Highlights
-
•
Hyperglycemia is known to accelerate oxidative stress-induced myocardial injury.
-
•
Mitochondrial energetics is an important mechanism to explore in the diabetic heart.
-
•
NAC protects against hyperglycemia-induced cardiomyocyte toxicity.
-
•
NAC improves mitochondrial energetics and enhances endogenous CoQ levels.
-
•
CoQ supports the process of bioenergetics in addition to its antioxidant activities.
Abbreviations: ATP, adenosine triphosphate; CoQ9/10, Coenzyme Q9/10; DCFH-DA, dichlorofluorescein diacetate; DMEM, Dulbecco’s Modified Eagle’s Medium; ECAR, extracellular acidification rates; FBS, fetal bovine serum; HPLC, high-performance liquid chromatograph; PBS, Phosphate buffered saline; MET, metformin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NAC, N-acetyl cysteine; ROS, reactive oxygen species
Keywords: N-Acetyl cysteine, Coenzyme Q, Diabetes, Hyperglycemia, Mitochondrial energetics, Reactive oxygen species
Abstract
The diabetic heart has been linked with reduced endogenous levels of coenzyme Q9/10 (CoQ), an important antioxidant and component of the electron transport chain. Although CoQ has displayed cardioprotective potential in experimental models of diabetes, the impact of N-acetyl cysteine (NAC) on mitochondrial energetics and endogenous levels of CoQ remains to be clarified. To explore these effects, high glucose-exposed H9c2 cardiomyocytes were used as an experimental model of hyperglycemia-induced cardiac injury. The results showed that high glucose exposure caused an increased production of reactive oxygen species (ROS), which was associated with impaired mitochondrial energetics as confirmed by a reduction of maximal respiration rate and depleted ATP levels. These detrimental effects were consistent with significantly reduced endogenous CoQ levels and accelerated cell toxicity. Although metformin demonstrated similar effects on mitochondrial energetics and cell viability, NAC demonstrated a more pronounced effect in ameliorating cytosolic and mitochondrial ROS production. Interestingly, the ameliorative effects of NAC against hyperglycemia-induced injury were linked with its capability to enhance endogenous CoQ levels. Although such data are to be confirmed in other models, especially in vivo studies, the overall findings provide additional evidence on the therapeutic mechanisms by which NAC protects against diabetes-induced cardiac injury.
1. Introduction
Reactive oxygen species (ROS)-mediated myocardial injury is a widely reported phenomenon in the diabetic state [[1], [2], [3]]. Chronically elevated ROS levels can drive several pathological conditions by depleting intracellular antioxidant defence systems [4]. In both humans and animals with diabetes mellitus, enhanced generation of oxidative stress due to excess ROS production has been associated with depleted glutathione (GSH) levels [5,6]. This consequence has been shown to be even worse in patients with microvascular complications [7], which could explain the accelerated myocardial injury in a diabetic state [8]. Alternatively, N-acetylcysteine (NAC) is a thiol containing antioxidant that is being investigated for its diverse therapeutic capabilities [9,10]. NAC is known to act broadly by neutralizing free radical-induced cellular injury and blocking excess ROS production [11]. Most importantly, NAC is a known precursor for GSH synthesis, and thus it could play a major role in ameliorating diabetes associated complications, especially those linked with excess generation of oxidative stress.
Indeed, increasing studies [[12], [13], [14]], including reviews from our group [9,10], have demonstrated that NAC displays a strong potential to attenuate a diverse group of metabolic complications, including hyperglycemia-induced myocardial injury. For example, NAC attenuates oxidative stress and cardiac remodeling in rats during transition from compensated left ventricular hypertrophy to heart failure [14]. NAC could also alleviate palmitic acid-induced myocardial apoptosis by blocking excess mitochondrial ROS production in cultured neonatal rat cardiomyocytes [15]. In fact, impaired mitochondrial energetics that is usually accompanied by excess ROS production is recognized as the key feature implicated in diabetes-induced myocardial injury [2,16,17]. Moreover, other critical components of the mitochondrial electron transport chain such as coenzyme Q9/10 (CoQ) that are essential in the process of bioenergetics have been shown to be compromised in the diabetic heart [18]. Beyond its importance in bioenergetics, CoQ remains a significant antioxidant able to protect the myocardium against oxidative stress-induced injury [19]. Interestingly, although NAC has displayed partial cardioprotective effects in experimental models of diabetes [10], the impact of NAC on mitochondrial energetics or endogenous levels of CoQ remains mostly unknown. Thus, the current study explored such effects in high glucose-exposed H9c2 cardiomyocytes as an experimental model of hyperglycemia-induced cardiac injury.
