Leaves and stems of the pantropical coastal herb Sesuvium portulacastrum exhibit low-level CAM that is enhanced by drought-stress, emphasizing the need for careful characterization of photosynthetic physiology when selecting species to study the evolution of photosynthetic pathways.
Keywords: Crassulacean acid metabolism, CO2 assimilation, C4 photosynthesis, facultative CAM, salt tolerance, Sesuvium portulacastrum, succulence
Abstract
Demonstration of crassulacean acid metabolism (CAM) in species with low usage of this system relative to C3-photosynthetic CO2 assimilation can be challenging experimentally but provides crucial information on the early steps of CAM evolution. Here, weakly expressed CAM was detected in the well-known pantropical coastal, leaf-succulent herb Sesuvium portulacastrum, demonstrating that CAM is present in the Sesuvioideae, the only sub-family of the Aizoaceae in which it had not yet been shown conclusively. In outdoor plots in Panama, leaves and stems of S. portulacastrum consistently exhibited a small degree of nocturnal acidification which, in leaves, increased during the dry season. In potted plants, nocturnal acidification was mainly facultative, as levels of acidification increased in a reversible manner following the imposition of short-term water-stress. In drought-stressed plants, nocturnal net CO2 exchange approached the CO2-compensation point, consistent with low rates of CO2 dark fixation sufficient to eliminate respiratory carbon loss. Detection of low-level CAM in S. portulacastrum adds to the growing number of species that cannot be considered C3 plants sensu stricto, although they obtain CO2 principally via the C3 pathway. Knowledge about the presence/absence of low-level CAM is critical when assessing trajectories of CAM evolution in lineages. The genus Sesuvium is of particular interest because it also contains C4 species.
Introduction
The Aizoaceae, a family of about 1600 species in the order Caryophyllales (Hartmann, 2017), contains more species with succulent leaves than any other eudicot family (Hernández-Ledesma et al., 2015). In three of the four sub-families of the Aizoaceae, the Aizooideae, Mesembryanthemoideae, and the Ruschioideae, succulence is associated with multiple lineages of crassulacean acid metabolism (CAM), a water-use efficient pathway of photosynthesis (von Willert et al., 1977, 1992; Smith and Winter, 1996; Arakaki et al., 2011).
The Sesuvioideae, the earliest diverging sub-family (Klak et al., 2003; Bohley et al., 2015), is unique in the Aizoaceae in that it is the only sub-family within which CAM has not yet been demonstrated unequivocally (Bohley et al., 2015) and it is the only sub-family that contains species with C4 photosynthesis, which is present in the genera Sesuvium (Bittrich, 1990; Bohley et al., 2015), Trianthema, and Zaleya (Carolin et al., 1978). Within Sesuvium, two clades have been recognized, one composed of species with C4-type anatomy, C4 biochemistry, and carbon isotope values of between –13.3 and –11.2‰ [S. congense, S. crithmoides, S. humifusum (formerly Cypselea humifusum), S. mesembryanthemoides, and S. sesuvoides) (Bohley et al., 2015, 2017), and one with isotope values of between −27.7 and −25.3‰ that are characteristic of plants with C3 photosynthesis (S. ayresii, S. dystylum, S. maritimum, S. microphyllum, S. portulacastrum, S. sessile, and S. verrucosum) (Bohley et al., 2015). For one species, S. edmonstonei, native to the Galapagos, a reported isotope value of −21.5‰ is intermediate between those characteristic of C3 plants and those of plants with C4 photosynthesis or full CAM (Winter and Holtum, 2002; Bohley et al., 2015; Alonso-Cantabrana and von Caemmerer, 2016), although for plants of a northern Venezuelan population the δ13C value is –24‰ (Medina et al., 2008).
Despite a lack of evidence for CAM-type CO2 dark fixation in the Sesuvioideae, the ~50 species in this small sub-family [Sesuvium, 14 spp. (Bohley et al., 2017); Trianthema, ~20 spp. (Hartmann, 2017); Tribulocarpus, two spp. (Thulin et al., 2012); Zaleya, six spp. (Gilbert et al., 2000)] are mostly succulent-leaved and drought-tolerant, traits common in CAM plants (Ogburn and Edwards, 2010). Bearing in mind recent reports of low-level CAM in other small succulent-leaved herbs within the Caryophyllales that exhibit C3- or C4-type isotopic signatures [e.g. Calandrinia (Montiaceae), Portulaca (Portulacaceae), and in the succulent-leaved vine Anredera baselloides (Basellaceae)] (Holtum et al., 2017a, 2017b, 2018; Winter and Holtum, 2017), we decided to test whether the ostensibly C3 or C4 isotope values reported for Sesuvium might mask the presence of low-level CAM. We were further encouraged to do so by a single observation of small nocturnal acidification in field-growing S. maritimum (Walter) Britton, Sterns & Poggenb. that exhibited a C3-type isotope value of –26‰ (Martin et al., 1982).
A plant exhibits the CAM photosynthetic pathway when, in chloroplast-containing cells, CO2 is incorporated at night into malic acid that is stored in vacuoles (Osmond, 1978). In the subsequent light-period, the CO2 is retrieved from malic acid and is used for growth. The manifestation of CAM is therefore defined as an ability of a plant to fix CO2 in the dark and to accumulate acid at night. Nocturnal acidification is typically determined by measuring the differences in titratable acidity in extracts of tissue harvested at the beginning and the end of the night.
In the majority of plants with CAM, it is expressed alongside C3 photosynthesis or, in a few species, C4 photosynthesis (Koch and Kennedy, 1980; Winter and Holtum, 2017). The contribution of CAM to net daily carbon gain varies considerably between species. Nocturnal acidification reported for plants with CAM ranges over two orders of magnitude from 3–4 μmol H+ g−1 fresh mass (FM; close to the lower limit of detectability) to over 400 μmol H+ g−1 FM. At one extreme of the continuum, CAM may be the principal carbon-contributing pathway in a plant (Winter and Holtum, 2014), whereas at the other extreme the contribution of CAM to carbon gain may be extremely small [e.g. Platycerium veitchii (Holtum and Winter, 1999); Jatropha curcas (Winter and Holtum, 2015); Yucca gloriosa (Heyduk et al., 2016)]. In the latter group, the machinery for CAM is nonetheless present and the pathway is operational, although nocturnal CO2 fixation may be masked by dark respiratory CO2 loss.
The expression of CAM may be constitutive, i.e. non-optional in that expression is part of the pre-set processes of development and growth, or it may be facultative, i.e. optional, in that it is not always present (Winter and Holtum, 2014; Winter et al., 2015). Facultative CAM involves an induction or up-regulation of CAM in response to a stress (typically water stress) that is fully or largely lost when the stress is removed. Facultative and constitutive CAM are not necessarily mutually exclusive in a given photosynthetic organ. In a plant in which CAM is constitutively expressed, transient stress may transiently up-regulate CAM (Winter et al., 2008). Because the contribution of dark CO2 fixation to lifetime carbon gain can be small in some facultative CAM plants and in species that exhibit low levels of constitutive CAM, such plants may exhibit isotope values similar to C3 or C4 plants and the detection of CAM in these species is best measured by quantifying both leaf acidification and nocturnal CO2 exchange during a wet-dry-wet sequence.
To further investigate the possibility of CAM in the Sesuvioideae we chose as a test species S. portulacastrum. Typically regarded as a C3 species even when growing under conditions of stress (Lüttge et al., 1989; Voznesenskaya et al., 2010; Bohley et al., 2015), a single value of overnight H+ increase of field-growing S. portulacastrum has been reported (Ting, 1989). The species is a pan-tropical succulent-leaved coastal herb with an extensive distribution that spans coastlines of Africa, North and South America, tropical and temperate Asia, Australia, and the Pacific islands between 35°N and 42°S (Gonçalves, 1978; Lonard and Judd, 1997; Bohley et al., 2017; Hartmann, 2017). In some regions, the salt-tolerating halophyte is cultivated for food and fodder, and used for dune stabilization and phytoremediation (Lokhande et al., 2013a, 2013b).
