Abstract
Although dry eye occurs mostly in adults, dry eye may be induced in teens receiving allogeneic hematological stem cell transplantations (AHSCT). Changes in meibum composition and structure, has been associated with dry eye. The structure of meibum from teens with dye eye or teens with dry eye and AHSCT has not been studied, so in this study, we compared the structure of meibum from teens receiving AHSCT that had dry eye with meibum from teens without AHSCT and without dry eye symptoms.
Keywords: Allogeneic Hematological Stem Cell Transplantations, Dry Eye, Infrared Spectroscopy, Lipid, Meibum
INTRODUCTION
Dry eye affects about 15% of individuals worldwide.1 A thin lipid layer 2 on the surface of tears contributes to tear film stability.3 The lipid layer is composed mostly of wax and cholesteryl esters from the meibomian glands in the eye lid that are deposited on the tear surface with each blink.
Although dry eye occurs mostly in adults, dry eye may be induced in teens receiving allogeneic hematological stem cell transplantations (AHSCT).4 Dry eye induced by AHSCT is associated with meibomian gland disease (MGD), in which meibomian glands are atrophic5, and also involves the lacrimal glands, resulting in aqueous deficient dry eye (ADDE).4 Dry eye related to chronic graft-versus-host disease occurs in 35% of children after AHSCT.6
Changes in the structure of meibum from teens receiving AHSCT or any form of dry eye has never been studied, so in this study, the meibum from a cohort of patients with dry eye and AHSCT (CAHSCT) were compared with meibum from a cohort of patients who did not have AHSCT or dry eye symptoms (Cn) as a control. Meibum from CAHSCT and Cn was abbreviated MAHSCT and Mn, respectively.
MATERIALS and METHODS
In this prospective comparative study, meibum from both eyes were collected only once and pooled. Participants were recruited from the Kentucky Lions Eye Center and the James Graham Brown Cancer Center in Louisville, Kentucky. Written informed consent was obtained from all donors and protocols and procedures were approved by the University of Louisville Institutional Review Board Institutional Review Board # 11.0319, August, 2016. All procedures were in accordance with the Declaration of Helsinki.
Participants were assigned to the cohort Cn (normal control) when the patient’s Meibomian gland orifices showed no evidence of keratinization or plugging with turbid or thickened secretions and no dilated blood vessels were observed on the eyelid margin. The participants did not recall having dry eye symptoms. Participants were assigned to the cohort CAHSCT if they had undergone AHSCT. Patients in CAHSCT underwent a full ophthalmic eye exam using slit lamp biomicroscopy or by pen light exam while at their oncology follow up appointment at the cancer center. Tear film break up time was measured at the slit lamp after instillation of one fluorescein drop. The diagnosis of dry eye was based on the clinical examination results, including fluorescein stain uptake of the cornea or conjunctiva, irregular tear film, low tear meniscus, as well as symptoms. Symptoms that were considered positive included foreign body sensation, excessive tearing, excessive blinking, burning of eyes or blurry vision. The Schirmer’s test was performed on all patients by placing a standard strip in the lower conjunctival sac without anesthesia for 5 minutes. Meibomian gland orifices, eyelid changes at the mucocutaneous junction and expression of meibum by gentle pressure were all evaluated for diagnosis of MGD.
Lipid phase transitions were measured as described previously.7,8 About 500 µL of sample in CDCl3 was applied to a AgCl infrared window. The solvent was evaporated under a stream of Argon gas and the window was placed in a lyophilizer for 4 hours to remove all traces of solvent. Infrared spectra were measured using a Fourier transform infrared spectrometer (Nicolet 5000 Magna Series; Thermo Fisher Scientific, Inc., Waltham MA). Lipid on the AgCl window was placed in a temperature-controlled infrared cell. The cell was jacketed by an insulated water coil connected to a circulating water bath (model R-134A; Neslab Instruments, Newton NH). The sample temperature was measured and controlled by a thermistor touching the sample cell window. The water bath unit was programmed to measure the temperature at the thermistor and to adjust the bath temperature so that the sample temperature could be set to the desired value. The rate of heating or cooling (1°C/15 minutes) at the sample was also adjusted by the water bath unit. Temperatures were maintained within ± 0.01°C. Exactly 100 interferograms were recorded and averaged. Spectral resolution was set to 1.0 cm−1. Infrared data analysis was then performed (GRAMS/386 software; Galactic Industries, Salem, NH).
