Abstract
Adolescents living with HIV (ALHIV, ages 10-19) are retained in care at low rates, resulting in poor clinical outcomes. We sought to define barriers and facilitators to retention experienced by perinatally-infected ALHIV in western Kenya. This qualitative study purposefully sampled hospitalized ALHIV (both engaged and not currently engaged in care), ALHIV engaged in outpatient care, and caregivers of ALHIV. In total, 116 ALHIV and caregivers participated in interviews or focus group discussions. Complex challenges related to the effects of both stigma and poverty at multiple socio-ecological levels pose the greatest barriers to adolescent retention in HIV care. Adolescents with positive relationships with family, clinic, and/or peers with the resources to support their care are facilitated to overcome these barriers. Conversely, adolescents with few of these supports due to orphanhood, caregiver illness, severe poverty, family conflicts, negative relationships with healthcare workers, or isolation, have the greatest challenges staying in care, and may be at risk of disengagement. Emerging from narratives of disengagement are experiences of trauma, which contribute to isolation, mental health challenges, and difficulties engaging in care. Retention of the most vulnerable adolescents will require interventions to mitigate the impacts of stigma, poverty, mental health issues, and limited social support on their engagement in HIV care.
Keywords: adolescents living with HIV, continuity of patient care, antiretroviral therapy, lost to follow-up, sub-Saharan Africa
Introduction
There are 1.8 million adolescents living with HIV (ALHIV, ages 10-19) globally (UNAIDS, 2018). ALHIV experience low rates of retention in care, resulting in poor clinical outcomes and HIV-associated mortality (Auld et al., 2014; Kariminia et al., 2018). ALHIV face challenges in care that are specific to adolescent development and transitions (Enane, Vreeman, & Foster, 2018c). Adolescents with perinatally-acquired HIV may have lost parents to HIV, and are frequently diagnosed late with advanced illness (Ferrand et al., 2007). During adolescence, they will need to learn of their HIV status and to navigate care independently; adhere well to ART; and cope with a life-threatening, heavily-stigmatized illness; while developing peer relationships and meeting the developmental tasks of adolescence (Enane, Vreeman, & Foster, 2018c).
To improve ALHIV care engagement, it is critical to examine adolescent-specific barriers and facilitators to retention in care (Kariminia et al., 2018). Few qualitative studies have focused on adolescent barriers to retention, separate from other challenges in the care cascade (Hall et al., 2016; Williams, Renju, Ghilardi, & Wringe, 2017). We sought to define barriers and facilitators to retention experienced by perinatally-infected ALHIV in western Kenya. We used a qualitative approach with key informant interviews (KIIs) and focus group discussions (FGDs) with a diverse set of ALHIV and their caregivers in both clinic and hospital settings.
Methods
This qualitative study was performed from April to November, 2017, at Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya, and the associated, co-located HIV treatment clinics, MTRH AMPATH Centre and Rafiki Centre for Excellence in Adolescent Health. MTRH is the major referral hospital in western Kenya and hosts the largest AMPATH pediatric clinic. MTRH and AMPATH recently established the Rafiki Centre to provide comprehensive adolescent-centered services, including HIV testing, prevention, and treatment. Non-disclosed ALHIV are treated in the pediatric clinic at MTRH AMPATH Centre, with transition to Rafiki Centre after HIV disclosure.
Procedures
We utilized a conceptual model incorporating adolescent developmental needs and concerns in HIV care within a socio-ecological framework, informed by literature review and previous work in this area (Figure 1) (Bekker, Johnson, Wallace, & Hosek, 2015; Enane, Mokete, Joel, Daimari, et al., 2018b; Enane, Vreeman, & Foster, 2018c; Mburu et al., 2014; Viner et al., 2012). Retention is conceived of as the long-term engagement of ALHIV in care services. Missed visits and visit adherence are measures of care engagement which correlate with HIV outcomes (Mugavero et al., 2014; Zinski et al., 2015). We considered disengagement from care to be a gap in kept visits of at least 6 months (Chi et al., 2011; Geng et al., 2010). While medication non-adherence is an outcome of missed visits and disengagement, it is considered a separate behavior and is not specifically investigated here. Interview and FGD guides elicited open-ended narratives regarding barriers to retention and how these are navigated, facilitators to care, reasons for missed appointments or disengagement, and facilitators to returning to care. Probes investigated relevance of stigma, mental health, disclosure, and transition. Areas of needed intervention were explored, focused around elicited barriers to retention.
Figure 1.
