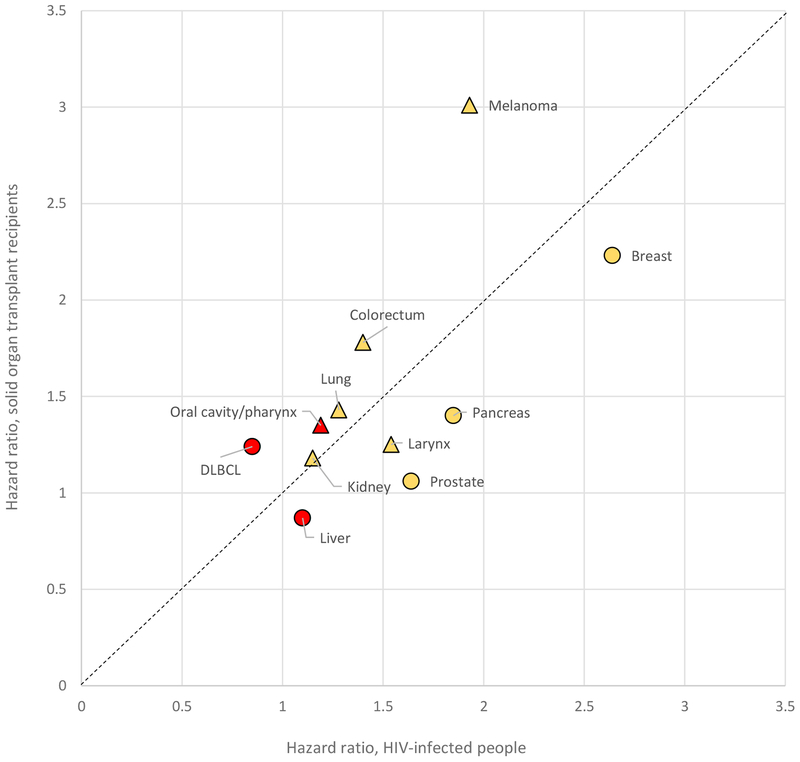
Two recent population-based epidemiologic studies that evaluated cancer-specific mortality are summarized in Supplemental Table 2 (54, 55). D’Arcy et al. described outcomes among 11,146 US SOTRs with cancer (54). SOTRs had higher cancer-specific mortality than patients without a transplant for multiple cancer types, including melanoma and cancers of the oral cavity/pharynx, colorectum, stomach, pancreas, lung, breast, bladder, and kidney. Regarding HIV infection, Coghill et al. demonstrated that cancer-specific mortality was higher among HIV-infected cancer patients in the US than comparable HIV-uninfected cancer patients, for cancers of the colorectum, pancreas, larynx, lung, and breast, and melanoma (Supplemental Table 2) (55). One challenge is that it can be difficult to assign a cause of death to patients with multiple medical problems, which may lead to errors in assessing cancer-specific mortality. Nonetheless, as shown graphically in Figure 2, solid organ transplantation and HIV infection are associated with somewhat parallel increases in cancer-specific mortality across different cancers (54, 55).
Figure 2.
Hazard ratios for cancer-specific mortality associated with the presence of HIV infection or solid organ transplant. Hazard ratios are from sources indicated in Supplemental Table 2. Results for virus-related cancers are shown in red and for virus-unrelated cancers in yellow. Results for cancers for which checkpoint inhibitor therapy has been approved by the US Food and Drug Administration (see Supplemental Table 3) are shown as triangles and for other cancers as circles. The dashed line indicates shows equality in hazard ratios associated with HIV and transplantation. Abbreviation: DLBCL diffuse large B-cell lymphoma.
Several additional observations highlight the importance of immunocontainment for certain cancers. First, among cervical cancer patients in Botswana and Brazil, HIV-infected and HIV-uninfected women had similar initial responses to treatment (56, 57), but HIV-infected women relapsed more often and had higher cancer-specific mortality, which would be predicted if immunocontainment is important for controlling small cancer foci remaining after initial treatment. Additionally, for immunosuppressed patients with KS, reduction of immunosuppression (e.g., through decrease in medication intensity for SOTRs) can result in resolution of tumors and long-term remission (58). Second, immunosuppressive therapy administered at transplantation can cause individuals with a prior cancer diagnosis to relapse, even after apparently curative treatment and several years of remission before transplantation (59). Finally, there are cases of cancer transmission to SOTRs from apparently healthy organ donors with a distant prior history of melanoma (60), implying that the donors had harbored asymptomatic foci of melanoma cells held in equilibrium by immunocontainment. With administration of immunosuppressive medications to the SOTR, the melanoma cells escaped and became clinically apparent as disseminated cancer. With these considerations in mind, clinicians usually try to reduce immunosuppression in SOTRs with cancer while balancing the need to control the cancer with preventing organ rejection.
