Version Changes
Revised. Amendments from Version 1
In Material & Method Section: We have mentioned that at what temperature and when DBS samples transported from Bangalore to Mumbai. In Discussion Section: We have explained about why we have used two methods for DNA extraction, also give justification on 260/280 ratio obtained by these methods.
Abstract
Background: Limited infrastructure is available to collect, store and transport venous blood in field epidemiological studies. Dried blood spot (DBS) is a robust potential alternative sample source for epidemiological studies & bio banking. A stable source of genomic DNA (gDNA) is required for long term storage in bio bank for its downstream applications. Our objective is to optimize the methods of gDNA extraction from stored DBS and with the aim of revealing its utility in large scale epidemiological studies.
Methods: The purpose of this study was to extract the maximum amount of gDNA from DBS on Whatman 903 protein saver card. gDNA was extracted through column (Qiagen) & magnetic bead based (Invitrogen) methods. Quantification of extracted gDNA was performed with a spectrophotometer, fluorometer, and integrity analyzed by agarose gel electrophoresis.
Result: Large variation was observed in quantity & purity (260/280 ratio, 1.8-2.9) of the extracted gDNA. The intact gDNA bands on the electrophoresis gel reflect the robustness of DBS for gDNA even after prolonged storage time. The extracted gDNA amount 2.16 – 24 ng/µl is sufficient for its PCR based downstream application, but unfortunately it can’t be used for whole genome sequencing or genotyping from extracted gDNA. Sequencing or genotyping can be achieved by after increasing template copy number through whole genome amplification of extracted gDNA. The obtained results create a base for future research to develop high-throughput research and extraction methods from blood samples.
Conclusion: The above results reveal, DBS can be utilized as a potential and robust sample source for bio banking in field epidemiological studies.
Keywords: Dried blood spot (DBS), Whatman 903 cards, FTA cards, Human genomic DNA, Bio-banking, Epidemiology
Introduction
The concept of using dried blood spot (DBS) in new born screening was presented by Guthrie and Susie in 1963 1. DBS has been used for the last 5 decades by researcher in medical research. In field epidemiological studies there is a need for robust sample sources so they can be stored for long periods without any damage or spoilage. DBS is a much better option as compared to venous blood in low resource field setups for large epidemiological studies. Biomarkers reveals biological information from normal to disease condition and provide information about the disease condition, as it also acts as a prognostic marker. The collection of DBS is simple compared to venous blood collection as it only requires a finger prick, compared to venous puncture via needle for venous blood collection. Today DBS samples are utilized to test for a variety of health related markers including; infectious pathogens, HbA1c, total cholesterol, creatinine, uric acid, low density lipoprotein (LDL), high density lipoprotein (HDL), very low density lipoprotein (VLDL), Triglyceride and many more 2– 4. DBS is prepared by spotting 40–50µl of whole blood on Whatman 903 protein saver cards, air dried for 2 hours by hanging or by placing in a rack, and then packed it in sealed ziplock bags with desiccant. Other cards available for blood sampling include Ahlstrom, Whatman No.6, DMPK, FTA etc. however the Whatman 903 card is US Food and Drug Administration (FDA) approved for medical research 5. In epidemiological research the protein saver cards act as information storage devices in terms of blood based analytes and provide genetic, environmental, immunological information. Genomic DNA (gDNA) is a very robust and stable biological sample when stored on paper cards, and has been used for many decades 3, 4. RNA, which is less stable than gDNA in solution, appears to also be stable on DBS 6. Due to the small amount of blood in DBS, the obtained concentration of genetic material is also low but this problem can be overcome by amplification of the whole genome, and yield high quality DNA for performing assays, such as sequencing and genotyping arrays, at low cost 7. Limited studies are available regarding the use of DBS for downstream SNP genotyping following whole genome amplification 6, 8.
This study was performed with the aim of extracting maximal gDNA using archived DBS cards obtained from the Centre for Global Health Research (CGHR) Bangalore unit to establish its feasibility for downstream applications and biobanking in large scale epidemiological studies.
Methods
Ethical considerations and consent
The study was ethically approved by Institutional Ethics Review Board (IERB) of St. John’s Medical College and Hospital, Bangalore (India) with approval number IERB/1/77/05. After explaining the study to participants, informed written consent (as per norms of Indian Council of Medical Research (ICMR) Government of India) was obtained from volunteer participants.