Here, we provide results from a detailed study of gas exchange and nocturnal acidification in plants from a Panamanian population of S. portulacastrum either cultivated in pots or growing in outdoor experimental plots throughout tropical wet-season/dry-season transitions. Conclusive evidence is provided for the presence of low-level CAM in both the stems and leaves of S. portulacastrum.
Materials and methods
Plant material
Cuttings were obtained from five plants of Sesuvium portulacastrum (L.) L. collected at Sarigua National Park, Azuero Peninsula, Republic of Panama (8.013348N, 80.485658W) and further propagated at the Smithsonian Tropical Research Institute. A voucher specimen was deposited at the herbarium of the National University of Panama (PMA) (J. Aranda, A. Virgo, M. García, K. Winter 4324; 25 October 2018).
Outdoor experiments
Two experiments were performed. In the first, at the beginning of September, 2016 (middle of the wet season), 10 cuttings were planted in forest soil in a 1.5×1.5×0.3 m raised garden bed surrounded by 3.5-cm thick wood panels at the Smithsonian Tropical Research Institute, Santa Cruz Experimental Research Facility, Gamboa, Republic of Panama (9.120085N, 79.701894W). Plants were exposed to full natural solar radiation and received natural rainfall. Plants were grown for 186 d. In the second experiment, which began on 1 June, 2017 (early wet season), plants derived from 25 cuttings were grown for 333 d in a mixture of 50:25:25% (v/v/v) of forest soil: potting mix (Miracle-Gro Lawn Products, Marysville, OH): sea-sand (Noveys, Panama) in a raised garden bed as described above (Fig. 1). From July to December 2017, in addition to natural rainfall, the plants received at monthly intervals 10 l of 10% seawater prepared from 3.5 g l−1 Ocean Salt (Instant Ocean, Blacksburg, VA (e.g. Reef et al., 2016). Leaves and stems were sampled at dusk and dawn at weekly or 2-weekly intervals, unless specified otherwise. Samples were weighed for fresh mass determination and stored in liquid nitrogen to be processed further for measurements of titratable acidity, as described below.
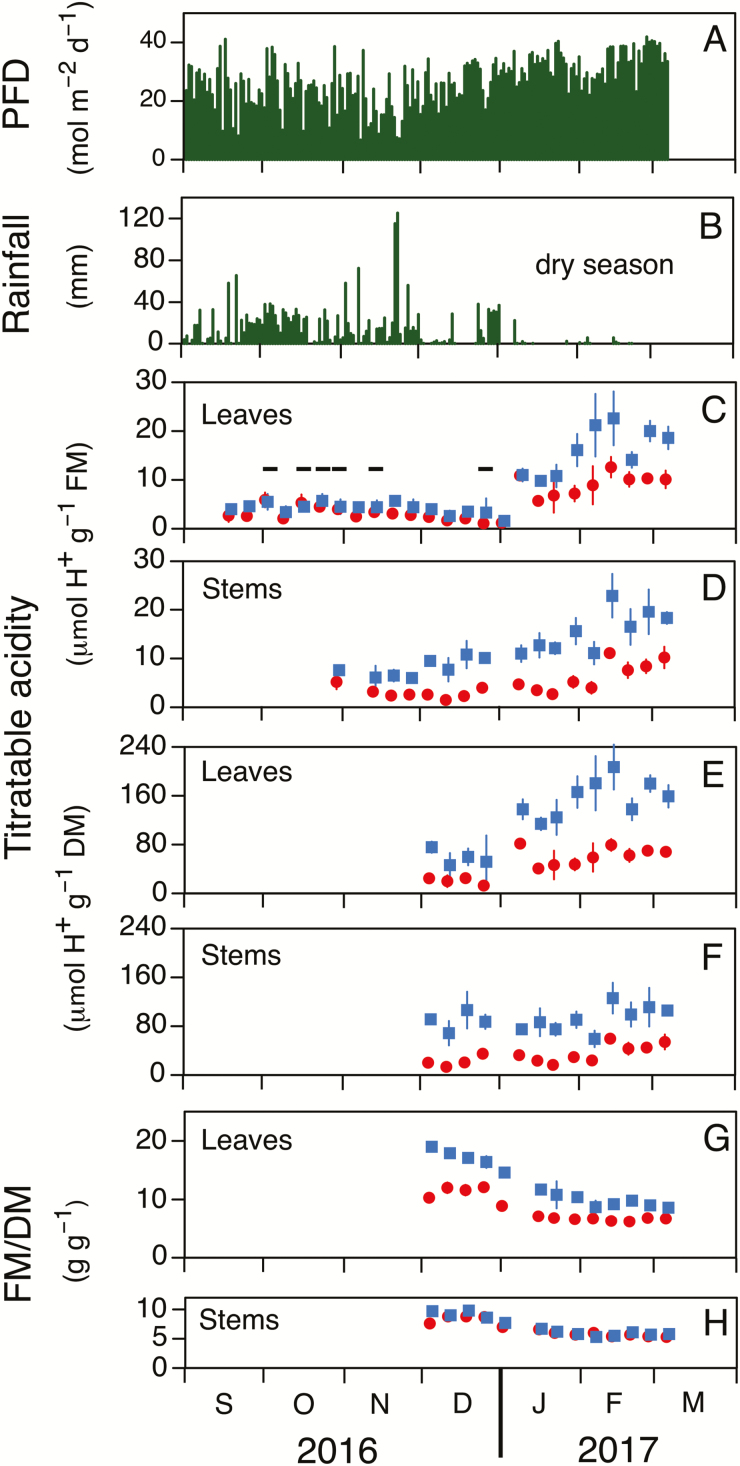
Fig. 1.
Sesuvium portulacastrum growing outside in a 1.5×1.5×0.3-m raised garden bed at the Smithsonian Tropical Research Institute, Republic of Panama in January 2018.
Pot experiments
Throughout 2016 and 2017, several experiments were conducted to study titratable acidity changes and gas-exchange responses during wet-dry-wet cycles. Plants were grown in terracotta pots (ranging from 0.5–3.5 l) containing potting mix (Miracle-Gro). Plants were maintained at the Tupper Centre of the Smithsonian Tropical Research Institute, Panama City (8.962568N, 79.543911W) under either full solar radiation or underneath rain shelters at 70% natural light. Growth conditions are specified in the relevant figure legends. As in the outdoor experiments, for acidity measurements mature leaves and stems excised from plants at dusk and dawn were weighed for determination of fresh mass and frozen in liquid nitrogen. In some experiments, prior to freezing the leaf area was determined using a LI-3100 leaf area meter (Li-Cor, Lincoln, NE).
Titratable acidity and dry mass
Frozen tissue was either extracted sequentially in boiling 50% ethanol and in water (Winter and Holtum, 2017), or was freeze-dried for 72 h (Labonco, Freezeone 4.5, Kansas City, MO) and reweighed for dry mass determination before extraction. All extracts were titrated with 5 mM KOH to pH 6.5.