Cancer immunotherapy
Although elevated incidence of cancer in immunosuppressed individuals is often cited as the motivation for development of cancer immunotherapy, the cancers that most clearly indicate a role for immunosurveillance—i.e., virus-related cancers—have not featured prominently in cancer immunotherapy trials. Instead, melanoma has been the most frequent target, first for interleukin-2 (61), then adoptive cell therapy (62), and most recently for checkpoint inhibitors (17).
The 2018 Nobel Prize in Physiology or Medicine was awarded to James Allison and Tasuku Honjo for development of checkpoint inhibitor therapies for cancer. Checkpoint inhibitors are therapeutic monoclonal antibodies that inactivate checkpoint proteins expressed in tumors, thereby unleashing anti-tumor T-cell responses. Checkpoint inhibitors have demonstrated efficacy for a range of cancers, including melanoma, NSCLC, renal cell carcinoma, urothelial carcinoma, cutaneous SCC, and MCC (Supplemental Table 3). Variable response rates are seen, but treatment can result in complete responses and prolonged remission for some patients.
A key predictor of response to checkpoint blockade is tumor mutational burden (TMB), defined as the number of nonsynonymous mutations in the tumor genome (18), which tracks with the number of neoantigens (63, 64). Cancer types that manifest high TMB on average (e.g., melanoma and NSCLC) are especially responsive to checkpoint blockade (64–66). Indeed, anti-PD-1 checkpoint blockade is highly efficacious against advanced-stage cutaneous SCC (67), which has among the highest average TMB of any cancer (68). Moreover, patients whose tumors show the highest TMB tend to have the best responses (63, 69–71). Another promising biomarker is PD-L1 expression on tumor cells, which is predictive for a range of cancers (e.g., melanoma, NSCLC, bladder cancer) (18).
A subset of colorectal, endometrial, and other cancers arise due to mutations caused by defective DNA mismatch repair (MMR). MMR-deficient cancers manifest genomic evidence of frequent DNA copying errors and extremely high TMB (72, 73). Pembrolizumab, an anti-PD-1 checkpoint inhibitor, is highly effective against MMR-deficient cancers (74).
Checkpoint inhibitors are also used to treat several virus-related cancers, including MCC, Hodgkin lymphoma, and head and neck cancer. These cancers can arise without the implicated virus. Remarkably, response to checkpoint inhibitor therapy for these cancers appears similar regardless of whether the tumors are virus-positive or virus-negative and instead is mainly predicted by high TMB (75–77). For virus-negative cases of these cancers, immunity is presumably directed against neoantigens generated by environmental exposures (66, 78, 79).
Cervical cancers, which are universally HPV-positive, also typically manifest high TMB (66), largely induced by APOBEC (i.e., apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like) (16), a family of innate antiviral proteins. These mutations in host genes generate non-viral neoantigens that may serve as important targets for immunosurveillance or immunocontainment of cervical cancer (80).
An immunosurveillance-immunocontainment model for cancer
The above review of epidemiologic evidence on cancer in immunosuppressed populations supports that cell-mediated immunity plays a role both in immunosurveillance and immunocontainment. Several important points can be highlighted.
The cancers for which the incidence is greatly elevated in immunosuppressed SOTRs and HIV-infected individuals are mainly those caused by viruses and differ from those targeted in cancer immunotherapy trials. Figure 1 highlights this disjunction by presenting separate symbols for cancers caused by viruses and those where checkpoint inhibitor therapy is approved.
Cancers with high TMB (e.g., melanoma, NSCLC, bladder cancer) commonly arise in the general population among individuals without obvious immunocompromise. Conversely, immunosuppressed individuals have only modestly elevated incidence for tumors that show high TMB (with the exception of cutaneous SCC in SOTRs).
SOTRs and HIV-infected people have elevated cancer-specific mortality for a broad range of cancers. Notably, most of these cancers are not thought to be caused by viruses. Figure 2 illustrates some correspondence, albeit incomplete, between cancers for which cancer-specific mortality is increased in immunosuppressed individuals and those for which checkpoint inhibitor therapy is approved.
