Abstract
Objectives:
This study compared hospital readmission and mortality for patients with sepsis who received ceftaroline or daptomycin as first-line MRSA therapy.
Methods:
This retrospective comparative-effectiveness study included adults ≥18 years old hospitalized in the United States Veterans Health Care System with sepsis between 10/1/2010–9/30/2014, who received ceftaroline or daptomycin within 14 days of hospital admission as the first antibiotic effective against methicillin resistant Staphylococcus aureus (MRSA). Patients with pneumonia, and those who received both study drugs, were excluded. Baseline characteristics were compared using Chi-square, Fischer’s exact, Student’s t, and Wilcoxon Rank Sum tests. Patient outcomes were compared with multivariable logistic regression models.
Results:
409 patients were included (ceftaroline=67, daptomycin=342). Ceftaroline patients were older, less likely to be Black, more likely to have diabetes with complications, and had higher Charlson comorbidity scores. Median (interquartile range) time from admission to drug initiation was 1 (0–1) day for ceftaroline and 1 (1–3) day for daptomycin (p=0.01). Unadjusted hospital readmission rates for ceftaroline and daptomycin, respectively, were: 30-day (25%/37%, p=0.06), 60-day (27%/44%, p=0.008), and 90-day (28%/46%, p=0.01). Unadjusted mortality rates were: in-hospital (7%/12%, p=0.4), 30-day (3%/9%, p=0.1), 60-day (6%/12%, p=0.2), and 90-day (7%/15%, p=0.1). In multivariable models with all divergent baseline characteristics included as covariates, patients treated with ceftaroline were less likely to experience (OR, 95% CI): 30/60/90-day hospital readmission (0.54, 0.29–0.98; 0.42, 0.23–0.76; 0.42, 0.23–0.75) and 30/60/90-day mortality (0.23, 0.04–0.82; 0.34, 0.10–0.93; 0.34, 0.11–0.86).
Conclusion:
In patients with sepsis, ceftaroline was associated with fewer hospital readmissions and lower mortality as compared to daptomycin. Prospective investigations in larger, more generalized cohorts are needed to examine outcomes with specific MRSA therapies.
Keywords: comparative-effectiveness, ceftaroline, daptomycin, MRSA, sepsis
1. Introduction
Sepsis is a leading cause of mortality due to infections, especially in patients with underlying comorbidities [1–6]. Adequate and timely drug therapy is critical to prevent multiple organ dysfunction, morbidity, and mortality associated with sepsis [7]. The ideal empiric antibiotic therapy for sepsis is currently unclear, as there is little evidence that supports one specific antibiotic regimen over another. Treatment of suspected sepsis should begin initially with broad, empiric antibiotic coverage, followed by antibiotic de-escalation upon return of cultures and susceptibilities [8].
Vancomycin is well-studied, inexpensive, and effective for most MRSA infections. However, the use of vancomycin is not without concerns: bacterial resistance to vancomycin has emerged, and adverse effects, such as nephrotoxicity, may limit its use in certain patients [9–11]. A review of the literature available through 1993, conducted by Cantu et al., determined that the frequency of nephrotoxicity due to vancomycin monotherapy was 5–7% [12]. Currently, the available evidence does not support widespread use of vancomycin alternatives to prevent nephrotoxicity. However, in selected patients we may need other antibiotics.
Results of prior studies suggest that ceftaroline and daptomycin may be effective, independent agents for the treatment of MRSA bacteremia. Use is associated with positive outcomes and lower rates of nephrotoxicity, especially in patients with nosocomial blood stream infections [13–15]. Although the findings from these studies support the use of ceftaroline and daptomycin, multicenter comparative studies of ceftaroline and daptomycin are absent from the literature. Furthermore, guidelines fail to address newer anti-MRSA agents, despite positive results for the use of ceftaroline and daptomycin in treating invasive infections.
Ceftaroline and daptomycin are utilized in the inpatient setting at hospitals within the Veteran’s Health Administration (VHA) network for the treatment of various infections, including sepsis. Both antibiotics are on the VHA formulary, but often require infectious disease consults for their use. These drugs represent potential antibiotics for the treatment of sepsis caused by methicillin-resistant Staphylococcus aureus (MRSA) and other hospital- and community-acquired pathogens including Staphylococcus and Streptococcus species; however, the comparative effectiveness of these agents has not been established.
