Abstract
Patients with mutations in the POLG-1 gene often are afflicted with drug-resistant seizures at an early age and have an increased risk of valproic acid-induced acute liver failure. Severe valproate hepatotoxicity most commonly arises in children within the first 3 months of treatment with an overall estimated incidence of 1 in 40,000 treated patients. Due to high mortality rates among transplanted children, many experts consider valproic acid-induced acute liver failure in patients with mitochondrial disorders to be a contraindication to liver transplant. We report the successful use of liver transplantation in a young man with valproic acid-associated acute liver failure harboring a previously unrecognized POLG-1 mutation.
Abbreviations: ALF, acute liver failure; DILI, drug-induced liver injury; ULN, upper limit of normal; VPA, valproic acid
Keywords: Liver transplantation, Drug hepatotoxicity, Anti-convulsants
Highlights
-
•
Patients with mutations in the POLG-1 gene often have an increased risk of valproic acid (VPA) induced acute liver failure.
-
•
Severe valproate hepatotoxicity most commonly arises in children within the first 3 months of treatment.
-
•
Many experts consider VPA induced acute liver failure with mitochondrial disorders a contraindication to liver transplant.
-
•
We report a case of acute liver failure associated with VPA treated successfully with a liver transplant
1. Introduction
Valproic acid (VPA) is a widely used branched, medium chain fatty acid that was approved as a treatment for patients with epilepsy in 1978. Due to its other benefits, VPA has also received United States Food and Drug Administration approval for use in patients with bipolar disorders and recurrent migraine headaches [1]. The use of VPA in epilepsy patients is guided by the clinical response, blood levels, and dose-dependent side effects. VPA can cause several distinctive forms of acute and chronic hepatic injury in treated patients [[2], [3], [4], [5]]. The most common form of injury to the liver is mild elevation in serum AST and ALT levels that occur in up to 5 to 10% of treated patients within 12 months. The frequency and severity of these self-limited aminotransferase elevations do not appear to be related to subject age, gender, or race nor the dose of VPA administered or peak serum VPA level. VPA can also interfere with intracellular ammonia metabolism and lead to a systemic hyperammonemia syndrome with associated mental status changes despite normal hepatic enzymes and bilirubin levels. Hyperammonemia is most commonly seen during the first 6 months of therapy but can occur later in treatment with dose escalations and usually resolves with either holding or reducing the dose of VPA or by giving l-carnitine supplementation [6]. The third and least common phenotype of VPA-mediated injury to the liver is acute hepatocellular injury with jaundice and/or coagulopathy. The liver biopsy in these cases typically shows microvesicular steatosis and varying degrees of centrolobular inflammation and necrosis. Studies demonstrate that children under the age of two, patients on multiple antiseizure drugs, and those with inherited mitochondrial disorders are particularly susceptible to this potentially severe form of liver injury with over 100 fatalities reported in the literature [[2], [3], [4], [5]]. Patients with mutations in the mitochondrial DNA (mtDNA) polymerase Ɣ (POLG-1) gene appear to be particularly susceptible to severe hepatic injury [7]. As a result, the prescribing information for VPA contains a BLACK-BOX warning stating that the medication is contraindicated in patients with known mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder. When the drug is used in children less than 2 years of age it should be given with extreme caution and as a sole agent [3].
