Portulaca cryptopetala is a C3–C4 intermediate species that exhibits facultative CAM suggesting that in Portulaca facultative CAM is ancestral to C4.
Keywords: C4 photosynthesis, crassulacean acid metabolism, Portulaca cryptopetala, Portulaca molokiniensis, Portulacaceae
Abstract
The Portulacaceae enable the study of the evolutionary relationship between C4 and crassulacean acid metabolism (CAM) photosynthesis. Shoots of well-watered plants of the C3–C4 intermediate species Portulaca cryptopetala Speg. exhibit net uptake of CO2 solely during the light. CO2 fixation is primarily via the C3 pathway as indicated by a strong stimulation of CO2 uptake when shoots were provided with air containing 2% O2. When plants were subjected to water stress, daytime CO2 uptake was reduced and CAM-type net CO2 uptake in the dark occurred. This was accompanied by nocturnal accumulation of acid in both leaves and stems, also a defining characteristic of CAM. Following rewatering, net CO2 uptake in the dark ceased in shoots, as did nocturnal acidification of the leaves and stems. With this unequivocal demonstration of stress-related reversible, i.e. facultative, induction of CAM, P. cryptopetala becomes the first C3–C4 intermediate species reported to exhibit CAM. Portulaca molokiniensis Hobdy, a C4 species, also exhibited CAM only when subjected to water stress. Facultative CAM has now been demonstrated in all investigated species of Portulaca, which are well sampled from across the phylogeny. This strongly suggests that in Portulaca, a lineage in which species engage predominately in C4 photosynthesis, facultative CAM is ancestral to C4. In a broader context, it has now been demonstrated that CAM can co-exist in leaves that exhibit any of the other types of photosynthesis known in terrestrial plants: C3, C4 and C3–C4 intermediate.
Introduction
An estimated 10% of terrestrial vascular plants express either crassulacean acid metabolism (CAM) or C4 photosynthesis (Smith and Winter, 1996; Winter et al., 2015; Sage, 2016). Both photosynthetic pathways have evolved independently over 60 times. CAM is documented in more than 30 angiosperm families, and in one family in each of the cycads, gnetophytes, ferns, and lycophytes (Smith and Winter, 1996), while C4 is known in 19 families of angiosperms (Sage and Sultmanis, 2016).
The CAM and C4 pathways are comparable in many respects (Osmond, 1978; Hatch, 1987). Using a similar complement of enzymes, each pathway concentrates CO2 in the vicinity of Rubisco thereby reducing the competitive inhibition by molecular oxygen of CO2 uptake. In both pathways, atmospheric CO2 initially fixed as HCO3− using oxygen-insensitive phosphoenolpyruvate carboxylase (PEPc) is incorporated into a four-carbon intermediate from which CO2 is ultimately liberated in the vicinity of Rubisco. At the site of Rubisco, CO2 attains concentrations that ensure that the enzyme functions overwhelmingly as a carboxylase.
Despite similarities, CAM and C4 differ in important aspects. In C4 plants, all of the processes associated with photosynthetic CO2 assimilation occur during the light. PEPc and Rubisco are simultaneously active but are separated spatially, usually in two distinct types of cells (for exceptions, see e.g. Edwards and Vozsenskaya, 2011). The primary carboxylation by PEPc typically occurs in thin-walled mesophyll cells that surround thicker walled bundle-sheath (BS) cells. The four-carbon intermediate is transferred via plasmodesmata to BS cells where CO2 is liberated and the refixation of the CO2 by Rubisco takes place. In contrast to C4 photosynthesis, CAM is essentially a single-cell phenomenon, during which the PEPc- and Rubisco-catalysed carboxylations operate at different times of the day–night cycle, i.e. their activity is separated temporally. During the night, CO2 is fixed by PEPc and the four-carbon intermediate, malic acid, is stored in large vacuoles. During the following light period, the stomata close, PEPc is inactivated, and CO2 released from the decarboxylation of malic acid is refixed by Rubisco.
Across the phylogenetic tree of angiosperms (Ogburn and Edwards, 2010, 2012), CAM and C4 origins cluster in numerous distinct clades suggesting that certain plant lineages are prone to evolve both pathways (Edwards and Ogburn, 2012). It has been proposed that the distinct anatomical requirements of the CAM and C4 pathways, coupled with differences in timing and regulation of their respective biochemical pathways, reduce the likelihood that both co-occur in the same organ. The possibility that parts of the C4 and CAM cycles take place in the same cell has been suggested to be even less likely (Sage, 2002). In accordance, the reported instances of co-expression of CAM and C4 within plants with Kranz anatomy are rare, with the only known cases being observed in Portulaca (Koch and Kennedy, 1980; Guralnick et al., 2002). Portulaca is the only genus of the Portulacaceae, a family assigned to the order Caryophyllales in which CAM and C4 have evolved multiple times (Christin et al., 2014). Phylogenetic relationships of families within the Caryophyllales and the currently known distribution of CAM and C4 photosynthesis among them have been recently featured in Holtum et al. (2018) (see their Fig. 3).
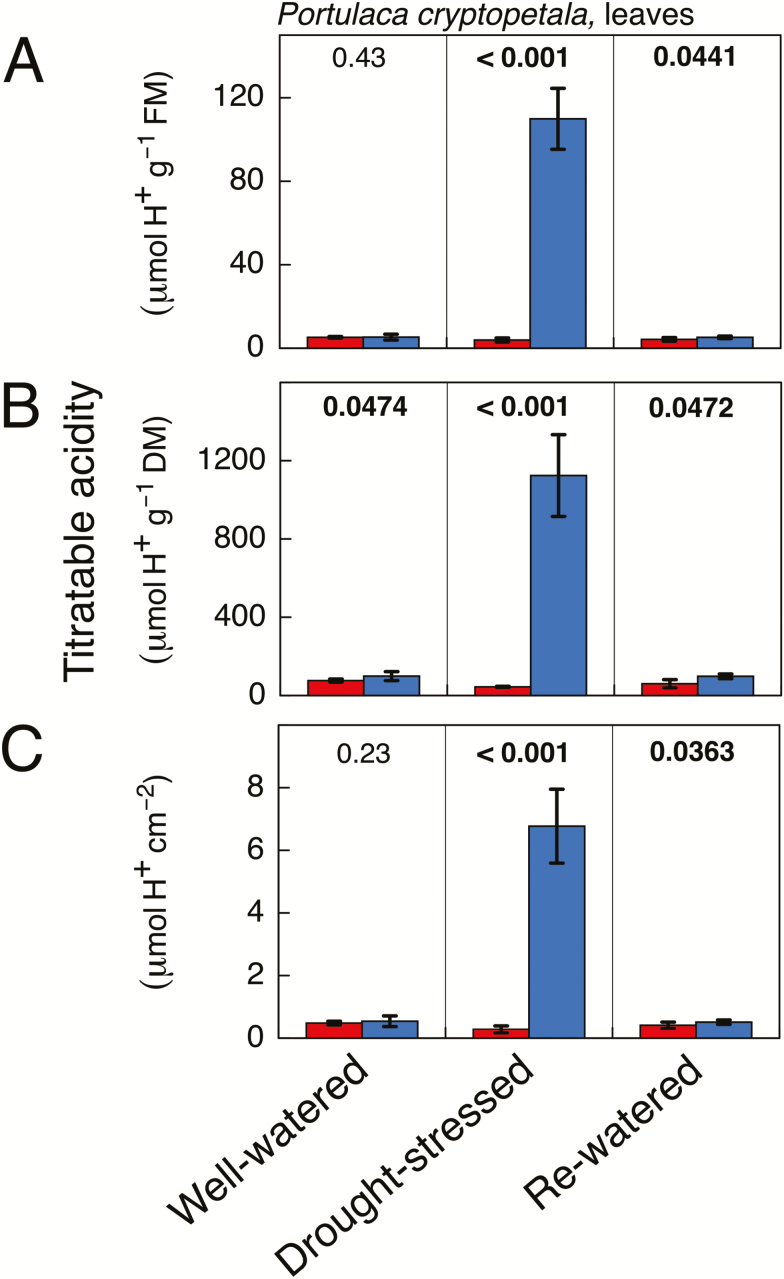
Fig. 3.