Subjects enrollment
This was part of a multicenter study involving the Centre for Global Health Research (CGHR) Bangalore unit and Tata Memorial Centre, Mumbai unit, who worked together to conduct study DBS. DBS samples were collected at health checkup camps in rural and urban areas of Bangalore city through a sample registration system (SRS). 3000 DBS samples were prepared through finger prick method by using lancet (Accu Chek Softclix Lancet, Roche), puncture the finger site using lancet, drop of blood form which is lightly touch the circle of filter paper cards (GE Health Care Life Science, Catalog no. 10534612) and form valid DBS during health checkup camp by CGHR at Bangalore unit and transported to Tata memorial Centre (TMC) Mumbai for further analysis. Samples were collected between the years 2005–2007, but samples transported to Mumbai from Bangalore at ambient temperature in year 2013 and laboratory experiments conducted in 2016. DBS samples were collected by trained staff. Systematic random samples (n=40) were selected from top to bottom order from collected DBS. The following anthropometric measurements were recorded; height, weight, waist-to-hip ratio, blood pressure with gender and age. The complete study was explained to the subjects, and only voluntary participants aged between 18–49 years were included in this study after obtaining written informed consent as per the norms of Indian Council of Medical Research (ICMR) Government of India.
Sample collection, transport and storage
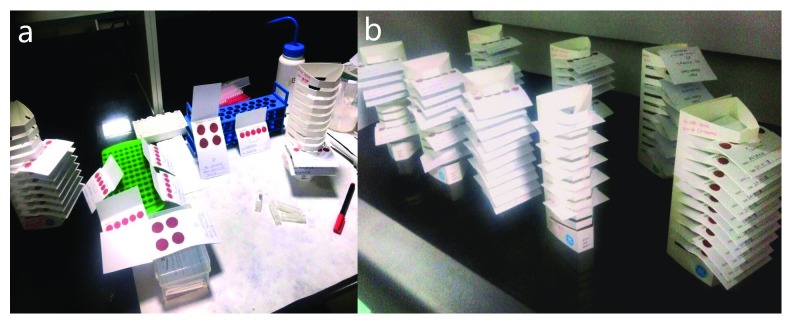
Finger prick blood DBS Preparation. Finger prick blood was collected using a lancet to puncture the fingertip. Once a full small drop of blood is formed it was lightly touched to the center of the circle on the filter paper (GE Health Care Life Science, Catalog no. 10534612) to form valid DBS. These were collected from study participants during health checkup organized at government schools, and at the center of villages. Cards were dried for 2 hours in velcro rack and packed in sealed ziplock bag with 1–2gm desiccant sachet ( Figure 1). Only valid DBS samples were used for gDNA extraction determined by the blood sample completely saturating each circle on the card, and not overlapping or merging with other blood circles. Prepared DBS samples were transported to the laboratory at the Centre for Cancer Epidemiology, Tata Memorial Centre, where they were stored in a -80°C refrigerator.
Figure 1. Preparation and drying of dried blood spot (DBS) cards.
( a) Prepared DBS cards. ( b) Drying of blood spots at room temperature.
Sample quality & validity
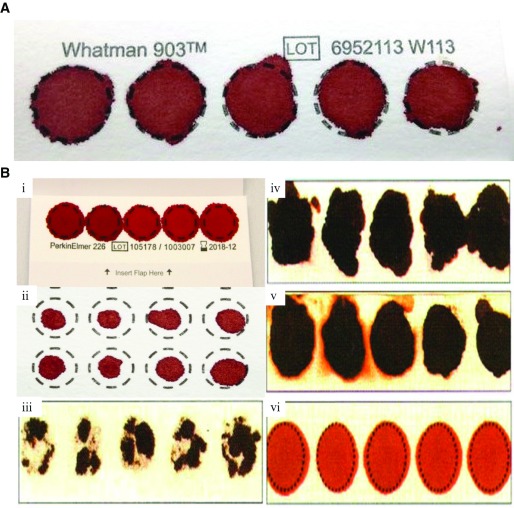
We have used only good quality DBS samples for gDNA extraction, it is defined as the complete saturation of whole blood over the complete circle of blood collection card 9, blood card should be labelled and blood should adsorb on both side of the card. We have used only valid samples for gDNA extraction ( Figure 2).
Figure 2. Valid / Invalid dried blood spot (DBS) specimens.