2. Case
A 20-year-old previously healthy caucasian male experienced multiple focal aware-motor seizures involving the left upper and lower extremity in July 2017 after initiating boot camp exercises as a military recruit. He subsequently developed generalized motor seizures. His past medical history and family history were negative for epilepsy and he was receiving no antiseizure medication. The patient underwent an extensive medical evaluation including an MRI of the brain that showed bilateral multifocal areas of gyriform-restricted diffusion with associated cortical thickening and T2/FLAIR hyperintensity most pronounced in the posterior right frontal lobe. He had an EEG performed that showed focal motor status epilepticus emanating from the right frontal region associated wtih myoclonic jerks. The patient underwent a lumbar puncture that showed elevated protein and B2 microglobulin. For the diagnosis of focal motor status epilepticus, the patient was treated with a series of antiseizure medications including levetiracetam, phenytoin, and lacosamide. However, due to progressive neurological symptoms including persistent left upper and lower extremity focal seizures, mental status fluctuations and weakness, he required hospitalization and was diagnosed with presumed autoimmune encephalopathy based on results of the above studies and after a PET scan showed large areas of hypermetabolic activity in the right parietal lobe, thalamus and left cerebellum. A cerebral angiogram was unremarkable without evidence of vasculitis. He was given immunoglobulin, plasma exchange, cyclophosphamide, mycophenolate mofetil and rituximab infusions as well as high dose corticosteroids. CSF testing for the presence of anti-NMDA antibodies was negative. The patient's seizures eventually were controlled with the addition of valproic acid that was initiated in October 2017 at a dose of 1000 mg/day. Of note, his pretreatment liver biochemical analyses were normal. Follow-up lab work in December 2017 demonstrated a serum AST of 28 IU/L and ALT of 73 IU/L with total bilirubin of 0.6 mg/dl and his valproate dose was increased to 1500 mg per day due to a low serum concentration (Fig. 1).
Fig. 1.
Serial serum ALT and bilirubin levels in a 20-year-old caucasian man with new onset seizures who started VPA in October 2017.
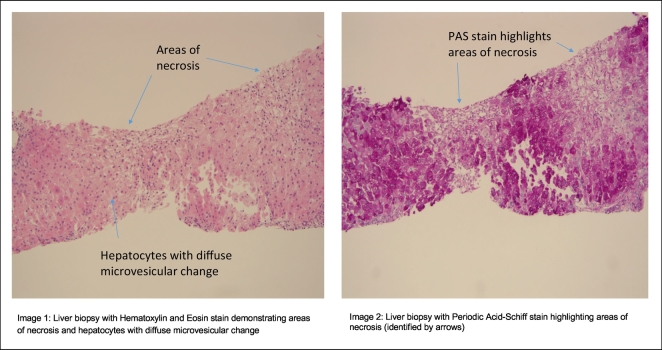
In January 2018 the patient developed extreme fatigue, lethargy, and nausea with vomiting and subsequent jaundice, mental status changes and coagulopathy. At the time of hospitalization he had a serum AST 161 IU/L, ALT 283 IU/L bilirubin 4.5 mg/dl and INR of 3.1 with no eosinophilia. His initial NH3 level was elevated at 70 μg/dl in Nov 2017 and remained elevated at 60 μg/dl in January 2018. Evaluation for hepatitis A, B, C and CMV/EBV infection was negative as was liver imaging. A liver biopsy showed marked microvascular steatosis and associated necrosis consistent with drug-induced hepatotoxicity. Despite discontinuation of VPA and use of N-acetylcysteine, his liver function continued to decline and he underwent emergency orthotopic liver transplantation in February 2018. His explanted liver weighed 1332 g and demonstrated regenerative nodules with extensive necrosis and early cirrhosis on trichrome stain (Fig. 2). Post-operative genetic testing demonstrated a homozygous pathogenic variant in the POLG-1 gene (A467T).
Fig. 2.
Liver biopsy with Hematoxylin and Eosin stain (image 1) as well as Periodic Acid-Schiff stain (image 2).
Early in the course following transplantation the patient experienced several focal aware-motor seizures that involved the left upper and lower extremity and were successfully managed with a combination of levetiracetam, lacosamide, pregabalin and topiramate. As of October 2019, he continues to have preserved allograft function with normal liver enzyme tests while receiving tacrolimus, prednisone, l-carnitine and Co-enzyme Q-10.