Titratable acidity in recently fully expanded leaves of Portulaca cryptopetala at the end of the 12 h light period (red) and the end of the 12 h dark period (blue) in plants that were well-watered (left-hand column), droughted (middle column; 9 d without irrigation) and droughted and rewatered (right-hand column; 3 d with irrigation). The data are expressed on a fresh mass basis (A), a dry mass basis (B) and a leaf area basis (C). Mean ±SD (n=5 leaves; at a given time point each leaf was harvested from a different plant). The numerical values shown above the bars are P values (one-tailed t-test). Bold letters indicate that the values at the end of the dark period were significantly greater than those at the end of the day at P≤0.05.
Originally, CAM and C4 were reported for two species of Portulaca (P. oleracea and P. grandiflora), and in each, it appeared that CAM and C4 were confined to different cell or tissue regions. More recent studies indicate that the co-existence of CAM and C4 in the same photosynthetic organ is common in Portulaca (Winter and Holtum, 2014, 2017; Holtum et al., 2017a; Winter, 2019). CAM has been demonstrated by CO2 gas exchange and quantification of nocturnal acidification in seven species from four of the six major phylogenetic clades of Portulaca. In each case of CAM and C4 co-expression, the expression of CAM was facultative (Guralnick et al., 2002; D’Andrea et al., 2014; Winter and Holtum, 2014, 2017; Holtum et al., 2017a). CAM-type gas-exchange patterns and nocturnal acidification were not detected in well-watered plants, but were induced when the plants were subjected to water stress. When stressed plants were rewatered, their physiology returned to the original well-watered pattern. The observation of widespread CAM in Portulaca is supported by the evolutionary history of PEPc genes in Portulaca (Christin et al., 2014). The putative gene encoding CAM-specific PEPc was apparently present before the divergence of Portulaca, and is similarly used for CAM in relatives of Portulaca, whereas PEPcs optimized for C4 metabolism in Portulaca originated from a duplication event of a different paralog, which occurred at the base of Portulaca.
The coexistence of C4 and CAM in leaves of C4Portulaca species raises interesting questions about the location of both pathways, i.e. whether they occur in different regions of the leaf or whether there is cell sharing. This issue is not yet fully resolved. In Portulaca oleracea, CAM-type nocturnal CO2 fixation presumably takes place in centripetally located large parenchyma cells, yet critical daytime reactions of the CAM cycle may occur in the C4 bundle-sheath cells (Lara et al., 2003, 2004). By contrast, for P. grandiflora separate operation of the C4 and CAM pathways in different regions of the leaf has been postulated, with C4 in mesophyll cells associated with the bundle sheath cells and the complete CAM cycle taking place in the centripetal parenchyma cells (Guralnick and Jackson, 2001; Guralnick et al., 2002; Holtum et al., 2017a).
Species in five of the six phylogenetic clades of Portulaca are thought to use C4 as the principal pathway of carbon acquisition (Ocampo et al., 2013; Voznesenskaya et al., 2017). All species examined exhibit C4-type δ13C values, Kranz anatomies, enzyme complements, and gas-exchange characteristics. Portulaca is not known to contain C3 species senso strictu, but three species in the Cryptopetala clade, P. cryptopetala, P. hirsutissima and P. mucronata, have been characterized as C3–C4 intermediates on the basis of C3-type δ13C values, anatomy, location of glycine decarboxylase, and CO2 compensation points (Voznesenskaya et al., 2010, 2017; Ocampo et al., 2013). It was inferred that the C3–C4Cryptopetala clade evolved from C4 progenitors and that it represents a reversion from a C4 state (Ocampo and Columbus, 2012; Ocampo et al., 2013). The reversion hypothesis was questioned by Christin et al. (2014) who argued, on the basis of the composition of PEPc genes, the distinct leaf anatomy in each major clade, and the diversity of the de-carboxylating enzymes used by the different clades, that C4 evolved multiple times in parallel. The Cryptopetala clade may therefore be a lineage of Portulaca with a photosynthetic complement that reflects a pre-C4 stage.
CAM in the Cryptopetala clade would strengthen the argument that CAM represents an ancestral state in Portulaca, being present prior to the evolution of C4 photosynthesis. If so, the relationship between C3–C4 metabolism and CAM remains unclear. In C3–C4 intermediates, CO2 is concentrated into BS-like compartments via the localization of the photorespiratory enzyme glycine decarboxylase (GDC) in the BS, and the shuttling of photorespiratory glycine into the BS for decarboxylation. This metabolism, termed C2 photosynthesis, can raise CO2 concentrations in the BS two to three times above the atmospheric value, but does not greatly alter δ13C values from what are present in C3 species (Keerberg et al., 2014; Sage et al., 2014). As proposed for C4 plants, dual expression of C2 metabolism and CAM could interfere with the optimal function of each, and hence it could be hypothesized that the two metabolic types are segregated either to different tissues or to different phases of development. Here we use gas exchange and measurements of titratable acidity to explore whether CAM is present in the annual/biennial P. cryptopetala, and in the perennial P. molokiniensis (Hobdy, 1987). Portulaca cryptopetala is a C3–C4 intermediate and a member of one of the three clades of Portulaca in which CAM has not yet been reported. Portulaca molokiniensis is a C4 species that belongs to the C4Oleracea clade that is sister to the Cryptopetala clade.
Materials and methods
Seeds of P. cryptopetala and P. molokiniensis were obtained from the laboratory stock of one of us (RFS). Plants were grown from seed in either 0.5 litre terracotta pots with an upper diameter of 10 cm, or in 1 litre terracotta pots with an upper diameter of 13 cm. Pots contained potting mix (Miracle-Gro Lawn Products, Marysville, OH, USA). Plants were 1–3 months old when studied.
