Crassulacean acid metabolism (CAM) photosynthesis is one of the principle carbon-concentrating mechanisms in terrestrial plants. A primary feature of the CAM photosynthetic pathway revolves around the night-time uptake of CO2 and its subsequent storage as organic acids for later daytime fixation into sugars. This unique, water-saving, and carbon-concentrating photosynthetic pathway is the major means by which land plants achieve superior levels of resource-use efficiency. As a result, CAM plants are increasingly recognized as among the world’s most important climate-resilient crops for food, forage, fodder, fiber, and fuel, as well as being key drivers of ecosystem function in dry regions.
CAM photosynthesis stands out as among the most prolific examples of complex trait evolution in the biosphere, with >60 independent evolutionary origins, and occurrence found in >38 families encompassing >400 genera of vascular plant species (Smith and Winter, 1996; Silvera et al., 2010; Winter et al., 2015). Most of the origins are correlated with increasing aridity and declines in atmospheric CO2 in the geological past, allowing examples of CAM evolution to serve as leading case studies for evolutionary responses to global climate change (Box 1). CAM also evolved with a series of co-adaptive traits such as stem or leaf succulence, water capture and storage strategies, thick cuticles and epicuticular wax deposition, low stomatal density, high stomatal responsiveness, and shallow rectifier-like roots, which allow plants to occupy harsh environments with limited or intermittent water availability (Niechayev et al., 2019a). The high number of origins provides repeated examples of how CAM arose from C3 photosynthesis ancestors, and thus can be exploited to provide new directions and genetic constructs for improving resource-use efficiency in ways that provide a wide range of societal benefits (Yang et al., 2015; Lim et al., 2019). Global warming is expected to result in increased surface drying and an expansion of drylands throughout the globe (Huang et al., 2015; Dai et al., 2018). However, some CAM species show positive growth responses to elevated CO2 (Drennan and Nobel, 2000; Ceusters and Borland, 2010) and can tolerate high temperatures (Nobel et al., 1986; Chetti and Nobel, 1988). Exploiting the efficiency potential of CAM photosynthesis is one of the most cost-effective and environmentally safe ways to simultaneously meet the overlapping challenges of global food security, scarce water supplies, increasing bioenergy resources, and low-input carbon sequestration in the face of global climate change (Osmond et al., 2008; Borland et al., 2009; Cushman et al., 2015; Davis et al., 2015, 2017).
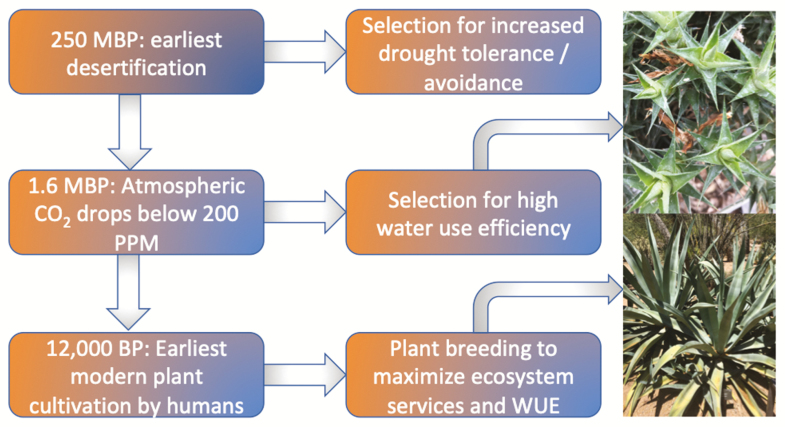
Box 1. The origins and evolution of the CAM photosynthetic pathway.
CAM is known in 38 plant families and, due to its broad systematic distribution, it appears that CAM has arisen independently several times. CAM is estimated to exist in ~6% of all terrestrial plant taxa as well as 6% of all aquatic plant taxa (Keeley, 1998). The precise origins of the earliest CAM plants are not well known, in part because evidence for CAM is largely depauperate in the fossil record (Ehleringer and Monson, 1993). Over broad geographical scales, aridity appears to be the primary driver of terrestrial CAM and is perhaps best known in desert succulents that are regularly exposed to high daytime temperatures and low relative humidities. However, selection for CAM should also correspond to conditions of low available CO2 due to the intensive carbon-concentrating mechanisms associated with the diurnal storage of organic acids during the CAM cycle. The presence of CAM in primitive aquatic plants such as those in the genus Isoëtes, for example, probably evolved due to the low diffusion coefficient of CO2 in water (Monson, 1989). Similarly, the evolution of CAM in terrestrial plant taxa probably accelerated in response to glacial episodes during the Pleistocene when reduced atmospheric CO2 concentrations favored photosynthetic pathways such as CAM and C4 (Ehleringer and Monson, 1993; Raven and Spicer, 1996; Keeley and Rundel, 2003).