Given the gap in clinical literature, the purpose of the present study was to compare the clinical outcomes in patients treated with either first-line ceftaroline or daptomycin for sepsis across the VHA system. The objective of this study was to compare the real-world effectiveness of first-line ceftaroline and daptomycin for the treatment of sepsis through assessment of 30-, 60-, and 90-day hospital readmission and 30-, 60-, and 90-day patient mortality rates.
2. Methods
2.1. Data Source
Data were obtained from the VHA electronic medical record (EMR), which includes administrative, clinical, laboratory, and pharmacy data. The VHA EMR is linked between all United States-based VHA sites. The data compiled for this study thus represents nationwide VHA use of ceftaroline and daptomycin within the study period. The Institutional Review Board of the University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development committee approved this study.
2.2. Study Design
This was a retrospective cohort, comparative-effectiveness study of adults aged ≥18 years in the United States (US) VHA with a diagnosis code for sepsis during their hospital stay between October 1, 2010 and September 30, 2014.
Variables were determined prior to study initiation and included patient characteristics (age, race, Hispanic ethnicity, comorbidities, prior medications, and concomitant medications), treatment setting, ceftaroline and daptomycin use, and treatment outcomes (length of stay, hospital readmission, patient mortality). International Classification of Diseases, 9th Revision (ICD-9) and Clinical Modification Diagnosis (CSS) codes were utilized to identify patients with sepsis and comorbidities.
2.3. Inclusion and Exclusion Criteria
This study was designed to assess effectiveness of ceftaroline and daptomycin as first-line MRSA therapy. Patients hospitalized within the study period with a diagnosis of sepsis and an order for ceftaroline or daptomycin within 14 days of hospital admission were included if they had not previously received a drug with activity against MRSA.
This study did not evaluate the use of either agent in the treatment of patients with pneumonia. It is not appropriate to evaluate the treatment success of daptomycin in treating sepsis secondary to pneumonia, as daptomycin is not effective in respiratory infections due to inactivation by lung surfactant [16]. Therefore, patients with pneumonia were excluded from this study.
Previous studies have described the combined use of ceftaroline and daptomycin in the treatment of infection after vancomycin failure; however, such use is related to an increased potential for antibiotic resistance and potential toxicity [17]. Fewer than five patients in our cohort received both ceftaroline and daptomycin simultaneously; those patients were excluded from all analyses.
2.4. Statistical Analysis
Two-way statistical tests, including Chi-square, Fisher’s exact, Student’s t, and Wilcoxon Rank Sum tests, were used to compare baseline characteristics of patients treated with each agent. Baseline characteristics found to be significantly different (p ≤ 0.05) with two-way statistical tests were selected as covariates for the multivariable models. Multivariable analyses were conducted to account for the effects of dissimilar baseline characteristics between treatment arms. JMP Pro 12.1.0 (SAS Corp) was used for all statistical analyses.
3. Results
A total of 409 patients met study criteria (ceftaroline=67 and daptomycin=342). Ceftaroline patients were older (median (interquartile range [ICR]) age: 63 (59–71) vs. 60 (55–68), p = 0.02), less likely to be Black (19% vs. 30%, p=0.03), more likely to have a history of diabetes with complications (30% vs. 19%, p=0.05), and had higher Charlson comorbidity scores (median [IQR] score: 6 (4–9) vs. 5 (3–7), p<0.01). See Table 1 for a full comparison of all baseline characteristics.
Table 1.