( A) Valid specimen. DBS with complete filled circle with proper air dry with no hemolyzed blood or serum ring. ( B) Invalid DBS Specimen. ( i) DBS with overlapping of spotted blood. ( ii) DBS with insufficient filled blood. ( iii) DBS with incomplete absorption which reduces blood volume. ( iv) DBS that is potentially rubbed and develop scratches. ( v) DBS with hemolyzed or contaminated blood. ( vi) DBS with improper air drying before packaging in ziplock bags.
DBS processing
A generic single hole 6mm punch plier was used to cut the blood spots from the Whatman 903 paper card. A punch of diameter 6mm represents approximately 8.7±1.9µl of blood spotted 10. 1 – 4 blood spots of size 6 mm punch was added to an eppendorf tube and incubated with 200µl PBS (readymade PBS buffer used with pH 7.4 supplied by Gibco with Ref No. 10010-023) overnight at room temperature. Our major aim was to extract the maximum amount of gDNA, therefore we have used 6mm × 1 spot to 6mm × 4 spots for extracting gDNA from DBS ( Table 1). We have used phosphate buffer saline (PBS) for extraction of completely dry blood matrix on Whatman 903 for easy gDNA extraction, because once the surface of blood spot becomes wet, it is easy to extract DNA from the adsorbed blood.
Table 1. Average genomic DNA (gDNA) concentration with different number of blood spots.
| Number of blood spots
used of size 6mm |
Average gDNA
concentration (ng/µl) |
Average
260/280 ratio |
Total elution
volume |
Total gDNA
Yield |
|---|---|---|---|---|
| 6mm × 1 spot (n=10) | 3.43 | 1.93 | 30µl | 102.9 ng |
| 6mm × 2 spots (n=10) | 6.38 | 2.18 | 30µl | 191.4 ng |
| 6mm × 3 spots (n=10) | 7.23 | 2.30 | 30µl | 216.9 ng |
| 6mm × 4 spots (n=10) | 8.91 | 2.84 | 30µl | 267.3 ng |
Genomic DNA extraction methods
We have applied 2 methods for gDNA extraction from DBS. ( 1) Column based (QIAamp DNA kit, Qiagen) ( 2) Magnetic bead based (ChargeSwitch Forensic DNA Purification Kit, Invitrogen).
Column based gDNA extraction from DBS. We used the QIAamp DNA kit (Qiagen, Catalog no. 56304). 1 – 4 blood spots 6mm in size were added with 180 µL of cell lysis buffer ATL (Lysis buffer supplied with Qiagen kit), and incubated in a waterbath (Trishul Equipment, Sr. No. 5460311) at 85°C for 10 min. 20 µL Proteinase K was added and incubated it at 56°C for 1 hour to denature the proteins. 3–4 µL RNAse was added immediately after to degrade RNA, then 200 µL buffer AL (Lysis buffer supplied with Qiagen kit) was added & mixed thoroughly by vortexing and incubated at 70°C in a waterbath (Trishul Equipment with Sr. No. 5460311) for 10 min. Buffer AL helps in complete cell lysis and binding of gDNA with the silica gel of the column provided in the Qiagen kit. gDNA was then immediately precipitated by adding 200 µL of 70% v/v ethanol. The solution was then transferred into a spin column (supplied with Qiagen kit) and centrifuged (Eppendorf 5810R) at 8000 rpm for 1 min. The spin column has the capacity to load approximately 600 microliter sample at a time, but generally we had approximately 1.2 or 1.4 ml of solution, we therefore performed the process 2–3 times. In this process gDNA becomes bound with the column, impurities are then washed out with 700 µL buffer AW1 (Wash buffer with a low concentration of quinidine) followed by 700 µL of AW2 (Wash buffer with Tris based ethanol solution used for removal of salts) buffer. Buffers AW1 & AW2 remove unwanted impurities from the gDNA. The empty tube was then centrifuged at 14000 rpm for 3 min for complete removal of ethanol from the gDNA. Finally the gDNA was eluted with 30 µl of pre-incubated elution buffer (AE).