Two laboratory gas-exchange systems were used to measure 24 h patterns of CO2 gas exchange of plants. Whole shoots were enclosed in either an 11×11×10 cm or an 11×11×16 cm Perspex cuvette. Roots plus pot remained outside the cuvette. The gas-exchange cuvettes were located inside controlled-environment chambers operating under 12 h light (28 °C):12 h dark (22 °C) cycles. Light was provided by LED grow lights (model LL4L-GP300, GrowPro300). Photon flux density at the level of the cuvettes is specified in the corresponding figure legends. Cuvettes were supplied with air containing 400 ppm CO2 at flow rates of either 1.26 or 2.5 l min−1. Net CO2 exchange was measured in flow-through gas-exchange systems consisting of Walz components (gas mixing units, air pumps, cold traps, dew point mirrors; Walz GmbH, Effeltrich, Germany), LI-6252 CO2 analyzers (Li-Cor, NE, USA) and CR-1000 data loggers (Campbell Scientific, UT, USA) (Holtum and Winter, 2003). For measurements at 2% O2, N2 flowing at 4.75 l min−1 was added to ambient air flowing at 0.5 l min−1. CO2 was removed by passing the mixture through soda-lime and then re-added via a mass-flow controller to obtain 400 ppm CO2 before the gas mixture entered the cuvette. Exposures to air containing 2% O2 lasted 30–60 min.
Well-watered plants were watered daily to field capacity. Drought treatments were imposed by withholding irrigation until net CO2 uptake in the light was reduced to close to, at most, 10% of the value for well-watered plants, after which the plants were rewatered daily.
In a separate set of experiments, nine plants of each species were grown in the laboratory under 12 h light–12 h dark cycles. Photosynthetically active photon flux density (PFD) was 600 μmol m−2 s−1 supplied by a LED grow light (300 W Diamond series, Advanced LED Lights, Hiwasse, AR, USA). Temperature was 26 °C during light periods and 24 °C during dark periods. Plants watered daily to field capacity were deprived of water for several days and then rewatered as specified in the corresponding figure legends. Mature leaves were excised at the end of the light and dark periods from each well-watered, drought-stressed and rewatered plants, and then the fresh mass (FM) obtained, and leaf area measured using a LI-3100 area meter (Li-Cor). Samples were then frozen in liquid nitrogen and freeze-dried. After determination of dry mass, samples were boiled in 80 ml of 50% ethanol until the volume had about halved. Water was then added to bring the volume back to 80 ml and the extract was boiled until the volume again decreased by about half. The extracts were brought to the original volume with water, cooled to room temperature, and titrated with 5 mM KOH to pH 6.5.
Results
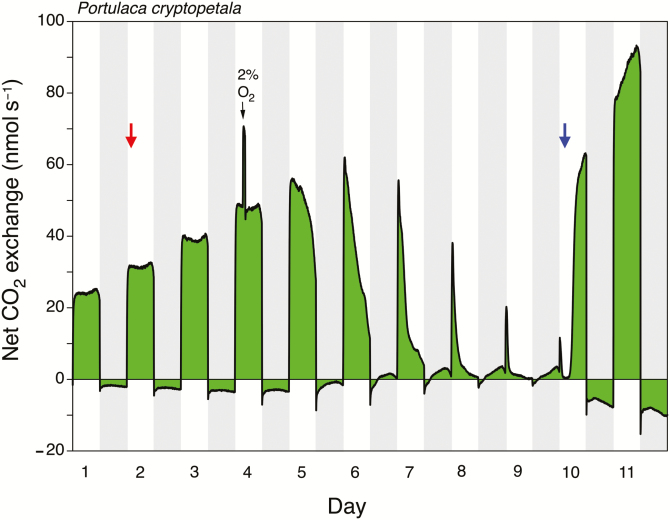
Well-watered plants of P. cryptopetala exhibited net CO2 uptake during the day and net CO2 loss at night (Fig. 1; see also Supplementary Fig. S1 at JXB online). The net rates of CO2 exchange during the day and night increased as the plants grew. In the experiment of Fig. 1, 3 d after watering ceased (day 5 of the experiment), net CO2 exchange began to decline in the light and the dark. The shape of the CO2 exchange curve in the dark was noticeably more curved, and nocturnal CO2 exchange approached the CO2 compensation point. On day 6, net CO2 uptake was present for the first time at night. Nocturnal uptake peaked during the night of day 7 and remained approximately constant until the night of day 9, the day prior to rewatering. Within 6 h of rewatering the plant on day 10, CO2 uptake during the light had almost recovered to the rates observed before the imposition of water stress. No nocturnal net CO2 uptake was present during the following dark periods.
Fig. 1.
Eleven days of net CO2 exchange by the shoot (leaves plus stems) of a Portulaca cryptopetala plant growing in a pot. Watering was withheld on day 2 (red arrow) and recommenced on day 10 (blue arrow). During the light period of day 4, the shoot was exposed to air containing 2% O2 (black arrow) for approx. 1 h. Shaded areas represent the 12 h dark periods. PFD incident to the top of the gas-exchange cuvette was 1000 μmol m−2 s−1. At the end of the experiment, total leaf area was 71.1 cm2, and leaf and stem dry masses were 0.26 and 0.095 g, respectively.
On day 4, when the P. cryptopetala plant shown in Fig. 1 was still exhibiting the well-watered pattern of CO2 uptake in the light and CO2 loss at night, the transfer of shoots during the light from an air-stream containing 21% O2 to an air-stream containing 2% O2 was accompanied by an increase in the rate of net CO2 uptake of up to 46%. When air containing 21% O2 was resupplied, the rate of CO2 uptake reattained the control pre-2% O2 rate. A total of nine 2% O2 treatments were performed on three plants and resulted in an increase of CO2 uptake by 39±7% (mean ±SD, n=3). The range was 31–46%.
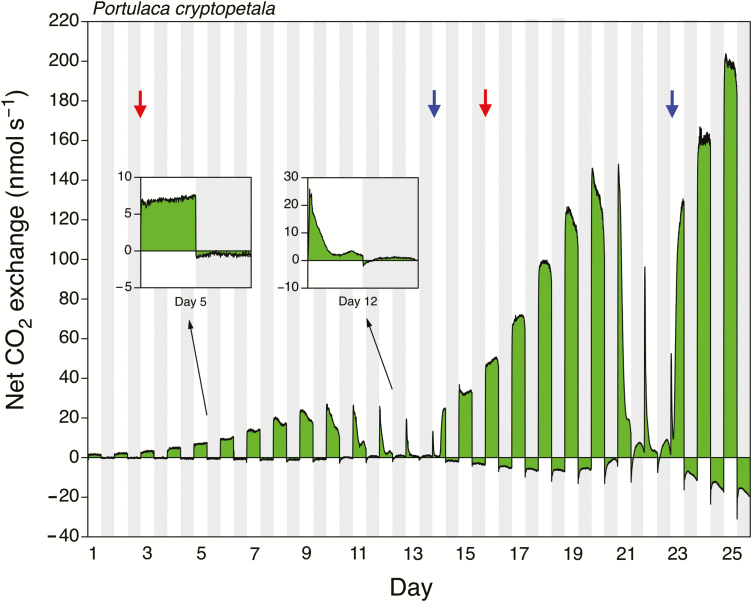
When a plant of P. cryptopetala was exposed to sequential watering, droughting, and rewatering cycles, the stress-related induction of net CO2 uptake in the dark was observed during each period of water stress (Fig. 2). The shoot inside the gas-exchange cuvette continued to grow during the experiment as evidenced by the progressive increase in net CO2 uptake during the light.