2. Materials and methods
2.1. Chemicals and reagents
Embryonic ventricular rat heart derived H9c2 cardiomyoblasts (CRL-1446) were from American Type Culture Collection (Manassas, USA); Penicillin-Streptomycin, Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and Phosphate Buffered Saline (PBS) were from Carlo Erba (Milano, Italy); dichlorfluorescein diacetate (DCFH-DA) and MitoSOX fluorescent probes were from ThermoFisher Scientific (Waltham, USA); Bradford and RC DC protein assay kit was from Bio-Rad Laboratories (Hercules, USA); ViaCount was from Merck-Millipore (Darmstadt, Germany). Seahorse XF96 microplate plates, Seahorse XF Assay media, Seahorse XF media and Mito Stress Kits were from Agilent (Santa Clare, USA). All other chemicals including NAC and metformin were obtained from Sigma-Aldrich (St. Louis, USA).
2.2. Cell culture and treatment conditions
H9c2 cardiomyoblasts were cultured in DMEM supplemented with 10% FBS, 100 μg/mL penicillin and 100 μg/mL streptomycin overnight under standard tissue culture conditions (37 °C in humidified air and 5% CO2). Cells were cultured at a seeding density of 25 000 cells/well in 24 multi-well plates, and maintained in growth media until confluency, while medium was refreshed every second day. Confluent H9c2 cardiomyoblasts were exposed to 33 mM glucose (high glucose) for 24 h before treatment for 6 h with NAC or metformin at 1 mM and 1 μM, respectively. Cells exposed to 5.5 and 33 mM glucose concentrations served as controls for normoglycemic and hyperglycemic states, respectively. All doses for treatment compounds and experimental controls were based on previously published studies [5,20].
2.3. Cytosolic and mitochondrial ROS detection assays
Cytosolic and mitochondrial ROS production was evaluated using flow-cytometry by means of DCFH-DA and MitoSOX fluorescent dyes, as per already described methods [[21], [22], [23]]. Briefly, treated cardiomyocytes or untreated control cells were incubated for 30 min or 15 min in a solution 5 μM of DCFH-DA or MitoSOX dye, respectively at 37 °C. After trypsinization, washed cells were analyzed on a Guava Easycite flow cytometer (Millipore; Darmstadt, Germany). Most importantly, flow-cytometry analysis of cytosolic ROS production was done together with cell viability assay using ViaCount (described below in Section 2.6), and this was based on single cell analysis for optimal estimation of ROS in viable cells.
2.4. Assessment of mitochondrial bioenergetics
Oxygen consumption rate (OCR) was measured using the XF96 Extracellular Flux analyzer (Agilent Technologies; Santa Clare, USA), as previously described [24]. Parameters that were measured included basal oxygen consumption rate, ATP production, maximal respiration rate, and spare capacity. OCR (pmol/min) was normalized with protein content, whereas OCR was reported as absolute rates (pmoles/min for OCR and mpH/min for ECAR/ mg protein).
2.5. CoQ quantification and oxidation status
The intracellular levels of CoQ9, which is the predominant form of ubiquinone in murine models [25], as well as the CoQ10, which is the well-known and predominant form in humans were quantified from treated H9c2 cardiomyocytes based on a previously described method [26]. Briefly, 50 μl cell suspension, approximately 200,000 cells, were extracted adding 250 μl isopropanol and vigorously mixing before centrifugation at 20,900 g for 2 min at 4 °C. Thereafter, extracts were injected into a high-performance liquid chromatograph (HPLC) with electro-chemical detector (ECD) (Shiseido Co. Ltd.; Tokyo, Japan). This system is characterized by a post-separation reducing column (Shiseido CQR) that is capable of fully reducing eluted ubiquinone. CoQ9/10 oxidative status was expressed as percentage of ubiquinone/total CoQ9/10, while total CoQ9/10 content in H9c2 cardiomyocytes was expressed as μg/mg CoQ9/10 protein.
2.6. Cell viability and metabolic activity assays
Viability was estimated flow-cytometry using Millipore™ Guava ViaCount Reagent cytotoxicity test as previously reported [21]. Briefly, the assay makes use of a mixture of cell membrane permeable and impermeable DNA-binding fluorescent probes, discriminated by red and yellow color, diluted 1:10 in PBS and used to stain approximately 20,000 cells immediately before reading. The analysis of the distribution allows the discrimination of the percentage of cell debris (R−/Y−), live cells (R+/Y−) and dead cells (R+/Y+). In addition, cell viability was confirmed by MTT using a previously described method [27]. The MTT assay uses colorimetric measurement of tetrazolium dye (MTT) conversion to its insoluble formazan product to estimate metabolic activity of viable cells. MTT absorbance was read at 570 nm using a BioTek ELx800 plate reader (Winooski, USA) and Gen 5 software for data acquisition.