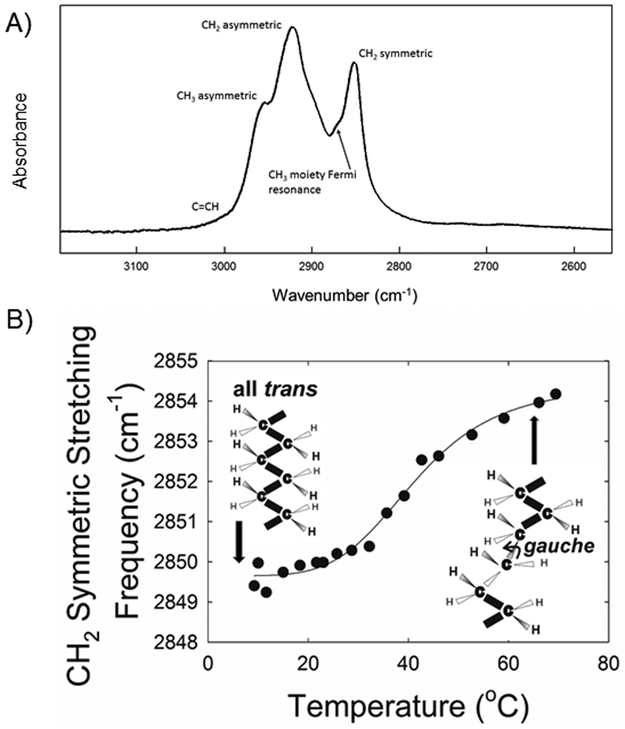
The frequency of the symmetric CH2 stretching band near 2850 cm−1 () was used to estimate the content of trans and gauche rotamers (lipid order) in the hydrocarbon chains as described.15 The data for percentage of trans rotomer were used to calculate the phase-transition enthalpy and entropy from the slopes of Arrhenius plots.
Meibum was collected and lipid phase transitions were measured as described previously.7,8 Curves were fit using Sigma plot 10 software (Systat Software, Inc., Chicago, IL, USA) and the confidence levels, were obtained from a critical value table of the Pearson product–moment correlation coefficient.
Two of the phase transition parameters, the minimum and maximum vibrational frequency of the C-H symmetric stretch (ṽsym), correspond to the most ordered and disordered states of hydrocarbon chains, respectively. Another parameter was the phase transition temperature, which is the temperature at which half of the lipid molecules undergo a change from the gel to liquid crystalline phase. The relative cooperativity of the phase transition describes how the order of a lipid influences that of neighboring lipids. Broad phase transitions have a relatively smaller absolute value of the cooperativity. Lipid order was calculated at 33.4 °C the temperature at the surface of the eye and 36 °C the temperature of the eye lid.
Data are reported as the mean plus or minus the standard error. The Student’s t-test was used to compare the parameters obtained from MAHSCT and Mn. A value of P < 0.05 was considered statistically significant.
RESULTS
Participant demographics are listed in Table 1. There were no gender or race differences between Cc and CAHSCT (Table 1). For CAHSCT, dry eye was classified as aqueous deficient dry eye (ADDE) for one participant, MGD for three participants, and combination of ADDE and MGD for one participant. Their average visual acuity was 20/27 ± 5 and their average Schirmer score was 26 ± 5 mm. Two of the participants in CAHSCT had chronic and one had acute GvHD.
Table 1.
