Abstract
Mucus clearance provides an essential innate defense mechanism to keep the airways and lungs free of particles and pathogens. Baseline and stimulated mucin secretion from secretory airway epithelial cells need to be tightly regulated to prevent mucus hypersecretion and mucus plugging of the airways. It is well established that extracellular ATP is a potent stimulus for regulated mucus secretion. Previous studies revealed that ATP acts via metabotropic P2Y2 purinoreceptors on goblet cells. Extracellular ATP, however, is also a potent agonist for ionotropic P2X purinoreceptors. Expression of several P2X isoforms has been reported in airways, but cell type-specific expression and the function thereof remained elusive. With this study, we now provide evidence that P2X4 is the predominant P2X isoform expressed in secretory airway epithelial cells. After IL-13 treatment of either human primary tracheal epithelial cells or mice, P2X4 expression is upregulated in vitro and in vivo under conditions of chronic inflammation, mucous metaplasia, and hyperplasia. Upregulation of P2X4 is strongest in MUC5AC-positive goblet cells. Moreover, activation of P2X4 by extracellular ATP augments intracellular Ca2+ signals and mucin secretion, whereas Ca2+ signals and mucin secretion are dampened by inhibition of P2X4 receptors. These data provide new insights into the purinergic regulation of mucin secretion and add to the emerging picture that P2X receptors modulate exocytosis of large secretory organelles and secretion of macromolecular vesicle cargo.
Keywords: goblet cells, IL-13, MUC5AC, mucin, mucus secretion, P2X4
INTRODUCTION
Mucus secretion in the airways is an essential mechanism for maintaining airway function and integrity. The mucus layer in the airways and mucociliary clearance provide a first line of defense and keep the airways and lungs free of particles and pathogens (33). To maintain and replenish the mucus layer in the airways, mucins are continuously synthesized and secreted from secretory cells along the airways (3, 15, 73, 74). In the upper airways goblet cells are the main secretory cells secreting mucins (MUC5AC and MUC5B), whereas club cells are the principal secretory cells in the distal airways, where goblet cells are absent under healthy, noninflammatory conditions (30). Mucin secretion (and clearance) needs to be tightly regulated under baseline as well as stimulated conditions (3, 73). Excessive secretion (or impaired clearance) leads to mucin aggregation and mucus plugging of the airways. This is particularly prevalent in inflammatory lung diseases including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and chronic bronchitis when club-to-goblet cell transformation, mucous metaplasia, and hyperplasia result in mucin hypersecretion (16). The mechanisms regulating mucin secretion have therefore been major research areas for almost half a century.
Stimulated mucin secretion from goblet cells occurs via Ca2+-dependent exocytosis of large secretory granules. Within recent years substantial progress has been achieved in identifying key molecular mechanisms of the trafficking and core and regulatory exocytic machinery (see reviews in Refs. 3, 10). It is now also well established that extracellular ATP is a potent agonist for stimulated mucin secretion (1, 32). ATP activates P2Y2 purinoreceptors on the apical membranes of goblet cells via phospholipase C pathways, resulting in generation of the second messengers inositol 1,4,5-trisphosphate and diacylglycerol and release of Ca2+ from intracellular stores (6).
Extracellular ATP, however, is also a potent agonist for P2X purinoreceptors, ligand-gated ion channels. Expression of several P2X receptors has been reported in lung tissues, with P2X4 and P2X7 being the most widely expressed isoforms (6, 21). To date, information on cell type-specific expression is mostly lacking. Also, apart from a well-established proinflammatory/proapoptotic role for P2X7 (41, 46, 56), little is known of the function of P2X receptors in airway epithelia. In recent years P2X receptors have attracted increasing attention as regulators of exocytosis and cellular secretion (24, 25, 28, 29, 45, 69). It has been shown that P2X receptor activation stimulates exocytosis directly via influx of Ca2+ from the extracellular space and elevation of cytoplasmic Ca2+ concentration ([Ca2+]c) (27, 29, 35, 60). More recently, we have reported that activation of P2X4 receptors after vesicle-plasma membrane (PM) fusion modulates the secretion and activation of pulmonary surfactant from alveolar type II (ATII) epithelial cells (12, 44, 65). Secretion of pulmonary surfactant in many aspects resembles mucin secretion. First, surfactant, similar to mucins, is a large macromolecular complex stored in large, lysosome-related secretory organelles termed lamellar bodies, and second, the regulatory machinery governing lamellar body exocytosis is similar to the regulatory machinery in mucin granule secretion (13, 19, 26, 36, 55). Therefore, it was the aim of this study to investigate whether P2X receptors are expressed in goblet cells and if activation of P2X receptors impacts mucin secretion.
With this study, we now provide evidence that P2X4 is the predominant P2X isoform expressed in secretory airway epithelial cells. Moreover, we show that P2X4 expression is upregulated in vitro and in vivo under conditions of chronic inflammation with mucous metaplasia and hyperplasia. Upregulation is strongest in MUC5AC+ cells. Activation and/or potentiation of P2X4 augments mucin secretion, whereas inhibition of P2X4 receptors dampens mucin secretion. These data provide new insights into the purinergic regulation of mucin secretion and add to the emerging picture that P2X receptors modulate exocytosis of large secretory organelles and secretion of macromolecular vesicle cargo.
METHODS
Materials.
The α-P2X4 antibody was designed by us against the human protein sequence CKKYKYVEDYEQGLASELDQ, raised in rabbits and affinity purified by Pineda (Berlin, Germany); α-tubulin β-IV mouse monoclonal antibody (catalog no. T7941) was purchased from Sigma-Aldrich (Steinheim, Germany), α-Club Cell Secretory Protein (CCSP) was either rabbit polyclonal antibody (catalog no. sc-9772) from Santa Cruz (Dallas, TX) or rat monoclonal antibody (catalog no. MAB4218) from R&D Systems (Minneapolis, MN), and the α-MUC5AC mouse monoclonal antibody (catalog no. MA1-21907) was from ThermoScientific/Pierce (Rockford, IL). Fluorescently labeled secondary antibodies were obtained from Molecular Probes (Life Technologies, Karlsruhe, Germany). All other chemicals were purchased from Sigma-Aldrich unless otherwise indicated.
Cell culture.
Human primary tracheal epithelial cells (HTECs) and growth medium were purchased from Promocell (Heidelberg, Germany). For differentiation, a 50:50 mixture of DMEM-H and LHC Basal (both from Thermo Fisher, Waltham, MA) was supplemented with Airway Epithelial Cell Growth Medium SupplementPack (Promocell) and different trace elements as described previously (20). Briefly, frozen primary HTECs derived from several donors were obtained at passage 2, thawed, and expanded in a T75 flask. Growth medium was replaced every 2 days. Once cells were 70–90% confluent, they were passaged with DetachKiT from Promocell and plated at a density of 3.5 × 104 cells per 6.5-mm Transwell filter (Corning Costar 3470; Corning, Corning, NY) precoated with collagen solution (StemCell Technologies, Cologne, Germany). Cells were plated with 200 µl of growth medium apically, and an additional 600 µl of growth medium was added basolaterally. The apical medium was replaced at 48 h and then removed completely at 72 h. The basolateral medium was switched to differentiation medium at the same time and replaced every 3 days. Cells were grown up to 32 days, and after day 14 apical Dulbecco’s phosphate-buffered saline (DPBS) washes were performed in the incubator for 30 min every 3 days. For cells treated with IL-13 (Millipore, Darmstadt, Germany), the IL-13 was added to the basolateral medium every 3 days to a final concentration of 10 ng/ml when air-liquid interphase (ALI) was imposed until day 21, when, after collection of samples, the basolateral medium was switched to normal differentiation medium without IL-13. Calu-3 cells were grown at ALI with supplemented minimal essential medium according to the manufacturer’s recommended conditions (ATCC, Manassas, VA).