More recently, cultivation of CAM plants for food, fiber, medicinal, and other uses may have enhanced diversification along a number of plant lineages. For example, plants within the genus Agave have been an important source of food and fiber for humans in Mesoamerica since at least 9000 years BP (Gentry, 1982). Intensive cultivation of Agave by pre-Columbian Native Americans has given rise to new species originating from North American ‘domestication centers’ selected for high water-use efficiency, sugar content, and productivity (Hodgeson et al., 2019). Modern agricultural practices are poised to follow the lead of pre-Columbian agronomists to further exploit the high water-use efficiency in CAM taxa to enhance crop yields relative to water allocation for food, fiber, and bioenergy production (Yang et al., 2015; Davis et al., 2019).
Timeline of the major geological/anthropological events that facilitated the evolution and diversification of CAM plants across the globe. Upper right photo: Deuterocohnia brevifolia (Griseb.), a perennial succulent-leaved shrub native to the Andes region of Argentina and Bolivia. Lower right photo: the widely cultivated Agave tequilana (Weber).
CAM plants are not only recognized as important crop plants (e.g. pineapple, Agave, Opuntia, Aloe, and vanilla) and valuable horticultural ornamentals (e.g. Agave, cactus, Kalanchoe, and orchids), they also include many of the most iconic and important foundation species of arid and semi-arid and epiphytic and atmospheric ecosystems. CAM metabolism is often, but not exclusively, associated with stem and leaf succulence: features that have evolved across a broad range of plant taxa in apparent coordination with the evolution of the CAM photosynthetic pathway among certain lineages (Arakaki et al., 2011; Horn et al., 2014). The co-evolution of succulence and subsequent internal water storage capacity and CAM metabolism has facilitated the radiation of many plant species into areas dominated by prolonged aridity and episodic drought (Box 1). Recent analysis within the Agavoideae (Asparagaceae) suggests that succulent leaf anatomy pre-dates the appearance of CAM (Heyduk et al., 2016). However, to what extent amplified aridity from climate change is impacting the productivity and distribution of CAM plants across the globe remains an open question. CAM-related genes were among many gene families associated with adaptation to harsh or extreme environments (Wang et al., 2019). Recent advances in functional genomics, stable isotope applications, and mechanistic niche modeling approaches are poised to shed light on the extent to which rapid environmental changes might alter the range and productivity of CAM plants and the resources that flow to dependent consumers and human populations.
Exploiting the productive potential of CAM for human enterprise while protecting CAM plants in naturally occurring environments requires the integration of a broad range of biological disciplines so that ecological and evolutionary discoveries can inform society in an era of global climate change. This special issue highlights this integration with reviews and original research reports that focus on cutting-edge topics that revolve around research on CAM, including the expression of CAM within phylogenetic contexts, opportunities surrounding the climate resilience of CAM plants, and advances in CAM genetics and genomics.
CAM expression within phylogenetic contexts
The classic model of CAM defined by inverted stomatal behavior and four phases of gas exchange and biochemical activity (Osmond, 1978) belies the diversity and complexity of CAM expression in nature. Many recent advancements in our understanding of CAM have come from studies at the margins of CAM expression, in the so-called ‘weak’, ‘facultative’, and ‘intermediate’ CAM plants. It is at the margins and across the spectrum of CAM types where the evolution, functional significance, and molecular and genomic features of CAM are fruitfully explored, and where targets for introduction of CAM traits into C3 and C4 crops might be found and successfully engineered. That many CAM plants express CAM together with some C3 or C4 photosynthesis and this expression often varies over different stages of development, or facultatively when exposed to drought or salinity stress, challenges our notion of what CAM is, how it evolved, and even how it is defined. In this special issue, Winter (2019) presents a review of the complexity of CAM expression and establishes core terminology and definitions to help frame and articulate research directions and findings (Winter, 2019). Winter demonstrates, using continuous monitoring of daily CO2 exchange and broad comparisons of night-time malate accumulation, how the often-overlooked subtleties of CAM expression can be central to further advancements in the field, especially when considering weakly expressed (Winter et al., 2019a) or stress-induced facultative CAM (including facultative CAM in C3–C4 intermediates; Winter et al., 2019b). These are key points that should be embraced more broadly and, although the level of evidence required to diagnose the full range of CAM expression is technically demanding, the payoffs are demonstrably large.
