Abstract
We report a case of a patient with drug-resistant epilepsy treated with deep brain stimulation of the anterior nucleus of the thalamus (ANT-DBS). The patient developed psychiatric side effects (PSEs), namely irritability, hostility, aggressiveness, and paranoia, after implantation and stimulation initiation. The stimulation was discontinued and the PSEs were mitigated, but the patient did not return to her pre-implantation state, as documented by repeated psychiatric reports and hospitalizations. To our knowledge, this is the first report of a patient who developed long-term PSEs that did not disappear after stimulation discontinuation. We suppose that ANT-DBS caused a persistent perturbation of the thalamic neuronal networks that are responsible for long-term PSEs.
Abbreviations: ANT, anterior nucleus of the thalamus; ANT-DBS, deep brain stimulation of the anterior nucleus of the thalamus; MRI, magnetic resonance imaging; PET, positron emission tomography; PSEs, psychiatric side effects
Keywords: Deep brain stimulation of the anterior nucleus of the thalamus, Long-term psychiatric side effects, Case report
Highlights
-
•
ANT-DBS can trigger long-term psychiatric side effects (PSEs).
-
•
ANT-DBS PSEs can persist despite stimulation discontinuation.
-
•
Long-term PSEs can be caused by alteration of thalamic circuits.
1. Introduction
Deep brain stimulation of the anterior nucleus of the thalamus (ANT-DBS) is a novel and promising treatment method for patients with drug-resistant epilepsy. More than 70% of patients implanted with ANT-DBS benefit significantly from this method, i.e., they report seizure-reduction rates higher than 50% [1]. We have only limited knowledge about short- and long-term ANT-DBS side effects because of relatively low numbers of implanted patients. When focusing on the adverse events reported in a study of stimulation of the anterior nuclei of thalamus (SANTE study), the patients reported paresthesia (18% patients), pain in the implant side (10.9% patients), and infection at the implant site (9.1% patients) [2]. During a five-year follow-up course, device-related adverse events were reported [1]. When focusing on adverse events, depression was present in 37.3% patients (3 events in 3 subjects were considered to be device-related), memory impairment was present in 27.3% patients (approximately a third of memory impairment was confirmed by neuropsychological examination), 11.8% patients reported suicidal ideation (one subject committed suicide; the suicide was not thought to be device related), and 7 patients died during the study — 1 probable sudden unexpected death in epilepsy (SUDEP), 2 definite SUDEP, and 1 possible SUDEP [1].
The anterior nucleus of the thalamus (ANT) has connections with limbic structures, anterior cingulate cortex, and orbitomedial prefrontal cortex; thus, it plays a vital role in memory processes and in emotional and executive functions [3]. A disruption or alteration in baseline circuits can be associated with ANT-DBS psychiatric side effects (PSEs). PSEs can appear immediately after stimulation initialization or can develop over a more extended period. However, in all previously published reports, the PSEs were time-related to stimulation. Järvenpää et al. [9] suggested decreasing the voltage or changing the stimulation contacts to suppress PSEs. At the moment, there is no official recommendation for the management of PSEs in patients with ANT-DBS. We report a patient in whom ANT-DBS caused PSEs that persisted despite stimulation discontinuation. To our knowledge, this is the first report of a patient in whom ANT-DBS caused PSEs not directly linked to the stimulation itself.