Net CO2 exchange
To determine daily CO2 exchange patterns during wet-dry-wet cycles whole plants, attached branches, or individual attached leaves were studied. In one of the experiments, the shoot of a small plant was enclosed inside a Perspex cuvette (internal dimensions 11×11×10 cm) that rested on the 0.5-l terracotta pot in which the plant grew in potting mix (Miracle-Gro). The roots and the pot remained outside the cuvette. In another experiment, an individual leaf attached to a plant growing in a 1-l terracotta pot containing potting mix was enclosed in a 4.5-cm diameter PMK-10 leaf cuvette (porometer head; Walz, Effeltrich, FRG). After initial daily irrigation with water or a solution equivalent to 10% seawater, a drought treatment was imposed by withholding irrigation until net CO2 uptake during the light period approached zero, after which plants were rewatered daily.
The gas-exchange cuvettes were located inside a controlled environment chamber (GC8, EGC, Chagrin Falls, OH) operating under 12/12 h light/dark, 28/22 °C cycles. Illumination was supplied by a LED grow light (GroPro300, Model LL4L-GP300, www.LL4L.com). Photon flux densities are specified in the relevant figure legends. The cuvette was supplied with air containing 400 ppm CO2 at a flow rate of 1.26 l min−1. Net CO2 exchange was measured in a flow-through gas-exchange system consisting of Walz components and a LI-6252 CO2 Analyzer (Li-Cor) (Holtum and Winter, 2003).
Results
Outdoor experiments in garden beds
Sesuvium portulacastrum experienced two distinct climatic phases when grown outside for 186 d in forest soil (Fig. 2). During the first 125 d (i.e. from September 2016 to early January 2017) it rained on many days, whereas for the final 61 d rainfall was essentially absent (Fig. 2B). As rainfall ceased, the average daily irradiance increased (Fig. 2A). By the end of March, all plants had died. During the rainy period, leaves contained low levels of titratable acidity, when expressed on a fresh mass basis, but the values were significantly greater at dawn than at dusk for 9 of 15 weekly measurements (Fig. 2C). Stem acidity measurements began in October. In all wet-season samples, stem acidity increased significantly overnight, with the dawn-to-dusk difference being greater in stems than in leaves (Fig. 2C, D).
Fig. 2.
Seasonal changes in (A) photon flux density (PFD), (B) rainfall, (C–F) variation of titratable acidity at dusk (circles) and dawn (squares), and (G, H) variation in fresh:dry mass ratio (FM/DM) at dusk and dawn in leaves and stems of Sesuvium portulacastrum. Plants were grown in non-saline soil during the latter half of the 2016 wet season (September–December) and as they transitioned into the 2017 dry season. Acidity levels are expressed per unit fresh mass for leaves (C) and stems (D) and per unit dry mass for leaves (E) and stems (F). Acidity and FM/DM values are means (±SD) (n=5; for leaves, each sample comprised eight leaves). In (C–F), horizontal bars indicate non-significant differences between dusk and dawn values (one-tailed t-test, P≤0.05).
Soon after initiating the experiment, we noted that leaves slightly wilted during the daytime and recovered overnight. This observation prompted us to determine fresh:dry mass ratios from December onwards, and to express leaf and stem acidities also on a dry mass basis (Fig. 2E, F).
Nocturnal acidification in leaves, on both a fresh mass (FM) and a dry mass (DM) basis, was greater during the dry season than in the rainy season (Fig. 2C, E). ΔH+ was 1.3±0.9 μmol g−1 FM (mean±SD, n=15 d) during the wet season and 7.7±3.2 μmol g−1 FM (n=8 d) during the dry season (P≤0.001). Corresponding values on a dry mass basis were 38±10 μmol g−1 DM (n=4 d; wet season) and 95±26 μmol g−1 DM (n=9 d; dry season) (P≤0.001). In stems, acidification was also higher during the dry season, but only when values were expressed on a fresh mass basis (5.1±2.2 μmol g−1 FM, n=8 d, wet season versus 9.1±1.8 μmol g−1 FM, n=9 d, dry season; P≤0.001) and not when expressed on a dry mass basis (Fig. 2D, F). As the soil dried out during the progression between seasons, the FM to DM ratios of both leaves and stems decreased (Fig. 2G, H). Diurnal reductions in leaf FM:DM were large during the wet season and decreased during the dry season (Fig. 2G). Diurnal variations in FM:DM were largely absent in stems.
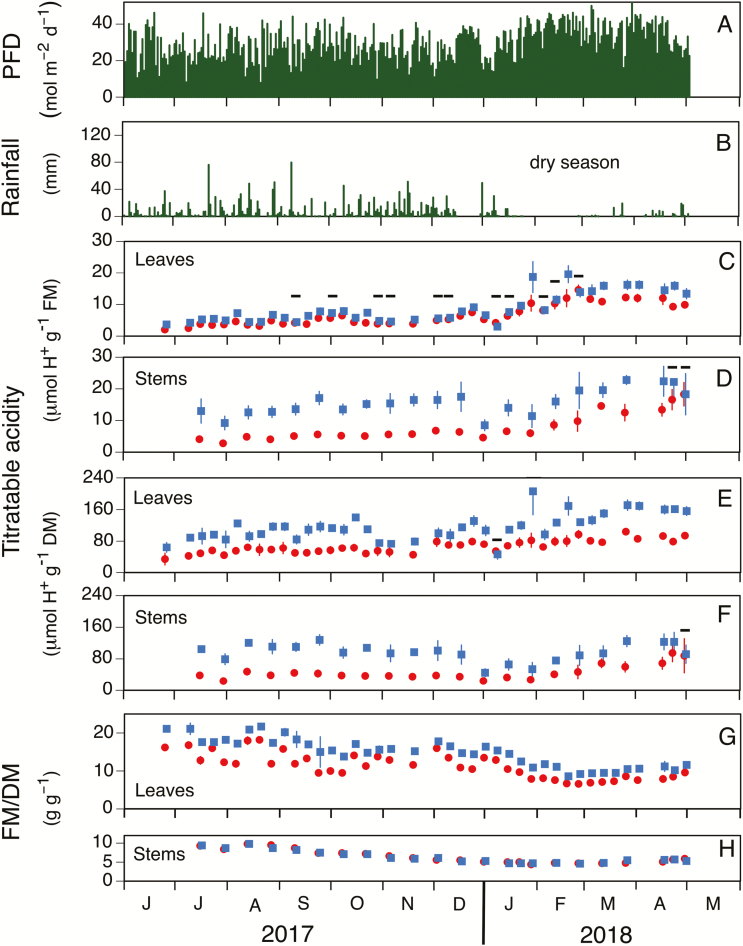
Since S. portulacastrum typically grows in coastal saline environments, a second outdoor experiment was conducted during the following 2017/2018 season in which plants were irrigated monthly with 10% seawater during the wet period. The input of extra moisture was small, equivalent to 4.4 mm precipitation per month. The salt was supplied to fulfil a hypothetical requirement for optimal growth, not to stress the plants per se.
The rainfall period lasted longer during the 2017/2018 season than during the 2016/2017 season of the first experiment (Fig. 3). Significant rainfall events extended into the middle of January 2018 (the wettest January since rainfall measurements started at the site 14 years previously). January 2018 rainfall was 80 mm, whereas January 2017 rainfall was 28 mm. In contrast to the previous experiment, plants survived the 2018 dry season.
Fig. 3.
Seasonal changes in (A) photon flux density (PFD), (B) rainfall, (C–F) variation of titratable acidity at dusk (circles) and dawn (squares), and (G, H) variation in fresh:dry mass ratio (FM/DM) at dusk and dawn in leaves and stems of Sesuvium portulacastrum grown in soil supplemented with sea-salt. Plants were grown for most of the 2017 wet season, and as they transitioned into the 2018 dry season. Acidity levels are expressed per unit fresh mass for leaves (C) and stems (D) and per unit dry mass for leaves (E) and stems (F). Acidity and FM/DM values are means (±SD) (n=5; for leaves, each sample comprised eight leaves). In (C–F), horizontal bars indicate non-significant differences between dusk and dawn values (one-tailed t-test, P≤0.05).