Magnetic bead based gDNA extraction from DBS. We used the ChargeSwitch Forensic DNA Purification Kit (Thermofisher, Catalog No. CS11200). Processed DBS samples were added to 1ml lysis buffer with 10 µL of Proteinase K in a tube, vortexed, and incubated at 55°C in a waterbath (Trishul Equipment Sr. No. 5460311) for 1 hour. Blood spots were removed after complete cell lysis and 200 µL of purification buffer added, followed by 20 µL magnetic beads. The solution was then gently mixed, left for 5 minutes, and then incubated on a Magna Rack (Thermofisher, catalog no. AM10027) for 1 minute. The supernatant was removed after complete binding of the pellet to the magnet of the Magna Rack. The pellet containing gDNA was then washed with 500 µL wash buffer (W12) 3 times and finally DNA eluted with 30–60 µL Elution Buffer (E5). We have followed the protocol as per manufacturer recommendation with some modifications; incubation time was increased from 4 hours to overnight for complete extraction of eluate from filter paper, and we also increased the time for proper binding of gDNA with the spin column.
Genomic DNA quantification
Concentration of extracted gDNA was measured using a Qubit 3.0 fluorometer and purity was measured by a spectrophotometer. Quality and integrity of gDNA was checked by performing a 0.8% Agarose gel electrophoresis.
Qubit 3.0 Fluorometer (Thermofisher, Catalog No. Q32850) was used to measure gDNA concentration by taking 1 µl gDNA sample in 0.2ml PCR tube with 200 µl buffer (199µl dsDNA BR buffer + 1 µl Ethidium Bromide dye) after proper mixing for 1min. Concentration was then measured with a fluorometer.
A spectrophotometer at wavelength 260/280 ratio (NanoDrop 2000 Thermofisher) was used to check purity of gDNA, by applying 1 µl gDNA sample directly to the device and measuring the 260/280 ratio.
Agarose gel electrophoresis was performed following preparation of a 0.8% agarose gel which was loaded with 5 µl gDNA in wells and run at 70–80 volts for 2 hours. A gel photograph was captured using a gel dock (Model: UVP EC3-Imaging System).
Results
Genomic DNA extraction efficiency
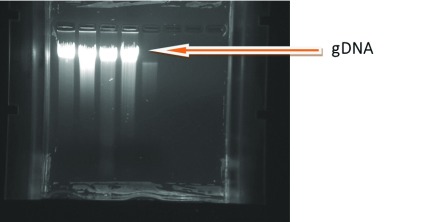
We obtained average gDNA concentrations of 6mm × 1 spot to 6mm × 4 spots from DBS cards ( Table 1). In our findings, we observed large variation in the concentration, from 2.16ng/µl to 24ng/µl, of the extracted gDNA ( Table 2). The integrity of gDNA was checked using 0.8% agarose gel electrophoresis, and highly intense single bands were observed on the gel ( Figure 3). The purity of gDNA was measured at an absorbance of 260/280nm (1.8–2.0) ratio. If it is less than 1.8 or greater than 2.0 it indicates the presence of impurities in the genetic material. gDNA concentration of each DBS with different number of blood spots, with their purity and total gDNA yield are presented in Table 3.
Table 2. Genomic DNA quantification (gDNA) by Fluorometer on stored dried blood spot (DBS) at standard condition.
| Type of
filter paper |
gDNA concentration
range (ng/µl) |
Elution
volume |
Total Yield (ng) |
|---|---|---|---|
| Whatman
903 card |
2.16 ng/µl - 24 ng/µl | 30 µl | 64.8 ng – 720 ng |
Figure 3. Agarose gel electrophoresis of extracted gDNA from dried blood spots.
Figure shows highly intense bands which are mostly intact with little smear.
Table 3. Genomic DNA (gDNA) concentration of each dried blood spot (DBS) from different number of blood spots.