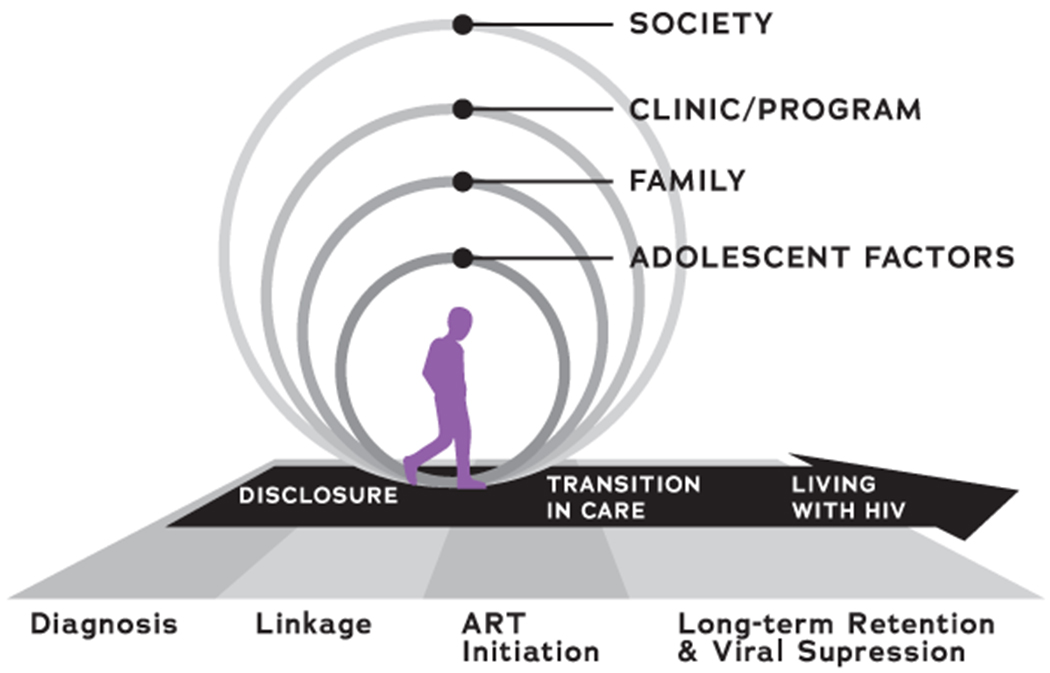
Conceptual model guiding the qualitative approach of this study.
We purposefully sampled ALHIV (ages 10-19, with documented HIV infection) and caregivers of ALHIV among the following: hospitalized ALHIV (engaged or not engaged in care) and ALHIV engaged in outpatient care. A comprehensive sample of hospitalized ALHIV and their caregivers was recruited from the pediatric and medical wards at MTRH. We did not recruit from the labor and delivery ward, because we sought to evaluate barriers to retention in adolescent HIV care separately from PMTCT care provision. Adolescents engaged in care were purposively sampled with attention to age, gender, and disclosure status. Non-disclosed adolescents were recruited for KIIs, and disclosed adolescents were recruited for FGDs. Caregivers of both groups of engaged adolescents were recruited for FGDs.
Questionnaires ascertained demographic variables. Clinical variables were abstracted from outpatient and hospital medical records. KIIs were performed separately for hospitalized adolescents and their caregivers. FGDs were conducted with disclosed adolescents (stratified by age 10-14 or 15-19, and gender), and with caregivers (separated by gender). A trained interviewer conducted KIIs; she partnered with a trained facilitator to conduct FGDs. All sessions were done in Kiswahili, audio-recorded, translated and transcribed into English. All recordings and transcripts were reviewed by both a professional translator/transcriber and a study team member to confirm accurate translation.
Sample size depended on thematic saturation within each participant cadre. We anticipated interviewing 15-30 hospitalized ALHIV and their caregivers, and 15-30 non-disclosed ALHIV engaged in care. We anticipated holding 3-4 FGDs/stratum with 5-10 ALHIV, and 4 FGDs with 5-10 caregivers.
Ethics
All participants provided informed consent; adolescent minors provided assent, and consent from their primary caregiver. Consent procedures included assurances that participation was voluntary and that declining to participate would not impact the adolescent’s clinical care. Strict procedures were maintained throughout to ensure confidentiality and minimize the risk of inadvertent disclosure to non-disclosed adolescents. Confidentiality was maintained by performing recruitment, consent, and procedures in a private space. Acutely ill adolescents were not interviewed; instead the primary caregiver was interviewed regarding the adolescent’s care. For adolescents who presented to clinic unaccompanied, their caregiver was called to request in-person consent. A disclosure screening tool was utilized to ascertain adolescent awareness of their HIV status. If full disclosure could not be ascertained, an abbreviated interview guide was used, which did not mention HIV. Only disclosed adolescents were enrolled in FGDs. Adolescents were fully informed that all FGD participants would be ALHIV, that questions would revolve around retention, and that they could decline participation if uncomfortable in such a group. No ALHIV declined for this reason. Most disclosed ALHIV were involved in group activities as part of care, and expressed feeling comfortable discussing challenges with peers. ALHIV were excluded if their medical teams determined a non-perinatal route of HIV infection to be likely. The study protocol was approved by the Institutional Research and Ethics Committee—constituted jointly by Moi University College of Health Sciences and by MTRH—and by the Institutional Review Board at Indiana University.
Analysis
Demographic and clinical data were analyzed through summary descriptive statistics using Stata 14.1 (StataCorp, LLC, College Station, Texas, USA). Review of transcripts concurrent with data collection allowed for preliminary thematic analysis and adjustments to guides to elicit detailed narratives. Open coding of select rich transcripts was done by two team members, and led to an initial set of codes. These were organized according to themes that emerged from the data and by the research questions and conceptual model. A codebook of code definitions and examples was generated. Coding of all transcripts was done by two team members, using Dedoose software (SocioCultural Research Consultants, LLC, Manhattan Beach, California, USA). Exploration of codes by adolescent characteristics [age, gender, disclosure, hospitalization, or (dis)engagement] was performed to refine the analysis by these factors. A set of overarching themes was formulated in discussion with the full study team.