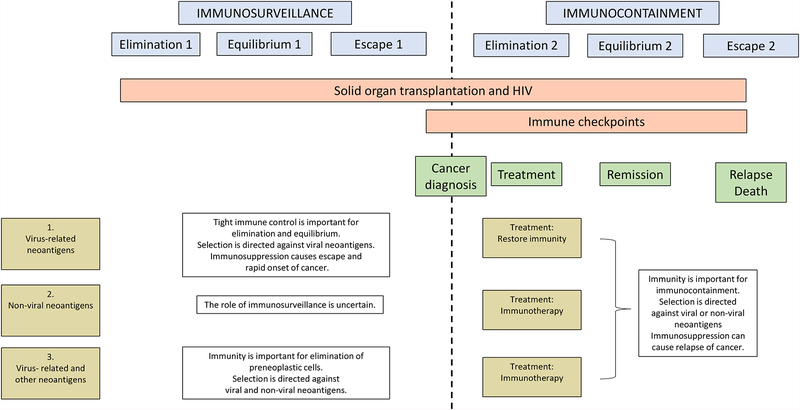
Based on these considerations, Figure 3 presents a theoretical model for immune control of cancer with two phases separated in time by the diagnosis of cancer. Under this model, immunosurveillance (before cancer diagnosis) and immunocontainment (at/after diagnosis) are each divided into three stages of elimination, equilibrium, and escape.
Figure 3.
Model for immunosurveillance and immunocontainment of cancer. The blue boxes depict two phases, before vs. at/after diagnosis of cancer, each divided into stages of elimination, equilibrium, and escape, reflecting the engagement of the immune system with premalignant or malignant cells. The red boxes depict examples of important processes that allow immune evasion: expression of immune checkpoint proteins (proposed to affect only the last part of phase 1 but having a much greater effect in phase 2) and immunosuppression associated with solid organ transplantation and HIV infection (affecting phases 1 and 2). Three example scenarios are depicted in the gold boxes and associated text: 1) cancers with virus-related neoantigens under very strong immune selection; 2) cancers with non-viral neoantigens; 3) cancers with both viral and non-viral neoantigens.
The first phase ends with escape of malignant cells and diagnosis of cancer. At the time of diagnosis, a patient’s immune system typically cannot eliminate the cancer without treatment. Instead, standard cancer treatment (e.g., surgery, radiotherapy, chemotherapy) or immunotherapy is required to eliminate the bulk of the tumor, leading in many instances to remission or stabilization of disease. Even with standard treatment in the absence of immunotherapy, the immune system plays a role in containing cancer cells and maintaining equilibrium. The cancer may ultimately escape immunocontainment, leading to relapse and death.
Immunosurveillance and immunocontainment appear relevant to a varying degree for different groups of malignancies. Three examples elaborate distinct scenarios regarding the role of immunity.
Scenario 1: Very strong immune selection against viral neoantigens
For KS and EBV-positive DLBCL, incidence is extremely elevated among immunosuppressed individuals. Onset is rapid after transplantation among SOTRs (81, 82), indicating that development of cancer crucially depends on expression of viral proteins, and that premalignant cells were previously maintained in equilibrium by strong immunosurveillance prior to transplantation. Tumors then arise when premalignant cells escape immunosurveillance due to the onset of immunosuppression. Immunocontainment is also relevant, as demonstrated by the effectiveness of immune reconstitution in causing KS to regress in SOTRs and HIV-infected patients, likely mediated by generation of strong immune selection against persistently expressed viral proteins.
Scenario 2: Immune selection directed against non-viral neoantigens
Cancers in this category include many for which immunotherapy has proven beneficial, including melanoma, kidney cancer, bladder cancer, NSCLC, and colorectal cancer. These cancers are notable for relatively high TMB, and several mechanisms generate neoantigens (e.g., exposure to tobacco or UVR, defective MMR). The frequent occurrence of these cancers in the general population among apparently immunocompetent individuals, and absence of markedly increased incidence in immunosuppressed populations, makes the role of immunosurveillance in preventing these cancers somewhat uncertain. However, the relevance of immunocontainment is supported by epidemiologic results related to cancer-specific mortality and the effectiveness of immunotherapy.
Scenario 3: Immune selection directed against both viral and non-viral neoantigens.