Baseline characteristics by treatment group
| Variables | Ceftaroline (n = 67) |
Daptomycin (n = 342) |
P-value |
|---|---|---|---|
| Age (years), median (IQR) | 63 (59–71) | 60 (55–68) | 0.0158 |
| Male, % | 96% | 95% | 1.000 |
| Married, % | 49% | 46% | 0.6177 |
| Race & Ethnicity, % | |||
| White, non-Hispanic | 68% | 59% | 0.1595 |
| Black, non-Hispanic | 19% | 30% | 0.0265 |
| Hispanic | 9% | 7% | 0.6864 |
| Other, non-Hispanic | 4% | 2% | 0.2242 |
| Missing | 0% | 2% | 0.3634 |
| Charlson comorbidity score, median (IQR) | 6 (4–9) | 5 (3–7) | 0.0093 |
| Comorbidities, % | |||
| Congestive heart failure | 33% | 23% | 0.0994 |
| COPD | 36% | 25% | 0.0784 |
| Cerebrovascular disease | 21% | 15% | 0.2603 |
| Dementia | 1% | 2% | 1.000 |
| Diabetes (complications) | 30% | 19% | 0.0468 |
| Diabetes (no complications) | 58% | 46% | 0.0789 |
| Hemi/paraplegia | 1% | 6% | 0.1482 |
| HIV | 0% | 1% | 1.000 |
| AIDS | 1% | 1% | 0.5932 |
| Liver (mild) | 3% | 6% | 0.5498 |
| Liver (mod/severe; cirrhosis) | 7% | 8% | 1.000 |
| Cancer | 24% | 16% | 0.1533 |
| Leukemia | 6% | 3% | 0.2838 |
| Metastatic cancer | 4% | 3% | 0.7104 |
| Myocardial infarction | 9% | 10% | 0.7467 |
| Peptic ulcer disease | 3% | 4% | 1.000 |
| Peripheral vascular disease | 25% | 20% | 0.3487 |
| Renal disease | 34% | 34% | 0.9852 |
| Rheumatic disease | 4% | 2% | 0.1696 |
| Dyslipidemia | 55% | 46% | 0.1499 |
| Hypertension | 82% | 73% | 0.1077 |
| Hemodialysis | 3% | 4% | 1.000 |
| Prior hospitalization (past 90 days), % | 31% | 31% | 0.955 |
| Prior antibiotics (past 90 days), % | 66% | 61% | 0.4524 |
| Intensive Care Unit (ICU), % | 22% | 29% | 0.2857 |
| Weight (lbs), median (IQR) | 202 (164–251) | 200 (169–240) | 0.832 |