Results
We conducted KIIs with 6 hospitalized ALHIV, 16 caregivers of hospitalized ALHIV, and 11 non-disclosed engaged ALHIV; 8 FGDs with 55 disclosed engaged ALHIV; and 4 FGDs with 28 caregivers of engaged ALHIV. In total, 116 participants provided data for this study regarding the care of 102 ALHIV (Table 1). Visit adherence was suboptimal across participant groups (averaging 67-74%), and history of disengagement was most common among hospitalized adolescents (54%). Viral suppression was lowest among hospitalized adolescents (14%). Major themes emerging from interviews and FGDs are presented, according to socio-ecological domains, with accompanying illustrative excerpts (Table 2).
Table 1.
Adolescent demographic and clinical characteristics according to site of enrollment of the adolescent and/or caregiver.†
| Characteristics | Hospital Wards | Pediatric Clinic | Adolescent Clinic | Total |
|---|---|---|---|---|
| Number, n (%) | 21 | 16 | 65 | 102 |
| Age group | ||||
| 10 to 14 | 12 (57.1) | 15 (93.8) | 31 (47.7) | 58 (56.9) |
| 15 to 19 | 9 (42.9) | 1 (6.3) | 34 (52.3) | 44 (43.1) |
| Female gender | 10 (47.1) | 8 (50.0) | 36 (55.4) | 54 (52.9) |
| Mother and/or father deceased, n=99 | 13 (61.9) | 5 (31.3) | 43 (66.2) | 61 (59.8) |
| Mother deceased (only) | 7 (53.9) | 1 (20.0) | 9 (20.9) | 17 (27.9) |
| Father deceased (only) | 3 (23.1) | 3 (60.0) | 19 (44.2) | 25 (41.0) |
| Both mother and father deceased | 3 (23.1) | 1 (20.0) | 15 (34.9) | 19 (31.2) |
| Primary caregiver | ||||
| Mother | 9 (42.9) | 11 (68.8) | 33 (50.8) | 53 (52.0) |
| Father | 2 (9.5) | 2 (12.5) | 9 (13.8) | 13 (12.7) |
| Other | 10 (47.6) | 3 (18.8) | 23 (35.4) | 36 (35.3) |
| School status | ||||
| Boarding school | 1 (5.0) | 0 | 9 (16.7) | 10 (11.1) |
| Day school | 12 (60.0) | 16 (100.0) | 43 (79.6) | 71 (78.9) |
| Neither | 7 (35.0) | 0 | 2 (3.7) | 9 (10.0) |
| Age at initiation of antiretroviral therapy, median years (IQR), n=91 | 8.0 (6.7-12.1) | 3.2 (1.6-5.7) | 7.9 (5.5-11.1) | 7.3 (5.1-10.4) |
| Time since initiation of antiretroviral therapy, median years (IQR), n=91 | 7.2 (1.6-11.7) | 7.7 (6.0-9.1) | 7.6 (4.5-9.7) | 7.6 (4.5-9.8) |
| New diagnoses of HIV (within 6 months), n=99 | 4 (21.1) | 0 | 1 (1.6) | 5 (5.1) |
| Viral load suppressed (<=40 copies/mL) | 3 (14.2) | 9 (56.3) | 31 (47.7) | 43 (42.2) |
| Visit adherence‡, mean % (sd), n=93 | 73.8 (5.5) | 67.1 (6.2) | 70.0 (7.6) | 70.0 (7.3) |
| Ever experienced a gap ≥180 days between kept visits, n=93 | 7 (53.9) | 3 (18.8) | 18 (28.1) | 28 (30.1) |
For some participants, both the adolescent and caregiver participated in a key informant interview and/or focus group discussion.
Visit adherence represents proportion of scheduled visits kept. Visits were considered kept if the adolescent attended clinic within +/− 7 days of their scheduled visit.
Table 2.