Examples include HPV-related cancers and MCC, where genetic alterations are related to somatic mutations in host genes as well as, for a variable fraction of cases, the presence of viral genetic material. Incidence is not as elevated in immunosuppressed individuals as for KS and EBV-positive NHLs. Cervical and anal cancers develop over a prolonged period during which HPV-infected cells advance from dysplasia to cancer, and there is a lag between onset of immunosuppression and cancer diagnosis. For instance, for anogenital cancers and MCC, incidence increases with prolonged time following solid organ transplantation (83, 84). Among HIV-infected people, anal cancer incidence increases with declining CD4 count, but this association is strongest with a 6–7-year lag (85).
These observations suggest that these tumors arise over an extended period during which immunosurveillance is directed against both viral and non-viral neoantigens. Elevated cancer-specific mortality for cervical cancer in HIV-infected women points to the importance of immunocontainment, and some relevant T-cells may be directed at non-viral neoantigens (80).
Future research
The forgoing considerations suggest some questions for future research. The strongly increased incidence of virus-related cancers in SOTRs and HIV-infected people implies that immunosurveillance plays a major role for these cancers. The question then arises: how do premalignant cells expressing viral neoantigens avoid immunosurveillance in immunocompetent individuals? This question could be addressed by comparing cancers from immunosuppressed and immunocompromised patients, for example, with respect to HLA expression and checkpoint proteins. Such comparisons could help identify new mechanisms used by cancers to avoid immunosurveillance, such as expression of additional checkpoint proteins including lymphocyte-activation gene 3 (LAG3) and T-cell immunoglobulin and mucin-domain containing 3 (TIM-3) (17).
For other cancers that prominently express neoantigens, incidence in immunosuppressed SOTR and HIV populations is not elevated as much as one might naively predict. Why is the incidence of oropharyngeal and nasopharyngeal cancers, both caused by viruses, not more elevated? Likewise, why is the incidence of some virus-unrelated cancers with high TMB not greatly increased? Possible explanations for the lack of strongly increased incidence of these cancers in immunosuppressed populations include expression of neoantigens only late in carcinogenesis (so that immunity only affects immunocontainment) or universal expression of immune evasion mechanisms (so that immunosuppression associated with transplantation or HIV has little added effect).
SCC incidence is markedly elevated in SOTRs, but incidence is elevated to a much smaller extent among immunosuppressed HIV-infected people as well as lymphoma patients. Therefore, additional research is required to understand the relevance of immunosurveillance and the mechanisms whereby an intact immune system may help prevent cutaneous SCC. For instance, there may be unique aspects of immunosuppression in SOTRs that make them exceptionally susceptible to this cancer. Alternatively, directly carcinogenic effects of immunosuppressive medications or other aspects of transplantation may contribute to cutaneous SCCs. Other cancers for which immunosurveillance appears to be very important (scenario 1) are caused by oncogenic viruses. Does a virus cause contribute to cutaneous SCC in SOTRs?
Is the high cancer-specific mortality in immunosuppressed cancer patients explained by impaired immunocontainment? This question could be addressed in a “dose-response” assessment of cancer-specific mortality in relation to immunosuppression, captured by intensity of immunosuppressive medications (in SOTRs) or CD4 count (in HIV-infected people).
One can hypothesize that cancers for which cancer-specific mortality is elevated in immunosuppressed populations are those for which immunotherapy would be most effective. Melanoma and breast cancer stand out in Figure 2. Melanoma is an established target of immunotherapy, but what about breast cancer? Breast cancers exhibit a wide range of TMB (66), with many mutations bearing an APOBEC signature (86, 87). Additionally, TILs in breast cancer are strongly predictive of treatment outcomes (11). Atezolizumab was recently approved for treatment of triple-negative breast cancer (i.e., tumors lacking expression of estrogen receptor, progesterone receptor, and human epidermal growth factor 2). Can effective immunotherapy be developed for other forms of breast cancer (88)?
Finally, there is substantial interest in using checkpoint inhibitors to treat SOTRs and HIV-infected people with cancer (89–91). One issue is that key characteristics of these tumors that predict response to checkpoint blockade (e.g., PD-L1 expression, TMB) have not been systematically assessed in these immunosuppressed populations. Indeed, it is possible that the loss of T-cell immunity due to other mechanisms (i.e., immunosuppressant medications, AIDS) reduces immune selective pressure, thus obviating the need for tumors to express checkpoint proteins, in which case checkpoint inhibitor treatment might be ineffective (18). Case reports of checkpoint blockade for treatment of advanced skin cancers in SOTRs have described modest efficacy but there appears to be substantial risk of graft rejection as a complication of treatment (89, 90). At least one phase 1 trial is planned to assess this approach further (https://clinicaltrials.gov ).