Donor demographics and phase transition parameters.
| Parameter | Cc | CAHSCT | P |
|---|---|---|---|
| Average Age (y) | 17.7 (0.9) | 15.0 (0.9) | > 0.05 |
| Age Range (y) | 13 to 20 | 13 to 18 | |
| Gender (% male) | 70 | 80 | |
| Race (%) | C (60) B (20) A (10) ? (10) |
C (60), B (20) A (20) |
|
| Tm | 31 (1) | 38 (2) | 0.004* |
| Cooperativity (Hill coefficient) | 8.4 (0.7) | 6.0 (0.4) | 0.04* |
| Order 36.0 °C (% trans) | 37 (3) | 48 (2) | 0.03* |
| Order 33.4 °C (% trans) | 41 (3) | 60 (2) | 0.001* |
| Δ enthalpy (kcal/mol) | 152 (15) | 134 (9) | > 0.05 |
| Δ entropy (kcal.mol/degree) | 0.50 (0.05) | 0.44 (0.04) | > 0.05 |
| Magnitude (cm−1) | 4.0 (0.3) | 4.85 (0.06) | > 0.05 |
| Minimum Frequency (cm−1) | 2849.6 (0.1) | 2849.35 (0.09) | > 0.05 |
| Maximum Frequency (cm−1) | 2853.6 (0.2) | 2853.9 (0.1) | > 0.05 |
| Δ Order 33.4 °C - 36.0 °C (% trans) | 5.3 (0.4) | 7.0 (0.6) | 0.03* |
| Number of Participants | 10 | 5 |
(SEM), C = Caucasian; A = Asian; B = African American; ? = unknown race.
Significant difference, P < 0.05.
The frequency of the symmetric CH2 stretching band near 2850 cm−1 () was used to estimate the trans to gauche rotamer content of the hydrocarbon chains (Fig. 1A),7,8 and it increased with an increase in temperature (Fig. 1B).
Figure 1.
a) Typical Infrared CH stretching region of meibum from a 17 year-old Caucasian male. b) Typical lipid phase transition of meibum from a 14 year-old Caucasian female who has dry eye induced by an allogeneic hematological stem cell transplantation. The larger the CH stretching frequency the larger the disorder (fluidity) of the lipid. The more trans rotamers the more ordered the lipid.
Lipid phase transition parameters for human meibum are listed in Table 1. The phase transition temperature (Tt), lipid order at 36°C and 34°C, and Δ order for MAHSCT were significantly higher (P < 0.05) compared with Mn (Table 1). The cooperativity for MAHSCT was significantly lower (P < 0.05) compared with Mn (Table 1).
DISCUSSION
The major finding of this study was that with dry eye, the Tt of MAHSCT, 38°C, was much higher compared with the Tt of Mn, 31°C. Tt is the temperature at which half of the lipid molecules undergo a change from the gel to liquid crystalline phase. One may speculate that the significance of a Tt being near the physiological temperature of the eye lid, 36°C, is that the meibum is fluid enough to flow out of the meibomian gland, and becomes more ordered at 34.4°C, the surface temperature of the eye,9 so it can withstand the shear stress of a blink. A small change in Tt may cause a large change in lipid order.8
Lipid structural order is similar to lipid fluidity. For instance, solid butter is more ordered than liquid olive oil. At lower temperatures lipids may become more ordered and their hydrocarbon chains arrange in an all trans conformation. In this conformation, the hydrocarbon chains are straight and they pack closely together (Fig. 1b); Van der Waal’s interactions between the chains are maximal. At higher temperatures, gauche rotamers are introduced into the hydrocarbon chains causing kinks (Fig. 1b). The chains are not able to pack tightly together and Van der Waal’s forces between the chains are minimal. The order of MAHSCT was 60 % trans, much higher than the 44 % trans order observed for MGD in adults8 and much higher than the 41% trans order for Mn measured for teens in this study. Some of the difference in order between MAHSCT in teens and meibum from adults with MGD may be due to the decrease in order with increasing age.7 It is reasonable that a consequence of MAHSCT being more ordered than Mn is that with dry eye, the ordered lipids block the meibomian glands causing inflammation. On the surface of the eye, the combination of increased % trans rotamers, raise in chain melting temperature and decrease in cooperativity for MAHSCT compared with Mn suggests that in vivo, gross changes in the structure and properties of meibomian films at the air/tear surface occur with AHSCT. Detailed reviews on the topic have been published.12,13 One would expect from the AHSCT induced alterations that a discontinuous patchy tear film lipid layer would form resulting in deteriorated spreading, decreased surface elasticity and attenuated capability to restore its structure between blinks.14
The cooperativity of the phase transition of MAHSCT was significantly lower than that for Mn. Cooperativity is related to how the melting of one lipid influences the melting of another lipid. Broad phase transitions show less cooperativity. The compositional differences between Mn and MAHSCT that contribute to lower cooperativity have yet to be determined. The homogeneity of the meibum lipid contributes to cooperativity as the phase transition cooperativity of pure waxes are orders of magnitude greater than that of meibum or mixtures of pure waxes.8 Unsaturation, contaminants and proteins could also lower the cooperativity.10 All of these factors could contribute to or be a marker of dry eye.