Fig. 2.
Twenty-five days of net CO2 exchange by the shoot (leaves and stems) of a potted Portulaca cryptopetala that was exposed to two wetting and drying cycles. Watering was withheld on days 3 and 16 (red arrows) and recommenced on days 14 and 23 (blue arrows). Shaded areas represent the 12 h dark periods. PFD incident to the top of the gas-exchange cuvette was 1350 μmol m−2 s−1. At the end of the experiment, total leaf area was 152 cm2 and leaf and stem dry masses were 0.578 and 0.372 g, respectively.
Leaves of well-watered P. cryptopetala either did not exhibit nocturnal acidification or, if it was present, the end of night/end of day differences in acidity were very low (Fig. 3). Following the imposition of water stress, strong nocturnal acidification was induced, reaching about 110 μmol H+ g−1 FM. At the end of the night, the absolute leaf H+ content was about 25-fold greater than in unstressed plants. Following rewatering, nocturnal leaf acidification was reduced markedly such that the end of the night–end of the day differences in H+ levels were close to zero. The expression in Fig. 3 of acid levels on fresh mass, dry mass, and leaf area bases enables the calculation of acid concentrations in leaves, permits estimation of the effects of changes in leaf-water content that occur during the droughting process, and facilitates comparison with gas-exchange measurements of CO2 exchange.
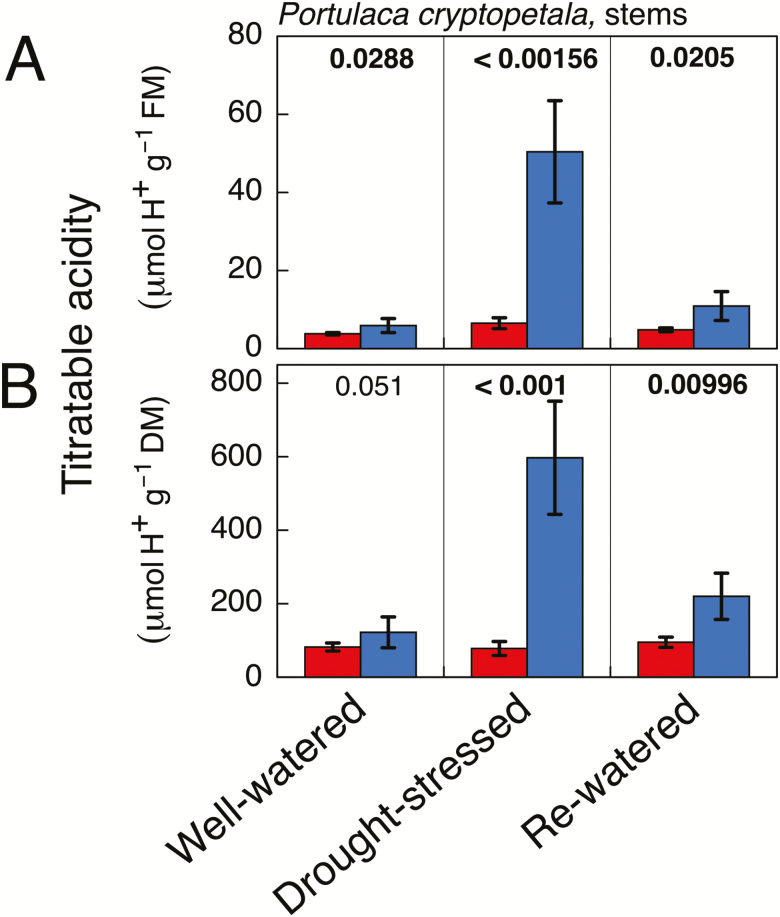
In stems of P. cryptopetala, in a manner similar to leaves, marked nocturnal acidification was induced when the plants were subjected to water stress (Fig. 4), with the end of the night acid pool increasing by about 10-fold in comparison to unstressed plants. In contrast to leaves, the stems of rewatered plants continued to exhibit nocturnal acidification, although the levels on a fresh mass basis were only about 14% of those observed in stems of droughted plants.
Fig. 4.
Titratable acidity in stems of Portulaca cryptopetala at the end of the 12 h light period (red) and the end of the 12 h dark period (blue) in plants that were well-watered (left-hand column), droughted (middle column; 10 d without irrigation), and droughted and rewatered (right-hand column; 2 d with irrigation). The data are expressed on a fresh mass basis (A) and a dry mass basis (B). Mean ±SD (n=5 stems; at a given time point each stem was harvested from a different plant). The numeric values shown above the bars are P values (one-tailed t-test). Bold letters indicate that the values at the end of the dark period were significantly greater than those at the end of the day at P≤0.05.
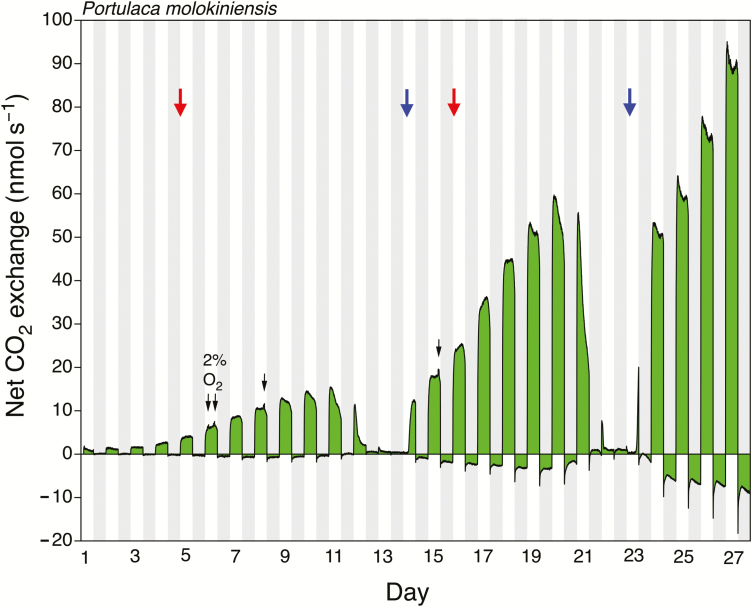
Well-watered shoots of P. molokiniensis exhibited net CO2 uptake during the light and net CO2 loss in the dark (Fig. 5). Following the imposition of water stress, a marked decrease in CO2 uptake was accompanied by the induction of net CO2 uptake in the dark. Rewatering was followed by a recovery of net CO2 uptake during the light and a loss of nocturnal net CO2 uptake. As was observed for P. cryptopetala, the exposure of shoots of P. molokiniensis to sequential watering, droughting, and rewatering cycles was accompanied by the stress-related induction of net CO2 uptake at night during each period of water stress. The continued increase in net CO2 uptake during the light demonstrated that the shoots of P. molokiniensis continued to grow during the experiment. The stress-induced, reversible induction of net dark CO2 fixation shown in Fig. 5 was fully confirmed in three additional gas-exchange experiments with three different P. molokiniensis plants (see Supplementary Figs S2–S4).