Conclusion
Epidemiologic studies of immunosuppressed SOTRs and HIV-infected individuals shed light on the immunosurveillance and immunocontainment of cancer. These processes appear important for distinct subsets of cancers and may have different mechanisms. Recent findings related to cancer-specific mortality in immunosuppressed populations may be most relevant for understanding and advancing cancer immunotherapy. This framework suggests new avenues of collaborative research among basic scientists, epidemiologists, and clinicians to advance cancer prevention and treatment.
Supplementary Material
Acknowledgements
I would like to thank Drs. David Berman, Christopher Buck, Allan Hildesheim, Lindsay Morton, Thomas O’Brien, Charles Rabkin, Meredith Shiels, Marcelo Soares, Minkyo Song, and Margaret Spitz for critical review and thoughtful comments on this manuscript. This work was supported by the Intramural Research Program of the National Cancer Institute.
References
- 1.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Current opinion in immunology. 2014;27:16–25. Epub 2014/02/18. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corthay A Does the immune system naturally protect against cancer? Frontiers in immunology. 2014;5:197 Epub 2014/05/27. doi: 10.3389/fimmu.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara H, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Seminars in cancer biology. 2015;35 Suppl:S185–s98. Epub 2015/03/31. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Burnet M Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. British medical journal. 1957;1(5023):841–7. Epub 1957/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. Epub 2011/03/08. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science (New York, NY). 2015;349(6255):1483–9. Epub 2015/09/26. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3(11):991–8. Epub 2002/10/31. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 8.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. Epub 2007/03/28. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–7. Epub 2007/11/21. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 10.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. Epub 2001/04/27. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 11.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nature reviews Clinical oncology. 2017;14(12):717–34. Epub 2017/07/26. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 12.Cohen CJ, Gartner JJ, Horovitz-Fried M, Shamalov K, Trebska-McGowan K, Bliskovsky VV, Parkhurst MR, Ankri C, Prickett TD, Crystal JS, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. The Journal of clinical investigation. 2015;125(10):3981–91. Epub 2015/09/22. doi: 10.1172/jci82416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science (New York, NY). 2014;344(6184):641–5. Epub 2014/05/09. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunology today. 1997;18(2):89–95. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 15.Shukla SA, Rooney MS, Rajasagi M, Tiao G, Dixon PM, Lawrence MS, Stevens J, Lane WJ, Dellagatta JL, Steelman S, Sougnez C, Cibulskis K, Kiezun A, Hacohen N, Brusic V, Wu CJ, Getz G. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nature biotechnology. 2015;33(11):1152–8. Epub 2015/09/16. doi: 10.1038/nbt.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378–84. Epub 2017/01/24. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–64. Epub 2012/03/23. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature reviews Cancer. 2016;16(5):275–87. Epub 2016/04/16. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, Lambert J, Kupper TS. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. The Journal of experimental medicine. 2008;205(10):2221–34. Epub 2008/09/17. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy K W C Janeway’s Immunobiology. New York: Garland Science, Taylor & Francis Group; 2017. [Google Scholar]
- 21.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–29. Epub 2004/12/24. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 22.Hoover R, Fraumeni JF Jr. Risk of cancer in renal-transplant recipients. Lancet. 1973;2(7820):55–7. Epub 1973/07/14. [DOI] [PubMed] [Google Scholar]
- 23.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. Epub 2007/07/10. doi: 10.1016/s0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 24.Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889–96. Epub 2010/07/28. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 26.Clarke CA, Morton LM, Lynch C, Pfeiffer RM, Hall EC, Gibson TM, Weisenburger DD, Martinez-Maza O, Hussain SK, Yang J, Chang ET, Engels EA. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109(1):280–8. doi: 10.1038/bjc.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahale P, Shiels MS, Lynch CF, Engels EA. Incidence and outcomes of primary central nervous system lymphoma in solid organ transplant recipients. Am J Transplant. 2018;18(2):453–61. doi: 10.1111/ajt.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung J, Munz C. Immune control of oncogenic gamma-herpesviruses. Current opinion in virology. 2015;14:79–86. Epub 2015/09/16. doi: 10.1016/j.coviro.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: An update. Int J Cancer. 2018;142(2):224–9. Epub 2017/09/03. doi: 10.1002/ijc.31027. [DOI] [PubMed] [Google Scholar]