Median (IQR) time from admission to drug initiation was 1 (0–1) day for ceftaroline and 1 (1–3) day for daptomycin (p=0.01). Concomitant antibiotic use was similar (≤5% different) for the ceftaroline and daptomycin groups, except for piperacillin/tazobactam (ceftaroline=1% vs. daptomycin=26%), ciprofloxacin (ceftaroline=1% vs. daptomycin=9%), and vancomycin (ceftaroline=24% vs. daptomycin=11%). Patients who received ceftaroline had a significantly shorter median length of hospital stay: 7 (3–15) vs. 10 (5–24), p = 0.03.
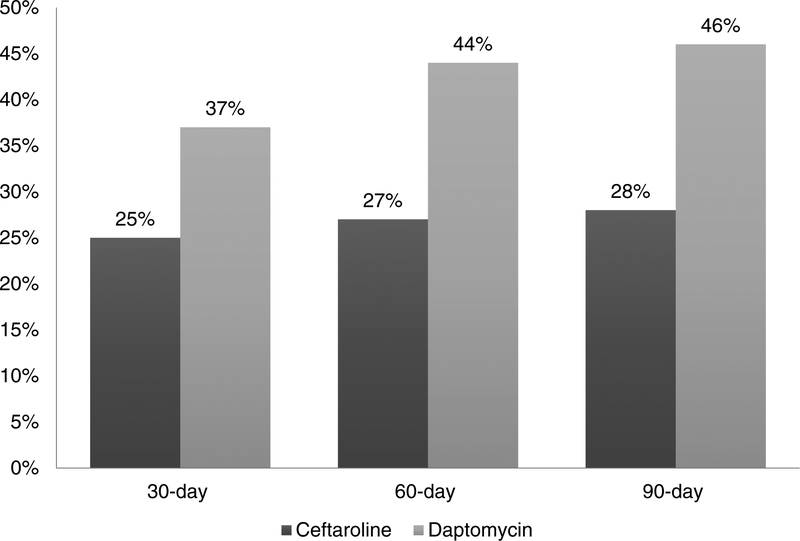
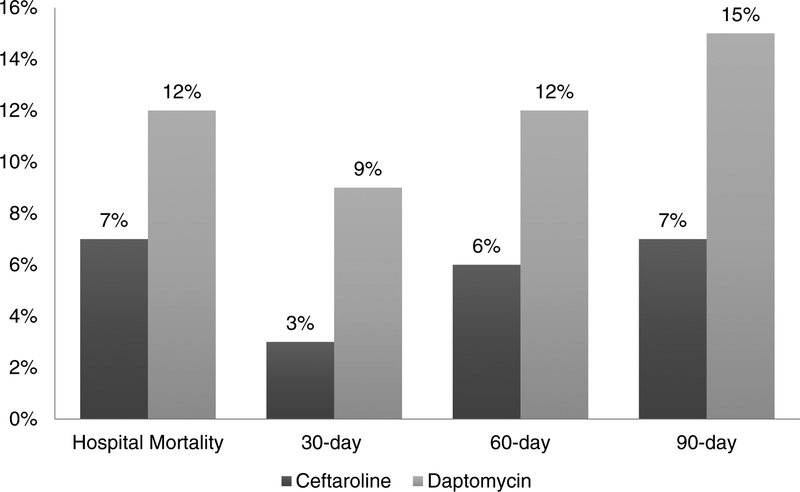
Regarding patient outcomes (Figures 1 and 2), unadjusted hospital readmission rates for ceftaroline and daptomycin were: 30-day (25% vs. 37%, p=0.06), 60-day (27% vs. 44%, p=0.008), and 90-day (28% vs. 46%, p=0.01). Unadjusted mortality rates were: in-hospital (7% vs. 12%, p=0.4), 30-day (3% vs 9%, p=0.1), 60-day (6% vs. 12%, p=0.2), and 90-day (7% vs. 15%, p=0.1).
Figure 1.
Hospital readmission: first-line ceftaroline versus first-line daptomycin
Figure 2.
Patient mortality: first-line ceftaroline versus first-line daptomycin
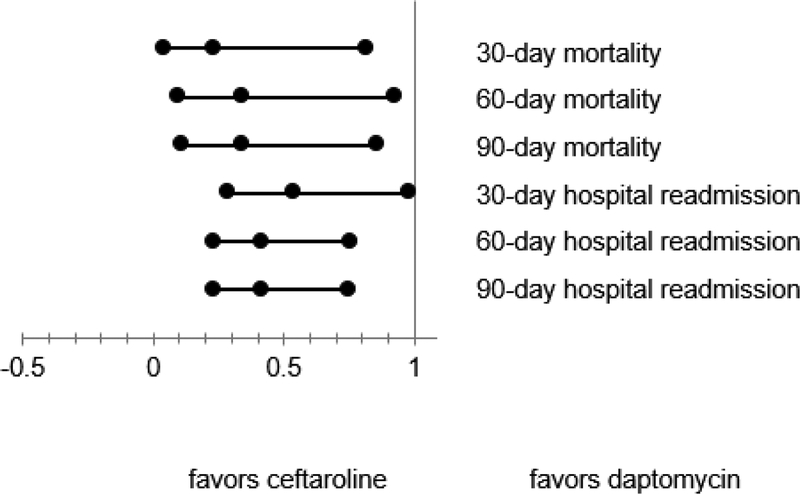
In multivariable models with all divergent baseline characteristics included as covariates (Figure 3), patients treated with ceftaroline were less likely than those treated with daptomycin to experience (OR, 95% CI): 30-,60-, and 90-day hospital readmission (0.54, 0.29–0.98; 0.42, 0.23–0.76; 0.42, 0.23–0.75) and 30-, 60-, and 90-day mortality (0.23, 0.04–0.82; 0.34, 0.10–0.93; 0.34, 0.11–0.86).
Figure 3.
Adjusted hospital readmission and patient mortality: first-line ceftaroline versus first-line daptomycin
4. Discussion
In this retrospective assessment of outcomes following real-world use of ceftaroline and daptomycin within the VHA for sepsis, multivariable models displayed lower mortality and readmission rates in patients treated with ceftaroline versus daptomycin at all time-points studied, 30-, 60-, and 90-days post-discharge.
