Abstract
The present research sought to examine whether hatha yoga, implemented as an adjunctive intervention for major depression, influences markers of inflammation. A subset of 84 participants who were enrolled in a randomized controlled trial (RCT) of hatha yoga vs. health education control provided blood samples at baseline (pre-treatment) and at 3- (during treatment) and 10-week (end of treatment) follow-up visits. To be eligible for the RCT, participants met criteria for a current or recent (past two year) major depressive episode, had current elevated depression symptoms, and current antidepressant medication use. Venous blood was drawn between 2 and 6pm and following at least one hour of fasting, and inflammatory markers (IL-6, CRP, and TNF-α) were assayed. Effects of participation in yoga relative to health education on inflammatory markers over time were examined with latent growth analyses. We observed a significant reduction in IL-6 concentrations in the yoga treatment group relative to the health education control group as demonstrated by a negative interaction between treatment group and slope of IL-6. TNF-α and CRP did not evidence significant interactions of treatment group by mean slope or intercept. In addition to the benefits of hatha yoga as an adjunctive intervention for individuals who have shown inadequate response to antidepressant medications, our findings point to possible benefits of yoga on IL-6 in depressed populations. Further research is needed to explore the effects of hatha yoga on immune function over time.
Keywords: hatha yoga, depression, cytokines, IL-6, CRP, TNF-α
INTRODUCTION
An estimated 16.2% of US adults report symptoms consistent with a diagnosis of major depressive disorder (MDD) in their lifetime, resulting in significant impairment and representing one of the most common psychological concerns faced by US adults.1 Importantly, only roughly half of depressed individuals are engaged in treatment and the majority of these individuals report receiving inadequate treatment.1 Pharmacologic treatment studies suggest that well over half of the individuals who receive antidepressant medication are either non-responders or only partial responders.2,3 Given the prevalence of MDD, as well as the reality that a majority of individuals who are engaged in treatment are receiving inadequate treatment, there is significant need for adjunctive interventions for MDD. Yoga is one promising intervention, with a meta-analysis of randomized controlled trials (RCTs) of yoga for clinical depression concluding that there is some evidence that yoga is better than usual care, relaxation exercises, or aerobic exercise in decreasing depressive symptoms.4
In the U.S., most people who practice yoga practice a modern form called hatha yoga, which involves training the body with the ultimate goal of promoting good physical and emotional health.5 Hatha yoga refers to any type of yoga that includes physical postures (asanas); hatha yoga practices can also include breath control (pranayama) and meditation. Most western medical research on yoga involves hatha yoga. Hatha yoga may have a positive impact on health outcomes through a synergistic combination of physical activity and cultivation of mindfulness (defined as non-judgmental attention to breath, physical sensations, thoughts, or feelings in the moment).6-8
In recently published results of an RCT of 122 adults with MDD who did not fully respond to pharmacological interventions,9 participants were randomly assigned to one of two adjunctive interventions: weekly hatha yoga classes or a health education control class. Although the groups did not differ at the end of the 10-week intervention period, the hatha yoga group had significantly lower depressive symptoms over time during a 6-month follow-up relative to the health education control group. Hatha yoga participants also showed greater improvements in social functioning and general health perceptions over time.9 These findings related to overall health benefits are particularly notable given the increasing evidence implicating inflammatory processes in the biological underpinnings of depressed mood.10
There are several lines of evidence that MDD may be associated with increased inflammation, including increased levels of pro-inflammatory cytokines (which amplify inflammatory processes) and other markers of inflammation in healthy depressed patients relative to controls and the finding that administration of pro-inflammatory cytokines frequently induces depression.11,12 Cytokines may be related to depression through their influence on the synthesis, release, and reuptake of relevant neurotransmitters in the brain, or by altering functioning of the hypothalamic-pituitary-adrenal (HPA) axis.11 There is both cross-sectional and longitudinal data that physical activity may reduce chronic inflammation,13 and preliminary evidence that mindfulness-based stress reduction 14,15 may have a positive impact on immune functioning.
Research with medical populations has shown the benefits of a number of forms of yoga on inflammatory processes believed to be critical to physical health outcomes. Some investigations have supported benefits of yoga on immune outcomes in breast cancer survivors.16-18 For example, breast cancer survivors participating in a 3-month RCT trial of hatha yoga evidenced lower interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin 1 beta (IL-1β) concentrations than a wait-list control group.17 Another investigation of breast cancer survivors revealed that participation in Iyengar yoga was associated with reduced nuclear factor kappa light chain enhancer of activated B cells (NF-κB) activity, reduced cAMP response element-binding protein (CREB) family transcription factor activity, and stable soluble tumor necrosis factor receptor II (sTNF-RII) expression in the yoga relative to control group, although there were no group differences in C-reactive protein (CRP) or IL-6.18 Similar benefits of yoga have been shown in chronic heart failure 19,20 and obese 21 populations. Another study of participants with chronic inflammatory diseases and overweight/obese status examined an intervention combining yoga, stress management, lecture, group discussion, and individual advice, and found reductions over time in participant levels of IL-6 and TNF-α.22 The benefits of yoga may further accrue over time: when compared with yoga novices, yoga experts show lower CRP as well as lower serum and lipopolysaccharide (LPS) stimulated IL-6, suggesting that yoga may dampen basal and induced levels of inflammation.23 These findings point to the potential promise of yoga related to immune function in populations with medical concerns; the importance of immune function, however, extends beyond medically compromised populations. One meta-analysis of mind-body therapies (including Yoga as well as Tai Chi, Qi Gong, meditation), observed an overall pattern supporting the benefits of mind-body therapies on immune indicators but reported that this pattern was strongest in medical (i.e., cancer, HIV, cardiovascular disease, etc) populations.24 One study of older adults with depression demonstrated a positive impact of Tai Chi on CRP 25 but we are unaware of any studies examining the effects of yoga on immune outcomes in a depressed sample.