Climate resilience of CAM plants: consequences and opportunities
The natural distribution of CAM species is dictated by their ability to persist in harsh climates. However, many CAM species, particularly those with stem succulence within the Old World Euphorbia and the New World Cactaceae, are threatened by human poaching activity and the unprecedented threat posed by ongoing global climate change. Hultine and colleagues review the challenges and opportunities associated with stable isotope analysis of the spines of large columnar cactus species, which serve as long-lived sentinels capable of documenting with exquisite accuracy changes in climatic conditions over tens to hundreds of years (Hultine et al., 2019). Many CAM species serve as climate-resilient crops possessing immense agricultural value capable of meeting the food, feed, fiber, biofuel, and pharmaceutical needs of the future. As reviewed by Davis et al. (2019), although a small number of CAM species and their products are globally traded commodities, including Agave tequilana, Ananas comosus (pineapple), Aloe spp., Vanilla spp., and Opuntia spp., these species have been traditionally undervalued and very little investment in agricultural improvements have been made in them. However, recent advances in genomic resources for many of these CAM crops promises to facilitate genetic improvement through targeted, molecular-assisted breeding and genome editing approaches (Davis et al., 2019). Among them, Agave spp. have received considerable attention as a bioenergy feedstock (Davis et al., 2017). Although Agave spp. have been cultivated by pre-Columbian Native Americans for centuries (Box 1), the capacity to produce fiber and biofuels on an industrial scale in dryland regions has stimulated growing interest in Agave production (Davis et al., 2019). To improve our understanding of the potential productivity of Agave americana in water-limited regions, a monthly environmental productivity index (EPI) was developed, which is based on light, temperature, and precipitation inputs (Niechayev et al., 2019b). Such modeling efforts will be fundamentally important for estimating the geographical range and productivity potential of this and other CAM crops under current and future climatic conditions.
Advances in CAM genetics and genomics
The application of high-throughput ‘omics technologies continues to provide novel insights into the mechanistic basis of CAM function. Exploration of the early stages of CAM induction by applying abscisic acid (ABA) to the leaves of the facultative CAM model species Talinum triangulare suggested new genes with possible functions in CAM including ABA signal transduction, amino acid metabolism, solute transport, protein degradation, and a set of putative transcriptional regulators involved in CAM induction (Maleckova et al., 2019). Comparative transcriptome and preliminary metabolomic analyses of a 24 h time course comparing Yucca (Asparagaceae) species with C3 photosynthesis (Y. filamentosa), C3–CAM [Y. gloriosa (hybrid)], and CAM (Y. aloifolia) under both well-watered and drought-stressed conditions in a common garden setting revealed clear-cut differences among the two parents and the hybrid, yet all three shared some common changes in steady-state mRNA abundance Such common expression patterns and resulting traits might have facilitated the convergent evolution of CAM within the Agavoideae (Heyduk et al., 2019). In the context of CAM evolution, Yang et al. (2019) discuss possible pathway scenarios for CAM evolution, anatomical modifications associated with CAM, and potential amino acid and temporal reprogramming changes that might sustain CAM evolution informed by comparative genomic analyses. Taking a phylogenetic approach and using δ 13C tissue analysis of herbarium specimens, Li et al. (2019) report multiple independent origins of CAM within the highly diverse orchid genus Dendrobium in Australasia (Li et al., 2019). A fine-scale understanding of the molecular genetic changes associated with CAM evolution will require a robust functional toolkit including the ability to readily transform favored model CAM species, RNAi, and genome editing using CRISPR/Cas (clustered regulatory interspaced short palindromic repeats/CRISPR-associated protein)-mediated genome editing approaches (Yang et al., 2019). Like most other plants, CAM species can be subjected to genome editing. In one of the first ‘proof of concept’ demonstrations of CRISPR/Cas-mediated genome editing in a CAM species, Liu et al. (2019) showed that knocking out the PHOTOTROPIN 2 (PHOT2) gene in Kalanchoë fedtschenkoi reduced stomatal conductance and CO2 fixation in the latter part of the photoperiod (Phase IV), whereas these behaviors were enhanced during the early evening and throughout the dark period (Phase I) of CAM, suggesting that blue-light signaling might be important for the proper functioning of the diel CAM cycle. To advance additional genome editing work, a genome-wide guide RNA (gRNA) database was developed for K. fedtschenkoi (Liu et al., 2019).