Illustrative qualitative excerpts regarding barriers and facilitators to adolescent retention in HIV care, from study participants.†
| Excerpt Number | Barrier or facilitator to retention | Excerpt |
|---|---|---|
| Adolescent- and family-level factors | ||
| 1 | Stigma | Stigma can even kill someone. My sister has been a victim of stigma. After our parents passed on, it came out that she has HIV. Even family members started sidelining her, telling her, ‘will you live?;’ mocking and discouraging her. It hurt her to a level where she was saying, ‘let me just die.’ It made her [school] performance go so far down. When you would ask her the reason, she couldn’t say, but it was all stigma. Sometimes when she had to go to clinic, she would say that she is going somewhere else, so that [family] would not know. Even if she is sick, she doesn’t go to [hospital]. It stigmatized her to a level that she saw that this thing is so bad, it is better to die. (Brother, adolescent female) |
| 2 | Poverty | You know, we rushed to this place of yours just to prolong our days, but some of us don’t have decent lives – tunakaa mbaya. (Mother, adolescent male) |
| 3 | Trauma, conflict, or abuse | I got married out of desperation. I didn’t have a caring parent. I was being mistreated [by a step-parent]. So I decided to start my life. I didn’t look at the consequences. That is why I rushed to marriage, because there was no one else who could help me. [My step-parent wouldn’t bring me to clinic] – never, not one day. (Adolescent female) |
| 4 | Mental health challenges, including suicidality and passive suicidality | [After a fight with the doctors,] I refused to come back to clinic. I didn’t know [death] will take longer; what I knew was I was leaving the clinic and we will die immediately. I was like someone who had given up on life and wished my child and I would die. I knew we will be gone because it is the medication which was prolonging our lives, because we had been told never to stop taking the medicines. I even started drinking. The child was okay [at that time. After two years out of care,] the child became very sick. (Mother, adolescent male) |
| Clinic-level factors | ||
| 5 | Relationships with clinic staff | The [clinic staff] are making her at least feel comfortable, because she feels someone cares for her. Sometimes she feels more loved than even being at home. When she goes to the clinic, she says that at least she gets someone who tells her, ‘I love you,’ or, ‘you are well, you will make it.’” (Brother, adolescent female) |
| 6 | Peer support interventions | Now that he is a peer, he won’t feel that he is alone. You know you may think that it is you alone and life has to end from there. So as you get people of your age, and they are telling you that life is still possible, life is positive and you can still make it. It can make someone think ahead [to the future]. (Father, adolescent male) |
| School-level factors | ||
| 7 | Disclosures at school | One day, I coughed in class and the teacher said, ‘You see this one, she has HIV.’ I almost cried. (Adolescent female) |
| 8 | Requesting absence from school | When she asked the teacher [for permission to] attend clinic, the teacher demanded which clinic it was for. She could not disclose this to the teacher. The teacher told her to finish the exams first and go to clinic later. So, it was difficult for her to go. (Brother, adolescent female) |
| Societal factors | ||
| 9 | Desire for financial interventions | [With a financial intervention], I would just start my business. Because with the job I do sometimes I am not given time to come to clinic, and you don’t feel like leaving it, and there are only two options, you leave the clinic or leave the job. (Adolescent female) |
| 10 | Desire for stigma reduction | [Stigma reduction] will make this child feel appreciated despite having HIV. If they feel they are part of the community, it will enable the child to continue well with his/her care. (Father, adolescent male) |
Numbered excerpts are referenced in the manuscript text.
Including inpatient and outpatient participants, we explored the challenges that most ALHIV experience and navigated to continue in care, and contrasted these descriptions with the severe challenges that ultimately resulted in disengagement from care.
Reasons for missed visits included common barriers in this context. Transportation costs were frequently cited, and required advanced planning, saving money, or walking long distances. Also frequent was being unable to miss school, and facing difficulties or stigma when requesting absence to attend clinic. Forgetting appointments was common. Despite these barriers, when visits were missed, participants reported being closely followed and rescheduled.
Reasons for disengagement were more complex, with multiple intersecting challenges for highly vulnerable adolescents (Figure 2). Stigma emerged as a dominant reality for ALHIV, with particular influence on the adolescent developmental tasks of investigating one’s identity, forming peer relationships, and developing autonomy in care. The ability of ALHIV and families to navigate and overcome stigma—anticipated, enacted, and internalized—emerged as a key element to retention in care. Poverty and economic issues influenced multiple levels of the socio-ecological model. Factors at the family, clinic, and community level impacted whether an adolescent was able to access care despite pervasive stigma and economic challenges.
Figure 2.
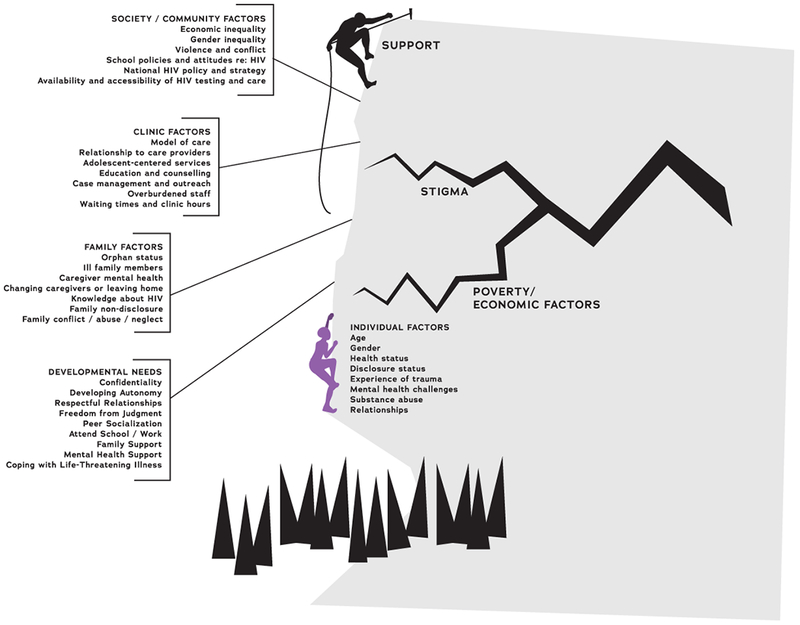
Multi-level factors influencing retention in or disengagement from HIV care, as experienced by perinatally-infected adolescents and their caregivers in western Kenya.
Broadly, retention in HIV care for these perinatally-infected adolescents was dependent on having supportive relationships and social networks, including among family, clinic staff, peers, teachers and classmates, and the community. Where these supports broke down, adolescents faced significant challenges continuing in care.