The present RCT focused on a sample of participants with significant levels of depression that were not responsive to pharmacological interventions. This unique sample provides an opportunity to assess the effects of hatha yoga on immune markers in an important and sizeable subpopulation of depressed individuals. Given extant research and theory, we focused our analyses on levels of CRP, IL-6, and TNF-α assessed at baseline, 3 weeks into treatment, and 10 weeks into treatment (at the end of treatment) and examined how these levels change over time as influenced by participation in either hatha yoga or the control group. We hypothesized that participants in the hatha yoga condition would evidence a decrease in pro-inflammatory immune markers relative to those in the health education control group.
METHODS
Participants
Inclusion criteria for the larger trial9 (n = 122) were: 1) met criteria for major depressive disorder (MDD) within the prior two years assessed via the Structured Clinical Interview for DSM-IV SCID; 26; 2) QIDS score ≥ 8 (mild depression) and ≤ 17 (moderately severe depression); 3) no history of bipolar disorder, schizophrenia, or psychotic symptoms, assessed via the SCID; 4) no current hazardous drug or alcohol use as assessed by cut-offs on the AUDIT 27 or DUDIT 28; 5) no suicidal ideation or behavior requiring immediate attention; 6) currently taking an antidepressant at a dose with demonstrated effectiveness per American Psychiatric Association practice guidelines 29 for at least 8 weeks; 7) antidepressant dose had not changed in the previous 4 weeks and no plans to change the dose in the next 10 weeks; 8) if in psychotherapy, therapy frequency had not changed in the past 6 weeks AND no plans to change it in the next 10 weeks; 9) medically cleared for moderate physical activity; 10) not pregnant or planning to become pregnant; 11) minimal recent exposure to yoga or meditation; 12) fluent in English; and 13) aged 18 or older. In addition, to be eligible for the biomarker sub-study, participants had to 1) agree to have their blood drawn; 2) not be taking daily oral steroids, insulin or oral hypoglycemic agents, oral antibiotics, 3) not have an acute illness, or new, unstable, or unclear health problem.
Procedures
As described previously9, participants were randomly assigned to either hatha yoga classes or a health education group using a 1:1 ratio. Research staff used a computer program that employed urn randomization.30 Participants were stratified on three variables: depression severity, current psychotherapy with visits more often than once per month (yes or no), and gender. Study staff had no way of knowing to which arm the next participant would be randomized. All research activities occurred at a private psychiatric hospital in Providence, RI. This research was approved by the hospital IRB. All participants provided informed consent prior to participation. Participants were recruited from the greater Providence area from July 2011 to June 2014, and some participants were active in the study until March 2015.
Interventions
Hatha yoga.
Instructors followed a detailed manualized hatha yoga program. Each participant received an introductory 20-30 minute individual meeting with a yoga instructor. We offered group classes twice per week; participants were expected to attend at least one class per week with the option of attending two per week for 10 weeks. Classes were 80 minutes. Classes included breathing exercises (pranayama) and seated meditation; warm-ups and half sun salutations; standing postures (asanas); seated postures; an inversion and a twist; shavasana (relaxation); and wrap-up and discussion of home practice. Classes accommodated rolling admission. Instructors were asked to encourage mindful attention to the present moment throughout class, and to repeatedly guide participants through the connection between breath and movement. To facilitate home practice, each participant received a yoga mat, descriptions of suggested practices, and relevant videos. All yoga instructors were Registered Yoga Teachers ® with the Yoga Alliance.
Healthy Living Workshop (HLW).
Group HLW classes were concurrent with hatha yoga classes. Instructors used a detailed manual. HLW included an initial individual orientation meeting between the instructor and participant. Subsequently, participants were invited to attend at least one and up to two HLW classes per week for 10 weeks. Classes were 60 minutes long. There were 20 different class topics that repeated every 10 weeks. Topics included: alcohol, nicotine, and caffeine; being a smart patient; brain diseases; cancer prevention; diabetes; nutrition (3 classes); germs, colds, and the flu; physical activity (2 classes); sleep; physical pain, prevalence and causes of depression; and protecting your heart. Classes were interactive, but instructors avoided focusing on personal problems of participants. To facilitate home learning, participant received a book about nutrition, handouts at each class, and lists of websites with relevant information. Instructors encouraged participants to read materials each week at home. HLW instructors were post-doctoral fellows in clinical psychology and a master’s level nurse.