Future directions
CAM photosynthesis was a leading topic in plant biology research in the two decades after its discovery in the 1960s. However, once the biochemical details of carbon flow in CAM photosynthetic pathways had been established, further progress in CAM research was dependent upon application of molecular genetic, phylogenetic, and isotopic approaches to investigate these metabolic adaptations. Recent advances in molecular phylogenetics, high-throughput sequencing, genome editing, and isotopic physiology have reinvigorated CAM research and contributed to major new initiatives in exploring CAM evolution, the development of novel C3 photosynthesis and CAM bioenergy crops, and the engineering of CAM pathways in non-CAM crop species for improved climate resilience. These initiatives are now producing exciting results that current and future CAM biologists will continue to build upon. Over the coming years, highly novel, transformative discoveries in CAM biology will have immediate significance towards commercial application and the conservation of natural ecosystems.
Acknowledgements
This editorial covers topics that were presented as part of a stimulating conference titled ‘The Biology of CAM Plants’ convened on 9–13 April 2018 at the Desert Botanical Garden in Phoenix, AZ, USA. The authors thank conference co-organizers Alberto Búrquez, Sarah Davis, Raul Puente, and Ryan Stewart for their assistance with organizing and convening the conference. Financial support was provided by Society for Environmental Biology and the National Science Foundation, Integrative Organismal Systems program (grant no. 1818560).
References
- Arakaki M, Christin P-A, Nyffeler R, Lendel A, Eggli U, Ogburn RM, Spriggs E, Moore MJ, Edwards E. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences, USA 108, 8379–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Hartwell J, Smith JAC. 2009. Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. Journal of Experimental Botany 60, 2879–2896. [DOI] [PubMed] [Google Scholar]
- Ceusters J, Borland AM. 2010. Impacts of elevated CO2 on the growth and physiology of plants with crassulacean acid metabolism. Progress in Botany 72, 163–181. [Google Scholar]
- Chetti MB, Nobel PS. 1988. Recovery of photosynthetic reactions after high-temperature treatments of a heat-tolerant cactus. Photosynthesis Research 18, 277–286. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Davis SC, Yang X, Borland AM. 2015. Development and use of bioenergy feedstocks for semi-arid and arid lands. Journal of Experimental Botany 66, 4177–4193. [DOI] [PubMed] [Google Scholar]
- Dai A, Zhao T, Chen J. 2018. Climate change and drought: a precipitation and evaporation perspective. Current Climate Change Reports 4, 301–312. [Google Scholar]
- Davis SC, Kuzmick ER, Niechayev N, Hunsake DJ. 2017. Productivity and water use efficiency of Agave americana in the first field trial as bioenergy feedstock on arid lands. Global Change Biology Bioenergy 9, 314–325. [Google Scholar]
- Davis SC, Ming R, LeBauer DS, Long SP. 2015. Toward systems‐level analysis of agricultural production from crassulacean acid metabolism (CAM): scaling from cell to commercial production. New Phytologist 208, 66–72. [DOI] [PubMed] [Google Scholar]
- Davis SC, Simpson J, Vega G, Del Carmen K, Niechayev NA, Tongerlo EV, Castano NH, Dever LV, Búrque A. 2019. Undervalued potential of crassulacean acid metabolism for current and future agricultural production. Journal of Experimental Botany 70, 6521–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan PM, Nobel PS. 2000. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant, Cell & Environment 23, 767–781. [Google Scholar]
- Ehleringer JR, Monson RK. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics 24, 411–439. [Google Scholar]
- Gentry HS. 1982. Agaves on Continental North America. Tucson, AZ: University of Arizona Press. [Google Scholar]
- Heyduk K, McKain MR, Lalani F, Leebens-Mack J. 2016. Evolution of a CAM anatomy predates the origins of Crassulacean acid metabolism in the Agavoideae (Asparagaceae). Molecular Phylogenetics and Evolution 105, 102–113. [DOI] [PubMed] [Google Scholar]
- Heyduk K, Ray JN, Ayyampalayam S, Moledina N, Borland AM, Harding SA, Tsai CJ, Leebens-Mack J. 2019. Shared expression of crassulacean acid metabolism (CAM) genes pre-dates the origin of CAM in the genus Yucca. Journal of Experimental Botany 70, 6597–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgeson WC, Salywon AM, Doelle WH. 2019. Hohokam lost crop found: a new Agave (Agavaceae) species only known from large-scale pre-Columbian agricultural fields in southern Arizona. Systemic Botany 43, 734–740. [Google Scholar]