HTEC cultures from two to four different donors were used for each experiment.
Mouse IL-13 instillation and FACS sorting.
Mucous metaplasia of tracheal epithelial cells was induced by intrapharyngeal instillation of 2 μg of IL-13 (Biolegend) in 50 μl of water on three consecutive days under isoflurane anesthesia. The lungs were harvested on the fifth day after the final IL-13 instillation. Airway epithelium cell sorting was performed essentially as described previously (8) with BD FACSAria Fusion/BD FACSDiva Software and adult mouse lung tissue digested in 5 mg/ml collagenase type I (Worthington, Lakewood, NJ). A global double-fluorescent Cre reporter mouse (48) crossed to a CCSP-iCre mouse (40) yielded green-only airway epithelial cells, which were selected with the four-way purity precision mode. Mice were bred within our colony, housed in specific pathogen-free conditions, and handled in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Semiquantitative RT-PCR.
Total RNA was isolated with the RNeasy MiniKit (Qiagen, Hilden, Germany). Reverse transcription was performed on 0.8–1.3 µg of total RNA with the SuperScript VILO cDNA Synthesis Kit according to the manufacturer’s protocol. The following validated QuantiTect primer assays (Qiagen) were used: HMBS [QT00014462 (hs), QT00494130 (ms)], MmP2Xr4_1_SG (QT00163149), MmP2X7_1_SG (QT00130900), MmMUC5AC_1_SG (QT001196006), and MmMUC5B_1_SG (QT01067899) for mouse samples and HsP2X1_1_SG (QT00194320), HsP2X2_1_SG (QT00427455), HsP2X3_1_SG (QT01083593), Hs P2X4_1_SG (QT00049693), HsP2X5_1_SG (QT00178297), HsP2X6_1_SG (QT00178297), HsP2X7_1_SG (QT00083643), HsMUC5AC_1_SG (QT00088991), HsMUC5B_1_SG (QT01529367), and Hs_FoxJ1_1_SG (QT01000797) for human samples. Amplification was performed on a Mastercycler RealPlex2 (Eppendorf, Hamburg, Germany) with the EXPRESS SYBR GreenER qPCR supermix. Each reaction was carried out on cDNA from three or more independent biological replicates (cDNAs were used at 1-, 10-, and 100-fold dilutions). Specificity of PCR reactions was confirmed by melting point analysis of PCR products. RealPlex software (Eppendorf) was used for data acquisition and analysis. Correction for PCR performance as well as quantification relative to housekeeping gene hydroxymethylbilane synthase (HMBS) was carried out as described previously (54). Stable expression of HMBS has been confirmed for all time points in control and IL-13 treated samples.
Western blot analysis.
HTECs were washed twice in DPBS, solubilized in lysis buffer, separated by SDS-PAGE, and transferred to nitrocellulose. Ponceau staining of blots was performed before immunolabeling to normalize for sample loading. Immunodetection of P2X4 was performed with the α-P2X4 antiserum (1:500) overnight and chromogenic detection of alkaline phosphatase-labeled secondary antibody (WesternBreeze anti-rabbit; Invitrogen, Karlsruhe, Germany).
Cell population analysis by FACS.
To analyze P2X4 expression in the respective cell populations (ciliated, secretory, and goblet cells), HTECS grown on Transwell filters were trypsinized and detached cells were fixed for 20 min in 4% paraformaldehyde in DPBS. Cells were stained for P2X4 and tubulin β-IV, MUC5AC, or CCSP as described in Immunofluorescence and analyzed on a BD Canto II flow cytometer with the FACSDiva software for a mean of 1 × 105 events. Cells were gated for different forward (FSC) and sideward light scatter characteristics, and doublet exclusion was achieved by gating in the FSC-A and FSC-W channels. Relative percentages of cell populations were calculated with FlowJo 10.4.1. Median P2X4 fluorescence intensity was derived from the respective 50% highest-expressing positive and 50% lowest-expressing negative populations.
Immunofluorescence.
For immunofluorescence staining of intact mucin vesicles, HTECs grown on Transwell filters were fixed for 20 min in 4% paraformaldehyde in DPBS containing 30 nM BAPTA to block mucin secretion due to handling (73). Cells were then permeabilized for 10 min with 0.2% saponin and 10% FBS (Thermo Scientific, Bonn, Germany) in DPBS. Cells were stained with primary (1:100) and secondary (1:400) antibodies in PBS, 0.2% saponin, and 10% FBS. Images were taken on an inverted confocal microscope (Leica TCS SP5; Leica) using a ×63 lens (Leica HCX PL APO lambda blue 63.0x1.40 OIL UV). Images for the blue (DAPI), green (Alexa Fluor 488), red (Alexa Fluor 568), and far red (Alexa Fluor 647) channels were taken in sequential mode with appropriate excitation and emission settings.
For formalin-fixed, paraffin-embedded (FFPE) sections of mouse lung samples, 2-μm sections were cut from the blocks for immunostaining. Antigen retrieval was performed with citrate buffer and blocked in Tris-buffered saline solution with 10% bovine serum albumin (BSA). Primary antibodies were added in the diluent of Tris-buffered saline-Tween 20 and 0.5% BSA for 1 h or overnight. Secondary antibodies were added in the diluent for 1 h at a concentration of 1:400.
Mucin ELISA.
The mucin ELISA assay was performed according to the protocol by Abdullah et al. (2), with the application of recently published improvements (73). Briefly, HTECs on filters were washed with DMEM every hour for four times to remove excess mucus. After initial washings, cells were incubated with DMEM two times for 1 h to collect samples for baseline secretion before cells were stimulated (15 min) and samples were collected for analysis. All samples were diluted 1:10 in PBS and treated with neuraminidase (0.625 mU/μg total protein) for 2 h at 37°C (9). One hundred microliters of the diluted sample was plated on an enzyme immunoassay high-binding 96-well plate (Corning) overnight at 4°C. The samples were blocked with 1% (wt/vol) BSA in PBS-Tween 20 (PBST) for 1 h at room temperature and then washed three times with PBST. The anti-MUC5AC antibody (catalog no. MA1-21907; ThermoScientific/Pierce) was added at 1:250 for 2 h at 37°C in 1% (wt/vol) BSA in PBST. Cells were then washed four times with PBST and incubated with horseradish peroxidase-conjugated secondary antibody goat anti-mouse (Jackson ImmunoResearch, West Grove, PA) at a final concentration of 1:1,000 in PBST for 2 h at 37°C. After washing, the samples were developed with 3,3′,5,5′-tetramethylbenzidine substrate (ThermoScientific/Pierce) and stopped with 1 M H2SO4 (Sigma-Aldrich). The samples were then immediately read in the ELISA plate reader (Tecan, Salzburg, Austria) for absorbance at 450 nm.
Fluo-4 measurements.