3. Discussion
Patients with mutations in the POLG-1 gene encoding mitochondrial DNA polymerase often are afflicted with drug-resistant seizures and progressive neurological dysfunction at a young age [8,9]. Both heterozygotes and homozygotes with point mutations in the POLG-1 gene are at increased risk of developing injury to the liver when given VPA especially in patients given the drug that tends to develop within the first 3 months of treatment and in those receiving multiple antiseizure drugs [2]. VPA may inhibit mitochondrial beta-oxidation through the microsomal production of toxic metabolites or via direct effects on mitochondrial function [10,11]. The estimated prevalence of nuclear and mitochondrial disorders in the adult general population is 7 per 100,000. Prior case series report a 1-year survival rate as low as 18% in children transplanted for VPA-induced ALF who have a known mitochondrial disorder [12]. As a result, many experts advise against proceeding with liver transplantation in children with VPA-related ALF who have known POLG1 mutations [13,14]. Our patient's POLG-1 mutation, A467T is the most common mutation for POLG-1-related disorders and the A467T and W748S mutations account for nearly 2/3 of patients with autosomal recessive POLG-related disorders [8]. These POLG-1 mutations are associated with ataxia–neuropathy syndrome and Alpers–Huttenlocher syndrome that manifests with seizures, liver disease and impaired cognitive ability at a young age. Prior studies in animals and human studies with historical controls have demonstrated that prompt administration of l-carnitine which is frequently depleted in patients on chronic VPA therapy may rescue some patients with severe acute VPA-induced liver injury [15,16]. Unfortunately, l-carnitine was not instituted prior to transplant in our patient due to the difficulty in establishing a diagnosis of idiosyncratic drug-induced hepatic injury due to VPA.
Due to its side-effect profile, VPA is often used as a second line antiseizure drug for adults and children with focal epilepsies. Nonetheless, it is reported by ClinCalc.com that in 2016 there were 5,484,270 total prescriptions of divalproex sodium identified in the United States. The incidence of mitochondrial diseases in the United Kingdom population is 1 in 4000 to 1 in 5000 [17]. Some authors have advocated for testing for POLG mutations in all children under the age of 2 prior to initiating VPA therapy although this is not routinely done in most medical centers in the United States [18]. Furthermore, the overall incidence of severe idiosyncratic drug-induced hepatic injury from VPA decreases with age and among adults the overall incidence is estimated at 1 in 40,000 [3,4]. However, a notable exception would be an adult patient presenting with focal status epilepticus without other explanation since this could be the first clinical manifestation of a subclinical congenital mitochondrial disorder as was seen in this case [18]. In those instances, whole exome sequencing to identify the entire POLG gene would be helpful in patients independent of their ethnicity. Epilepsy patients that harbor an unrecognized POLG-1 mutation may be at increased risk for severe VPA hepatotoxicity [19,20].
In the past, acute liver failure attributed to VPA therapy in patients with known POLG1 mutations has been considered a contraindication to liver transplantation among children [[12], [13], [14]]. However, the current case illustrates that liver transplantation can result in favorable clinical outcomes among adult patients with presumably milder forms of mitochondrial disorders. It is possible that our patient's older age at presentation and lack of features suggestive of Alpers–Huttenlocher syndrome may have contributed to his more favorable outcome [9].
Disclaimer statement
Some of the authors of this manuscript are active duty U.S. Navy service members. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. 101 defines United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person's official duties.
Ethics statement
Our manuscript “Acute Liver failure in a military recruit treated with valproic acid and harboring a previously unrecognized POLG-1 mutation” does adhere to ethics in publishing and ethical guidelines for journal publication.
Declaration of competing interest
The authors John T. Bassett, Sanford Health, Fargo, ND; Benjamin Rodriguez, Naval Hospital Jacksonville, FL; and Lisa Mulligan, Naval Medical Center Portsmouth, Portsmouth, VA declare that they have no conflicts of interest/disclosures.
The author Robert J. Fontana, University of Michigan, Ann Arbor, MI has received research grants from Abbvie, Gilead and Bristol-Myers Squibb. He also consults for Sanofi.
References
- 1.Accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf. Published 2019. Accessed July 5, 2019.