As occurred in plants grown outdoors in non-saline soil, nocturnal acidification in S. portulacastrum was observed in both leaves and stems in plants grown outdoors under rainfall supplemented with 10% seawater (Fig. 3). During the rainy period, acidification in stems was consistently greater than in leaves, about 2-fold on a DM basis and 3-fold on a FM basis. Nevertheless, the absolute levels of nocturnal acidification in leaves and stems, irrespective on what basis they were expressed, were similar to those observed in plants grown during the previous year.
Again, during the wet season, leaves exhibited low levels of acidity on a FM basis that were significantly higher at dawn than at dusk on 22 of 28 wet-season days. On a DM basis, dawn values were higher on all wet-season days. Nocturnal acidification in leaves significantly increased during the dry season (collection dates from 27 January to 22 April 2018), from 1.4±0.8 μmol H+ g−1 FM (n=28 d) during the wet season to 3.8±3.0 μmol H+ g−1 FM (n=11 d) during the dry season (P≤0.05), and from 41±17 μmol H+ g−1 DM (n=28 d) during the wet season to 68±27 μmol H+ g−1 DM (n=11 d) during the dry season (P≤0.01). No significant dry-season increase in nocturnal acidification was observed in stems, either on a FM or a DM basis.
Experiments with potted plants
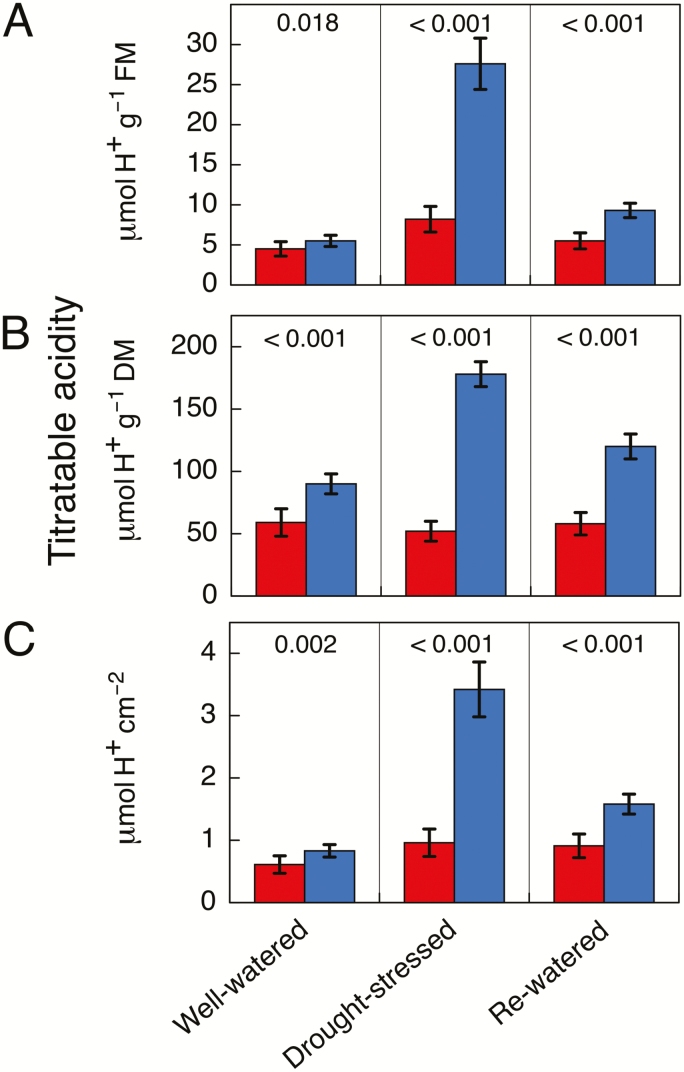
Several pot experiments were conducted to study nocturnal acidification patterns in response to short-term drought stress. Leaves of well-irrigated plants supplied with either water or 10% seawater exhibited either no or a small nocturnal acidification. In the case of the experiment shown in Fig. 4, nocturnal acidification was below 2 μmol H+ g−1 FM under high initial soil moisture conditions. Following the imposition of water stress, the acid accumulation at night was stimulated ~19-fold. Upon rewatering, acidification was markedly reduced but was not eliminated, remaining at ~4 μmol H+ g−1 FM.
Fig. 4.
Titratable acidity at dusk (red) and dawn (blue) in leaves of Sesuvium portulacastrum grown in 3.5-l pots in soil supplemented with sea-salt underneath a rain shelter at 70% of natural sunlight. Samples were taken from eight plants. The experiment was performed from the end of October to early December 2017. Data are shown for well-watered plants, droughted plants (20 d without irrigation), and plants that had been droughted and rewatered (20 d with irrigation). The data are expressed on the basis of (A) fresh mass, (B) dry mass, and (C) leaf area, and are means (±SD) (n=8; each sample comprised eight leaves). P-values from one-tailed t-tests are shown.
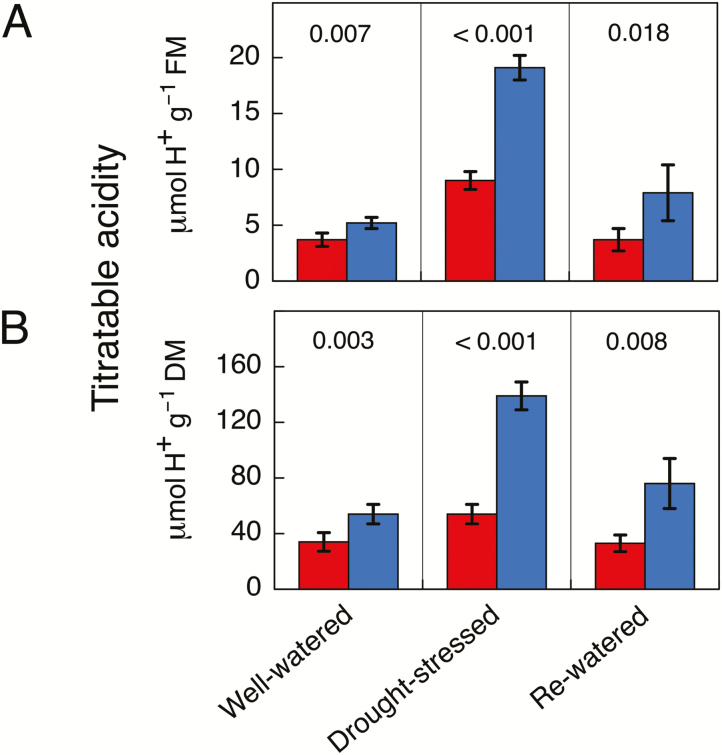
In a separate experiment, stems of well-watered S. portulacastrum exhibited low levels of nocturnal acidification of less than 2 μmol H+ g−1 FM (Fig. 5). Following the cessation of watering, nocturnal acidification increased ~7-fold on a FM basis. When the plants were rewatered, the nocturnal accumulation was reduced markedly but was still present at a level that was ~3-fold greater than the values at the beginning of the experiment. The wet-dry-wet acidification patterns were similar when H+ was expressed on a DM or area basis for leaves or on a DM basis for stems. In all cases, a strong drought-induced up-regulation of nocturnal acid accumulation was observed, which was largely, albeit not fully, reversible.
Fig. 5.
Titratable acidity at dusk (red) and dawn (blue) in stems of Sesuvium portulacastrum grown in 2.5-l pots in non-saline soil under full sunlight. The experiment was performed during January/February 2017. Samples were randomly taken from eight plants. Data are shown for well-watered plants, droughted plants (6 d without irrigation), and plants that had been droughted and rewatered (7 d with irrigation). The data are expressed on the basis of (A) fresh mass, and (B) dry mass, and are means (±SD) (n=4 stems; at a given time-point each stem was harvested from a different plant). P-values from one-tailed t-tests are shown.