For measurement of changes in intracellular calcium levels, HTECs on filters were loaded with 3 µM fluo-4 AM (ThermoScientific, Karlsruhe, Germany) for 45 min in DMEM, washed twice in bath solution (in mM: 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 glucose, and 10 HEPES; pH 7.4) and maintained in bath solution until the start of the experiments. In addition, HTECs were also stained with LysoTracker Deep Red (50 nM; ThermoScientific) to identify secretory cells. With a two-dimensional imaging system cells were illuminated for 50 ms at a rate of 1 Hz at 480 nm (26) and images were acquired with MetaFluor (Molecular Devices, Ismaning, Germany). Images were analyzed with MetaFluor Analyst (Molecular Devices) as described previously (44) To compare changes in the Ca2+ response, the integrated Ca2+ signal (area under the curve) was analyzed (19).
Adenoviral transfection.
Adenoviruses expressing either P2X4-EGFP [wild type (wt)] or P2X4(C353W)-EGFP [dominant negative (dn)] were produced as recently described (42) with the ViraPower Adenoviral Expression System (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions. HTECs were infected on days 14 and 18 after the start of ALI culture.
Statistics.
All statistics were analyzed and graphs plotted with GraphPad Prism 5 (San Diego, CA). Data are described as means ± SE, unless noted differently. Unless indicated otherwise, an unpaired, nonparametric Mann-Whitney test was applied to compare control vs. IL-13-treated samples. Data were considered significant if the P value was <0.05.
RESULTS
P2X4 is the dominant P2X isoform expressed in HTECs grown at ALI.
In initial experiments we examined the expression of P2X receptors in HTECs. RT-PCR performed on HTECs revealed that P2X4 is the most highly expressed isoform in fully differentiated (day 21) HTECs grown at ALI. P2X1 and P2X6 receptors were also expressed in differentiated HTEC cultures, whereas expression of P2X2, P2X3, P2X5, and P2X7 receptors was weak or negligible (<0.1-fold) compared with expression of the housekeeping gene HMBS (Fig. 1). Fully differentiated HTEC cultures contain ciliated as well as secretory cells, and we cannot conclude from these data whether P2X4 expression exhibits a cell type-specific pattern. Results from Calu-3 cells, however, suggested that P2X4 is expressed in secretory cells. A large proportion of Calu-3 cells contain MUC5AC granules with a goblet cell-like phenotype, but no ciliated cells are present (23, 37). P2X4 receptors were the most highly expressed isoform in Calu-3 cells, and relative expression was higher in Calu-3 cells than in fully differentiated HTECs [2.83 ± 0.21 (n = 24)- vs. 0.75 ± 0.08 (n = 12)-fold HMBS expression, respectively; P < 0.001]. Expression of all other P2X isoforms was <0.2-fold expression of housekeeping gene HMBS (Fig. 1), suggesting that P2X4 receptors are the predominantly expressed P2X isoform in gobletlike cells.
Fig. 1.
P2X4 is the dominant P2X isoform expressed in human primary tracheal epithelial cells (HTECs) grown at air-liquid interphase (ALI): real-time RT-PCR analysis of P2X purinergic receptor transcripts in HTECs grown at ALI for 21 days and Calu-3 cells, respectively. Data are expressed as fold expression of housekeeping gene hydroxymethylbilane synthase (HMBS). Values are means from 5–24 individual cultures. Significant differences between P2X4 expression levels and all other P2X isoforms: *HTEC (P < 0.019) and #Calu-3 cells (P < 0.015) (statistical significance was assessed by Kruskal-Wallis test for multiple comparisons). In this and subsequent figures, box plots show median, minimum, and maximum.
IL-13 induced goblet cell hyperplasia results in upregulation of P2X4 expression in HTECs.
In light of these findings we next investigated whether increasing secretory cell numbers and mucous differentiation results in increased P2X4 expression in HTEC cultures. It is well established that IL-13 treatment induces goblet cell hyperplasia and metaplasia in vitro (4, 37, 68) and in vivo (70, 73). Therefore, confluent HTEC cultures were grown for 21 days in the absence (= control) or presence of 10 ng/ml IL-13 (basolaterally), and then on day 21 IL-13 was removed. Samples were collected at various time points after ALI conditions were established (day 0). In line with previous results, ALI culture induced a significant increase in FOXJ1 expression, a marker for ciliated cells, in control but not IL-13-treated cells (5, 72). IL-13 also significantly delayed the increase of MUC5B expression that occurs during differentiation of control cells (Fig. 2B; Ref. 14) and induced a massive increase in MUC5AC expression from day 8 onward, when goblet cell differentiation begins (66), resulting in >200 times higher MUC5AC expression (compared with baseline or control without IL-13) from day 4 onward. Removal of IL-13 resulted in rapid downregulation of MUC5AC expression (Fig. 2C). Together, these data demonstrate that IL-13 treatment of HTECs results in goblet cell hyperplasia and metaplasia with increased MUC5AC expression.
Fig. 2.
IL-13 induces upregulation of MUC5AC and inhibits expression of FoxJ1 and MUC5B in human primary tracheal epithelial cells (HTECs). Confluent HTEC air-liquid interphase cultures (reaching confluence = day 0) were grown for 21 days (d) in the absence [control (ctrl)] or presence of 10 ng/ml IL-13 basolaterally and then for 4 more days after IL-13 was removed. Cells were collected at various time points for analysis of gene expression by RT-PCR. IL-13 treatment resulted in a sustained decrease in FoxJ1 (A), a transient decrease in MUC5B (B), and a >200-fold increase in MUC5AC expression (C). Removal of IL-13 on day 21 resulted in immediate downregulation of MUC5AC expression. Data are expressed as fold expression of housekeeping gene hydroxymethylbilane synthase (HMBS). Values are means from 6 or 7 individual donors. *Significant differences between control and IL-13-treated cells at individual time points; #significant difference between end of IL-13 treatment (day 21) and time point after IL-13 removal (day 25). *P < 0.05; **P < 0.01.
IL-13 treatment of HTECs resulted in significantly increased P2X4 transcript expression, with a time course similar to the upregulation of mucin gene expression. Akin to MUC5AC expression, removal of IL-13 resulted in downregulation of P2X4 expression back to control levels (Fig. 3A). P2X7 receptor expression, which has previously been reported as a marker for airway inflammation (56), however, was unaffected by IL-13 treatment (Fig. 3A). In accordance with the time course of IL-13-induced upregulation of P2X4 transcription, a significant increase in P2X4 protein levels was observed by Western blotting from day 14 onward. Again, removal of IL-13 on day 21 resulted in a decrease in P2X4 protein levels to near-control levels at day 32, whereas cultures maintained for 32 days in the presence of IL-13 maintained high levels of P2X4 (Fig. 3, B and C).
Fig. 3.