Fig. 5.
Twenty-seven days of net CO2 exchange by shoots of a potted Portulaca molokiniensis that was exposed to two wetting and drying cycles. Watering was withheld on days 5 and 16 (red arrows) and recommenced on days 14 and 23 (blue arrows). During the light periods of days 6, 8, and 15 the shoots were exposed to air containing 2% O2 (black arrows). Shaded areas represent the 12 h dark periods. PFD incident to the top of the gas-exchange cuvette was 1000 μmol m−2 s−1. At the end of the experiment, total leaf area was 97.5 cm2, and leaf and stem dry masses were 0.405 and 0.060 g, respectively.
In well-watered P. molokiniensis, the transfer of shoots during the light from an air-stream containing 21% O2 to an air-stream containing 2% O2 was accompanied by an increase in the rate of net CO2 uptake by 8±3% (mean ±SD, n=3 different plants; total of 10 measurements) (e.g. Fig. 5; Supplementary Fig. S2). The range was 5–14%. As with P. cryptopetala, when air containing 21% O2 was resupplied, the rate of CO2 uptake reattained the control pre-2% O2 rate.
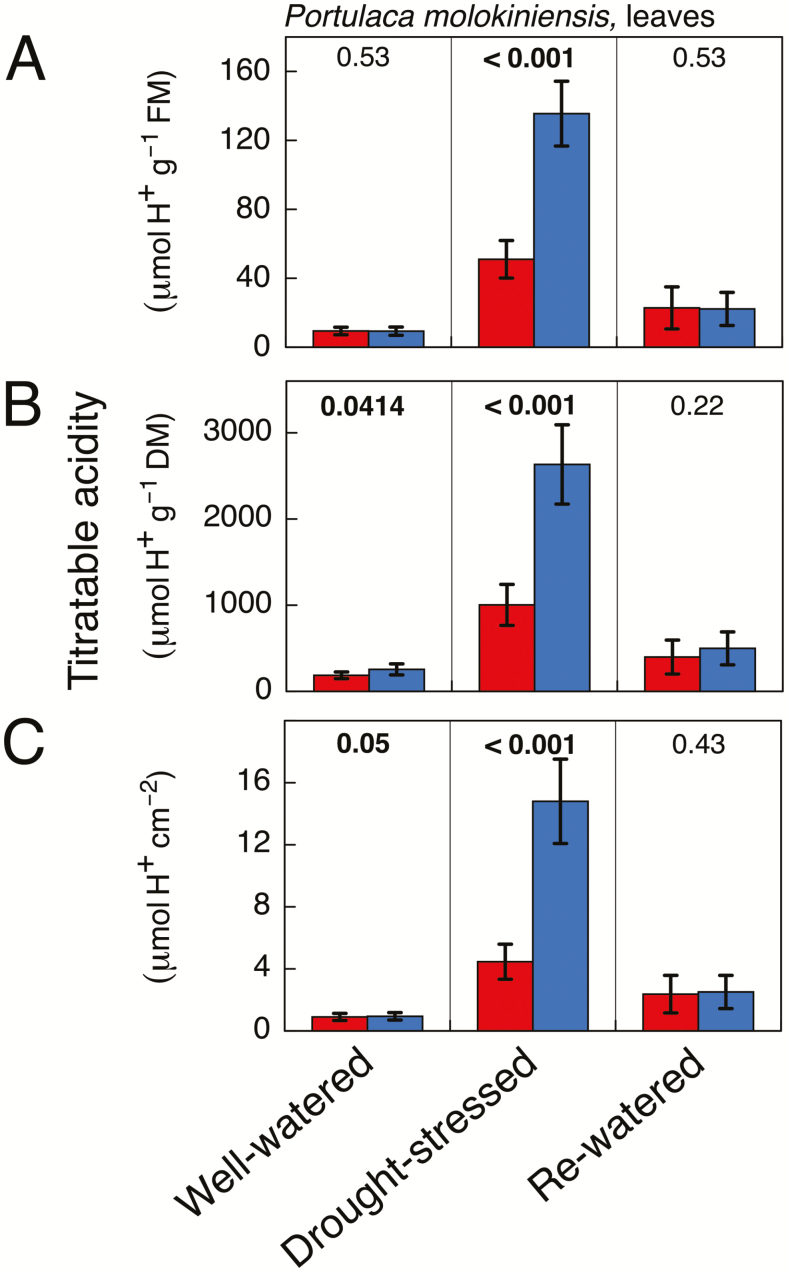
In a manner similar to P. cryptopetala, nocturnal acidification was either not present or barely detectable in leaves of well-watered P. molokiniensis (Fig. 6). Leaf acidity increased at the end of the light and the dark periods when plants were stressed. The increase in acidity at the end of the dark was much greater than at the end of the light period, resulting in substantial net acidification during the night. Nocturnal acidification of similar magnitude in droughted P. molokiniensis has been observed previously (L. Guralnik, unpublished data, personal communication). The nocturnal acidification was completely lost following rewatering, although the background [H+] remained somewhat greater than acidity levels at the beginning of the experiment.
Fig. 6.
Titratable acidity in recently fully expanded leaves of Portulaca molokiniensis at the end of the 12 h light period (red) and at the end of the 12 h dark period (blue) in plants that were well-watered (left-hand column), droughted (middle column; 12 d without irrigation), and droughted and rewatered (right-hand column; 6 d with irrigation). The data are expressed on a fresh mass basis (A), a dry mass basis (B), and a leaf area basis (C). Mean ±SD (n=5 leaves; at a given time point each leaf was harvested from a different plant). The numeric values shown above the bars are P values (one-tailed t-test). Bold letters indicate that the values at the end of the dark period were significantly greater than those at the end of the day at P≤0.05.