Adolescent and family-level factors
Family-level factors exerted greatest influence for younger or non-disclosed adolescents, though also impacted engagement throughout adolescence. Illness or death of caregivers commonly preceded disengagement. Orphanhood required ALHIV to live with other caregivers, who may not have been aware of their HIV status or adequately educated regarding their care. Stigma influenced disengagement of adolescents whose caregiver(s) were ill or deceased. Anticipated stigma within families caused ALHIV and caregivers to avoid disclosing to family or peers, despite needing support to continue care. Enacted stigma within families, directed toward the adolescent or caregiver, also influenced disengagement (Excerpt 1).
Family poverty resulted in financial barriers and food insecurity; severe poverty was further compounded by social isolation (Excerpt 2).
Narratives described prominent, frequent experiences of trauma, particularly for adolescents with history of disengagement. Trauma included ordeals living with HIV – such as learning one’s HIV status after the death of a parent, or being hospitalized – or experiences of conflict or abuse. Limited social support experienced by these adolescents and families exacerbated trauma, resulting in isolation, disengagement from care, and even passive suicidality for both adolescents and caregivers (Excerpt 3).
Mental health challenges, including isolation, depression, and alcohol abuse, were commonly described for adolescents and caregivers, particularly for those with history of disengagement. For caregivers, mental health challenges related to their own illness and history of trauma, deaths of loved ones, and experiences caring for a child with HIV. Isolation was common among adolescents. Multiple levels of stigma, interpersonal conflicts and lack of family/social support, resulted in serious mental health challenges. Suicidality and passive suicidality among caregivers and adolescents influenced disengagement from care (Excerpt 4).
Challenges with disclosure to the adolescent or others presented barriers. Lack of disclosure to close family members precluded seeking help for either the adolescent’s or the mother’s condition. Non-disclosed ALHIV have limited knowledge about their health and need for lifelong treatment. While they described following instructions regarding care, and expressed motivation to continue care to be healthy, multiple non-disclosed adolescents expressed that an adolescent may discontinue care after they “recover.” From narratives of disengagement, non-disclosed ALHIV’s care engagement depended on caregivers’ education and support, and disengagement could result from changes in their caregivers’ health, orphanhood, or needing to live with other relatives.
Despite these many difficult challenges, critical factors at the level of the adolescent and family supported retention. The motivation to be healthy and to receive HIV education were central to retention. For adolescents with history of disengagement, worsening illness often precipitated return to care. Supportive family relationships were essential, and in most cases, caregivers or relatives were identified as primary sources of encouragement. Disclosure to the adolescent and to family, and the support that this enables, also facilitated retention in care.
Clinic-level factors
Poor relationships with healthcare workers (HCW) were central in several narratives of disengagement. Conversely, supportive HCW relationships promoted retention. Time spent by HCW on counseling was valued (Excerpt 5).
Regarding areas of intervention, participants desired greater access to education about HIV, peer groups, disclosure support, and nutritional and financial support (Table 3). Peer interventions were particularly highly supported and desired. Peer support was seen as breaking through isolation, providing positive peer relationships and social support, offering respite from stigma and motivation to be healthy, and creating a venue for HIV education (Excerpt 6).
Table 3.
Areas of clinic- and program-level intervention addressing barriers and facilitators to adolescent retention in HIV care raised by participants.
| Socio-ecological level | Area of clinic- or program-level intervention | Examples |
|---|---|---|
| Adolescent intrapersonal needs | Mental health support | • Mental health counseling • Peer interventions |
| Family and adolescent | Strengthening family support | • Family counseling and mental health support for caregivers • HIV education • Supported disclosure to the adolescent or to family members |
| Financial and nutritional support | • Job training • Micro loans • Nutritional programs |
|
| Clinic and healthcare program | Improving relationships with care providers | • Dedicated training in adolescent HIV care • Increased time available to clinicians for visits with adolescent patients • Stigma reduction among healthcare workers and clinic staff |
| Community and peers | Peer support | • Peer support groups • Peer navigators • Peer mentors or educators |
| School support | • Liaisons between the care program and schools • Addressing school-related stigma and barriers to care • Improved HIV curricula |
|
| Stigma reduction in the community | • Stigma reduction campaigns |
School factors
School-related barriers to retention were common; centering around school-based stigma and inaccurate or stigmatizing teaching around HIV. ALHIV reported disclosures of their HIV status in school (Excerpt 7). Further barriers included the need to request absence from school to attend clinic and anticipated stigma if clinic attendance is discovered (Excerpt 8). A facilitator to retention was clinic scheduling of visits during school breaks. We did not identify school-level facilitators to care.
Societal factors
Social barriers impacting retention reflected pervasive effects of stigma and poverty. Limited employment opportunities and job discrimination impacted ALHIV engagement. Many participants favored financial interventions, job training, or community-level stigma reduction efforts (Excerpts 9 and 10).
Discussion
This qualitative study highlights the influence of family, clinic, and social support – or the lack thereof – on the retention or disengagement of perinatally-infected adolescents in HIV care. Given the influences of social determinants, developmental concerns, and adolescent trajectories in HIV care, we find that our adapted socio-ecological model provides an excellent framework in which to investigate ALHIV retention and disengagement. It is crucial for ALHIV to have positive and reliable relationships with family members, peers, HCW, and teachers, and that these individuals have the resources to support their care. When these supportive relationships are broken down or challenged by illness or death of caregivers, poverty, anticipated or enacted stigma, trauma, or violence; ALHIV or their caregivers may suffer from severe isolation, mental health issues, or suicidality, and risk disengagement from care and consequent poor outcomes or death.