Measures
Blood draws were scheduled to occur between 2 and 6 pm. All participants were instructed to fast for one hour prior to the blood draw. A research nurse obtained blood samples. Plasma was extracted by centrifugation.
Assays.
Quantikine high sensitivity ELISA Immunoassays (R&D Systems, Minneapolis, MN) were used to quantify IL-6 and TNF-α levels according to the manufacturer’s protocols. Each plate contained 96 wells and a standard curve was generated on each plate. Standards and samples were run in duplicate on each plate using 100 μL for IL-6 and 200 μL for TNF-α per well. The intra- and inter-assay coefficients of variation are 7.4% and 7.8% for IL-6 5.4% and 8.3% for TNF-α. The minimum detectable dose was 0.016 pg/mL for IL-6 and 0.038 pg/mL for TNF- α. High sensitivity CRP was assayed using quantitative rate nephelometry (Beckman Coulter IMMAGE). The analytical sensitivity is 0.2 mg/L and the intra-assay coefficient of variation is <5.0%.
Depression Symptoms.
Participants were interviewed by trained, treatment-blinded interviewers using the Quick Inventory of Depression Symptomatology - Clinician Ratings (QIDS) 31 at all assessments. The measure assesses nine DSM symptoms of depression and scores of 6-10 reflect mild depression symptoms, scores 11-15 reflect moderate depression symptoms, and scores 16 or greater reflect severe or very severe symptoms. Reliability of interviews, assessed using an interrater reliability of a random selection of 61 interviews, was excellent (ICC = 0.96).
Analysis
Treatment effects on inflammatory measures were estimated using a latent growth curve (LGC) modeling approach. We fit separate linear growth models to assess change in each inflammatory marker (i.e., IL-6, CRP, and TNF-α) across the three time points (baseline, three weeks, and ten weeks post-baseline). The LGC approach aggregates individual trajectories across time, allowing for a model that directly tests for significant variation around growth (i.e., change) and intercept (i.e., baseline level) parameters. As such, this approach can be used to determine if there is (a) significant variation in mean level of each inflammatory measure at baseline among participants (i.e., significant intercept variance), (b) whether the mean level significantly changes over time (i.e., significant mean slope), and (c) whether there is significant variation in the rate of change across individuals (i.e., significant slope variance).
To assess change in these measures, we first fit unconditional latent growth models to estimate growth factors (i.e., intercept, slope) in each outcome separately. To account for potential association of body mass index (BMI) with inflammatory markers, all models incorporated BMI (calculated at each assessment) as a time varying covariate on the repeated inflammatory marker. Then, treatment group was entered as a time-invariant predictor of the growth factors to estimate the conditional effect of treatment group on inflammatory measures over time. Finally, for any model that either (1) showed significant change over time or (2) a significant treatment effect on the trajectory of change, we conducted a sensitivity analysis to examine the effects of gender, age, race/ethnicity, medication use, and baseline depression on the slope and intercept parameters. All analyses were conducted in MPlus version 7 using full information maximum likelihood estimation (FIML) to account for missing data. At baseline, 84 individuals had data for IL-6 and TNF-α (none missing) and 77 had data for CRP (7.2% missing). At the three-week follow-up, 65 individuals had data for IL-6 and TNF-α (22.6% missing) and 63 had data for CRP (24.1% missing). At the ten-week follow-up, 62 individuals had data for IL-6 and TNF-α (26.2% missing) and 61 had data for CRP (26.5% missing). FIML was used to accommodate cases with partially missing data,32 resulting in an analytic sample of 84 for IL-6 and TNF-α and 83 for CRP. Models were specified with loadings that reflected the time in weeks since baseline (i.e., 0, 3, and 10). In the conditional model, latent growth factors were regressed on treatment effect (i.e., HLW coded as 0, hatha yoga coded at 1). In sensitivity analysis models, latent growth factors were regressed on treatment effect as well as each covariate of interest in separate models and a full model containing all covariates. This approach was chosen to investigate the conditional effect of each covariate in isolation as well as jointly in the full model. Due to slight departures in normality of the inflammatory measures (i.e. kurtosis values were all above 3, ranging from a low of 3.39 [baseline IL-6] and a high of 14.71 [baseline IL-6]), Robust Maximum Likelihood Estimation was used to adjust the model Chi-Squared (χ2) statistic and standard errors for all models. Model fit was evaluated using the χ2 statistic, Comparative Fit Index (CFI; >0.9 indicate good fit), and Root Mean Square Error of Approximation (RMSEA; <0.08 indicate good fit).33,34
RESULTS
Demographics
Analyses were restricted to a set of 87 (NYoga = 48; NHLW = 39) individuals who had at least one inflammatory measure across each of the three time points (baseline, 3 week follow-up, 10 week follow-up). Across both treatment groups, participants in the study were primarily female (84%), White/Caucasian (88%), and had a mean age of 45.20 (SD = 12.72). All participants were taking antidepressant medications; slightly more than half of participants (52%) were taking an SSRI, 23% were taking an SNRI, 17% were taking an Aminoketone (e.g., bupropion), and the remaining 8% were taking either an anticonvulsant, tricyclic antidepressant, or tetracyclic antidepressant. See Table 1 for a breakdown of demographics and medications across each treatment group. Table 2 presents a summary of inflammatory protein means across time for each treatment group. A small number of blood samples were collected outside of the 2-6pm scheduled window (e.g., 16.27% of blood draws at baseline, 10.94% of blood draws at week 3, and 22.58% of blood draws at week 10 were taken outside of the 2-6pm time window). Consequently, we repeated all analyses in a subsample of individuals whose blood draws at all three assessments were taken within the 2-6pm time window.