- 30.Mawhorter S, Yamani MH. Hypogammaglobulinemia and infection risk in solid organ transplant recipients. Current opinion in organ transplantation. 2008;13(6):581–5. Epub 2008/12/09. doi: 10.1097/MOT.0b013e3283186bbc. [DOI] [PubMed] [Google Scholar]
- 31.Claas FH. Clinical relevance of circulating donor-specific HLA antibodies. Current opinion in organ transplantation. 2010;15(4):462–6. Epub 2010/07/09. doi: 10.1097/MOT.0b013e32833b9c38. [DOI] [PubMed] [Google Scholar]
- 32.Moir S, Fauci AS. B cells in HIV infection and disease. Nature reviews Immunology. 2009;9(4):235–45. Epub 2009/03/26. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, Gange SJ, Moore RD, Kitahata MM, Gebo KA, Martin J, Justice AC, Horberg MA, Hogg RS, Sterling TR, Cescon A, Klein MB, Thorne JE, Crane HM, Mugavero MJ, Napravnik S, Kirk GD, Jacobson LP, Brooks JT. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157(5):325–35. Epub 2012/09/05. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–e504. doi: 10.1016/S2352-3018(17)30125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–91. Epub 2003/04/25. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 36.Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer. 2013;132(6):1429–38. Epub 2012/08/14. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 37.Krynitz B, Olsson H, Lundh Rozell B, Lindelof B, Edgren G, Smedby KE. Risk of basal cell carcinoma in Swedish organ transplant recipients: a population-based study. Br J Dermatol. 2016;174(1):95–103. Epub 2015/09/04. doi: 10.1111/bjd.14153. [DOI] [PubMed] [Google Scholar]
- 38.Levine DE, Karia PS, Schmults CD. Outcomes of Patients With Multiple Cutaneous Squamous Cell Carcinomas: A 10-Year Single-Institution Cohort Study. JAMA dermatology. 2015;151(11):1220–5. Epub 2015/07/16. doi: 10.1001/jamadermatol.2015.1702. [DOI] [PubMed] [Google Scholar]
- 39.Lam JKS, Sundaresan P, Gebski V, Veness MJ. Immunocompromised patients with metastatic cutaneous nodal squamous cell carcinoma of the head and neck: Poor outcome unrelated to the index lesion. Head & neck. 2018;40(5):985–92. Epub 2018/01/24. doi: 10.1002/hed.25069. [DOI] [PubMed] [Google Scholar]
- 40.Arron ST, Ruby JG, Dybbro E, Ganem D, Derisi JL. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. J Invest Dermatol. 2011;131(8):1745–53. Epub 2011/04/15. doi: 10.1038/jid.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouwes Bavinck JN, Feltkamp MCW, Green AC, Fiocco M, Euvrard S, Harwood CA, Nasir S, Thomson J, Proby CM, Naldi L, Diphoorn JCD, Venturuzzo A, Tessari G, Nindl I, Sampogna F, Abeni D, Neale RE, Goeman JJ, Quint KD, Halk AB, Sneek C, Genders RE, de Koning MNC, Quint WGV, Wieland U, Weissenborn S, Waterboer T, Pawlita M, Pfister H. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am J Transplant. 2018;18(5):1220–30. Epub 2017/10/13. doi: 10.1111/ajt.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuschal C, Thoms KM, Schubert S, Schafer A, Boeckmann L, Schon MP, Emmert S. Skin cancer in organ transplant recipients: effects of immunosuppressive medications on DNA repair. Experimental dermatology. 2012;21(1):2–6. Epub 2011/12/14. doi: 10.1111/j.1600-0625.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 43.O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science (New York, NY). 2005;309(5742):1871–4. Epub 2005/09/17. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inman GJ, Wang J, Nagano A, Alexandrov LB, Purdie KJ, Taylor RG, Sherwood V, Thomson J, Hogan S, Spender LC, South AP, Stratton M, Chelala C, Harwood CA, Proby CM, Leigh IM. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nature communications. 2018;9(1):3667 Epub 2018/09/12. doi: 10.1038/s41467-018-06027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dziunycz PJ, Lefort K, Wu X, Freiberger SN, Neu J, Djerbi N, Iotzowa-Weiss G, French LE, Dotto GP, Hofbauer GFL. The oncogene ATF3 is potentiated by cyclosporine A and ultraviolet light A. J Invest Dermatol. 2014;134(7):1998–2004. Epub 2014/02/11. doi: 10.1038/jid.2014.77. [DOI] [PubMed] [Google Scholar]