IL-13 induces upregulation of P2X4 expression in human primary tracheal epithelial cells (HTECs). A: confluent HTEC air-liquid interphase (ALI) cultures were grown for 21 days (d) in the absence [control (ctrl)] or presence of 10 ng/ml IL-13 basolaterally. On day 21 IL-13 was removed. Left: IL-13 treatment significantly increased P2X4 transcript expression, which returned to control levels upon removal of IL-13. Right: P2X7 receptor expression was unaffected by IL-13 treatment. Values are the means from 7 or 8 individual donors. *Significant differences between control and IL-13-treated cells at individual time points; #significant difference between end of IL-13 treatment (day 21) and time point after IL-13 removal (day 25). B, top: Western blot of confluent HTEC ALI cultures grown for 32 days in the absence (−) or presence (+) of IL-13, except for a sample grown for 21 days in the presence of IL-13 and then another 11 days without IL-13 (day 32, 0–21). P2X4 receptor expression was detectably increased in IL-13-treated HTECs from day 14 onward, and removal of IL-13 resulted in a return to near control levels [sample day 32 (0–21)]. Bottom: quantitative analysis of P2X4 protein expression from 5 independent experiments (donors) as illustrated at top. P2X4 expression was normalized to total protein. a.u., Arbitrary units. C: representative immunoblot to validate specificity of α-P2X4 antibody. Left: Ponceau staining of blot to control for equal loading of lanes. Right: Western blot for P2X4 from untreated HeLa cells (control) and HeLa cells infected with P2X4 short hairpin (sh)RNA for 48 h. P2X4 shRNA adenovirus was constructed based on the shRNA cassette design described by Gou et al. (22) and recently utilized by Fois et al. (18). *Significant differences between control and IL-13-treated cells at individual time points. *P < 0.05; **P < 0.01.
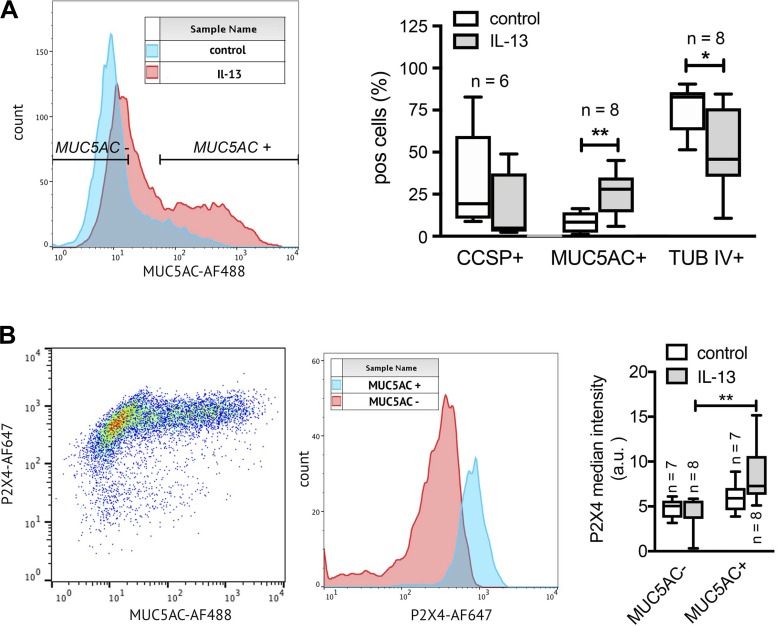
To further delineate the correlation between increases in MUC5AC expression, goblet cell metaplasia, and increased P2X4 expression, we used FACS analysis to investigate relative changes in P2X4 expression in goblet (MUC5AC+), club (CCSP+) and ciliated (tub IV+) cells upon IL-13 treatment. Initial FACS analysis of the percentage of the respective cell populations further confirmed that IL-13 treatment results in a significant increase in MUC5AC+ cells [25.64 ± 4.53% (n = 8) (IL-13) vs. 8.28% ± 2.10 (control), P = 0.007] (Fig. 4A). The percentage of tub IV+ and CCSP+ cells, on the other hand, either was significantly downregulated or showed a trend toward reduction upon IL-13 treatment, respectively. Our data further revealed that P2X4 expression significantly increased in MUC5AC+ cells (n = 8) after IL-13 treatment, whereas P2X4 expression was not significantly altered in MUC5AC− cells (Fig. 4B). In summary, these data provide strong evidence that the IL-13-induced increase of P2X4 expression in HTECs results from increased goblet cell numbers and increased P2X4 expression within these goblet cells.
Fig. 4.
IL-13-induced upregulation of P2X4 expression is primarily induced in MUC5AC+ cells. A, left: FACS analysis histograms for MUC5AC fluorescence intensity of human primary tracheal epithelial cells (HTECs) cultured in the absence (control) or presence of IL-13. Overlay illustrates that the proportion of MUC5AC+ cells is increased in IL-13-treated samples. Right: quantitative analyses of goblet (MUC5AC+), club cell [α-Club Cell Secretory Protein (CCSP)+], and ciliated cell [tubulin β-IV (tub IV)+] populations in control and IL-13-treated HTEC cultures reveal a significant upregulation of MUC5AC+ cells and downregulation of tub IV+ cells after IL-13 treatment. The percentage of CCSP+ was not significantly changed. pos, Positive. B: P2X4 median intensity is increased in MUC5AC+ cells. Left: FACS blot depicting P2X4 vs. MUC5AC intensity in IL-13-treated HTECs. High MUC5AC expression in cells is associated with high P2X4 expression. Center: P2X4 expression levels in MUC5AC- and MUC5AC+ cells from blot on left. Right: quantitative analysis of P2X4 expression in MUC5AC− and MUC5AC+ cell populations in control and IL-13-treated HTEC cultures. Median P2X4 expression is significantly increased in MUC5AC+ cells after IL-13 treatment. n, No. of individual experiments. *P < 0.05; **P < 0.01.
P2X4 is expressed on PM and in vesicular structures in goblet cells.
We next investigated the subcellular localization of P2X4 receptors in goblet cells. P2X4 receptors have been shown to be expressed at the PM but predominantly reside on lysosomes and lysosome-related organelles (47), including lamellar bodies in ATII epithelial cells (42), affecting lysosomal trafficking and secretion. First of all, immunofluorescence staining of HTECs confirmed that the strongest P2X4 signal is found in MUC5AC+ cells (Fig. 5A). Again, IL-13 treatment significantly increased the percentage of MUC5AC+ cells and accordingly P2X4+ cells (Fig. 5A). High-resolution confocal microscopy further revealed that the MUC5AC+ structures in IL-13-treated HTECs indeed resemble granular structures identical in size and shape to mucin granules in goblet cells (∼1–3 μm in diameter). However, at close examination, P2X4 receptors were closely associated with the mucin vesicles but did not strictly colocalize with the granular structures (Fig. 5B, top). Analysis of individual confocal sections confirmed that P2X4 receptors are localized on circular structures devoid of MUC5AC (Fig. 5B, middle), and xy-sectioning further supported that P2X4 receptors are, in addition to some PM staining, localized on intracellular organelles located basolaterally of the apical MUC5AC+ mucin granule pool (Fig. 5B, bottom). In line with this, when P2X4-EGFP is expressed in IL-13-treated cultures of HTECs, P2X4-EGFP is localized in vesicular, intracellular structures distinct from Muc5AC-containing granules (Fig. 5C).
Fig. 5.
P2X4 is expressed on the plasma membrane (PM) and in vesicular structures in goblet cells. A: immunofluorescence staining of human primary tracheal epithelial cells (HTECs) cultured in the absence (control) or presence of IL-13 for 21 days. IL-13 treatment significantly increased % of MUC5AC-positive cells. Overlay reveals that P2X4 (red) expression is almost exclusively restricted to MUC5AC (green)-positive cells. Scale bars, 25 μm. B: confocal images reveal that P2X4 (red) is, in addition to PM localization (arrows), expressed on vesicular structures (arrowheads) devoid of MUC5AC (green) residing basolateral to mucin granules. Top: maximum projection of confocal z-sections through apical region of a single goblet cell. Middle: individual confocal section beneath the apical pool of mucin granules (as indicated in scheme on left). Bottom: xz-section of the same cell. Scale bars, 5 μm. C: confocal cross section of HTECs, expressing wild-type (wt) P2X4-EGFP (green) and treated for 21 days with IL-13. Cells were stained for MUC5AC (red). Scale bar, 10 μm.