Given the inherent potential for morbidity and mortality related to sepsis, selection of an appropriate first-line MRSA therapy is critical; this study reinforces use of ceftaroline as a viable first-line MRSA therapy agent in the treatment of such infections, one that may be more effective than daptomycin.
The results of this study parallel the findings of a previously reported small-scale case series [18]. Ho et al. found, through a small case series (n=6), that ceftaroline, when administered to patients with MRSA bacteremia or endocarditis due to persistent or recurrent bacteremia while on standard antibiotics (vancomycin or daptomycin), patients experienced sterilization after 13 days [18]. Both clearance and microbiological cure are strong indicators for potential clinical efficacy [19]. These results emphasize the potential use of ceftaroline in treating resistant infections, leading to the assumption that ceftaroline has the potential to be effective as first-line MRSA therapy in the treatment of diverse bacterial illness.
Lin et al., described 10 patients treated with ceftaroline for severe MRSA infections, including sepsis, endocarditis, and other deep-seated infections [20]. Seventy-percent of patients included achieved microbiologic cure and sixty-percent of patients experienced clinical cure after treatment with ceftaroline. Though the study lacks a comparator, these findings suggest that ceftaroline is safe and effective for the treatment of severe MRSA infections.
Our study goes beyond assessment of microbiologic outcomes and instead evaluates the clinical effectiveness of ceftaroline and daptomycin. In addition, it provides a direct comparison of ceftaroline and daptomycin. No other published study to date has evaluated such a comparison.
Fowler et al. compared daptomycin versus standard therapy (initial low-dose gentamicin plus either an antistaphylococcal penicillin or vancomycin) for bacteremia and endocarditis caused by Staphylococcus aureus and concluded that daptomycin was non-inferior to standard therapy [21]. Fowler et al. did not include patients on ceftaroline. In our study, ceftaroline had better outcomes than daptomycin, and the reason might be that ceftaroline is a beta-lactam. Sakoulas et al. indicated that beta-lactams offer adjunctive properties that other antibiotics do not have in enhancing immune clearance of bacteria and patients with penicillin-allergies who were denied beta-lactam antibiotics had inferior outcomes [22]. In addition, Geriak et al. demonstrated that first-line daptomycin plus ceftaroline combination improved mortality in MRSA bacteremia over standard of care [23].
By nature of the patient population served by the VHA, there are some limitations to the external validity. First, 95% of the patients included in this study were male. Black patients were more highly represented in the daptomycin treatment arm and may not reflect the outcome for the general patient population. However, the ethnic groups represented in this study closely parallel the patient population served by the VHA and we included patient race in our multivariable model. Second, patients treated within the VHA system benefit from the use of an integrated EHR system, including medical, clinic, prescription, and administrative data; as compared to patients treated at non-VHA facilities, the records are likely to be more complete. Furthermore, underlying disease states and comorbidities have the potential to affect hospital readmission and patient mortality. In this study, patients in each treatment arm were relatively similar at baseline. To eliminate bias, multivariable analyses were conducted to account for dissimilar baseline characteristics between treatment arms. Patients in the ceftaroline treatment arm had a higher baseline Charlson risk score, indicating patients were at higher risk prior even before drug treatment. Thus, in addressing this variable alone, it is likely that the patient’s condition would be more resilient in the daptomycin treatment arm, as their baseline condition is better than those treated with ceftaroline. Median time from admission to drug initiation was 1 day in both groups, but the variability in time to initiation of drug therapy was greater for daptomycin, leading to a statistically significant difference in time to initiation in favor of ceftaroline. Time to initiation of drug therapy is important, so we were quite pleased that our median time to initiation was short, and only 1 day, in both groups. We do not believe the greater variability in the time to initiation of daptomycin is responsible for the differences in outcomes between groups; nevertheless, this is another limitation of our study. Some clinical variables were unavailable and were therefore not included in the study. For example, the etiology of sepsis was unknown, and we did not exclude gram-negative infections because we did not have microbiology data.
Finally, a retrospective study innately invites bias; a controlled clinical trial would be more appropriate to assess the efficacy of each of these agents. However, real-world use of these medications reflects prescribing patterns and appropriate trends in patient response to treatment.