We highlight barriers and facilitators to retention that evolve across the trajectories of adolescent development and transitions in care. Transportation costs were challenging across participant groups, and can range from $0.25-$7.44 USD, often far outpacing family earnings (Wachira, Middlestadt, Vreeman, & Braitstein, 2012). Younger adolescents may specifically benefit from family-level interventions promoting caregiver mental health, social or financial support, or supported disclosure to relatives to support care. Non-disclosed adolescents may benefit from disclosure support interventions which allow them to take part in peer groups—a key facilitator to retention—and become more knowledgeable about their care and need for life-long treatment. Peer support was widely endorsed to facilitate retention.
We did not identify key factors related to adolescent gender. This is likely due to our focus on perinatally-infected adolescents, whose engagement may be dominated by experiences surviving perinatal HIV, which may not necessarily differ greatly according to gender. We also may not have had sufficient numbers in each stratum to detect gender differences. Further, we excluded pregnant adolescents, who likely experience unique barriers to retention (Enane, Davies, Leroy, Edmonds, et al., 2018a; Ronen et al., 2017). Our findings would likely have been different had we included recently-infected adolescents.
Multiple studies support the central role of social support in adolescent engagement with HIV services (Busza, Dauya, Bandason, Mujuru, & Ferrand, 2014; Cluver et al., 2015; Williams et al., 2017). A study of lost to follow-up older adolescents and youth in western Kenya reported findings that correlate with this study: relationships with family, teachers, and clinic staff, and their mitigating effects on stigma, influenced disengagement (Wolf et al., 2014). Retention of the most vulnerable adolescents will require interventions to mitigate the impacts of stigma, poverty, and limited social support on their engagement in HIV care (Enane, Vreeman, & Foster, 2018c). Participants in this study supported key areas of intervention to address gaps in social support for vulnerable adolescents at risk for disengagement (Table 3). Intensive interventions in these areas would need to be targeted for at-risk adolescents (MacPherson et al., 2015).
Peer interventions were highly desired by participants to support adolescent engagement and mental health. Research is needed to evaluate the impact of and expand access to peer interventions for ALHIV (Funck-Brentano et al., 2005; Genberg et al., 2016; MacPherson et al., 2015; Ruria et al., 2017). There is a need for disclosure support to allow adolescents to engage in peer groups and to facilitate HIV education that promotes retention (Dahourou, Raynaud, & Leroy, 2018; Menon, Glazebrook, Campain, & Ngoma, 2007). The urgent need for expanded mental health support for both ALHIV and caregivers is supported by this study (Dow et al., 2016; Vreeman, McCoy, & Lee, 2017).
Relationships with HCW influenced disengagement or retention among participants. Adolescents and families who are already vulnerable to stigma and who may have few avenues to voice concerns about negative clinic interactions may instead become disengaged and suffer poor outcomes. Interventions supporting effective engagement of HCW with adolescents and families, and mechanisms for patient advocacy, should be promoted.
Stigma reduction interventions were supported by participants, who described pervasive influences of stigma on adolescent engagement in HIV care. Such interventions may include community-wide campaigns, or may focus on ALHIV environments, through engagement with schools or clinics for dedicated training to reduce stigma and increase awareness of stigmatizing practices. While peer support may help ALHIV engage in care despite stigma; community-, school-, or clinic-level interventions may directly combat the stigmatizing attitudes that impact ALHIV.
Experiences of violence have recently been shown to be associated with decreased adherence among ALHIV (Kim et al., 2017). Other childhood traumatic experiences are frequent among ALHIV, particularly the death of one or both parents (Tepper, Zaner, & Ryscavage, 2017). Childhood adversity has been examined in association with multiple chronic illnesses (Oh et al., 2018; Wan et al., 2018). It remains to be explored how trauma in the HIV experience impacts care engagement or outcomes. In this study, traumatic experiences emerged in narratives of disengagement. The experience of trauma and its influence on HIV outcomes should be further investigated, and interventions to identify and support adolescents who have experienced trauma should be explored.
There are important limitations to this study. It should be emphasized that this study focused on the experiences of perinatally-infected ALHIV and their caregivers, and that we did not include adolescents on labor/delivery or maternity wards, or in PMTCT care. There is a need to specifically explore the experiences of pregnant ALHIV, recently-infected adolescents, and adolescents from key populations as they relate to retention in care (Enane, Davies, Leroy, Edmonds, et al., 2018a). While they experience many of the same challenges identified by this study, they have distinct vulnerabilities that need to be explored and addressed.
This study took place at a single center of a large HIV treatment program and referral hospital. However, the inclusion of hospitalized adolescents (some referred from other treatment programs or not in care), adolescents from both pediatric and adolescent clinics, and disclosed and non-disclosed adolescents, provided much variation in care experiences. In this respect, a strength of this study is the inclusion of both engaged, well ALHIV and very ill adolescents and their caregivers; this facilitated exploration of contrasting experiences and vulnerabilities that may precipitate disengagement from care.
In summary, long-term retention of the most vulnerable adolescents in HIV care will require interventions to mitigate the impacts of stigma, poverty, family-level challenges, and limited social support. Interventions increasing social support for vulnerable ALHIV may most directly support their retention in care. In particular, access to peer support is highly desired and valued by ALHIV and their caregivers as a facilitator to ongoing retention in care. There is a need for research to investigate the effect of peer interventions on retention of ALHIV and to study implementation of these interventions at scale.