| Total number
of blood spots used |
DNA Yield
(ng/µl) |
260/280
ratio |
Total Elution
Volume |
Total
Yield |
|---|---|---|---|---|
| 6mm × 1 spot | 3.4 | 1.84 | 30 | 102 |
| 6mm × 1 spot | 3.52 | 1.9 | 30 | 105.6 |
| 6mm × 1 spot | 4.52 | 1.8 | 30 | 135.6 |
| 6mm × 1 spot | 4.7 | 1.39 | 30 | 141 |
| 6mm × 1 spot | 2.16 | 2.4 | 30 | 64.8 |
| 6mm × 1 spot | 2.19 | 1.98 | 30 | 65.7 |
| 6mm × 1 spot | 2.12 | 2.1 | 30 | 63.6 |
| 6mm × 1 spot | 4.66 | 2.23 | 30 | 139.8 |
| 6mm × 1 spot | 3.56 | 2.21 | 30 | 106.8 |
| 6mm × 1 spot | 3.47 | 1.5 | 30 | 104.1 |
| 6mm × 2 spots | 4.98 | 2.4 | 30 | 149.4 |
| 6mm × 2 spots | 5.95 | 2.2 | 30 | 178.5 |
| 6mm × 2 spots | 6.36 | 2.23 | 30 | 190.8 |
| 6mm × 2 spots | 6.4 | 2.13 | 30 | 192 |
| 6mm × 2 spots | 8.98 | 2.28 | 30 | 269.4 |
| 6mm × 2 spots | 8.06 | 2.32 | 30 | 241.8 |
| 6mm × 2 spots | 5.4 | 2.1 | 30 | 162 |
| 6mm × 2 spots | 5.49 | 1.82 | 30 | 164.7 |
| 6mm × 2 spots | 6.4 | 2.15 | 30 | 192 |
| 6mm × 2 spots | 5.82 | 2.21 | 30 | 174.6 |
| 6mm × 3 spots | 6.86 | 2.45 | 30 | 205.8 |
| 6mm × 3 spots | 8.08 | 2.61 | 30 | 242.4 |
| 6mm × 3 spots | 5.72 | 2.28 | 30 | 171.6 |
| 6mm × 3 spots | 7.13 | 2.41 | 30 | 213.9 |
| 6mm × 3 spots | 6.94 | 2.21 | 30 | 208.2 |
| 6mm × 3 spots | 7.18 | 2.51 | 30 | 215.4 |
| 6mm × 3 spots | 8.7 | 1.98 | 30 | 261 |
| 6mm × 3 spots | 6.43 | 2.31 | 30 | 192.9 |
| 6mm × 3 spots | 8.12 | 2.38 | 30 | 243.6 |
| 6mm × 3 spots | 7.19 | 1.95 | 30 | 215.7 |
| 6mm × 4 spots | 9.9 | 2.9 | 30 | 297 |
| 6mm × 4 spots | 10.9 | 2.84 | 30 | 327 |
| 6mm × 4 spots | 7.6 | 2.93 | 30 | 228 |
| 6mm × 4 spots | 8.23 | 2.74 | 30 | 246.9 |
| 6mm × 4 spots | 9.25 | 2.62 | 30 | 277.5 |
| 6mm × 4 spots | 24 | 2.89 | 30 | 720 |
| 6mm × 4 spots | 3.36 | 2.84 | 30 | 100.8 |
| 6mm × 4 spots | 7.62 | 2.91 | 30 | 228.6 |
| 6mm × 4 spots | 4.17 | 2.86 | 30 | 125.1 |
| 6mm × 4 spots | 4.12 | 2.96 | 30 | 123.6 |
This amount of gDNA extracted can be used for polymerase chain reaction (PCR), and PCR based molecular assays such as PCR based sequencing, PCR based genotyping, but can’t be used for whole genome sequencing or genotyping 11.
Discussion
For the last 5 decades, DBS sample have been collected and stored in bio-banks to conduct field epidemiological studies worldwide. DBS collection on filter paper is more applicable and acceptable method in epidemiological research as compared with standard venous blood. The advantage of DBS over venous blood collection include less discomfort for the subject, especially if many samples are needed within a short period of time, only a small amount of blood is needed to perform the assay. Our findings shows that, we can extract the gDNA from dried blood spots. Previously, studies have been performed to compare genomic DNA extraction methods to examine its feasibility in genetic studies 12. As per obtained results, we have found good concentration of total gDNA, In this study, our target was to improve the maximum extraction of gDNA from DBS. We followed 2 methods; one column based and one magnetic bead based. Before proceeding to cell lysis process, we had treated the blood spots with PBS (pH 7.4) & kept it overnight at 37°C to elute the complete matrix from the Whatman for efficient & complete cell lysis. DNA samples can be stable on filter paper for many years if it is stored in dry conditions 8. Our main objective was to extract the maximum amount of gDNA from DBS irrespective of methodology use, therefore we have used 2 methods to extract gDNA to evaluate from which method we have got more gDNA but unfortunately, we have not found any difference in gDNA concentration with between both methods. We did assay randomly from both methods with full focus on maximum quantity of gDNA extraction from DBS. We have tried gDNA extraction with direct cell lysis of DBS by using lysis buffer and also blood spots treated with PBS overnight to complete elution of eluate. We have done these experiments to evaluate gDNA concentration but unfortunately there are no such yield increases with these modifications. As our results show, there is large variation in the concentration and purity (260/280) of extracted gDNA in both the methods. This variation might be due to the small volume of blood, long term storage, loss during assays, cell debris, cellulosic component of the Whatman card etc. In the case of column (QIAamp) based gDNA extraction, 5% loss is predicted by the manufacturer, where as in magnetic bead based DNA extraction, some 5–10% beads are lost during assay, results there is loss of gDNA. The obtained purity also shows variation, 260/280 ratio 1.8–2.9, this might be due to interference of cellulosic components of Whatman paper. It is true that a 260/280 ratio >2.0 indicates impurities. But due to limitation of blood spots we have not increased the number of spots beyond 4. As I have mentioned that we have got DNA concentration in a range 64.8ng – 720ng. This amount of DNA can be used in downstream applications and we can remove the impurities by gel purification method.