Discussion
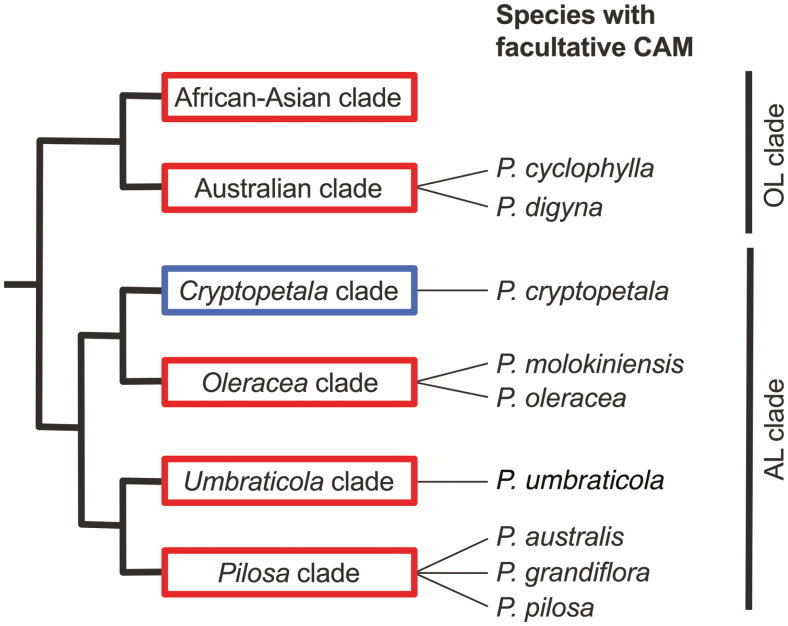
The demonstration of CAM in P. cryptopetala and in P. molokiniensis adds a new facet to our understanding of the diversity in origins, functioning, expression, and interrelationships of C3, C4, and CAM photosynthesis. CAM, long known to be co-expressed alongside C3 photosynthesis in plants with succulent tissues, is now documented in eight C4 species, all within Portulaca (Koch and Kennedy, 1980; Guralnick et al., 2002; Christin et al., 2014; Winter and Holtum, 2014, 2017; Holtum et al., 2017a). With the evidence presented here for CAM in P. cryptopetala, we can now conclude that CAM can also co-occur in leaves with C3–C4 photosynthesis. In Portulaca, the C4 and C3–C4 intermediate species that express CAM are dispersed across five of the six clades of Portulaca (Fig. 7). CAM has been detected in species with all of the forms of anatomy described for Portulaca (Atriplicoid, Pilosoid, Portulacelloid and C3–C4) and in both NAD-ME C4 species (P. oleracea and P. molokiniensis) and in NADP-ME C4 species (P. pilosa, P. grandiflora and P. umbraticola) (Voznesenskaya et al., 2010, 2017; Ocampo et al., 2013).
Fig. 7.
Phylogenetic relationships of species within the genus Portulaca, based upon the analyses of Ocampo et al. (2013) and Moore et al. (2018), showing the currently known distribution of C4 (red) and C3–C4 (blue) photosynthesis among them. The C4 distribution is from Ocampo et al. (2013), and the CAM distribution is from Koch and Kennedy (1980), Guralnick et al. (2002), Winter and Holtum (2017), Holtum et al. (2017a), Winter (2019), and the current study.
In P. cryptopetala and P. molokiniensis, as in other Portulaca with CAM, the expression of CAM is unmistakably facultative. Compared with rates of C3 and C4 photosynthesis in unstressed plants, the magnitudes of CAM-type dark CO2 uptake and nocturnal acidification are relatively low, but both characters are clearly present in water-stressed plants and are absent, or close to absent, in well-watered plants (Figs 1–6). The observation that CAM can be repeatedly induced or lost following cycles of water supply and water stress in P. cryptopetala and P. molokiniensis (Figs 2, 5) reveals a tight relationship between the environmental trigger, in this case water stress, and the physiological reaction of the plants, independent of ontogeny (Winter and Holtum, 2007).
At present, in the absence of field studies, we can only speculate as to how a combination of C4 and CAM traits in a single plant might potentially be of adaptive significance. The most obvious conclusion is that C4 and C2 provide a capacity for enhanced productivity and that CAM increases the ability to cope with water stress (Winter and Ziegler, 1992). Particularly in warmer climates, the C4 component could enable rapid growth and high nitrogen-use efficiency, and CAM could contribute to survival via its ability to reduce carbon and water loss when the supply of water is constrained. The rapid switching from CAM back to C4 would be expected to enable a prompt response to rainfall events, an ability of relevance to species that are fast-growing, generally annual, weedy ecological opportunists of disturbed sites, e.g. P. cryptopetala, P. grandiflora, P. oleracea, and P. pilosa.
While an intermediate CO2 compensation point and other characteristics (Voznesenskaya et al., 2010, 2017; Ocampo et al., 2013) support the notion that P. cryptopetala is not a C4 species but rather a C3–C4 species, the stimulation of photosynthesis by up to 46% when P. cryptopetala was exposed to air containing 2% O2 (Fig. 1; Supplementary Fig. S1), together with C3-type δ13C values, suggests that it is an intermediate in which, at current ambient CO2 concentrations, uptake of atmospheric CO2 in the light is catalysed largely by Rubisco. Presumably, this Rubisco signal is contributed to by Rubisco in C3–C4 tissue and CAM tissue.
In contrast to P. cryptopetala, the exposure of P. molokiniensis to 2% O2 resulted in up to a 14% stimulation of photosynthesis (Fig. 5; Supplementary Fig. S2), a response more similar to that of C4 plants. In C4 plants, photosynthesis is typically unaffected by a transfer from 21 to 2% O2 but, at current ambient [CO2], it is not uncommon for plants to exhibit a small stimulation in photosynthesis as [O2] is lowered from 21 to 5–10% followed by a small inhibition as [O2] is further reduced to 2% or lower (Maroco et al., 1997, 1998). The inhibition is thought to be related to a greater requirement for O2-dependent ATP generation by C4 photosynthesis compared with C3 photosynthesis. The ATP is required to regenerate PEP, the primary substrate of the C4 cycle. The [O2] at which the stimulation-to-inhibition transition occurs is apparently species-specific and may be anywhere between 10 and 2%. The small stimulation in CO2 uptake in well-watered plants of P. molokiniensis following exposure to 2% O2 is probably not an effect of O2 on C4 metabolism; rather it reflects the effect of [O2] on reducing photosynthesis in the large-celled chloroplast-containing parenchyma (Kim and Fisher, 1990) in which C3 photosynthesis presumably occurs in well-watered plants, and in which CAM is induced when the plants are drought-stressed.
Christin et al. (2014) suggest that the occurrence of CAM and C4 in Portulaca is the product of a partially shared evolutionary trajectory in which Portulaca was ancestrally a C3–CAM plant. C4 photosynthesis subsequently evolved multiple times while a functional CAM cycle was maintained. For enzymes other than PEPc, Portulaca co-opted the ancestral CAM genes for C4 photosynthesis, but the C4 PEPc genes appear to have arisen via Portulaca-specific gene duplication, and were independently optimized in each Portulaca clade. It is possible that the Cryptopetala clade may represent an ancestral C3–CAM state common to all extant Portulaca (but see Hancock and Edwards (2014) for challenges to this type of inference). Nevertheless, the presence of CAM but not full C4 in P. cryptopetala is consistent with the notion that in Portulaca CAM is an ancestral state that has persisted despite the subsequent repeated evolution of C4 photosynthesis.