2. Case report
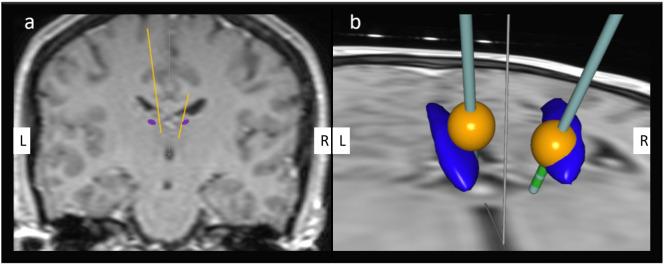
The patient is a female born in 1970 with a family history of mesial temporal lobe epilepsy. The patient was treated only for lower back pain and had no history of psychiatric illness. Family history regarding psychiatric disease was also absent. The patient developed epilepsy at the age of 17; the epilepsy was characterized as focal with independent left and right temporal interictal epileptiform discharges and bitemporal seizure onsets during scalp EEG monitoring. Magnetic resonance imaging (MRI) revealed right-sided hippocampal sclerosis. Positron emission tomography (PET) showed bitemporal hypometabolism. The Wada test demonstrated a crucial role of right-sided mesial temporal structures for verbal memory. The patient was judged to be a poor surgical candidate and neuromodulation was favored. Therefore, the implantation of a vagus nerve stimulator (VNS) was performed at the age of 29. The vagus nerve stimulator was ineffective, which led to its explantation after seven years. The patient was offered ANT-DBS implantation at the age of 41 years. The patient experienced 8 focal seizures with impairment of awareness per month. Before implantation, the psychological examination revealed no severe mental pathology, such as anxiety, depression, or psychosis. However, there were some psychological problems, specifically echolalia, perseveration, and difficulties with management of stressful situations, as well as global deterioration of cognitive function (IQ 72, the most severe alteration in execution and memory). The patient was on stable doses of antiseizure drugs for two years before implantation. She was treated with pregabalin 600 mg/day, zonisamide 400 mg/day, and lacosamide 400 mg/day. The monopolar stimulation of proximal contacts (contact 3 on the left and contact 11 on the right) was initiated one month after ANT-DBS implantation (Fig. 1a and b); the stimulation parameters were as follows: amplitude 2.5 V, stimulation frequency 140 Hz, pulse width 90 ms, 5-minute off-time, 1-minute on-time.
Fig. 1.
The position of deep brain stimulation (DBS) electrodes to anterior nucleus thalami (ANT).
Panel a illustrates the relation between DBS electrodes and ANT in the coronal section. There are 4 contacts on each electrode (contacts are labeled from the most distal to the most proximal as 0, 1, 2, and 3 on the left, and as 8, 9, 10, and 11 on the right). We stimulated the most proximal contacts on both sides, i.e., contact 3 on the left and contact 11 on the right.
Panel b shows the estimated distribution of the electrical field (yellow) and its relation to ANT (blue). On the right, the correct contact for stimulation was chosen. On the left, the more distal contact was more suitable. The figures were obtained using SureTune software (Medtronic, Minneapolis, MN, USA). L — left, R — right.
The patient did not note a decrease in seizure frequency, but she did report reduction in seizure severity and duration. Behavioral changes started to appear gradually three months after implantation. The family complained about patient irritability, hostility, and aggressiveness, which were accompanied by newly evolved paranoia. The patient reported that DBS influenced her manners and forced her to walk backward, which worsened when someone was speaking about DBS. A psychological and psychiatric examination performed five months after stimulation initialization revealed incoherent thoughts and behavioral (unrest, agitation) and emotional alteration. Personality and behaviorial disorders were diagnosed. Any possibility of a new structural abnormality was excluded by post-operative magnetic resonance imaging (MRI). We discontinued stimulation and introduced quetiapine (50 mg/day) and subsequently risperidone (1.5 mg/day). This resulted in partial alleviation of the PSEs, but the patient did not reach her pre-implantation state over the next seven years documented by repeated psychological and psychiatric examinations. The patient's state required recurrent psychiatric hospitalizations conditioned by intermittent worsening with psychosis. The patient subjectively reported persistent reduction in seizure severity.
3. Discussion
The anterior nucleus of the thalamus plays a crucial role in seizure generation, as shown in animal and human studies. Stimulation of the anterior nucleus of the thalamus leads to substantial seizure reduction in the majority of patients. The results of two studies suggest that the positioning of the DBS electrode has a significant impact on seizure reduction [4,5]. Patients with electrodes located more anterior and superior [5] or in the antero-ventral part of the thalamus tend to have better responses [4].