Despite its limitations, our study also has notable, important strengths. This study offers clinical data from a larger population than in previous studies; to date it is the largest comparative efficacy trial of ceftaroline and daptomycin in the real-world treatment of sepsis. Previous studies have been done evaluating each agent independently, especially for those conditions in which there are FDA-approved indications, yet there are no real-world comparative-effectiveness studies in sepsis.
The VHA is the largest integrated health care system in the United States, operating facilities in all 50 states. The VHA EMR system includes medical and clinic visits, pharmacy records, and administrative data. These repositories provide a comprehensive source for evaluating patient mortality even when it occurs outside of the hospital. As a result, this study offers information on the clinical-effectiveness of the selected antimicrobial agents in a real-world setting as opposed to clinical trials through use of comprehensive VHA medical records.
In evaluating the comparative efficacy of each agent, this study included patients of varying health status with various underlying comorbidities; patients with the potential for poor outcomes post-treatment were included regardless of anticipated lifespan, leading to the assumption that both agents have the potential to be effective, even when patients are severely immunocompromised.
5. Conclusion
This real-world effectiveness study of patients with sepsis demonstrates that first-line ceftaroline is associated with lower rates of hospital readmissions and lower patient mortality than first-line daptomycin. While this study provided preliminary information on the use of ceftaroline and daptomycin for MRSA infections, these findings must be confirmed or refuted in a larger, more diverse patient population.
Acknowledgements
This work was supported by TEF-IT-41, an investigator-initiated grant to Dr. Frei’s institution, from Actavis Pharmaceuticals (formerly Forest and Allergan Pharmaceuticals). Dr. Frei was supported in part by a NIH Clinical Research Scholar (KL2) career development award (National Center for Research Resources KL2 RR025766 [2010–2012] and National Center for Advancing Translational Sciences KL2 TR000118 [2013]) and a NIH Clinical and Translational Science Award (UL1 TR002645 and TL1 TR002647) during part of the time during which this study was conducted. This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
Footnotes
Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011;140:1223–31. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546–54. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- 4.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc 2015;12:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: a population-based study. Crit Care Med 2013;41:1450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttunen R, Aittoniemi J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J Infect 2011;63:407–19. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- 9.Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 2014;58 Suppl 1:S20–7. [DOI] [PubMed] [Google Scholar]
- 10.Fowler VG Jr., Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006;355:653–65. [DOI] [PubMed] [Google Scholar]
- 11.Culos KA, Cannon JP, Grim SA. Alternative agents to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Am J Ther 2013;20:200–12. [DOI] [PubMed] [Google Scholar]
- 12.Cantú TG, Yamanaka-Yuen NA, Lietman PS. Serum vancomycin concentrations: reappraisal of their clinical value. Clin Infect Dis 1994;18:533–43. [PubMed] [Google Scholar]
- 13.Moise PA, Culshaw DL, Wong-Beringer A, et al. Comparative effectiveness of vancomycin versus daptomycin for MRSA bacteremia with vancomycin MIC >1 mg/L: a multicenter evaluation. Clin Ther 2016;38:16–30. [DOI] [PubMed] [Google Scholar]
- 14.Casapao AM, Davis SL, Barr VO, et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother 2014;58:2541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–17. [DOI] [PubMed] [Google Scholar]
- 16.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52. [DOI] [PubMed] [Google Scholar]
- 17.Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014;36:1317–33. [DOI] [PubMed] [Google Scholar]
- 18.Ho TT, Cadena J, Childs LM, Gonzalez-Velez M, Lewis JS 2nd., Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother 2012;67:1267–70. [DOI] [PubMed] [Google Scholar]
- 19.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JC, Aung G, Thomas A, Jahng M, Johns S, Fierer J. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother 2013;19:42–9. [DOI] [PubMed] [Google Scholar]
- 21.Fowler VG Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. [DOI] [PubMed] [Google Scholar]
- 22.Sakoulas G, Geriak M, Nizet V. Is a Reported Penicillin Allergy Sufficient Grounds to Forgo the Multidimensional Antimicrobial Benefits of β-Lactam Antibiotics? Clin Infect Dis 2019;68:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geriak M, Haddad F, Rizvi K, et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother 2019;63:e02483–18. [DOI] [PMC free article] [PubMed] [Google Scholar]