Table 1.
Demographics, medications, depression severity and BMI by treatment group.
| Yoga (N=48) |
HLW (N=39) |
Total (N=87) |
||||
|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % |
| Gender | ||||||
| Male | 4 | 8.33% | 10 | 25.64% | 14 | 16.09% |
| Female | 44 | 91.67% | 29 | 74.36% | 73 | 83.91% |
| Race/Ethnicity | ||||||
| American Indian/Alaskan Native | 1 | 2.08% | 0 | 0.00% | 1 | 1.15% |
| Asian/Native Hawaiian/Pacific Islander | 1 | 2.08% | 0 | 0.00% | 1 | 1.15% |
| Black/African American | 0 | 0.00% | 1 | 2.56% | 1 | 1.15% |
| White/Caucasian | 43 | 89.58% | 34 | 87.18% | 77 | 88.51% |
| Other | 3 | 6.25% | 4 | 10.26% | 7 | 8.05% |
| Medication Type* | ||||||
| SSRI | 25 | 53.19% | 20 | 51.28% | 45 | 52.33% |
| SNRI | 12 | 25.53% | 8 | 20.51% | 20 | 23.26% |
| Aminoketone | 8 | 17.02% | 6 | 15.38% | 14 | 16.28% |
| Anti-convulsant | 0 | 0.00% | 1 | 2.56% | 1 | 1.16% |
| Tricyclic | 1 | 2.13% | 2 | 5.13% | 3 | 3.49% |
| Tetracyclic | 1 | 2.13% | 2 | 5.13% | 3 | 3.49% |
| M | SD | M | SD | M | SD | |
| Age | 45.52 | 12.72 | 44.79 | 13.79 | 45.20 | 12.72 |
| Baseline Depression | 12.67 | 2.83 | 12.87 | 2.70 | 12.76 | 2.76 |
| BMI | ||||||
| Baseline | 30.13 | 6.31 | 31.26 | 7.84 | 30.64 | 7.02 |
| Week 3 | 30.49 | 6.33 | 30.26 | 8.87 | 30.41 | 7.19 |
| Week 10 | 30.70 | 6.44 | 30.45 | 9.26 | 30.61 | 7.47 |
For one person in the yoga group, medication type was unknown.
Table 2.
Inflammatory protein means (standard deviations) across time for each treatment group.
| IL-6 |
TNF-α |
CRP |
||||
|---|---|---|---|---|---|---|
| Time Point |
Yoga | HLW | Yoga | HLW | Yoga | HLW |
| Baseline | 2.82 (2.04) | 2.22 (2.10) | 3.17 (2.18) | 3.81 (2.31) | 4.62 (5.87) | 4.65 (7.69) |
| 3 Weeks | 2.96 (2.79) | 2.21 (2.17) | 3.62 (3.11) | 3.75 (2.09) | 4.32 (5.15) | 3.59 (5.00) |
| 10 Weeks | 1.95 (1.67) | 3.79 (3.91) | 3.43 (2.32) | 3.99 (2.97) | 4.41 (6.17) | 3.70 (4.90) |
Change in inflammatory proteins across time
Change over time for each inflammatory marker was assessed using linear growth curve models. See Table 3 for parameter estimates for each outcome for unconditional and conditional growth models.
Table 3.