As is the case for leaves in the C3–C4P. cryptopetala, the stems of Portulaca species in general lack Kranz anatomy and the C4 pathway (Voznesenskaya et al., 2010). Observations of nocturnal acidification in stems as well as leaves of the C3–C4P. cryptopetala (Figs 3, 4) and the C4 species P. oleracea (Koch and Kennedy, 1980) and P. grandiflora (Guralnick et al., 2002) may also be taken as evidence in support of the concept of a pre-C4 presence of CAM in Portulaca.
There is little sign that the evolution of C4 in organs with CAM has systematically enhanced or retarded the CAM phenotype in Portulaca. Nocturnal acidification in leaves of the C3–C4P. cryptopetala does not overly differ in magnitude from acidification in the C4–CAM species in the other three clades known with CAM. Nocturnal acid accumulation in leaves of P. cryptopetala of ca. 100 μmole H+ g−1 FM (Fig. 3A) is comparable to values reported for P. oleracea (Koch and Kennedy, 1980) and P. grandiflora (Guralnick et al., 2002), but greater than levels of ca. 75 μmole H+ g−1 FM reported for P. australis, P. digyna, P. molokiniensis, and P. pilosa, and far in excess of the 8 μmole H+ g−1 FM reported for P. cyclophylla (Holtum et al., 2017b; Winter and Holtum, 2017). Although water-stressed P. molokiniensis (Fig. 6) accumulated less acid at night than did water-stressed P. cryptopetala, in terms of the absolute acidity stored in tissues, the acid levels in P. molokiniensis were greater. The reason for the difference was that following the imposition of stress, the background levels of acid increased in P. molokiniensis but not in P. cryptopetala. To further address the question of possible differences in the capacity for nocturnal acid accumulation between different species of Portulaca, a rigorous comparison of acid levels from a wide range of species growing under identical conditions is warranted.
Similarities exist between the expression of CAM in Portulaca (Portulacaceae) and in the Australian Calandrinia (Montiaceae) (Winter and Holtum, 2011; Holtum et al., 2017b; Hancock et al., 2018). Both are located in the sub-order Portulacineae (Carophyllales) where they nest among lineages in which CAM and succulence are common (Moore et al., 2018; Ogburn and Edwards, 2013), and both are mainly composed of small, short-lived, succulent-leaved herbs of open arid to semi-arid sites (Eggli 2004; Nyffeler et al., 2008; Kapitany, 2007). Indeed, in Australia it is not uncommon to see species of Portulaca and Calandrinia growing alongside each other. Facultative CAM appears widespread in both groups but, although full C4 is present in Portulaca, there is currently no evidence of strong constitutive CAM in either lineage, despite both having diverged from their respective progenitors around 30 Ma ago (Arakaki et al., 2011; Hancock et al., 2018). In each of the lineages, it is unclear why full CAM has not evolved but facultative CAM has. The answer undoubtedly lies in historical contingencies that are the products of interactions between genetic composition and ecological opportunity over space and time (Edwards and Donoghue, 2013; Christin et al., 2014, 2015).
In the case of the C4 pathway, detailed analyses of phylogeny, anatomy, genes, and physiological phenotypes in the ~40 C3–C4 intermediates known from ca. 20 monocot and eudicot genera has markedly assisted conceptualization of the importance of parallel and convergent evolution to the multiple emergence of the C4 pathway, and of the processes that constrain and enable it (Sage et al., 2011, 2014; Christin et al. 2015). If plants with low-level CAM or facultative CAM are the CAM equivalent of C3–C4 intermediates, then many more C3–CAM intermediates are known than are C3–C4 intermediates (Winter et al., 2015). Presumably, as has been demonstrated for the C4 pathway intermediates, the C3–CAM intermediates contain a subset of the anatomical and biochemical components of the CAM CO2 pump that improve physiological performance over the C3 system in the places where the plants are found (Heckmann, 2016). Addressing the core questions of CAM origins and expression will benefit from rigorous comparisons across lineages of genes and traits that have been acquired repeatedly during evolution of CAM.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Fourteen days of net CO2 exchange of Portulaca cryptopetala during a wet–dry–wet cycle.
Fig. S2. Eleven days of net CO2 exchange of Portulaca molokiniensis during a wet–dry–wet cycle.
Fig. S3. Sixteen days of net CO2 exchange of Portulaca molokiniensis during a wet–dry–wet cycle.
Fig. S4. Twelve days of net CO2 exchange of Portulaca molokiniensis during a wet–dry–wet cycle.
Acknowledgements
The research was supported by Australian Research Council Discovery Grant DP160100098, US National Science Foundation grant DEB-1252901, and by the Smithsonian Tropical Research Institute.