IL-13 treatment induces P2X4 expression in goblet cells in vivo.
We next confirmed whether this goblet cell-specific expression of P2X4 could also be observed in vivo. Goblet cell metaplasia was induced in a murine model using IL-13 instillation. Subsequent RT-PCR analysis of mucin expression on FACS-sorted secretory cells confirmed that many of the club cells had transitioned to mucin-producing goblet cells. Compared with naive animals, IL-13 induced a significant increase in Muc5ac expression [1.11 ± 0.20 (n = 7)-fold vs. 3.30 ± 0.95 (n = 5)-fold Hmbs expression, respectively; P = 0.01] and an increase in Muc5b expression [1.37 ± 0.42 (n = 7)-fold vs. 5.09 ± 2.97 (n = 5)-fold Hmbs expression, respectively; P = 0.11] (Fig. 6A). In line with findings in HTECs, P2X4 expression increased after IL-13 treatment [1.02 ± 0.07 (n = 7)-fold vs. 1.47 ± 0.18 (n = 5)-fold Hmbs expression, respectively; P = 0.04], whereas no change was detected for P2X7 expression [1.16 ± 0.27 (n = 7)-fold vs. 1.30 ± 0.33 (n = 5)-fold Hmbs expression, respectively; P = 0.76] (Fig. 6B).
Fig. 6.
IL-13 treatment in vivo induces P2X4 expression in mouse secretory cells. A: RT-PCR analysis of mucin gene expression in airway epithelial cells isolated by FACS from control mice and mice treated with intratracheal instillation of IL-13. B: RT-PCR analysis of P2X4 and P2X7 expression in the same cells as those in A reveal that P2X4 but not P2X7 expression is upregulated in animals treated with IL-13. C: immunofluorescence staining of airway epithelia from control (top) and IL-13 treated (middle and bottom) animals. Sections were stained for P2X4 and markers of club cells [open arrows, α-Club Cell Secretory Protein (CCSP)], goblet cells (filled arrows, Muc5ac), and ciliated cells [arrowheads, tubulin β-IV (tubIV)]. P2X4 receptors are localized to secretory cells with both club (CCSP) and goblet (Muc5ac) phenotypes but not to ciliated cells. Asterisk denotes alveolar type II cell. Scale bars, 15 μm. *P < 0.05, significant difference between groups.
To verify these findings, we also performed additional immunofluorescence staining on tissue sections from control and IL-13-treated animals. Immunofluorescence confirmed the absence of Muc5ac+ goblet cells in control animals and revealed expression of P2X4 in CCSP+ club cells (Fig. 6C, top). After IL-13 treatment several of the CCSP+ cells were also positive for Muc5ac (Fig. 6C, middle), and CCSP staining was less pronounced (being restricted to the apical region), indicating club-to-goblet cell transition. These goblet cells were positive for P2X4 receptors. with P2X4 receptor distribution resembling a vesicular distribution, basolateral from the Muc5ac+ granules. In comparison, P2X4 receptor staining was exclusive of tubulin β-IV staining (Fig. 6C, bottom), a marker for ciliated cells. In keeping with previous findings (44, 65), P2X4 expression can also be detected in ATII cells. In summary, these data further substantiate that P2X4 receptors are expressed in secretory cells of the airways and that inflammation-induced mucous metaplasia entails upregulation of P2X4 expression.
Upregulation and activation of P2X4 augment intracellular Ca2+ signals and mucin secretion.
We previously reported that P2X4 activation facilitates secretion of pulmonary surfactant in primary ATII epithelial cells (42, 45). Therefore, we reasoned that P2X4 expression in goblet cells might also be involved in regulating mucin secretion. Mucins are released upon Ca2+-dependent exocytosis of large secretory granules, and it has been shown that PM P2X receptor activation stimulates exocytosis directly via Ca2+ entry and elevation of [Ca2+]c.
We therefore analyzed the contribution of P2X4 activation to the generation of intracellular Ca2+ signals in individual secretory cells (Fig. 7A) as well as its impact on overall mucin secretion.
Fig. 7.
Upregulation and activation of P2X4 augments ATP-induced Ca2+ signals. A: staining of human primary tracheal epithelial cells (HTECs) with fluo-4 and LysoTracker Deep Red (LTR) to analyze Ca2+ signals in secretory cells (dashed line) upon stimulation with ATP. Scale bar, 15 μm. B: fluo-4 traces from individual untreated (top) or IL-13-treated (bottom) secretory HTECs. Cells were grown at air-liquid interphase conditions, treated with respective inhibitors of P2Y2 (suramin) or P2X4 [expression of P2X4(C353W)-mcherry], and stimulated with 100 μM ATP at time = 5 s. a.u., Arbitrary units; dn, dominant negative. C: quantitative analysis of changes in the intracellular Ca2+ signal under different conditions. Changes in intracellular Ca2+ were analyzed by calculating the integrated increase in fluo-4 fluorescence (area under the curve in B) upon stimulation with 100 μM ATP. Data are from 12–726 individual cells per condition. Statistical significance was assessed by nonparametric tests (Mann-Whitney for comparison of 2 groups or Kruskal-Wallis test for multiple comparisons as indicated by ### and ***, respectively).
Under control conditions, stimulation with ATP resulted in a transient rise in intracellular Ca2+ that was almost completely inhibited (>94.7%) by the P2Y2 receptor inhibitor suramin (Fig. 7, B and C). In IL-13 treated cells, the ATP-induced integrated Ca2+ signal was significantly higher than in control cells [21.3 ± 0.52 arbitrary units (n = 726 cells) and 16.7 ± 0.55 arbitrary units (n = 323 cells), respectively]. Moreover, in IL-13-treated cells, suramin treatment resulted in partial inhibition of the intracellular Ca2+ signal (54.0%) only. The remaining Ca2+ increase likely resulted from activation of P2X4 receptors. Overexpression of the dn mutant P2X4(C353W)-EGFP, which dramatically decreases currents of P2X4 receptor units when coexpressed with wt P2X4 (61), resulted in significant inhibition (55.4%) of the Ca2+ signal. Combined inhibition of P2Y2 (suramin) and P2X4 [P2X4(C353W)-EGFP] receptors almost completely abolished (>93%) the ATP-induced rise in intracellular Ca2+ (Fig. 7, B and C).
To assess mucin secretion from HTEC cultures we used a previously developed MUC5AC ELISA assay (2, 73). Initially, baseline secretion was determined for untreated HTECs and HTECs treated with IL-13 for 21 days, and then stimulated secretion was compared to baseline secretion (Fig. 8A). Not surprisingly, in control cultures with almost no expression of mucins (see Fig. 2, B and C), stimulation with 100 μM ATP or 100 μM UTP, known agonists for mucin secretion, resulted in no to weak increase in MUC5AC secretion [1.14 ± 0.17-fold (P = 0.50) and 1.55 ± 0.11-fold (P = 0.03) increase over baseline, respectively; n = 6 for all]. In IL-13-treated cultures, both agonists resulted in significantly increased MUC5AC secretion [1.80 ± 0.18-fold (P < 0.01) and 2.59 ± 0.21-fold (P < 0.001), respectively; n = 8 for all], again confirming that IL-13 induces goblet cell metaplasia and that increased mucin expression leads to increased mucus secretion after stimulation.