The thalamus is a key structure in two core neurocognitive networks, the salience network and the default-mode network, that were proven to play important roles in cognition, emotion, and execution, and that are altered in many psychiatric conditions, including depression, bipolar disorder, schizophrenia, substance use disorder, obsessive–compulsive disorder, and anxiety disorder [6,7]. It is therefore not surprising that interference with the functioning of this structure could be associated with PSEs. ANT-DBS was found to be independently associated with de novo psychopathology in a group of surgically treated patients [8]. Järvenpää et al. [9] reported a group of 22 patients treated with ANT-DBS; four patients developed reversible PSEs. The stimulation induced depression in two patients. The other two patients had symptoms of paranoia and anxiety. In all their patients, the PSEs completely disappeared when they lowered the output current or changed the stimulated contacts [9]. Based on their experience, the authors supposed the stimulation of other thalamic nuclei or tracts close to the ANT to be responsible for PSEs, as the stimulated contacts were not in the ANT itself but beneath.
These findings contrast with our experience. As shown in Fig. 1, the appropriate contact on the left was chosen, the more distal contact was more suitable on the right. Moreover, the PSEs did not disappear after stimulation discontinuation. They persisted for seven years despite intensive treatment with repeated hospitalization. The explanation for this situation is complicated; we can merely speculate about possible reasons. Our hypothesis is that the insertion of electrodes and subsequent stimulation can cause alteration in thalamic circuitry with potential long-term consequences in predisposed individuals such as our patient. We suspect some lesional effect because the PSEs persisted despite stimulation discontinuation similar to thalamic lesions caused by ischemia. Most authors localized the ANT with surrounding nuclei to the territory of the tuberothalamic artery, the closure of which is characterized by severe wide-ranging neuropsychological deficits [10]. Strokes in this arterial territory are associated with the fluctuation of consciousness in the early stage of ischemia. Subsequently, disorientation, euphoria, lack of insight, apathy, lack of spontaneity, and emotional unconcern can develop [11]. We excluded an acute surgical complication and doubt an adverse effect due to antiseizure drugs was suddenly responsible for the psychiatric complaints in our patient.
ANT-DBS is a promising novel technique for drug-resistant epilepsy treatment, but may be occasionally associated with significant PSEs. It is necessary to inform patients about the possibility of PSEs when offering ANTI-DBS. Because only limited information is available about chronic ANT-DBS PSEs more experience in larger groups of patients is necessary to identify those who are at risk for long-term consequences.
Funding
The results of this research have been acquired with the support of the Ministry of Health of the Czech Republic, grant no. NV19-04-00343.
Ethical statement
We confirm that we have read the Journal's position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
Declaration of competing interest
Neither of the authors has any conflict of interest to disclose.
References
- 1.Salanova V., Witt T., Worth R., Henry T.R., Gross R.E., Nazzaro J.M. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher R., Salanova V., Witt T., Worth R., Henry T., Gross R. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 3.Child N.D., Benarroch E.E. Anterior nucleus of the thalamus: functional organization and clinical implications. Neurology. 2013;81:1869–1876. doi: 10.1212/01.wnl.0000436078.95856.56. [DOI] [PubMed] [Google Scholar]
- 4.Krishna V., King N.K., Sammartino F., Strauss I., Andrade D.M., Wennberg R.A. Anterior nucleus deep brain stimulation for refractory epilepsy: insights into patterns of seizure control and efficacious target. Neurosurgery. 2016;78:802–811. doi: 10.1227/NEU.0000000000001197. [DOI] [PubMed] [Google Scholar]
- 5.Lehtimaki K., Mottonen T., Jarventausta K., Katisko J., Tahtinen T., Haapasalo J. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. 2016;9:268–275. doi: 10.1016/j.brs.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Peters S.K., Dunlop K., Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novais F., Pestana L.C., Loureiro S., Andrea M., Figueira M.L., Pimentel J. Predicting de novo psychopathology after epilepsy surgery: a 3-year cohort study. Epilepsy Behav. 2019;90:204–208. doi: 10.1016/j.yebeh.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Järvenpää S., Peltola J., Rainesalo S., Leinonen E., Lehtimaki K., Jarventausta K. Reversible psychiatric adverse effects related to deep brain stimulation of the anterior thalamus in patients with refractory epilepsy. Epilepsy Behav. 2018;88:373–379. doi: 10.1016/j.yebeh.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Bogousslavsky J., Regli F., Assal G. The syndrome of unilateral tuberothalamic artery territory infarction. Stroke. 1986;17:434–441. doi: 10.1161/01.str.17.3.434. [DOI] [PubMed] [Google Scholar]
- 11.Schmahmann J.D. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]