Acknowledgments
We acknowledge and thank the adolescents and families who participated in this research. Salim Bakari, Jacqueline Gavana, and Mark Omollo performed data verification. Edward Kipkorir performed transcription and translation. Carole McAteer assisted with code book development. Figures courtesy of Dustin Lynch, Indiana Clinical and Translational Sciences Institute.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD095778. This work was additionally supported by the Thrasher Research Fund, the Indiana Center for AIDS Research, and the Indiana Clinical and Translational Sciences Institute, funded, in part by Award number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Dr. Vreeman’s time was supported in part by the East Africa International Epidemiology Databases to Evaluate AIDS (IeDEA) regional consortium, which is funded by multiple institutes of the National Institutes of Health, through award U01 AI069911. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests
The authors declare no conflicts of interest.
References
- Auld AF, Agolory SG, Shiraishi RW, Wabwire-Mangen F, Kwesigabo G, Mulenga M, et al. (2014). Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults--seven African countries, 2004-2013. MMWR. Morbidity and Mortality Weekly Report, 63(47), 1097–1103. [PMC free article] [PubMed] [Google Scholar]
- Bekker L-G, Johnson L, Wallace M, & Hosek S (2015). Building our youth for the future. Journal of the International AIDS Society, 18(2(Suppl 1)), 1–7. 10.7448/IAS.18.2.20027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busza J, Dauya E, Bandason T, Mujuru H, & Ferrand RA (2014). “I don’t want financial support but verbal support.” How do caregivers manage children’s access to and retention in HIV care in urban Zimbabwe? Journal of the International AIDS Society, 17(1), 1–9. 10.7448/IAS.17.1.18839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, et al. (2011). Universal Definition of Loss to Follow-Up in HIV Treatment Programs: A Statistical Analysis of 111 Facilities in Africa, Asia, and Latin America. PLoS Medicine, 8(10), e1001111–12. 10.1371/journal.pmed.1001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluver LD, Hodes RJ, Sherr L, Orkin FM, Meinck F, Lim Ah Ken P, et al. (2015). Social protection: potential for improving HIV outcomes among adolescents. Journal of the International AIDS Society, 18(7 (Suppl 6)), 1–7. 10.7448/IAS.18.7.20260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahourou D, Raynaud J-P, & Leroy V (2018). The challenges of timely and safe HIV disclosure among perinatally HIV-infected adolescents in sub-Saharan Africa. Current Opinion in HIV and AIDS, 13(3), 220–229. 10.1097/COH.0000000000000462 [DOI] [PubMed] [Google Scholar]
- Dow DE, Turner EL, Shayo AM, Mmbaga B, Cunningham CK, & O’Donnell K (2016). Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS Care, 28(7), 825–833. 10.1080/09540121.2016.1139043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enane LA, Davies M-A, Leroy V, Edmonds A, Apondi E, Adedimeji A, & Vreeman RC (2018a). Traversing the cascade: urgent research priorities for implementing the “treat all” strategy for children and adolescents living with HIV in sub-Saharan Africa. Journal of Virus Eradication, 4(Suppl 2), 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enane LA, Mokete K, Joel D, Daimari R, Tshume O, Anabwani G, et al. (2018b). “We did not know what was wrong-”Barriers along the care cascade among hospitalized adolescents with HIV in Gaborone, Botswana. PLoS ONE, 13(4), e0195372 10.1371/journal.pone.0195372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enane LA, Vreeman RC, & Foster C (2018c). Retention and adherence: global challenges for the long-term care of adolescents and young adults living with HIV. Current Opinion in HIV and AIDS, 13(3), 212–219. 10.1097/COH.0000000000000459 [DOI] [PubMed] [Google Scholar]
- Ferrand RA, Luethy R, Bwakura F, Mujuru H, Miller RF, & Corbett EL (2007). HIV Infection Presenting in Older Children and Adolescents: A Case Series from Harare, Zimbabwe. Clinical Infectious Diseases, 44(6), 874–878. 10.1086/511873 [DOI] [PubMed] [Google Scholar]
- Funck-Brentano I, Dalban C, Veber F, Quartier P, Hefez S, Costagliola D, & Blanche S (2005). Evaluation of a peer support group therapy for HIV-infected adolescents. Aids, 19(14), 1501–1508. [DOI] [PubMed] [Google Scholar]
- Genberg BL, Shangani S, Sabatino K, Rachlis B, Wachira J, Braitstein P, & Operario D (2016). Improving Engagement in the HIV Care Cascade: A Systematic Review of Interventions Involving People Living with HIV/AIDS as Peers. AIDS and Behavior, 20(10), 2452–2463. 10.1007/s10461-016-1307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. (2010). Understanding Reasons for and Outcomes of Patients Lost to Follow-Up in Antiretroviral Therapy Programs in Africa Through a Sampling-Based Approach. JAIDS Journal of Acquired Immune Deficiency Syndromes, 53(3), 405–411. 10.1097/QAI.0b013e3181b843f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Sou K-L, Beanland R, Lacky M, Tso LS, Ma Q, et al. (2016). Barriers and Facilitators to Interventions Improving Retention in HIV Care: A Qualitative Evidence Meta-Synthesis. AIDS and Behavior, 1–13. 