Fig. 8.
Upregulation and activation of P2X4 augment mucin secretion. MUC5AC secretion was assessed by ELISA. A: stimulation of mucin secretion with 100 μM ATP or 100 μM UTP in control (untreated) and IL-13-treated cultures. Agonist treatment resulted in significantly increased MUC5AC secretion in IL-13-treated cells. B: stimulation of mucin secretion with 100 μM ATP in control and IL-13-treated cultures where P2X4 activity was modulated by overexpression of wild-type (wt) P2X4-EGFP, the P2X4 potentiator ivermectin (iverm, 3 μM), the P2X4 inhibitor 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD, 30 μM), or overexpression of the dominant-negative (dn) mutant P2X4(C353W)-EGFP. No effects were observed in control cultures, whereas P2X4 activation resulted in a significant increase and P2X4 inhibition in a significant decrease of mucin secretion in IL-13-treated cultures. C: stimulation of mucin secretion with 100 μM UTP in control and IL-13-treated cultures where P2X4 activity was modulated. The effects of modulating P2X4 activity on mucin secretion in IL-13 treated cultures were absent in cells stimulated with 100 μM UTP, which stimulates mucin secretion but does not activate P2X4 receptors. Means are derived from 5–12 individual experiments. Statistical significance was assessed by Kruskal-Wallis test for multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.001.
We next aimed at elucidating the impact of P2X4 receptor activation on stimulated mucin secretion. Not surprisingly, modulation of P2X4 activity had no impact on mucin secretion in control cultures after stimulation with either 100 μM ATP (Fig. 8B) or 100 μM UTP (Fig. 8C). However, increasing P2X4 activity by either overexpression of wt P2X4-EGFP or treatment with the P2X4 potentiator ivermectin (3 μM) (34, 42, 65) resulted in significantly increased mucin secretion in IL-13-treated HTECs after stimulation with 100 μM ATP [4.40 ± 1.30-fold (n = 6, P < 0.05) and 4.43 ± 0.79-fold (n = 8, P < 0.01) increase over baseline, respectively] compared with stimulation with 100 μM ATP alone (Fig. 8B). In contrast, inhibition of P2X4 activity with either 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (30 μM) or overexpression of the dn mutant P2X4(C353W)-EGFP, which significantly reduced Ca2+ signals in these cultures (Fig. 7B), resulted in dampened mucin secretion after stimulation with 100 μM ATP (0.57 ± 0.21-fold and 0.99 ± 0.34-fold increase over baseline, respectively; n = 6 for all) compared with stimulation with 100 μM ATP alone (Fig. 8B). The effects of modulating P2X4 activity on mucin secretion in IL-13-treated cultures were absent in cells stimulated with 100 μM UTP (Fig. 8C), which stimulates mucin secretion via P2Y2 receptors but does not activate P2X4 receptors.
Together, these data support that upregulation and activation of P2X4 receptors in IL-13-treated cells augment ATP-induced Ca2+ signals, modulate the rate of mucin secretion, and result in a rapid and increased mucin secretion response.
DISCUSSION
It is well established that extracellular ATP stimulates mucin release from airway goblet cells via P2Y2 purinergic receptors (6, 32). Ion-gated P2X purinergic receptors, on the other hand, have so far been neglected in regulation of mucin secretion. With this study we now provide evidence that P2X4 receptors are expressed in secretory airway epithelial cells, in particular in goblet cells, and activation of P2X4 receptors augments mucin secretion. In recent years P2X receptors have attracted increasing attention as regulators of exocytosis and cellular secretion (24, 25, 28, 29, 45, 69). Activation of P2X can stimulate granule exocytosis either directly via influx of Ca2+ from the extracellular space and elevation of [Ca2+]c (27, 29, 35, 60) or by modulating vesicle content release from fused granules during the so-called exocytic “postfusion” phase (12, 44, 65). From our data we cannot discriminate the exact role of P2X4 activation in mucin secretion.
Our data suggest that P2X4 receptors are localized at the PM and in vesicular structures inside the goblet cells that are devoid of MUC5AC and located basolaterally of the apical MUC5AC+ mucin granule pool. Our data further indicate that activation of P2X4 augments the ATP-induced rise in intracellular Ca2+ in cells where P2X4 receptor expression is upregulated upon IL-13 treatment. It is easily conceivable that this results from Ca2+ entry via P2X4 receptors at the PM, which amplifies the Ca2+ signal generated by P2Y activation. However, it is also tempting to speculate whether the vesicular P2X4 receptors contribute to generating a Ca2+ signal. This has been reported from various secretory systems where vesicular P2X4 receptors modulate secretion (18, 42, 44, 57). However, the identity of the P2X4-expressing organelles in MUC5AC+ cells is still elusive and the subject of ongoing studies. P2X4 receptors are predominantly expressed on intracellular, mainly lysosomal or lysosome-related, organelles (47, 57). The size of the P2X4+ structures (∼1 μm) in goblet cells is much larger than the average size of “classical” lysosomes (100–500 nm) (71), indicating that these vesicles represent a distinct class of organelles. Recently, a VNUT-dependent, ATP-loaded vesicular pool, different from the pool associated with mucin granules, has been reported in Calu-3 cells (59). It is hence possible that the P2X4+ vesicles observed in this study contain the P2X4 agonist ATP. Alkalinization of these vesicles could therefore lead to P2X4-dependent Ca2+ release facilitating mucus secretion similar to mechanisms recently described in ATII cells (18). Alternatively, it is possible that the P2X4+ organelles observed in our images, although completely devoid of MUC5AC, belong to the mucin granule maturation pathway containing MUC5AC precursors that are not recognized by the 45M1 antibody used or simply constitute a compartment of the recycling pathway to resensitize P2X4 receptors again (17).
The intracellular localization potentially also indicates a different role for P2X4 receptor activation in addition to amplifying the Ca2+ signal for the initial exocytotic response to extracellular ATP. After stimulation of the exocytic response, P2X4+ granules could fuse with the PM, resulting in surface expression of P2X4 receptors and, in the continued presence of extracellular ATP, also activation of P2X4 receptors. The relatively high Ca2+ permeability of P2X4 receptors (50) will then result in a substantial rise of subplasmalemmal [Ca2+]c. Such a Ca2+ signal could then either induce a sustained exocytotic response in an amplifying feedback mechanism or, alternatively, facilitate release of the macromolecular mucin glycoproteins from fused mucin granules. Similar to ATII cells, the P2X4 receptor on vesicles may control the kinetics of mucin release during the exocytic postfusion phase. In ATII cells P2X4-mediated Ca2+ signals around fused lamellar bodies induce dilation of the exocytic fusion pore and thereby facilitate secretion of pulmonary surfactant (43–45, 49, 65). Such a model requires sufficiently high levels of extracellular ATP at the time of P2X4 surface expression. The ATP concentration outside resting cells has been shown to be in the 5–20 nM range (38, 39, 51), well below the EC50 values for P2X4 activation (50). However, it has been shown that ATP is released from mucin granules (53) as well as via vesicular mechanisms not associated with mucin granule secretion (52). The tight spatio-temporal coupling of mucin granule exocytosis, ATP release, and P2X4 surface expression could therefore result in sufficiently high ATP concentrations within microdomains around newly inserted P2X4 receptors on the apical side of goblet cells to activate the P2X4 receptors. Alternatively, ATP could be released directly from the P2X4+, MUC5AC−, granules (59). In both scenarios, P2X4 activation would be tightly linked and locally restricted to mucin granule exocytosis and provide an ideal feedback loop to induce a “fusion-activated,” local Ca2+ signal to impact on the postfusion phase of mucin secretion (42, 65).