Unstandardized parameter estimates from growth models for each inflammatory marker.
| Parameter | IL-6 | TNF-α | CRP |
|---|---|---|---|
| Unconditional Model | |||
| Mean Intercept | 0.59 (0.92) | 3.82 (0.99)* | −3.67 (2.19) |
| Mean Slope | 0.01 (0.20) | 0.04 (0.16) | −0.01 (0.24) |
| Intercept Variance | 2.25 (1.48) | 1.54 (0.98) | 20.36 (8.36)* |
| Slope Variance | 0.03 (0.04) | 0.04 (0.05) | 0a |
| Intercept/Slope Covariance | −0.21 (0.17) | 0.05 (0.11) | 0a |
| Residual Variance: Baseline | 1.84 (1.39) | 3.48 (1.14)* | 28.39 (12.97)* |
| Residual Variance: Week 3 | 5.04 (2.25)* | 5.49 (2.45)* | 1.17 (2.24) |
| Residual Variance: Week 10 | 6.62 (4.40) | 0.38 (3.92) | 7.10 (3.27)* |
| Conditional Model | |||
| Mean Intercept | −0.12 (0.88) | 4.22 (0.99)* | −3.78 (2.04) |
| Mean Slope | 0.18 (0.20) | 0.04 (0.18) | <0.01 (0.27) |
| Intercept Variance | 1.88 (1.41) | 1.57 (0.97) | 20.34 (8.31)* |
| Slope Variance | 0.02 (0.03) | 0.04 (0.05) | 0a |
| Intercept/Slope Covariance | −0.16 (0.16) | 0.03 (0.10) | 0a |
| Intercept on treatment | 0.86 (0.42)* | −0.54 (0.46) | 0.15 (1.11) |
| Slope on treatment | −0.24 (0.09)* | −0.01 (0.08) | −0.02 (0.10) |
| Residual Variance: Baseline | 2.10 (1.31) | 3.35 (1.12)* | 28.41 (13.07)* |
| Residual Variance: Week 3 | 5.06 (2.24) | 5.54 (2.47)* | 1.17 (2.25) |
| Residual Variance: Week 10 | 5.66 (3.89) | 0.15 (3.89) | 7.10 (3.30)* |
= parameter fixed to 0 due to low variance,
= p < .05
Unconditional models.
Unconditional models, which ignored possible treatment effects, demonstrated adequate model fit (IL-6: χ2 = 7.72 (7), p = 0.358, CFI = 0.94, RMSEA [90% Confidence Interval (CI)] = 0.03 [0.00, 0.14]; TNF-α: χ2 = 16.93(7), p = 0.018, CFI = 0.58, RMSEA = 0.13 [0.05, 0.21]; CRP: χ2 = 10.87 (9), p = 0.285, CFI = 0.98, RMSEA = 0.05 [0.00, 0.15]). Due to very low variation across individuals for slope in the CRP model, the slope variance estimate was fixed to 0 in order to reach convergence. Across all models, parameter estimates revealed a significant intercept variance for CRP, but not for IL-6 or TNF-α, signifying that there was significant variation around mean level of CRP at baseline. None of the inflammatory markers evidenced a significant mean slope or slope variance estimate.
Conditional models.
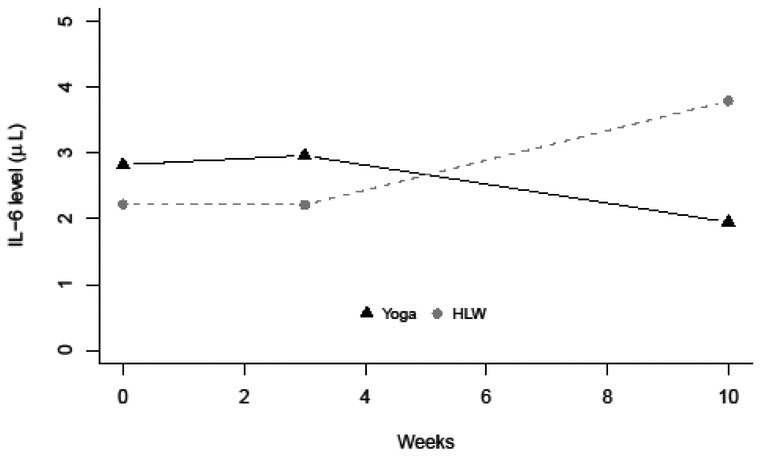
Conditional models incorporated treatment effect into estimations of means and variances of latent growth factors and also had adequate model fit (IL-6: χ2 = 12.46 (11), p = 0.330, CFI = 0.93, RMSEA = 0.04 [0.00, 0.12]; TNF-α: χ2 = 16.17 (11), p = 0.135, CFI = 0.76, RMSEA = 0.07 [0.00, 0.15]; CRP: χ2 = 13.36 (13), p = 0.420, CFI = 1.00, RMSEA = 0.02 [0.00, 0.11]). Findings supported a significant negative interaction between treatment group and slope of IL-6 (βs-IL-6 = −0.24, SE = 0.09) This demonstrates that individuals in the hatha yoga treatment group had a significant reduction in IL-6 levels relative to the HLW group. In fact, as depicted graphically in Figure 1, IL-6 level in the hatha yoga group decreased by week 10 while IL-6 level in the HLW group increased. TNF-α and CRP did not evidence significant interactions of treatment group by mean slope or intercept. Analyses conducted in the subsample of individuals of individuals whose blood draws at all three assessments were taken within the 2-6pm time window (Supplementary Table 1) revealed similar results, with the significant negative interaction between treatment group and slope of IL-6 remaining significant (βs-IL-6 = −0.32, SE = 0.14).