gDNA concentration depends on the blood matrix on spots. In a study using DBS stored for 6 years and they found reduced gDNA concentration in quality and quantity. 13, but some other studies reported that gDNA is stable for at least 11 years under ambient tropical conditions 8. We have performed some modification to the protocol for the extraction, we increased the time for cell lysis, binding of DNA with column & beads, washing with buffer & final elution. Due to regular successive research on DBS, today DBS samples are used for genetic analysis, proteome research, vitamins estimation, infection agent, epigenetic research, nucleic acid research 14– 17.
A punch of diameter 6mm represents approximately 8.7±1.9µl of blood spotted. This difference in blood volume from a single spot might be due to the presence of hematocrit, because due to increased percentage of hematocrit in blood, the blood becomes very viscous and it can’t spread homogenously over the Whatman circle which results the concentration of DNA and blood on 6mm spot changes accordingly. Composition of hematocrit value influence the gDNA concentration with different number of blood spots. Filter paper contains cellulosic fibers, probably referred as cotton linters, while extracting gDNA from Whatman card, the cellulosic composition of filter paper interfere the concentration and purity of gDNA because during thermal agitation and vortex steps in protocol, these cotton linters are also present in supernatant and interfere with final DNA elution steps.
Our findings show, approximately 64.8ng – 720ng gDNA is extracted from Whatman 903 card >50 ng is sufficient for PCR based applications 18. DBS extracted gDNA can be used in downstream applications such as polymerase chain reaction (PCR), PCR based sequencing, PCR based genotyping, disease diagnosis, molecular basis of disease etiology and study of genetic variants. the extracted amount of gDNA, however, cannot be used for whole genome sequencing, but this can be overcome by whole genome amplification of extracted gDNA, as this will increase the concentration of gDNA by increasing the copy number of templates. DBS is a suitable and applicable sample source in large scale epidemiological studies and biobanking. Further study is warranted to explore DBS efficiency in high throughput research to reveal other biochemical analytes stability on filter paper card to replace venous blood collection in future epidemiological studies.
Conclusion
Analyte stability on filter paper in dry form is a good biological sample source to perform molecular epidemiological based assays. Dried blood spot on paper card act as a potential and robust sample source for biobanking in large scale epidemiological studies.
Data availability
Data underlying this study is available from Open Science Framework. Dataset 1: Optimization of extraction of gDNA from DBS: Potential application in epidemiological research & biobanking. http://doi.org/10.17605/OSF.IO/FZYTM 19
Data is available under a CC0 1.0 Universal license.
Acknowledgements
Authors are thankful to the Director, Tata Memorial Centre, Mumbai, for providing the necessary facilities, Centre for Global Health Research (CGHR) Canada for support to carry out the research on Dried Blood Spots. This work was supported by the Bill and Melinda Gates Foundation [OPP1148667].