Irrespective of the molecular mechanisms linking P2X4 activation and increased mucin secretion, upregulation of P2X4 during inflammatory and hypersecretion pathologies may facilitate rapid secretion of MUC5AC into the airway surface liquid (ASL), thereby contributing to mucus plaque formation. Additionally, an inward flux of Na+ into the cell may promote water absorption from the ASL as well, furthering this probability (31, 50, 65). Effective mucociliary clearance depends on maintaining a thin (7 μm) periciliary layer lining airway surfaces, which provides a viscous solution for ciliary beating and acts as a lubricant layer for mucus transport (7, 11, 63). ASL volume production reflects a balance between Cl− secretion and Na+ absorption. Cl− secretion via cystic fibrosis transmembrane regulator (CFTR), a cAMP-regulated Cl− channel (62), is a major element in regulating ASL levels. CFTR activity is also controlled by the A2b adenosine receptor, and, under resting conditions, rapid degradation of extracellular ATP results in adenosine levels capable of promoting A2b receptor activation (64). Therefore, fine control mechanisms are required in the lung to establish local nucleotide/nucleoside concentrations that maintain mucociliary clearance. Under inflammatory conditions, however, ATP release is enhanced (52, 53), which, in combination with increased P2X4 expression, could result in increased Na+ currents via P2X4 receptors, counteracting fluid secretion and leading to decreases in ASL, mucus hypohydration, and mucus plaque formation—prominent features of chronic lung diseases including asthma, COPD, and CF (58, 67).
In summary, our data provide strong evidence that P2X4 receptors are predominantly expressed in secretory cells of the airways. Furthermore, P2X4 expression is upregulated in those cells under inflammatory conditions, and activation of P2X4 receptors augments mucin secretion and potentially contributes to mucus hypersecretion and mucus plaque formation. Although the molecular mechanisms by which P2X4 activation leads to increased mucin secretion are still elusive and currently under investigation, P2X4 could constitute a potential pharmaceutical target to attenuate mucus hypersecretion in patients with mucous metaplasia.
GRANTS
This work was supported by a grant from the Ministry of Science, Research and the Arts of Baden-Württemberg, Grant Az: 32-7533.-6-10/15/5 (to M. Frick) and by the Deutsche Forschungsgemeinschaft Grants D-1402/3-1 (to P. Dietl) and SFB1149/1 (A05) (to M. Frick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.E.W., K.E.T., B.F.D., and M.F. conceived and designed study; V.E.W., K.E.T., K.N., A.-M.J., G.F., H.S., W.H., M.J.T., and M.F. performed experiments; V.E.W., K.E.T., K.N., G.F., and M.F. analyzed data; V.E.W., K.E.T., G.F., O.H.W., B.F.D., and M.F. interpreted results of experiments; V.E.W., K.E.T., and M.F. prepared figures; V.E.W., K.E.T., P.D., and M.F. drafted manuscript; V.E.W., K.E.T., O.H.W., M.J.T., B.F.D., P.D., and M.F. edited and revised manuscript; V.E.W., G.F., B.F.D., and M.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Carolin Schilpp for help with primary cell culture, Michael Fauler for help with FACS analysis, S. Britsch and C. Wiegreffe for access to and support for the Leica SP5 confocal microscope, and Jochen Lennertz for FFPE sections.
REFERENCES
- 1.Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, Davis CW. P2u purinoceptor regulation of mucin secretion in SPOC1 cells, a goblet cell line from the airways. Biochem J 316: 943–951, 1996. doi: 10.1042/bj3160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdullah LH, Wolber C, Kesimer M, Sheehan JK, Davis CW. Studying mucin secretion from human bronchial epithelial cell primary cultures. In: Mucins: Methods and Protocols, edited by McGuckin MA, Thornton DJ. Totowa, NJ: Humana, 2012, p. 259–277. doi: 10.1007/978-1-61779-513-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler KB, Tuvim MJ, Dickey BF. Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne) 4: 129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest 126: 2367–2371, 2016. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brekman A, Walters MS, Tilley AE, Crystal RG. FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro. Am J Respir Cell Mol Biol 51: 688–700, 2014. doi: 10.1165/rcmb.2013-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G, Brouns I, Adriaensen D, Timmermans JP. Purinergic signaling in the airways. Pharmacol Rev 64: 834–868, 2012. doi: 10.1124/pr.111.005389. [DOI] [PubMed] [Google Scholar]
- 7.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337: 937–941, 2012. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, Chen J. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci USA 110: 18042–18051, 2013. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chorley BN, Crews AL, Li Y, Adler KB, Minnicozzi M, Martin LD. Differential Muc2 and Muc5ac secretion by stimulated guinea pig tracheal epithelial cells in vitro. Respir Res 7: 35, 2006. doi: 10.1186/1465-9921-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 11.Dickey BF. Biochemistry. Walking on solid ground. Science 337: 924–925, 2012. doi: 10.1126/science.1227091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietl P, Haller T, Frick M. Spatio-temporal aspects, pathways and actions of Ca2+ in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 52: 296–302, 2012. doi: 10.1016/j.ceca.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Dietl P, Haller T, Mair N, Frick M. Mechanisms of surfactant exocytosis in alveolar type II cells in vitro and in vivo. News Physiol Sci 16: 239–243, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol 205: 621–631, 2014. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 31: 382–394, 2004. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fois G, Föhr KJ, Kling C, Fauler M, Wittekindt OH, Dietl P, Frick M. P2X4 receptor re-sensitization depends on a protonation/deprotonation cycle mediated by receptor internalization and recycling. J Physiol 596: 4893–4907, 2018. doi: 10.1113/JP275448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fois G, Winkelmann VE, Bareis L, Staudenmaier L, Hecht E, Ziller C, Ehinger K, Schymeinsky J, Kranz C, Frick M. ATP is stored in lamellar bodies to activate vesicular P2X4 in an autocrine fashion upon exocytosis. J Gen Physiol 150: 277–291, 2018. doi: 10.1085/jgp.201711870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frick M, Eschertzhuber S, Haller T, Mair N, Dietl P. Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am J Respir Cell Mol Biol 25: 306–315, 2001. doi: 10.1165/ajrcmb.25.3.4493. [DOI] [PubMed] [Google Scholar]
- 20.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183–206, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stühmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol 51: 109–118, 1997. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- 22.Gou D, Mishra A, Weng T, Su L, Chintagari NR, Wang Z, Zhang H, Gao L, Wang P, Stricker HM, Liu L. Annexin A2 interactions with Rab14 in alveolar type II cells. J Biol Chem 283: 13156–13164, 2008. doi: 10.1074/jbc.M801532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res 23: 1482–1490, 2006. doi: 10.1007/s11095-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 24.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 389: 749–753, 1997. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez-Martín Y, Bustillo D, Gómez-Villafuertes R, Sánchez-Nogueiro J, Torregrosa-Hetland C, Binz T, Gutiérrez LM, Miras-Portugal MT, Artalejo AR. P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells. J Biol Chem 286: 11370–11381, 2011. doi: 10.1074/jbc.M110.139410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller T, Auktor K, Frick M, Mair N, Dietl P. Threshold calcium levels for lamellar body exocytosis in type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 277: L893–L900, 1999. doi: 10.1152/ajplung.1999.277.5.L893. [DOI] [PubMed] [Google Scholar]