Figure 1.
Change in IL-6 mean level by treatment group over time.
Sensitivity Analyses
Given the significant treatment effect on IL-6, sensitivity analyses were conducted to examine the individual and combined effects of covariates on IL-6, including age, gender (coded as male or female), race (coded as nonwhite or white), baseline depression level, and medication type (coded as taking SSRI medication or taking any other antidepressant/anti-convulsant medication). Table 4 provides the parameter estimates of the regression of slope and intercept on treatment group and covariate for each of these models (see Supplementary Table 2 for a summary of model fit statistics for all models). Overall, the significant treatment effect on the slope was maintained when the covariates were entered into the model individually as well as all at once. No single covariate was significantly related to IL-6 in any of the models tested.
Table 4.
Summary of sensitivity analyses showing covariate effects on intercept and slope of IL-6.
| Covariate | Latent Variable | Parameter | Estimate (SE) |
|---|---|---|---|
| Medications (other than SSRI) | Intercept | Treatment | 0.89 (0.41)* |
| Meds | 0.46 (0.40) | ||
| Slope | Treatment | −0.23 (0.09)* | |
| Meds | 0.01 (0.07) | ||
| Male | Intercept | Treatment | 0.97 (0.42)* |
| Male | 0.64 (0.50) | ||
| Slope | Treatment | −0.24 (0.09)* | |
| Male | −0.02 (0.12) | ||
| Nonwhite | Intercept | Treatment | 0.86 (0.42)* |
| Nonwhite | 0.21 (0.87) | ||
| Slope | Treatment | −0.23 (0.09)* | |
| Nonwhite | 0.09 (0.14) | ||
| Age | Intercept | Treatment | 0.84 (0.42)* |
| Age | 0.01 (0.01) | ||
| Slope | Treatment | −0.24 (0.09)* | |
| Age | <0.01 (<0.01) | ||
| Baseline depression | Intercept | Treatment | 0.61 (0.34) |
| Depression | 0.04 (0.05) | ||
| Slope | Treatment | −1.36 (0.76) | |
| Depression | 0.05 (0.09) | ||
| All covariates | Intercept | Treatment | 1.04 (0.39)* |
| Meds | 0.41 (0.41) | ||
| Nonwhite | 0.14 (1.03) | ||
| Male | 0.58 (0.54) | ||
| Age | 0.01 (0.01) | ||
| Depression | 0.05 (0.08) | ||
| Slope a | Treatment | −0.25 (0.09)* | |
| Meds | −0.06 (0.07) | ||
| Nonwhite | 0.08 (0.15) | ||
| Male | −0.08 (0.14) | ||
| Age | 0.01 (<0.01) | ||
| Depression | 0.01 (0.02) |
Note: Each model includes treatment group and one covariate as predictors of the Intercept/Slope.
= Slope variance of model set to 0 due to low variance;
= p < 0.05. The medication variable was coded such that anyone who taking a medication other than an SSRI was coded with 1 and anyone who was taking an SSRI was coded with a 0.
DISCUSSION
Given extant research and theory supporting relationships between depression, inflammation, and physical activity- and mindfulness-based treatments, we expected to observe reductions of inflammatory markers in participants in the hatha yoga condition relative to the control group. Our findings supported a greater reduction of IL-6 among hatha yoga participants relative to the health education control participants in this group of persistently depressed patients with an incomplete response to antidepressants. Group differences were not observed for TNF-α and CRP. The present findings are consistent with recent evidence that physical exercise (participation in a 12 week intervention involving exercise) is associated with simultaneous decreases in depression and IL-6.35 Animal and human studies demonstrate the exponential and rapid influence of exercise on IL-6.36 Changes in IL-6 are an especially salient marker of inflammatory processes and plays a critical and unique role in local and systemic acute inflammatory responses by controlling proinflammatory cytokines which cannot be compensated for by IL-10 or other IL-6 family members.37 Relevant to this discussion, there is reason to believe that IL-6 is associated more closely with clinical reduction in depressive symptoms than TNF-alpha, and IL-6 levels are associated with refractoriness to antidepressant treatment.38,39 Together, these results suggest that IL-6 may be a mechanism by which hatha yoga helps decrease depressive symptoms.
We expected to observe an effect of treatment on TNF-α and especially CRP (in addition to IL-6) given meta-analytic evidence providing greatest support for a positive impact of mind-body interventions on CRP (pooled effect size = 0.58) followed by small but significant effects on IL-6 (pooled effect size = 0.35). 24 Given that the effects of hatha yoga on depressive symptoms were not present at 10 weeks but emerged during the 6 months following the intervention, it is possible that changes in CRP and TNF-α might have been observed if we had continued to measure these markers at later follow-up assessments, as changes to these markers may take longer before they are observable.