Funding Statement
This work was supported by the Bill and Melinda Gates Foundation [OPP1148667]
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved with reservations]
References
- 1. Guthrie R, Susi A: A simple phenylalanine method for detecting phenylketonuria in large populations of Newborn infants. Pediatrics. 1963;32(3):338–43. [PubMed] [Google Scholar]
- 2. Lakshmy R, Gupta R: Measurement of glycated hemoglobin A1c from dried blood by turbidimetric immunoassay. J Diabetes Sci Technol. 2009;3(5):1203–6. 10.1177/193229680900300527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakshmy R: Analysis of the use of dried blood spot measurements in disease screening. J Diabetes Sci Technol. 2008;2(2):242–3. 10.1177/193229680800200211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lakshmy R, Mathur P, Gupta R, et al. : Measurement of cholesterol and triglycerides from a dried blood spot in an Indian Council of Medical Research-World Health Organization multicentric survey on risk factors for noncommunicable diseases in India. J Clin Lipidol. 2012;6(1):33–41. 10.1016/j.jacl.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 5. Mei JV, Alexander JR, Adam BW, et al. : Innovative Non- or Minimally-Invasive Technologies for Monitoring Health and Nutritional Status in Mothers and Young Children Use of Filter Paper for the Collection and Analysis of Human Whole Blood Specimens 1.2001;1631–6. Reference Source [Google Scholar]
- 6. Gauffin F, Nordgren A, Barbany G, et al. : Quantitation of RNA decay in dried blood spots during 20 years of storage. Clin Chem Lab Med. 2009;47(12):1467–9. 10.1515/CCLM.2009.351 [DOI] [PubMed] [Google Scholar]
- 7. Hollegaard MV, Grauholm J, Børglum A, et al. : Genome-wide scans using archived neonatal dried blood spot samples. BMC Genomics. 2009;10(1):297. 10.1186/1471-2164-10-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaisomchit S, Wichajarn R, Janejai N, et al. : Stability of genomic DNA in dried blood spots stored on filter paper. Southeast Asian J Trop Med Public Health. 2005;36(1):270–3. [PubMed] [Google Scholar]
- 9. Blood D, Dbs S: Module 14 Blood Collection and Handling - Dried Blood Spot (DBS).2005;1–13. Reference Source [Google Scholar]
- 10. Hewawasam E, Liu G, Jeffery DW, et al. : Estimation of the Volume of Blood in a Small Disc Punched From a Dried Blood Spot Card. Eur J Lipid Sci Technol. 2018;120(3):1700362 10.1002/ejlt.201700362 [DOI] [Google Scholar]
- 11. Rajatileka S, Luyt K, El-Bokle M, et al. : Isolation of human genomic DNA for genetic analysis from premature neonates: a comparison between newborn dried blood spots, whole blood and umbilical cord tissue. BMC Genet. 2013;14:105. 10.1186/1471-2156-14-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molteni CG, Terranova L, Zampiero A, et al. : Comparison of manual methods of extracting genomic DNA from dried blood spots collected on different cards: implications for clinical practice. Int J Immunopathol Pharmacol. 2013;26(3):779–83. 10.1177/039463201302600324 [DOI] [PubMed] [Google Scholar]
- 13. Hollegaard MV, Thorsen P, Norgaard-Pedersen B, et al. : Genotyping whole-genome-amplified DNA from 3- to 25-year-old neonatal dried blood spot samples with reference to fresh genomic DNA. Electrophoresis. 2009;30(14):2532–5. 10.1002/elps.200800655 [DOI] [PubMed] [Google Scholar]
- 14. Eyles D, Anderson C, Ko P, et al. : A sensitive LC/MS/MS assay of 25OH vitamin D 3 and 25OH vitamin D 2 in dried blood spots. Clin Chim Acta. 2009;403(1–2):145–51. 10.1016/j.cca.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 15. Haak PT, Busik JV, Kort EJ, et al. : Archived unfrozen neonatal blood spots are amenable to quantitative gene expression analysis. Neonatology. 2009;95(3):210–6. 10.1159/000155652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boppana SB, Ross SA, Novak Z, et al. : Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–82. 10.1001/jama.2010.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong N, Morley R, Saffery R, et al. : Archived Guthrie blood spots as a novel source for quantitative DNA methylation analysis. Biotechniques. 2008;45(4):423–4, 426, 428 passim. 10.2144/000112945 [DOI] [PubMed] [Google Scholar]
- 18. Bhagya CH, Wijesundera Sulochana WS, Hemamali NP: Polymerase chain reaction optimization for amplification of Guanine-Cytosine rich templates using buccal cell DNA. Indian J Hum Genet. 2013;19(1):78–83. 10.4103/0971-6866.112898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar A: Optimization of extraction of gDNA from DBS: Potential application in epidemiological research & biobanking.2018. 10.17605/OSF.IO/FZYTM [DOI] [PMC free article] [PubMed] [Google Scholar]