Glossary
Abbreviations:
- BS
bundle sheath
- FM
fresh mass
- PEPc
phosphoenolpyruvate carboxylase
- PFD
photosynthetic photon flux density
References
- Arakaki M, Christin PA, Nyffeler R, Lendel A, Eggli U, Ogburn RM, Spriggs E, Moore M, Edwards EJ. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences, USA 108, 8379–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Arakaki M, Osborne CP, et al. 2014. Shared origins of a key enzyme during the evolution of C4 and CAM metabolism. Journal of Experimental Botany 65, 3609–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Arakaki M, Osborne CP, Edwards EJ. 2015. Genetic enablers underlying the clustered evolutionary origins of C4 photosynthesis in angiosperms. Molecular Biology and Evolution 32, 846–858. [DOI] [PubMed] [Google Scholar]
- D’Andrea RM, Andreo CS, Lara MV. 2014. Deciphering the mechanisms involved in Portulaca oleracea (C4) response to drought: metabolic changes including crassulacean acid-like metabolism induction and reversal upon re-watering. Physiologia Plantarum 152, 414–430. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Donoghue MJ. 2013. Is it easy to move and easy to evolve? Evolutionary accessibility and adaptation. Journal of Experimental Botany 64, 4047–4052. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM. 2012. Angiosperm responses to a low CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. International Journal of Plant Science 173, 724–733. [Google Scholar]
- Edwards GE, Vozsenskaya E. 2011. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht: Springer, 29–61. [Google Scholar]
- Eggli U. 2004. Illustrated handbook of succulent plants: dicotyledons. Berlin, Heidelberg: Springer Verlag. [Google Scholar]
- Guralnick LJ, Edwards GE, Ku MSB, Hockema B, Franceschi VR. 2002. Photosynthetic and anatomical characteristics in the C4-crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Functional Plant Biology 29, 763–773. [DOI] [PubMed] [Google Scholar]
- Guralnick LJ, Jackson MD. 2001. The occurrence and phylogenetics of crassulacean acid metabolism in the Portulacaceae. International Journal of Plant Sciences 162, 257–262. [Google Scholar]
- Hancock L, Edwards EJ. 2014. Phylogeny and the inference of evolutionary trajectories. Journal of Experimental Botany 65, 3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock LP, Obbens F, Moore AJ, Thiele K, de Vos JM, West J, Holtum JAM, Edwards EJ. 2018. Phylogeny, evolution, and biogeographic history of Calandrinia (Montiaceae). American Journal of Botany 105, 1021–1034. [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis, a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Heckmann D. 2016. C4 photosynthesis evolution: the conditional Mt. Fuji. Current Opinion in Plant Biology 31, 149–154. [DOI] [PubMed] [Google Scholar]
- Hobdy RW. 1987. Portulaca molokiniensis (Portulacaceae); a new species from the Hawaiian Islands. Pacific Science 41, 64–67. [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K. 2017a Optional use of CAM photosynthesis in two C4 species, Portulaca cyclophylla and Portulaca digyna. Journal of Plant Physiology 214, 91–96. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K. 2017b Facultative CAM photosynthesis (crassulacean acid metabolism) in four species of Calandrinia, ephemeral succulents of arid Australia. Photosynthesis Research 134, 17–25. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K. 2018. Crassulacean acid metabolism in the Basellaceae (Caryophyllales). Plant Biology 20, 409–414. [DOI] [PubMed] [Google Scholar]
- Holtum JA, Winter K. 2003. Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta 218, 152–158. [DOI] [PubMed] [Google Scholar]
- Kapitany A. 2007. Australian succulent plants. Boronia, Victoria, Australia: Kapitany Concepts. [Google Scholar]
- Keerberg O, Pärnik T, Ivanova H, Bassüner B, Bauwe H. 2014. C2 photosynthesis generates about 3-fold elevated leaf CO2 levels in the C3–C4 intermediate species Flaveria pubescens. Journal of Experimental Botany 65, 3649–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Fisher DG. 1990. Structural aspects of the leaves of seven species of Portulaca growing in Hawaii. Canadian Journal of Botany 68, 1803–1811. [Google Scholar]
- Koch K, Kennedy RA. 1980. Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiology 65, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara MV, Disante KB, Podestá FE, Andreo CS, Drincovich MF. 2003. Induction of a Crassulacean acid like metabolism in the C4 succulent plant, Portulaca oleracea L.: physiological and morphological changes are accompanied by specific modifications in phosphoenolpyruvate carboxylase. Photosynthesis Research 77, 241–254. [DOI] [PubMed] [Google Scholar]
- Lara MV, Drincovich MF, Andreo CS. 2004. Induction of a crassulacean acid-like metabolism in the C4 succulent plant, Portulaca oleracea L: study of enzymes involved in carbon fixation and carbohydrate metabolism. Plant & Cell Physiology 45, 618–626. [DOI] [PubMed] [Google Scholar]
- Maroco JP, Ku MSB, Edwards GE. 1997. Oxygen sensitivity of C4 photosynthesis: evidence from gas exchange and fluorescence analysis with different C4 sub-types. Plant, Cell & Environment 20, 1525–1533. [Google Scholar]
- Maroco JP, Ku MSB, Lea PJ, Dever LV, Leegood RC, Furbank RT, Edwards GE. 1998. Oxygen requirement and inhibition of C4 photosynthesis. An analysis of C4 plants deficient in the C3 and C4 cycles. Plant Physiology 116, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AJ, De Vos JM, Hancock LP, Goolsby E, Edwards EJ. 2018. Targeted enrichment of large gene families for phylogenetic inference: phylogeny and molecular evolution of photosynthesis genes in the Portullugo clade (Caryophyllales). Systematic Biology 67, 367–383. [DOI] [PubMed] [Google Scholar]
- Nyffeler R, Eggli U, Ogburn M, Edwards EJ. 2008. Variations on a theme: repeated evolution of succulent life forms in the Portulacineae (Caryophyllales). Haseltonia 14, 26–36. [Google Scholar]
- Ocampo G, Columbus JT. 2012. Molecular phylogenetics, historical biogeography, and chromosome number evolution of Portulaca (Portulacaceae). Molecular Phylogenetics and Evolution 63, 97–112. [DOI] [PubMed] [Google Scholar]
- Ocampo G, Koteyeva NK, Voznesenskaya EV, Edwards GE, Sage TL, Sage RF, Columbus JT. 2013. Evolution of leaf anatomy and photosynthetic pathways in Portulacaceae. American Journal of Botany 100, 2388–2402. [DOI] [PubMed] [Google Scholar]
- Ogburn RM, Edwards EJ. 2010. The ecological water-use strategies of succulent plants. Advances in Botanical Research 55, 179–225. [Google Scholar]
- Ogburn RM, Edwards EJ. 2012. Quantifying succulence: a rapid, physiologically meaningful metric of plant water storage. Plant, Cell & Environment 35, 1533–1542. [DOI] [PubMed] [Google Scholar]
- Ogburn RM, Edwards EJ. 2013. Repeated origin of three-dimensional leaf venation releases constraints on the evolution of succulence in plants. Current Biology 23, 722–726. [DOI] [PubMed] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29, 379–414. [Google Scholar]
- Sage RF. 2002. Are crassulacean acid metabolism and C4 photosynthesis incompatible? Functional Plant Biology 29, 775–785. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67, 4039–4056. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage RF, Khoshravesh R, Sage TL. 2014. From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. Journal of Experimental Botany 65, 3341–3356. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sultmanis S. 2016. Why are there no C4 forests? Journal of Plant Physiology 203, 55–68. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Winter K. 1996. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Berlin, Heidelberg: Springer Verlag, 427–436. [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2010. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3–C4 intermediate in genus Portulaca L. (Portulacaceae). Journal of Experimental Botany 61, 3647–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2017. Unique photosynthetic phenotypes in Portulaca (Portulacaceae): C3–C4 intermediates and NAD-ME C4 species with Pilosoid-type Kranz anatomy. Journal of Experimental Botany 68, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany, doi: 10.1093/jxb/erz002. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JA. 2007. Environment or development? Lifetime net CO2 exchange and control of the expression of crassulacean acid metabolism in Mesembryanthemum crystallinum. Plant Physiology 143, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2011. Induction and reversal of crassulacean acid metabolism in Calandrinia polyandra: effects of soil moisture and nutrients. Functional Plant Biology 38, 576–582. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JA. 2014. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 65, 3425–3441. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2017. CO2-exchange patterns demonstrate facultative CAM photosynthesis (crassulacean acid metabolism) in four small Australian C3 and C4 leaf-succulents. Australian Journal of Botany 65, 103–108. [Google Scholar]
- Winter K, Holtum JA, Smith JA. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208, 73–78. [DOI] [PubMed] [Google Scholar]
- Winter K, Ziegler H. 1992. Induction of crassulacean acid metabolism in Mesembryanthemum crystallinum increases reproductive success under conditions of drought and salinity stress. Oecologia 92, 475–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.