The present findings should be considered in the context of the public health significance of an intervention that may benefit a large number of individuals with significant depression-related impairments and, furthermore, has the potential for simultaneous beneficial influences on psychological and physical health. Given that only half of depressed individuals are engaged in treatment and the majority of these individuals report receiving inadequate treatment,1 the availability of a lifestyle intervention that is an effective adjunctive intervention is exciting. Moreover, hatha yoga may not only serve as an adjunctive intervention but may also benefit individuals who may avoid traditional psychiatric approaches. Additionally, whereas psychotherapy is often time-limited in frequency and duration, hatha yoga may be integrated into individuals’ lifestyles. For example, among students experiencing examination stress, those randomly assigned to yoga showed relative stability in psychological stress, cortisol, and IFN-γ as compared to the control group.40
Epigenetic processes which result in alterations in gene expression (without changes to DNA sequence) have been proposed as one mechanism whereby yoga may influence immune outcomes. One recent investigation of IL-6, TNF and CRP in a small subsample (n=28) of participants in a yoga intervention reported reduced methylation of the region encoding TNF in the yoga group relative to the waitlist control group.41 Inflammatory gene expression differences have been observed in caregivers of people with dementia42 and expressed gene alterations in influential pathways of cellular metabolism and response to oxidative stress have been identified in daily practitioners of mind-body practices.43
Although findings from the present research provide compelling evidence for the benefits of hatha yoga on IL-6 in persistently depressed participants with incomplete response to antidepressants, further research is needed to replicate and extend lines of evidence supporting the benefits of yoga on pro-inflammatory processes. Whereas some research has shown evidence of long term maintenance of immune improvements among individuals participating in yoga interventions relative to control conditions,23 the present investigation did not assess inflammatory markers at later post-treatment follow-ups. Future investigations aimed at understanding the influence of yoga participation on immune outcomes would benefit from continued follow-ups to assess whether improvements are maintained. It would be especially interesting to examine whether improvements are maintained only among individuals who have continued to incorporate regular hatha yoga practice into their lifestyles or whether time-limited involvement in yoga will result in improvements that are maintained even in the absence of continued yoga practice. Another limitation of this research is the relatively small sample size, with inadequate power representing one possible explanation for the lack of findings for CRP and TNF-α. All participants were taking an antidepressant medication, so the generalizability to people not taking medications for depression is unknown. We note that, although there is evidence that antidepressant treatment can reduce cytokine levels including IL-6,44,45 the current study was focused on medication non-responders and participants were taking stable medications when interventions were initiated, so the use of antidepressants is unlikely to explain the intervention effect. Finally, future research may also benefit from systems biology approaches to characterize these pro-inflammatory processes by examining epigenetic changes that may play a role in the reduced inflammatory marker levels observed here.
CONCLUSIONS
Findings from the present investigation provide further support for a growing literature related to the immune benefits of hatha yoga. IL-6, in particular, emerged as one inflammatory marker that was observed to decrease over time in persistently depressed participants in the hatha yoga condition relative to health education control participants. Further research is needed to continue to explore the combined psychological and physical health benefits of hatha yoga.
Supplementary Material
Acknowledgments
Funding: This project was supported by grant NR012005 (Uebelacker). Dr. Nugent’s effort is supported by R01MH108641 and 105379. Dr. Brick’s effort is supported by T32MH019927. Dr. Tyrka’s effort is supported by R01MH1011071 and R01HD086487. Dr. Ridout’s effort was supported by R25MH101076.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT01384916
Disclosures: Dr. Uebelacker’s spouse is employed by Abbvie Pharmaceuticals.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama. 2003;289(23):3095–3105. [DOI] [PubMed] [Google Scholar]
- 2.Corey-Lisle PK, Nash R, Stang P, Swindle R. Response, partial response, and nonresponse in primary care treatment of depression. Arch Intern Med. 2004;164(11):1197–1204. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. [DOI] [PubMed] [Google Scholar]
- 4.Cramer H, Lauche R, Langhorst J, Dobos G. Yoga for depression: a systematic review and meta-analysis. Depression and Anxiety. 2013;30(11):1068–1083. [DOI] [PubMed] [Google Scholar]
- 5.Feuerstein G Yoga: An Essential Introduction to the Principles and Practice of an Ancient Tradition. Shambala Publications, Inc.: Boston, MA; 1996. [Google Scholar]
- 6.van der Velden AM, Kuyken W, Wattar U, et al. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin Psychol Rev. 2015;37:26–39. [DOI] [PubMed] [Google Scholar]
- 7.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uebelacker LA, Epstein-Lubow G, Gaudiano BA, Tremont G, Battle CL, Miller IW. Hatha yoga for depression: critical review of the evidence for efficacy, plausible mechanisms of action, and directions for future research. Journal of psychiatric practice. 2010;16(1):22–33. [DOI] [PubMed] [Google Scholar]