- 2.Bryant A., Dreifuss F. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465–469. doi: 10.1212/wnl.46.2.465. [DOI] [PubMed] [Google Scholar]
- 3.Dreifuss F., Langer D. Hepatic considerations in the use of antiepileptic drugs. Epilepsia. 1987;28(s2):S23–S29. doi: 10.1111/j.1528-1157.1987.tb05768.x. [DOI] [PubMed] [Google Scholar]
- 4.Dreifuss F., Santilli N., Langer D., Sweeney K., Moline K., Menander K. Valproic acid hepatic fatalities: a retrospective review. Neurology. 1987;37(3):379. doi: 10.1212/wnl.37.3.379. [DOI] [PubMed] [Google Scholar]
- 5.Mindikoglu A.L., Magder L.S., Regev A. Outcome of liver transplantation for drug-induced acute liver failure in the United States: analysis of the United Network for Organ Sharing database. Liver Transpl. 2009;15:719–729. doi: 10.1002/lt.21692. [PubMed: 19562705] [DOI] [PubMed] [Google Scholar]
- 6.Nanau R., Neuman M. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323–1338. doi: 10.1016/j.clinbiochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 7.McFarland R., Hudson G., Taylor R. Reversible valproate hepatotoxicity due to mutations in mitochondrial DNA polymerase (POLG1) Case Reports. 2009 doi: 10.1136/bcr.12.2008.1303. 2009(may10 1):bcr1220081303-bcr1220081303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynynen J., Komulainen T., Tukiainen E., Nordin A., Arola J., Kälviäinen R. Acute liver failure after valproate exposure in patients with POLG1mutations and the prognosis after liver transplantation. Liver Transpl. 2014;20(11):1402–1412. doi: 10.1002/lt.23965. [DOI] [PubMed] [Google Scholar]
- 9.McKiernan P. Acute liver failure after valproate exposure: liver transplantation may be indicated beyond childhood. Liver Transpl. 2014;20(11):1287–1289. doi: 10.1002/lt.23988. [DOI] [PubMed] [Google Scholar]
- 10.Ishikura H., Matsuo N., Matsubara M., Ishihara T., Takeyama N., Tanaka T. Valproic acid overdose and l-carnitine therapy. J Anal Toxicol. 1996;20(1):55–58. doi: 10.1093/jat/20.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Aires C., Ruiter J., Luis P. Studies on the extra-mitochondrial CoA-ester formation of valproic and Δ4-valproic acids. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2007;1771(4):533–543. doi: 10.1016/j.bbalip.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Mindikoglu A., King D., Magder L., Ozolek J., Mazariegos G., Shneider B. Valproic acid-associated acute liver failure in children: case report and analysis of liver transplantation outcomes in the United States. J Pediatr. 2011;158(5):802–807. doi: 10.1016/j.jpeds.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W., Sokol R. Mitochondrial hepatopathies: advances in genetics, therapeutic approaches, and outcomes. J Pediatr. 2013;163(4):942–948. doi: 10.1016/j.jpeds.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKiernan P. Liver transplantation and cell therapies for inborn errors of metabolism. J Inherit Metab Dis. 2013;36(4):675–680. doi: 10.1007/s10545-012-9581-z. [DOI] [PubMed] [Google Scholar]
- 15.Mock C., Schwetschenau K. Levocarnitine for valproic-acid-induced hyperammonemic encephalopathy. Am J Health Syst Pharm. 2012;69(1):35–39. doi: 10.2146/ajhp110049. [DOI] [PubMed] [Google Scholar]
- 16.Perrott J., Murphy N., Zed P. l-Carnitine for acute valproic acid overdose: a systematic review of published cases. Annals of Pharmacotherapy. 2010;44(7–8):1287–1293. doi: 10.1345/aph.1P135. [DOI] [PubMed] [Google Scholar]
- 17.Gorman G., Schaefer A., Ng Y., Gomez N., Blakely E., Alston C. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015;77(5):753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saneto R., Lee I., Koenig M. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure. 2010;19(3):140–146. doi: 10.1016/j.seizure.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debray F., Lambert M., Mitchell G. Disorders of mitochondrial function. Curr Opin Pediatr. 2008;20(4):471–482. doi: 10.1097/MOP.0b013e328306ebb6. [DOI] [PubMed] [Google Scholar]
- 20.Stewart J., Horvath R., Baruffini E. Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52(5):1791–1796. doi: 10.1002/hep.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]