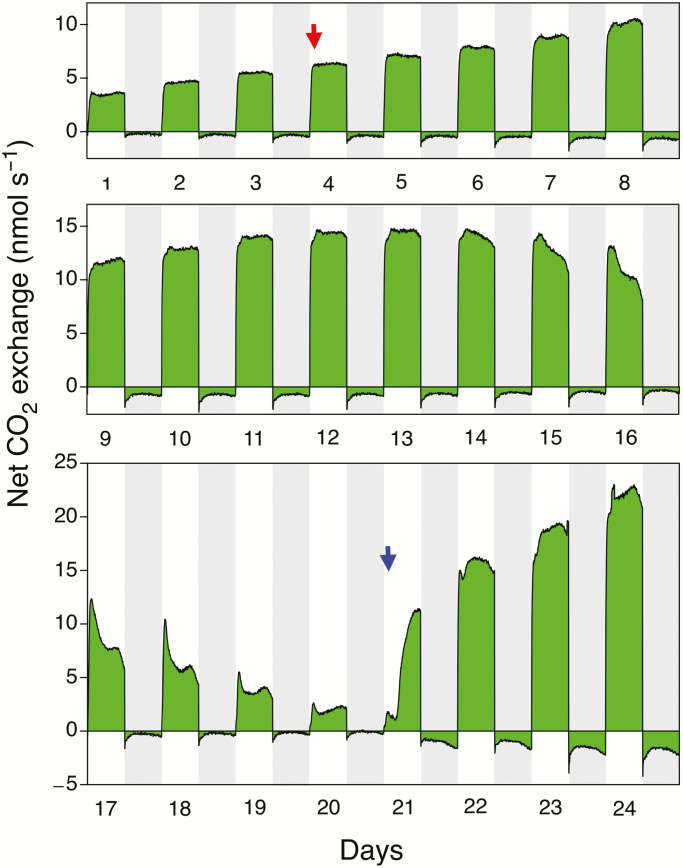
Seven gas-exchange experiments were conducted with essentially identical results, two of which are depicted in Figs 6 and 7. For a well-watered plant of S. portulacastrum, net CO2 uptake by its shoot was restricted to the 12-h light period (Fig. 6). CO2 efflux was relatively constant throughout the night following an initial overshoot and period of equilibration to the night temperature, although a small curvature in the nocturnal CO2 release pattern was detectable with lower rates in the middle than at the end of the dark period. Following cessation of watering on day 4, CO2 uptake during the day continued to increase as the shoot continued to grow, utilizing water remaining in the pot. From day 14 onwards there was a progressive reduction in both CO2 uptake during the day and CO2 loss at night. Daytime CO2 exchange developed a prominent mid-day depression of uptake. Nocturnal CO2 exchange approached the CO2 compensation point but never transitioned to net CO2 gain.
Fig. 6.
Net CO2 exchange over a 24-d period by the shoot of a Sesuvium portulacastrum plant growing in a 0.5-l pot containing non-saline soil. Watering was withheld on day 4 (red arrow) and recommenced on day 21 (blue arrow). The grey shaded areas represent the 12-h dark periods. Photon flux density was 700 µmol m−2 s−1 at the level of the shoot. On the last day of the experiment, the total leaf area was 6 cm2, the leaf dry mass was 0.129 g, and the stem dry mass was 0.024 g.
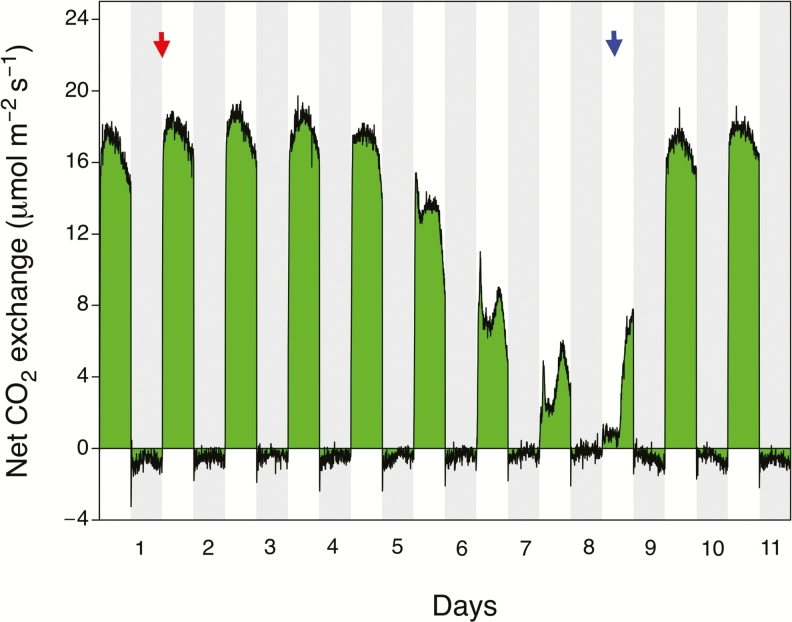
Fig. 7.
Net CO2 exchange over an 11-d period by an individual leaf of a Sesuvium portulacastrum plant growing in soil supplemented with sea-salt. Watering was withheld on day 2 (red arrow) and recommenced on day 9 (blue arrow). The grey shaded areas represent the 12-h dark periods. Photon flux density was 480 µmol m−2 s−1. Gas exchange was measured on a leaf area of 2.98 cm2.
Following rewatering, a recovery of CO2 uptake in the light was observed within 4 h and dark respiration increased during the subsequent night. Within 48 h after rewatering, the shoot exhibited a pattern of CO2 exchange similar to that observed in the original well-watered conditions. The kinetics of nocturnal CO2 efflux in the shoots of the rewatered plants were more curved than the efflux pattern in the original well-watered plants. The rate of CO2 loss was also greater. The daytime CO2 uptake rates following rewatering exceeded those at the onset of the experiment because the plants continued to grow inside the gas-exchange cuvette throughout the experiment, although at a reduced rate during the period of drought. The CO2 exchange pattern during a watering-droughting-rewatering cycle of a leaf of a plant, in this particular case irrigated with 10% seawater (Fig. 7), was similar to that of the shoot of a plant that was irrigated with water only (Fig. 6). The leaf of a well-watered plant exhibited net CO2 uptake during the day and net CO2 loss at night. At 3 d after watering ceased, daytime net CO2 exchange began to decrease, exhibiting a progressively larger mid-day depression of uptake. CO2 efflux at night also decreased, such that the CO2 compensation point was reached but net nocturnal CO2 uptake was not detected.
Within a few hours of rewatering on day 8, the rates of daytime CO2 uptake began to recover. During the following night, nocturnal CO2 loss gradually increased. By day 11, the patterns and extent of daytime CO2 uptake and night-time CO2 loss were similar to the patterns observed on day 1.