- 9.Uebelacker LA, Tremont G, Gillette LT, et al. Adjunctive yoga v. health education for persistent major depression: a randomized controlled trial. Psychological Medicine. 2017;47:2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(3):730–743. [DOI] [PubMed] [Google Scholar]
- 11.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. Journal of Affective Disorders. 2014;169:15–20. [DOI] [PubMed] [Google Scholar]
- 13.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunology and allergy clinics of North America. 2009;29(2):381–393. [DOI] [PubMed] [Google Scholar]
- 14.Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain, behavior, and immunity. 2008;22(6):969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, behavior, and immunity. 2007;21(8):1038–1049. [DOI] [PubMed] [Google Scholar]
- 16.Rao RM, Nagendra H, Raghuram N, et al. Influence of yoga on mood states, distress, quality of life and immune outcomes in early stage breast cancer patients undergoing surgery. International journal of yoga. 2008;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. Journal of Clinical Oncology. 2014;32(10):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullen PR, Nagamia SH, Mehta PK, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. Journal of cardiac failure. 2008;14(5):407–413. [DOI] [PubMed] [Google Scholar]
- 20.Pullen PR, Thompson WR, Benardot D, et al. Benefits of yoga for African American heart failure patients. Medicine and science in sports and exercise. 2010;42(4):651–657. [DOI] [PubMed] [Google Scholar]
- 21.Sarvottam K, Magan D, Yadav RK, Mehta N, Mahapatra SC. Adiponectin, interleukin-6, and cardiovascular disease risk factors are modified by a short-term yoga-based lifestyle intervention in overweight and obese men. The journal of alternative and complementary medicine. 2013;19(5):397–402. [DOI] [PubMed] [Google Scholar]
- 22.Yadav RK, Magan D, Mehta N, Sharma R, Mahapatra SC. Efficacy of a short-term yoga-based lifestyle intervention in reducing stress and inflammation: preliminary results. The journal of alternative and complementary medicine. 2012;18(7):662–667. [DOI] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Christian L, Preston H, et al. Stress, inflammation, and yoga practice. Psychosomatic medicine. 2010;72(2):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PloS one. 2014;9(7):e100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavretsky H, Alstein LL, Olmstead RE, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. The American Journal of Geriatric Psychiatry. 2011;19(10):839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, Research version, Patient edition with Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 27.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for use in primary care, 2nd edition. World Health Organization;2001. [Google Scholar]
- 28.Berman AH, Bergman H, Palmstierna T, Schlyter F. DUDIT Manual: The Drug Use Disorders Identification Test, Version 1.0. Stockholm: Karolinska Institutet, Department of Clinical Neuroscience;2005. [Google Scholar]
- 29.Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder, third edition. American Psychiatric Association;2010. [Google Scholar]
- 30.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;S12:70–75. [DOI] [PubMed] [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. [DOI] [PubMed] [Google Scholar]
- 32.Arbuckle JL. Full information estimation in the presence of incomplete data. . In: Marcoulides GA, Schumacker RE, eds. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Lawrence Erlbaum Associates; 1996: 243–277. [Google Scholar]
- 33.Browne MW, Cudeck R. Alternative ways of assessing model fit. Sage focus editions. 1993;154:136–136. [Google Scholar]
- 34.Kline RB, Santor DA. Principles & practice of structural equation modelling. Canadian Psychology. 1999;40(4):381. [Google Scholar]
- 35.Lavebratt C, Herring MP, Liu JJ, et al. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatry Research. 2017;252:270–276. [DOI] [PubMed] [Google Scholar]
- 36.Petersen A, Pedersen B. The role of IL-6 in mediating the anti inflammatory. J Physiol Pharmacol. 2006;57:43–51. [PubMed] [Google Scholar]
- 37.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. The Journal of clinical investigation. 1998;101(2):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannestad J, DellaGioia N, Bloch M. The Effect of Antidepressant Medication Treatment on Serum Levels of Inflammatory Cytokines: A Meta-Analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI-or SNRI-refractory depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(4):722–726. [DOI] [PubMed] [Google Scholar]
- 40.Gopal A, Mondal S, Gandhi A, Arora S, Bhattacharjee J. Effect of integrated yoga practices on immune responses in examination stress–A preliminary study. International journal of yoga. 2011;4(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harkess K, Ryan J, Delfabbro P, Cohen-Woods S. Preliminary indications of the effect of a brief yoga intervention on markers of inflammation and DNA methylation in chronically stressed women. Translational psychiatry. 2016;6(11):e965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black DS, Cole SW, Irwin MR, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38(3):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dusek JA, Otu HH, Wohlhueter AL, et al. Genomic counter-stress changes induced by the relaxation response. PloS one. 2008;3(7):e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jha MK, Trivedi MH. Personalized antidepressant selection and pathway to novel treatments: clinical utility of targeting inflammation. International Journal of Molecular Science. 2018;19(1): 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiedloch M, Marcinowicz P, Krupa R, et al. Effect of antidepressant treatment on peripheral inflammation markers – a meta-analysis. Progress in Neuropsychopharmacology and Biological Psychiatry. 2018; 80(Pt C): 217–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.