- 27.Hayoz S, Jia C, Hegg C. Mechanisms of constitutive and ATP-evoked ATP release in neonatal mouse olfactory epithelium. BMC Neurosci 13: 53, 2012. doi: 10.1186/1471-2202-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC, Roper SD. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci 31: 13654–13661, 2011. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques-Silva MC, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman DM, Kenyon NS, Ricordi C, Pileggi A, Molano RD, Berggren PO, Caicedo A. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci USA 107: 6465–6470, 2010. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery PK, Gaillard D, Moret S. Human airway secretory cells during development and in mature airway epithelium. Eur Respir J 5: 93–104, 1992. [PubMed] [Google Scholar]
- 31.Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors—recent progress and persisting challenges. Purinergic Signal 8: 375–417, 2012. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemp PA, Sugar RA, Jackson AD. Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol 31: 446–455, 2004. doi: 10.1165/rcmb.2003-0211OC. [DOI] [PubMed] [Google Scholar]
- 33.Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol 6: 379–392, 2013. doi: 10.1038/mi.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci 19: 7289–7299, 1999. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Nam JH, Kim MH, Koh DS, Choi SJ, Kim SJ, Lee JE, Min KM, Uhm DY, Kim SJ. Purinergic receptors coupled to intracellular Ca2+ signals and exocytosis in rat prostate neuroendocrine cells. J Biol Chem 279: 27345–27356, 2004. doi: 10.1074/jbc.M313575200. [DOI] [PubMed] [Google Scholar]
- 36.Kim K, Petrova YM, Scott BL, Nigam R, Agrawal A, Evans CM, Azzegagh Z, Gomez A, Rodarte EM, Olkkonen VM, Bagirzadeh R, Piccotti L, Ren B, Yoon JH, McNew JA, Adachi R, Tuvim MJ, Dickey BF. Munc18b is an essential gene in mice whose expression is limiting for secretion by airway epithelial and mast cells. Biochem J 446: 383–394, 2012. doi: 10.1042/BJ20120057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584: 245–259, 2007. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9: 262–267, 2009. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Cho SN, Akkanti B, Jin N, Mao J, Long W, Chen T, Zhang Y, Tang X, Wistub II, Creighton CJ, Kheradmand F, DeMayo FJ. ErbB2 pathway activation upon Smad4 loss promotes lung tumor growth and metastasis. Cell Rep 15: 00142–00144, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucattelli M, Cicko S, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol 44: 423–429, 2011. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 42.Miklavc P, Frick M. Vesicular calcium channels as regulators of the exocytotic post-fusion phase. Commun Integr Biol 4: 796–798, 2011. doi: 10.4161/cib.17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miklavc P, Frick M, Wittekindt OH, Haller T, Dietl P. Fusion-activated Ca2+ entry: an “active zone” of elevated Ca2+ during the postfusion stage of lamellar body exocytosis in rat type II pneumocytes. PLoS One 5: e10982, 2010. doi: 10.1371/journal.pone.0010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, Timmler M, Frick M. Fusion-activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc Natl Acad Sci USA 108: 14503–14508, 2011. doi: 10.1073/pnas.1101039108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miklavc P, Thompson KE, Frick M. A new role for P2X4 receptors as modulators of lung surfactant secretion. Front Cell Neurosci 7: 171, 2013. doi: 10.3389/fncel.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller T, Vieira RP, Grimm M, Dürk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, Ferrari D, Di Virgillio F, Idzko M. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol 44: 456–464, 2011. doi: 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- 47.Murrell-Lagnado RD, Qureshi OS. Assembly and trafficking of P2X purinergic receptors. Mol Membr Biol 25: 321–331, 2008. doi: 10.1080/09687680802050385. [DOI] [PubMed] [Google Scholar]
- 48.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 49.Neuland K, Sharma N, Frick M. Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J Cell Sci 127: 5218–5227, 2014. doi: 10.1242/jcs.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 51.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada SF, Ribeiro CM, Sesma JI, Seminario-Vidal L, Abdullah LH, van Heusden C, Lazarowski ER, Boucher RC. Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways. Am J Respir Cell Mol Biol 49: 814–820, 2013. doi: 10.1165/rcmb.2012-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol 45: 253–260, 2011. doi: 10.1165/rcmb.2010-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren B, Azzegagh Z, Jaramillo AM, Zhu Y, Pardo-Saganta A, Bagirzadeh R, Flores JR, Han W, Tang YJ, Tu J, Alanis DM, Evans CM, Guindani M, Roche PA, Rajagopal J, Chen J, Davis CW, Tuvim MJ, Dickey BF. SNAP23 is selectively expressed in airway secretory cells and mediates baseline and stimulated mucin secretion. Biosci Rep 35: e00220, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 57.Robinson LE, Murrell-Lagnado RD. The trafficking and targeting of P2X receptors. Front Cell Neurosci 7: 233, 2013. doi: 10.3389/fncel.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86: 245–278, 2006. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 59.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, Lazarowski ER. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol 304: C976–C984, 2013. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shigetomi E, Kato F. Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci 24: 3125–3135, 2004. doi: 10.1523/JNEUROSCI.0090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silberberg SD, Chang TH, Swartz KJ. Secondary structure and gating rearrangements of transmembrane segments in rat P2X4 receptor channels. J Gen Physiol 125: 347–359, 2005. doi: 10.1085/jgp.200409221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352: 628–631, 1991. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- 63.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280: 35751–35759, 2005. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–604, 2006. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson KE, Korbmacher JP, Hecht E, Hobi N, Wittekindt OH, Dietl P, Kranz C, Frick M. Fusion-activated cation entry (FACE) via P2X4 couples surfactant secretion and alveolar fluid transport. FASEB J 27: 1772–1783, 2013. doi: 10.1096/fj.12-220533. [DOI] [PubMed] [Google Scholar]
- 66.Thornton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L1118–L1128, 2000. doi: 10.1152/ajplung.2000.278.6.L1118. [DOI] [PubMed] [Google Scholar]
- 67.Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans 37: 877–881, 2009. doi: 10.1042/BST0370877. [DOI] [PubMed] [Google Scholar]
- 68.Turner J, Roger J, Fitau J, Combe D, Giddings J, Heeke GV, Jones CE. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am J Respir Cell Mol Biol 44: 276–284, 2011. doi: 10.1165/rcmb.2009-0304OC. [DOI] [PubMed] [Google Scholar]
- 69.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28: 11263–11268, 2008. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 282: 2258–2261, 1998. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 71.Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol 77: 57–80, 2015. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, Brody SL. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L650–L657, 2004. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA, Forest MG, Lethem MI, Dickey BF, Davis CW. Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS One 10: e0127267, 2015. doi: 10.1371/journal.pone.0127267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 586: 1977–1992, 2008. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]