Discussion
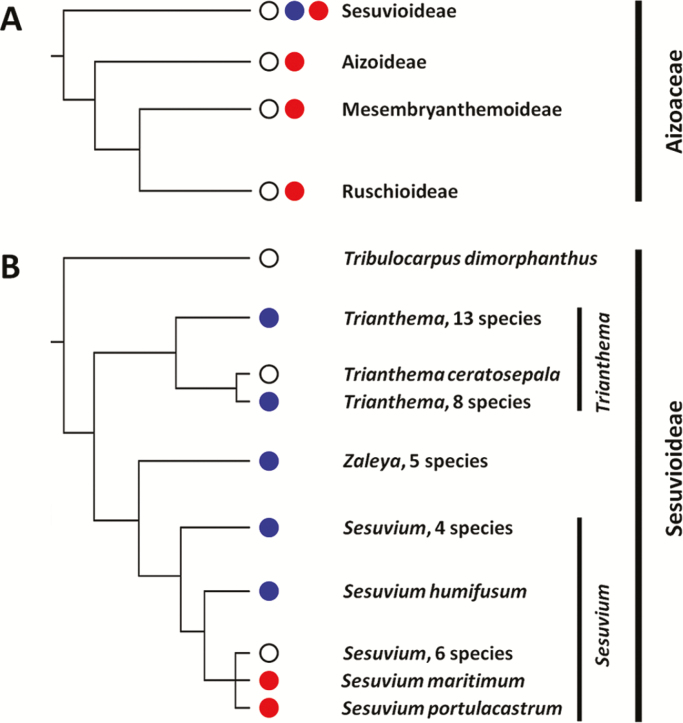
The purpose of this study was to clarify whether CAM is present or absent in S. portulacastrum. To this end, plants grown outdoors in the ground or potted plants grown under more controlled conditions were subjected to a range of conditions, including supplementation with NaCl and drought stress. Under most conditions, small nocturnal increases in acid content were measured in both leaves and stems, suggesting that CAM is present in Sesuvium and thus in the Sesuvioideae, the only sub-family of the Aizoaceae for which CAM had not yet been conclusively documented (Fig. 8). Given the ability to perform low-level CAM, S. portulacastrum joins a growing number of species that cannot be considered C3 plants sensu strictu, although they obtain CO2 principally via the C3-pathway.
Fig. 8.
Distribution of CAM (red) and C4 (blue) within (A) the four sub-families of the Aizoaceae, and (B) genera of the sub-family Sesuvioides (adapted from Smith and Winter, 1996; Klak et al., 2003; Muhaidat et al., 2007; Bohley et al., 2015, 2017, and the current study). Phylogenetic relationships in (A) are based upon analysis of three chloroplastic and nuclear loci (Klak et al., 2003), and relationships in (B) are based upon analysis of five chloroplastic and nuclear loci (Bohley et al., 2015). The open circles indicate plants with C3-type isotope values. Without measurements of acidification and gas exchange during a watering-droughting-rewatering regime, it cannot yet be determined whether such individuals exhibit C3senso strictu, low-level CAM, or are C3-C4 intermediates. In (B), the presence of CAM in S. maritimum is based upon nocturnal acidification reported during a single night for a plant growing in the field (Martin et al., 1982).
Whilst CAM photosynthesis is present in S. portulacastrum, in comparison to C3 photosynthesis, the contribution of nocturnal CO2 assimilation to the carbon gain of the plant is extremely small. Whole-leaf carbon isotope values of plants in the field of generally around –24 to –26‰ (Winter et al., 1981; Lüttge et al., 1989; Medina et al., 2008; Bohley et al., 2015), indicate that, averaged over the life of the plant, the vast majority of carbon is assimilated by C3 photosynthesis during the light (Winter and Holtum, 2002). In the gas-exchange time-courses for shoots and leaves shown in Figs 6 and 7 (as well as in the additional experiments for which data are not shown), the rates of dark CO2 fixation were not high enough to compensate fully for respiratory CO2 loss. CO2 exchange at night approached but did not exceed the CO2 compensation point.
Similar levels of nocturnal acidification accumulated in plants grown in outdoor raised garden plots and in plants grown in pots under more controlled conditions. Acidification was detected effectively throughout the life of the plants, even during the wet season, although acidification levels in leaves were greater during the dry season. In contrast to the evidence from the outdoor experiments, examination of the pot experiment acidification data (Figs 4, 5) would suggest that most, if not all, of the CAM expression in leaves was facultative as acidification in non-stressed leaves was extremely low. The evidence for mostly facultative CAM in the pot experiments with controlled irrigation and for longer-term low-level CAM in the outdoor experiments is not necessarily contradictory. Observation of leaves of S. portulacastrum on outwardly healthy, fast-growing plants growing in the outdoor plots subject to natural daylight during the wet season indicated that the relatively elastic leaves (low elastic modulus) often appeared flaccid in the afternoons. The resultant leaf water deficit was evidently sufficient to lead to low-level CAM that persisted throughout the experiment. A similar phenomenon has been reported to induce CAM in leaves of well-watered plants of the facultative CAM plant M. crystallinum (Winter and Holtum, 2007), especially in plants grown under non-saline conditions.
Observations in the two outdoor experiments of increased nocturnal acidification in leaves during the dry season are consistent with a strong facultative CAM component in leaves, while the absence of a consistent dry-season increase in nocturnal stem acidification suggests that low-level CAM in stems has a substantial constitutive component. The ability of S. portulacastrum to maintain nocturnal acidification levels well into the dry season, even as FM/DM ratios fell below 5 for stems and below 10 in succulent leaves, provides an indication of the relative drought-tolerance of the species. Indeed, plants growing on a saline plain in northern Venezuela have been reported to operate at xylem tensions of as low as –5.6 MPa (Lüttge et al., 1989).
CAM is now documented in all four sub-families of the Aizoaceae (Fig. 8A). Pronounced CAM, undoubtedly overwhelmingly constitutive, is present in the sub-families Azooideae, Mesembryanthemoideae, and Ruschioideae (Smith and Winter, 1996), and facultative CAM is present in the Mesembryanthemoideae [e.g. Mesembranthemum crystallinum (Winter and Holtum, 2007) and Aptenia cordifolia (Treichel, 1975)] and now in the Sesuvioideae (S. portulacastrum). All of the sub-types of CAM, constitutive, facultative, pronounced and weak, are also known in the only other clade of the Caryophyllales with CAM plants, the sub-order Portulacineae. In the portullugo (Portulacineae plus Molluginaceae; Ogburn and Edwards, 2010; Edwards and Ogburn, 2012), pronounced CAM predominates in most genera within the Cactaceae and Didiereaceae (Alluaudia, Alluaudiopsis, Decarya, Didierea), whereas weak and facultative CAM are more commonly expressed in the Anacampserotaceae (Guralnick and Jackson, 2001; Winter and Holtum, 2017), Portulacaria within the Didiereaceae (Ting and Hanscom 1977; Guralnick and Ting, 1986), Montiaceae (Winter and Holtum, 2011; Martin et al., 1988), Talinaceae (Herrera et al., 1991), Basellaceae (Holtum et al., 2018), and in the C4 and C3-C4 Portulacaceae (Koch and Kennedy, 1980; Winter and Holtum, 2017; K. Winter et al., unpublished results). In the thin-leaved Molluginaceae, C4 and C3 are present but CAM has not been reported (Christin et al., 2011).
CAM lineages and C4 lineages tend to cluster in certain regions of the angiosperm phylogenetic tree (Sage et al., 2011a; Edwards and Ogburn, 2012; Christin et al., 2015), with particularly closely related CAM and C4 lineages identified within the Aizoaceae, Euphorbiaceae, and Portulacineae (Klak et al., 2003, 2017; Sage et al., 2011b; Horn et al., 2012, 2014; Ocampo et al., 2013; Christin et al., 2014). Following the discovery of CAM and C4 photosynthesis in Euphorbia and Portulaca, Sesuvium becomes only the third genus known to contain both types of carbon-concentrating photosynthesis.
In the Sesuvioides, the most basal of the sub-families in the Aizoaceae, CAM is reported here in Sesuvium, the most derived clade (Fig. 8B). C4 is present in Zaleya and Trianthema (Muhaidat et al., 2007; Koteyeva et al., 2015), whereas neither C4 nor CAM have been reported in the most basal clade, Tribulocarpus (Bohley et al., 2015, 2017). Succulence is present in Sesuvium, Trianthema, and to a lesser extent in Zaleya and Tribulocarpus.