We are conducting a more extensive study of 60 participants to quantify the moieties in meibum and to determine their effect on structure, and to determine if AHSCT is the driving factor behind the reported changes in meibum or if the changes are simply due to dry eye disease.
In this study of teens post AHSCT, meibum lipid conformational and thermodynamic differences were observed between teens with dry eye post AHSCT and teens without dry eye or AHSCT. The phase transitional parameters could be markers of MGD (or atrophy) or contribute to dry eye as more ordered lipids with dry eye could block the meibomian glands causing inflammation and on the surface of the eye they may aggregate keeping tears from spreading.
Acknowledgments
Sources of Support
Major support was obtained from the National Institute of Health EYO RO126180 (DB) and an unrestricted grant from Research to Prevent Blindness, Inc. New York, NY, USA.
REFERENCES
- 1.Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–88. [DOI] [PubMed] [Google Scholar]
- 2.King-Smith PE, Fink BA, Fogt N, et al. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2000;41:3348–59. [PubMed] [Google Scholar]
- 3.Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–74. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic Stem Cell Transplantation. Cornea. 2003;22:S19–S27. [DOI] [PubMed] [Google Scholar]
- 5.Engel LA, Wittig S, Bock F et al. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplant. 2015;50:961–7. [DOI] [PubMed] [Google Scholar]
- 6.Kinori M, Bielorai B, Souroujon D, et al. Ocular complications in children after hematopoietic stem cell transplantation without total body irradiation. Graefes Arch Clin Exp Ophthalmol. 2015;253:1397–402. [DOI] [PubMed] [Google Scholar]
- 7.Borchman D, Foulks GN, Yappert MC, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010;44:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreau K, Callan C, Kottaiyan R, et al. Temperatures of the ocular surface, lid, and periorbital regions of Sjögren’s, evaporative, and aqueous-deficient dry eyes relative to normals. Ocul Surf. 2016;14:64–73. [DOI] [PubMed] [Google Scholar]
- 10.Sledge S, Henry C, Borchman D, et al. Human meibum age, lipid-lipid interactions and lipid saturation in meibum from infants. Int J Mol Sci. 2017;18:E1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borchman D, Foulks GN, Yappert MC, et al. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers 2007;87:124–133. [DOI] [PubMed] [Google Scholar]
- 12.Georgiev G, Eftimov P, Yokoi N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp Eye Res. 2017;163:17–28. [DOI] [PubMed] [Google Scholar]
- 13.Georgiev G, Yokoi N, Ivanova S, et al. , Surface relaxations as a tool to distinguish the dynamic interfacial properties of films formed by normal and diseased meibomian lipids. Soft Matter 2014;10:5579–88. [DOI] [PubMed] [Google Scholar]
- 14.Nencheva Y, Ramasubramanian A, Eftimov P, et al. , Effects of Lipid Saturation on the Surface Properties of Human Meibum Films. Int J Mol Sci. 2018;19: E2209. [DOI] [PMC free article] [PubMed] [Google Scholar]