- 46.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP Jr., Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105(5):350–60. Epub 2013/01/08. doi: 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triplette M, Crothers K, Mahale P, Yanik EL, Valapour M, Lynch CF, Schabath MB, Castenson D, Engels EA. Risk of lung cancer in lung transplant recipients in the United States. Am J Transplant. 2018. Epub 2018/12/20. doi: 10.1111/ajt.15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert review of anticancer therapy. 2008;8(4):605–15. Epub 2008/04/12. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 49.Coghill AE, Engels EA, Schymura MJ, Mahale P, Shiels MS. Risk of Breast, Prostate, and Colorectal Cancer Diagnoses Among HIV-Infected Individuals in the United States. J Natl Cancer Inst. 2018. doi: 10.1093/jnci/djy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekstrom Smedby K, Vajdic CM, Falster M, Engels EA, Martinez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori Costantini A, Bracci PM, Holly EA, Willett E, Spinelli JJ, La Vecchia C, Zheng T, Becker N, De Sanjose S, Chiu BC, Dal Maso L, Cocco P, Maynadie M, Foretova L, Staines A, Brennan P, Davis S, Severson R, Cerhan JR, Breen EC, Birmann B, Grulich AE, Cozen W. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–38. Epub 2008/02/12. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanik EL, Pfeiffer RM, Freedman DM, Weinstock MA, Cahoon EK, Arron ST, Chaloux M, Connolly MK, Nagarajan P, Engels EA. Spectrum of Immune-Related Conditions Associated with Risk of Keratinocyte Cancers among Elderly Adults in the United States. Cancer Epidemiol Biomarkers Prev. 2017. doi: 10.1158/1055-9965.EPI-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koljonen V, Kukko H, Pukkala E, Sankila R, Bohling T, Tukiainen E, Sihto H, Joensuu H. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer. 2009;101(8):1444–7. Epub 2009/09/17. doi: 10.1038/sj.bjc.6605306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: a search for common mechanisms. Br J Cancer. 2001;85(7):997–1005. Epub 2001/10/11. doi: 10.1054/bjoc.2001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Arcy M, Coghill AE, Lynch CF, Koch L, Li J, Pawlish K, Morris C, Rao C, Engels EA. Survival after cancer diagnosis among solid organ transplant recipients in the United States. Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol. 2015;33(21):2376–83. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, Ramogola-Masire D, Kebabonye-Pusoentsi M, Clayman R, Mapes AC, Tapela N, Asmelash A, Medhin H, Viswanathan AN, Russell AH, Lin LL, Kayembe MKA, Mmalane M, Randall TC, Chabner B, Lockman S. HIV Infection and Survival Among Women With Cervical Cancer. J Clin Oncol. 2016;34(31):3749–57. Epub 2016/08/31. doi: 10.1200/jco.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira MP, Coghill AE, Chaves CB, Bergmann A, Thuler LC, Soares EA, Pfeiffer RM, Engels EA, Soares MA. Outcomes of cervical cancer among HIV-infected and HIV-uninfected women treated at the Brazilian National Institute of Cancer. AIDS. 2017;31(4):523–31. doi: 10.1097/QAD.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duman S, Toz H, Asci G, Alper S, Ozkahya M, Unal I, Celik A, Ok E, Basci A. Successful treatment of post-transplant Kaposi’s sarcoma by reduction of immunosuppression. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2002;17(5):892–6. Epub 2002/05/01. [DOI] [PubMed] [Google Scholar]
- 59.Penn I The effect of immunosuppression on pre-existing cancers. Transplantation. 1993;55(4):742–7. Epub 1993/04/01. [DOI] [PubMed] [Google Scholar]
- 60.Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 2010;11(8):790–6. Epub 2010/05/11. doi: 10.1016/s1470-2045(10)70024-3. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. Epub 2014/06/08. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. Epub 2011/04/19. doi: 10.1158/1078-0432.ccr-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. Epub 2014/11/20. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nature reviews Cancer. 2017;17(4):209–22. Epub 2017/02/25. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–1. Epub 2017/12/21. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van ‘t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. Epub 2013/08/16. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379(4):341–51. Epub 2018/06/05. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 68.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome medicine. 2017;9(1):34 Epub 2017/04/20. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY). 2015;348(6230):124–8. Epub 2015/03/15. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Duran I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE 3rd, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thastrom A, Abidoye OO, Fine GD, Bajorin DF. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. Epub 2016/12/13. doi: 10.1016/s0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D’Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nature genetics. 2019;51(2):202–6. Epub 2019/01/16. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacology & therapeutics. 