CAM and C4 are present in Sesuvium but they are yet to be demonstrated in the same species. The genus circumscribes a C3 clade and a C4 clade (Bohley et al. 2015, 2017). Both S. portulacastrum and the closely related S. maritimum studied by Martin et al. (1982) are in the C3 clade. It would be informative to explore whether CAM is present within members of C4Sesuvium clade. In contrast to what is currently known about Sesuvium, in Portulaca CAM is expressed within at least three clades of C4 species and thus, in those species with CAM, it is co-expressed with C4 in the same plant (Koch and Kennedy, 1980; Holtum et al., 2017b; Winter and Holtum, 2017). In P. cryptopetala, facultative CAM is present in a C3-C4 intermediate species that exhibits a C2-cycle (Muhaidat et al., 2007; Vozsenskaya et al., 2010, 2017; K. Winter et al., unpublished results). Within the genus Euphorbia, which contains C3, C4, and CAM species (Webster et al., 1975; Sage et al., 2011b; Yang and Berry, 2011), there are no reports as yet of CAM within the C4 clade (subg. Chamaesyce), although it appears to have repeatedly evolved alongside succulence from C3 lineages (Horn et al., 2014). Together, the Euphorbia, Portulaca, and Sesuvium lineages with their differing evolutionary histories of CAM and C4 photosynthesis provide promise for exploring the biochemical, anatomical, and ecological trajectories that favour the selection of one carbon-concentrating mechanism over the other, or permit the co-existence of both.
The ecological significance of the low-level contribution of carbon contributed by CAM to S. portulacastrum is as yet unassessed, but as is evident from the outdoor experiments described here, low-level and facultative CAM can be expressed over extensive periods. As a species that is a coloniser of the fringes of saline flats, S. portulacastrum probably spends much of its life growing slowly under conditions of salinity, drought stress, high light, and high temperatures, as evidenced by the vividly red-coloured fleshy stems and wilted leaves frequently exhibited by plants in the field. Under such conditions one might expect CO2 assimilation in the light to be constrained by water limitation. The reductions in water loss and respiratory carbon loss enabled by the low-level CAM, an ability to accumulate compatible solutes, and to accumulate the anion oxalate (Lüttge et al. 1989), coupled with other physiological mechanisms (Lokhande et al., 2013b), presumably assist plants to cope with the stresses imposed by the environment it inhabits.
In terms of carbon-isotope composition and gas-exchange pattern, S. portulacastrum is C3-like. Yet our multiple measurements of plants in pots and long-term measurements in the field demonstrate that S. portulacastrum consistently exhibits CAM in both leaves and stems. Since the levels of nocturnal acidification are low and easy to overlook, caution is required when assessing such plants for photosynthetic pathway. This study also highlights the care required when selecting C3 control species in evolutionary studies of lineages in which CAM or C4 has evolved. CAM and C4 origins may not always represent completely independent evolutionary phenomena, and may partially share evolutionary trajectories in that one photosynthetic type can be co-opted to evolve the other (Christin et al., 2014).
Acknowledgements
This research was supported by the Smithsonian Tropical Research Institute (STRI) and the Australian Research Council Discovery Project (DP160100098). We acknowledge the assistance of Jorge Aranda who maintained the experimental plots at Gamboa.
References
- Alonso-Cantabrana H, von Caemmerer S. 2016. Carbon isotope discrimination as a diagnostic tool for C4 photosynthesis in C3–C4 intermediate species. Journal of Experimental Botany 67, 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki M, Christin P-A, Nyffeler R, Lendel A, Eggli U, Ogburn RM, Spriggs E, Moore MJ, Edwards EJ. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences, USA 108, 8379–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittrich V. 1990. Systematic studies in Aizoaceae. Mitteilungen aus dem Institut für Allgemeine Botanik in Hamburg 23b, 491–507. [Google Scholar]
- Bohley K, Joos O, Hartmann H, Sage R, Liede-Schumann S, Kadereit G. 2015. Phylogeny of Sesuvioideae (Aizoaceae) – Biogeography, leaf anatomy and the evolution of C4 photosynthesis. Perspectives in Plant Ecology, Evolution and Systematics 17, 116–130. [Google Scholar]
- Bohley K, Winter PJD, Kadereit G. 2017. A revision of Sesuvium (Aizoaceae, Sesuvioideae). Systematic Botany 42, 124–147. [Google Scholar]
- Carolin RC, Jacobs SWL, Vesk M. 1978. Kranz cells and mesophyll in the Chenopodiales. Australian Journal of Botany 26, 683–698. [Google Scholar]
- Christin PA, Arakaki M, Osborne CP, et al. 2014. Shared origins of a key enzyme during the evolution of C4 and CAM metabolism. Journal of Experimental Botany 65, 3609–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Arakaki M, Osborne CP, Edwards EJ. 2015. Genetic enablers underlying the clustered evolutionary origins of C4 photosynthesis in angiosperms. Molecular Biology and Evolution 32, 846–858. [DOI] [PubMed] [Google Scholar]
- Christin PA, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF. 2011. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65, 643–660. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn M. 2012. Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. International Journal of Plant Sciences 173, 724–733. [Google Scholar]
- Gilbert MG, Hartmann HEK, Edwards S. 2000. Aizoaceae. In: Edwards S, Tadesse S, Demissew S, Hedberg I, eds. Flora of Ethiopia & Eritrea. Vol. 2 Addis Ababa and Uppsala: National Herbarium/Uppsala University, 240–248. [Google Scholar]
- Gonçalves ML. 1978. Aizoaceae. In Launert E, ed. Flora Zambesiaca. Vol. 4 London: Flora Zambesiaca Managing Committee, 508–521. [Google Scholar]
- Guralnick LJ, Jackson MD. 2001. The occurrence and phylogenetics of crassulacean acid metabolism in the Portulacaceae. International Journal of Plant Sciences 162, 257–262. [Google Scholar]
- Guralnick LJ, Ting IP. 1986. Seasonal response to drought and rewatering in Portulacaria afra (L.) Jacq. Oecologia 70, 85–91. [DOI] [PubMed] [Google Scholar]
- Hartmann HEK. 2017. Illustrated handbook of succulent plants: Aizoaceae. 2nd edn. Heidelberg: Springer. [Google Scholar]
- Hernández-Ledesma P, Berendsohn WG, Borsch T, et al. 2015. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 45, 281–383. [Google Scholar]
- Herrera A, Delgado J, Paraguatey I. 1991. Occurrence of inducible crassulacean acid metabolism in leaves of Talinum triangulare (Portulacaceae). Journal of Experimental Botany 42, 493–499. [Google Scholar]
- Heyduk K, Burrell N, Lalani F, Leebens-Mack J. 2016. Gas exchange and leaf anatomy of a C3-CAM hybrid, Yucca gloriosa (Asparagaceae). Journal of Experimental Botany 67, 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K. 2017a. Facultative CAM photosynthesis (crassulacean acid metabolism) in four species of Calandrinia, ephemeral succulents of arid Australia. Photosynthesis Research 134, 17–25. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K. 2017b. Optional use of CAM photosynthesis in two C4 species, Portulaca cyclophylla and Portulaca digyna. Journal of Plant Physiology 214, 91–96. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K. 2018. Crassulacean acid metabolism (CAM) in the Basellaceae (Caryophyllales). Plant Biology 20, 409–414. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Winter K. 1999. Degrees of crassulacean acid metabolism in tropical epiphytic ferns. Australian Journal of Plant Physiology 26, 749–757. [Google Scholar]