- Horn JW, Xi Z, Riina R, Peirson JA, Yang Y, Dorsey BL, Berry PE, Davis CC, Wurdack KJ. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68, 3485–3504. [DOI] [PubMed] [Google Scholar]
- Huang J, Yu H, Guan X, Wang G, Guo R. 2015. Accelerated dryland expansion under climate change. Nature Climate Change 6, 166–171. [Google Scholar]
- Hultine KR, Dettman DL, English NB, Williams DG. 2019. Giant cacti: isotopic recorders of climate variation in warm deserts of the Americas. Journal of Experimental Botany 70, 6509–6519. [DOI] [PubMed] [Google Scholar]
- Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. Botanical Reviews 64, 121–175. [Google Scholar]
- Keeley JE, Rundel PW. 2003. Evolution of CAM and C4 carbon-concentrating mechanisms. International Journal of Plant Sciences 164, S55–S77. [Google Scholar]
- Li M-H, Zhang G-Q, Deng H, Liu D-K, Tu X-D, Wang Y, Lan S-R, Liu Z-J. 2019. A perspective on crassulacean acid metabolism photosynthesis evolution of orchids on different continents: Dendrobium as a case study. Journal of Experimental Botany 70, 6611–6619. [DOI] [PubMed] [Google Scholar]
- Lim SD, Lee S, Yim WC, Choi W-G, Cushman JC. 2019. Laying the foundation for crassulacean acid metabolism (CAM) biodesign: expression of the C4 metabolism cycle genes of CAM in Arabidopsis. Frontiers in Plant Science 10, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Mendoza B, Cheng H, Hu R, Li l, Trinh CT, Tuskan GA, Yang X. 2019. CRISPR/Cas9-mediated targeted mutagenesis for functional genomics research of crassulacean acid metabolism plants. Journal of Experimental Botany 70, 6621–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleckova E, Brilhaus D, Wrobel TJ, Weber APM. 2019. Transcript and metabolite changes during the early phase of abscisic acid-mediated induction of crassulacean acid metabolism in Talinum triangulare. Journal of Experimental Botany 70, 6581–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK. 1989. On the evolutionary pathways resulting in C4 photosynthesis and crassulacean acid metabolism (CAM). Advances in Ecological Research 19, 57–92. [Google Scholar]
- Niechayev NA, Jones AM, Rosenthal DM, Davis SC. 2019b A model of environmental limitations on production of Agave americana L. grown as a biofuel crop in semi-arid regions. Journal of Experimental Botany 70, 6549–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niechayev NA, Pereira PN, Cushman JC. 2019a Understanding trait diversity associated with crassulacean acid metabolism (CAM). Current Opinion in Plant Biology 49, 74–85. [DOI] [PubMed] [Google Scholar]
- Nobel PS, Geller GN, Kee SC, Zimmerman AD. 1986. Temperatures and thermal tolerances for cacti exposed to high temperatures near the soil surface. Plant, Cell & Environment 9, 279–287. [Google Scholar]
- Osmond B, Neales T, Stange G. 2008. Curiosity and context revisited: crassulacean acid metabolism in the Anthropocene. Journal of Experimental Botany 59, 1489–1502. [DOI] [PubMed] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29, 379–414. [Google Scholar]
- Raven JA, Spicer RA. 1996. The evolution of Crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag, 360–385. [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC. 2010. Evolution along the crassulacan acid metabolism continuum. Functional Plant Biology 37, 995–1010. [Google Scholar]
- Smith JAC, Winter K. 1996. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag, 427–436. [Google Scholar]
- Wang N, Yang Y, Moore MJ, et al. 2019. Evolution of Portulacineae marked by gene tree conflict and gene family expansion associated with adaptation to harsh environments. Molecular Biology and Evolution 36, 112–126. [DOI] [PubMed] [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70, 6495–6508. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Virgo A, Holtum JA. 2019a Operating at the very low end of the crassulacean acid metabolism spectrum: Sesuvium portulacastrum (Aizoaceae). Journal of Experimental Botany 70, 6561–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208, 73–78. [DOI] [PubMed] [Google Scholar]
- Winter K, Sage RF, Edwards EJ, Virgo A, Holtum JAM. 2019b Facultative CAM in a C3–C4 intermediate. Journal of Experimental Botany 70, 6571–6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cushman JC, Borland AM, et al. 2015. A roadmap for research on crassulacean acid metabolism to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytologist 207, 491–504. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu D, Tschaplinsk TJ, Tuskan G. 2019. Comparative genomics can provide new insights into the evolutionary mechanisms and gene function in CAM plants. Journal of Experimental Botany 70, 6539–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]