2018;189:45–62. Epub 2018/04/19. doi: 10.1016/j.pharmthera.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Kloor M, von Knebel Doeberitz M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer. 2016;2(3):121–33. Epub 2016/03/01. doi: 10.1016/j.trecan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 74.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20. Epub 2015/06/02. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542–52. Epub 2016/04/20. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–65. Epub 2016/06/02. doi: 10.1016/s1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 77.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. Epub 2014/12/09. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong SQ, Waldeck K, Vergara IA, Schroder J, Madore J, Wilmott JS, Colebatch AJ, De Paoli-Iseppi R, Li J, Lupat R, Semple T, Arnau GM, Fellowes A, Leonard JH, Hruby G, Mann GJ, Thompson JF, Cullinane C, Johnston M, Shackleton M, Sandhu S, Bowtell DD, Johnstone RW, Fox SB, McArthur GA, Papenfuss AT, Scolyer RA, Gill AJ, Hicks RJ, Tothill RW. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015;75(24):5228–34. Epub 2015/12/03. doi: 10.1158/0008-5472.can-15-1877. [DOI] [PubMed] [Google Scholar]
- 79.Desrichard A, Kuo F, Chowell D, Lee KW, Riaz N, Wong RJ, Chan TA, Morris LGT. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. J Natl Cancer Inst. 2018. Epub 2018/04/17. doi: 10.1093/jnci/djy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stevanovic S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, Hinrichs CS. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science (New York, NY). 2017;356(6334):200–5. Epub 2017/04/15. doi: 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cahoon EK, Linet MS, Clarke CA, Pawlish KS, Engels EA, Pfeiffer RM. Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int J Cancer. 2018. doi: 10.1002/ijc.31735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibson TM, Engels EA, Clarke CA, Lynch CF, Weisenburger DD, Morton LM. Risk of diffuse large B-cell lymphoma after solid organ transplantation in the United States. American journal of hematology. 2014;89(7):714–20. Epub 2014/04/23. doi: 10.1002/ajh.23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13(12):3202–9. doi: 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, Hernandez BY, Fraumeni JF Jr., Madeleine MM, Engels EA. Risk of merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107(2). doi: 10.1093/jnci/dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schoni-Affolter F, Bouchardy C, Dehler S, Levi F, Jundt G, Ess S, Pawlita M, Kovari H, Wandeler G, Calmy A, Cavassini M, Stockle M, Clifford G. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178(6):877–84. Epub 2013/08/01. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
- 86.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL, Saksena G, Harris S, Shah RR, Resnick MA, Getz G, Gordenin DA. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nature genetics. 2013;45(9):970–6. Epub 2013/07/16. doi: 10.1038/ng.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Middlebrooks CD, Banday AR, Matsuda K, Udquim KI, Onabajo OO, Paquin A, Figueroa JD, Zhu B, Koutros S, Kubo M, Shuin T, Freedman ND, Kogevinas M, Malats N, Chanock SJ, Garcia-Closas M, Silverman DT, Rothman N, Prokunina-Olsson L. Association of germline variants in the APOBEC3 region with cancer risk and enrichment with APOBEC-signature mutations in tumors. Nature genetics. 2016;48(11):1330–8. Epub 2016/10/28. doi: 10.1038/ng.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, Bear H, McArthur HL, Frank E, Perlmutter J, Page DB, Vincent B, Hayes JF, Gulley JL, Litton JK, Hortobagyi GN, Chia S, Krop I, White J, Sparano J, Disis ML, Mittendorf EA. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol. 2019. Epub 2019/04/12. doi: 10.1001/jamaoncol.2018.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Bruyn P, Van Gestel D, Ost P, Kruse V, Brochez L, Van Vlierberghe H, Devresse A, Del Marmol V, Le Moine A, Aspeslagh S. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Current opinion in oncology. 2019;31(2):54–64. Epub 2019/01/30. doi: 10.1097/cco.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 90.Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, Lin H, Wong MK, Abdelrahim M, Gaber AO, Suarez-Almazor ME, Diab A. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. Journal for immunotherapy of cancer. 2019;7(1):106 Epub 2019/04/18. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang E, Sabichi AL, Kramer JR, Hartman C, Royse KE, White DL, Patel NR, Richardson P, Yellapragada SV, Garcia JM, Chiao EY. Nivolumab Treatment for Cancers in the HIV-infected Population. Journal of immunotherapy (Hagerstown, Md : 1997). 2018;41(8):379–83. Epub 2018/07/19. doi: 10.1097/cji.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.