- Holtum JA, Winter K. 2003. Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta 218, 152–158. [DOI] [PubMed] [Google Scholar]
- Horn JW, van Ee BW, Morawetz JJ, Riina R, Steinmann VW, Berry PE, Wurdack KJ. 2012. Phylogenetics and the evolution of major structural characters in the giant genus Euphorbia L. (Euphorbiaceae). Molecular Phylogenetics and Evolution 63, 305–326. [DOI] [PubMed] [Google Scholar]
- Horn JW, Xi Z, Riina R, Peirson JA, Yang Y, Dorsey BL, Berry PE, Davis CC, Wurdack KJ. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68, 3485–3504. [DOI] [PubMed] [Google Scholar]
- Klak C, Hanacek P, Bruyns PV. 2017. Disentangling the Aizooideae: new generic concepts and a new subfamily in Aizoaceae. Taxon 66, 1147–1170. [Google Scholar]
- Klak C, Khunou A, Reeves G, Hedderson T. 2003. A phylogenetic hypothesis for the Aizoaceae (Caryophyllales) based on four plastid DNA regions. American Journal of Botany 90, 1433–1445. [DOI] [PubMed] [Google Scholar]
- Koch K, Kennedy RA. 1980. Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiology 65, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Edwards GE. 2015. An assessment of the capacity for phosphoenolpyruvate carboxykinase to contribute to C4 photosynthesis. Plant Science 235, 70–80. [DOI] [PubMed] [Google Scholar]
- Lokhande VH, Gor BK, Desai NS, Nikam TD, Suprasanna P. 2013a. Biochemical and physiological adaptations of the halophyte Sesuvium portulacastrum (L.) L., (Aizoaceae) to salinity. Archives of Agronomy and Soil Science 59, 1373–1391. [Google Scholar]
- Lokhande VH, Gor BK, Desai NS, Nikam TD, Suprasanna P. 2013b. Sesuvium portulacastrum, a plant for drought, salt stress, sand fixation, food and phytoremediation. A review. Agronomy for Sustainable Development 33, 329–348. [Google Scholar]
- Lonard RI, Judd FW. 1997. The biological flora of coastal dunes and wetlands. Sesuvium portulacastrum (L.) L. Journal of Coastal Research 13, 96–104. [Google Scholar]
- Lüttge U, Popp M, Medina E, Cram WJ, Diaz M, Griffiths H, Lee HSJ, Schäfer C, Smith JAC, Stimmel KH. 1989. Ecophysiology of xerophytic and halophytic vegetation of a coastal alluvial plain in northern Venezuela. V. The Batis maritima-Sesuvium portulacastrum vegetation unit. New Phytologist 111, 283–291. [DOI] [PubMed] [Google Scholar]
- Martin CE, Higley M, Wang WZ. 1988. Ecophysiological significance of CO2-recycling via Crassulacean acid metabolism in Talinum calycinum Engelm. (Portulacaceae). Plant Physiology 86, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Lubbers AE, Teeri JA. 1982. Variability in crassulacean acid metabolism: a survey of North Carolina succulent species. Botanical Gazette 143, 491–497. [Google Scholar]
- Medina E, Francisco AM, Wingfield R, Casañas OL. 2008. Halofitismo en plantas de la costa caribe de Venezuela: halófitas y halotolerantes. Acta Botanica Venezuelica 31, 49–80. [Google Scholar]
- Muhaidat R, Sage RF, Dengler NG. 2007. Diversity of Kranz anatomy and biochemistry in C4 eudicots. American Journal of Botany 94, 362–381. [DOI] [PubMed] [Google Scholar]
- Ocampo G, Koteyeva NK, Voznesenskaya EV, Edwards GE, Sage TL, Sage RF, Columbus JT. 2013. Evolution of leaf anatomy and photosynthetic pathways in Portulacaceae. American Journal of Botany 100, 2388–2402. [DOI] [PubMed] [Google Scholar]
- Ogburn RM, Edwards EJ. 2010. The ecological water-use strategies of succulent plants. Advances in Botanical Research 55, 179–225. [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29, 379–414. [Google Scholar]
- Reef R, Slot M, Motro U, Motro M, Motro Y, Adame MF, Garcia M, Aranda J, Lovelock CE, Winter K. 2016. The effects of CO2 and nutrient fertilisation on the growth and temperature response of the mangrove Avicennia germinans. Photosynthesis Research 129, 159–170. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ. 2011a. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, Heckel MA. 2011b. The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae). Journal of Experimental Botany 62, 3183–3195. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Winter K. 1996. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Berlin, Heidelberg: Springer, 427–436. [Google Scholar]
- Thulin M, Thiede J, Liede-Schumann S. 2012. Phylogeny and taxonomy of Tribulocarpus (Aizoaceae): a paraphyletic species and an adaptive shift from zoochorous trample burrs to anemochorous nuts. Taxon 61, 55–66. [Google Scholar]
- Ting IP. 1989. Photosynthesis of arid and subtropical succulent plants. Aliso 12, 387–406. [Google Scholar]
- Ting IP, Hanscom Z. 1977. Induction of acid metabolism in Portulacaria afra. Plant Physiology 59, 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treichel S. 1975. Crasulaceensäuerstoffwechsel bei einem salztoleranten Vertreter der Aizoaceae: Aptenia cordifolia. Plant Science Letters 4, 141–144. [Google Scholar]
- von Willert DJ, Eller BM, Werger MJA, Brinckmann E, Ihlenfeldt HD. 1992. Life strategies of succulents in deserts. Cambridge: Cambridge University Press. [Google Scholar]
- von Willert DJ, Thomas DA, Lobin W, Curdts E. 1977. Ecophysiological investigations in the family of the Mesembryanthemaceae. Oecologia 29, 67–76. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2010. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3-C4 intermediate in genus Portulaca L. (Portulacaceae). Journal of Experimental Botany 61, 3647–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2017. Unique photosynthetic phenotypes in Portulaca (Portulacaceae): C3-C4 intermediates and NAD-ME C4 species with Pilosoid-type Kranz anatomy. Journal of Experimental Botany 68, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GL, Brown WV, Smith BN. 1975. Systematics of photosynthetic carbon fixation pathways in Euphorbia. Taxon 24, 27–33. [Google Scholar]
- Winter K, Garcia M, Holtum JA. 2008. On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. Journal of Experimental Botany 59, 1829–1840. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JA. 2002. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129, 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JA. 2007. Environment or development? Lifetime net CO2 exchange and control of the expression of crassulacean acid metabolism in Mesembryanthemum crystallinum. Plant Physiology 143, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2011. Induction and reversal of crassulacean acid metabolism in Calandrinia polyandra: effects of soil moisture and nutrients. Functional Plant Biology 38, 576–582. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2014. Facultative CAM plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 65, 3425–3441. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2015. Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas L. Functional Plant Biology 42, 711–717. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2017. CO2-exchange patterns demonstrate facultative CAM photosynthesis (crassulacean acid metabolism) in four small C3 and C4 leaf-succulents. Australian Journal of Botany 65, 103–108. [Google Scholar]
- Winter K, Holtum JA, Smith JA. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208, 73–78. [DOI] [PubMed] [Google Scholar]
- Winter K, Osmond CB, Pate JS. 1981. Coping with salinity. In: Pate JS, McComb AJ, eds. The biology of Australian plants. Perth: University of Western Australia Press, 88–113. [Google Scholar]
- Yang Y, Berry PE. 2011. Phylogenetics of the Chamaesyce clade (Euphorbia, Euphorbiaceae): reticulate evolution and long-distance dispersal in a prominent C4 lineage. American Journal of Botany 98, 1486–1503. [DOI] [PubMed] [Google Scholar]