10.1007/s10461-016-1537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariminia A, Law M, Davies M-A, Vinikoor M, Kaloustian KW, Leroy V, et al. (2018). Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. Journal of the International AIDS Society, 21(12), e25215 10.1002/jia2.25215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Mazenga AC, Yu X, Ahmed S, Paul ME, Kazembe PN, & Abrams EJ (2017). High self-reported non-adherence to antiretroviral therapy amongst adolescents living with HIV in Malawi: barriers and associated factors. Journal of the International AIDS Society, 20(1), 1–12. 10.7448/IAS.20.1.21437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson P, Munthali C, Ferguson J, Armstrong A, Kranzer K, Ferrand RA, & Ross DA (2015). Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIV care. Tropical Medicine & International Health, 20(8), 1015–1032. 10.1111/tmi.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mburu G, Ram M, Oxenham D, Haamujompa C, Iorpenda K, & Ferguson L (2014). Responding to adolescents living with HIV in Zambia: A social–ecological approach. Children and Youth Services Review, 45(C), 9–17. 10.1016/j.childyouth.2014.03.033 [DOI] [Google Scholar]
- Menon A, Glazebrook C, Campain N, & Ngoma M (2007). Mental health and disclosure of HIV status in Zambian adolescents with HIV infection: implications for peer-support programs. JAIDS Journal of Acquired Immune Deficiency Syndromes, 46(3), 349–354. 10.1097/QAI.0b013e3181565df0 [DOI] [PubMed] [Google Scholar]
- Mugavero MJ, Westfall AO, Cole SR, Geng EH, Crane HM, Kitahata MM, et al. (2014). Beyond Core Indicators of Retention in HIV Care: Missed Clinic Visits Are Independently Associated With All-Cause Mortality. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America, 59(10), 1471–1479. 10.1093/cid/ciu603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DL, Jerman P, Marques SS, Koita K, Boparai SKP, Harris NB, & Bucci M (2018). Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatrics, 18(1), 1–19. 10.1186/s12887-018-1037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen K, McGrath CJ, Langat AC, Kinuthia J, Omolo D, Singa B, et al. (2017). Gaps in Adolescent Engagement in Antenatal Care and Prevention of Mother-to-Child HIV Transmission Services in Kenya. Journal of Acquired Immune Deficiency Syndromes (1999), 74(1), 30–37. 10.1097/QAI.0000000000001176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruria EC, Masaba R, Kose J, Woelk G, Mwangi E, Matu L, et al. (2017). Optimizing linkage to care and initiation and retention on treatment of adolescents with newly diagnosed HIV infection. Aids, 31 Suppl 3, S253–S260. 10.1097/QAD.0000000000001538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper V, Zaner S, & Ryscavage P (2017). HIV healthcare transition outcomes among youth in North America and Europe: a review. Journal of the International AIDS Society, 20(Suppl 3), 21490 10.7448/IAS.20.4.21490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2018). UNAIDS Estimates. Retrieved July 26, 2018, from http://aidsinfo.unaids.org/
- Viner RM, Ozer EM, Denny S, Marmot M, Resnick M, Fatusi A, & Currie C (2012). Adolescence and the social determinants of health. Lancet, 379(9826), 1641–1652. 10.1016/S0140-6736(12)60149-4 [DOI] [PubMed] [Google Scholar]
- Vreeman RC, McCoy BM, & Lee S (2017). Mental health challenges among adolescents living with HIV. Journal of the International AIDS Society, 20(3), 1–10. 10.7448/IAS.20.4.21497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachira J, Middlestadt SE, Vreeman R, & Braitstein P (2012). Factors underlying taking a child to HIV care: implications for reducing loss to follow-up among HIV-infected and -exposed children. SAHARA-J: Journal of Social Aspects of HIV/AIDS, 9(1), 20–29. 10.1080/17290376.2012.665255 [DOI] [PubMed] [Google Scholar]
- Wan Y, Chen R, Ma S, McFeeters D, Sun Y, Hao J, & Tao F (2018). Associations of adverse childhood experiences and social support with self-injurious behaviour and suicidality in adolescents. The British Journal of Psychiatry, 1–7. 10.1192/bjp.2018.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Renju J, Ghilardi L, & Wringe A (2017). Scaling a waterfall: a meta-ethnography of adolescent progression through the stages of HIV care in sub-Saharan Africa. Journal of the International AIDS Society, 20(1), 1–17. 10.7448/IAS.20.1.21922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf HT, Halpern-Felsher BL, Bukusi EA, Agot KE, Cohen CR, & Auerswald CL (2014). “It is all about the fear of being discriminated [against]…the person suffering from HIV will not be accepted”: a qualitative study exploring the reasons for loss to follow-up among HIV-positive youth in Kisumu, Kenya. BMC Public Health, 14(1), 1565–11. 10.1186/1471-2458-14-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinski A, Westfall AO, Gardner LI, Giordano TP, Wilson TE, Drainoni M-L, et al. (2015). The Contribution of Missed Clinic Visits to Disparities in HIV Viral Load Outcomes. American Journal of Public Health, 105(10), 2068–2075. 10.2105/AJPH.2015.302695 [DOI] [PMC free article] [PubMed] [Google Scholar]