2.7. Statistical analysis
Results were expressed as the mean of three independent biological experiments with each experiment containing at least three technical replicates. GraphPad Prism software version 6.0 (GraphPad Software, Inc., La Jolla, USA) was used to analyze data. Comparisons between groups were performed using one-way multivariate ANOVA followed by a Tukey post hoc test and Student t-test where appropriate. A p-value of ≤0.05 was considered statistically significant.
3. Results
3.1. NAC ameliorates excess ROS production in high glucose-exposed cardiomyocytes
The hyperglycemic state is consistently linked with excessive cytosolic and mitochondrial ROS production, and this may lead to the generation of oxidative stress and subsequent cellular damage. The current findings showed that exposing H9c2 cardiomyocytes to high glucose as a model of hyperglycemia caused a significant increase (p < 0.001) in cytosolic and mitochondrial ROS production when compared to the normal glucose control (Fig. 1A, B). Treatment with NAC showed a more pronounced effect (p ≤ 0.01) than metformin (p < 0.05) in reducing excess ROS production (Fig. 1A, B).
Fig. 1.
The impact of N-acetyl cysteine (NAC) treatment on mitochondrial and cytosolic production of reactive oxygen species (ROS) in cultured H9c2 cardiomyocytes. Briefly, H9c2 cardiomyocytes were exposed to 33 mM high glucose (HG) for 24 h before treatment with NAC or metformin (MET) at a dose 1 μM for 6 h. Graphs cytosolic (A) and mitochondrial (B) ROS production. Results are expressed as the mean of at least two independent experiments. ***p < 0.001 depicts statistical significance versus normal glucose (NG) control (5.5 mM). #p < 0.05, ##p ≤ 0.01 statistical significance versus HG control.
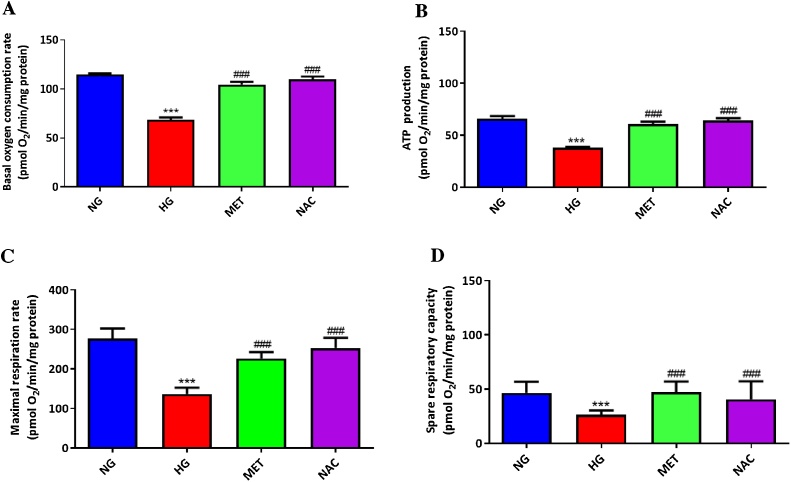
3.2. NAC improves mitochondrial energetics in high glucose-exposed cultured cardiomyocytes
A competent mitochondrial energetic process is essential for normal myocardial function. High glucose-exposed cardiomyocytes demonstrated compromised mitochondrial energetics, as depicted by markedly reduced oxygen consumption rate that was accompanied by suppressed ATP production and spare respiratory capacity, when compared to cells treated with normal glucose (Fig. 2 A–D). Treatment with NAC showed consistent results (p ≤ 0.001) with metformin (p ≤ 0.001) in improving mitochondrial energetics (Fig. 2A–D).
Fig. 2.
The impact of N-acetyl cysteine (NAC) treatment on mitochondrial energetics in cultured H9c2 cardiomyocytes. Briefly, H9c2 cardiomyocytes were exposed to 33 mM high glucose (HG) for 24 h before treatment with NAC or metformin (MET) at a dose 1 μM for 6 h. Graphs depict basal oxygen consumption rate (A), ATP production (B), maximal respiration rate (C), and spare capacity (D). Results are expressed as the mean of at least two independent experiments. ***p < 0.001 depicts statistical significance versus normal glucose (NG) control (5.5 mM). ###p ≤ 0.001 statistical significance versus HG control.
3.3. NAC enhances endogenous CoQ levels in high glucose-exposed cultured cardiomyocytes
In addition to its antioxidant properties, CoQ plays an important role in the process of mitochondrial energetics by acting as an electron carrier during oxidative phosphorylation. Although as expected that CoQ9 levels were relatively higher than that of CoQ10 in these murine derived cardiomyocytes, high glucose exposure showed consistent effect (p < 0.05) in reducing the endogenous levels for both forms of this enzyme, when compared to normal glucose treated cells (Fig. 3A, B). Interestingly, only treatment with NAC, not metformin, showed significant enhancement of endogenous CoQ levels after high glucose exposure (Fig. 3A, B). However, it was evident that treatment with neither NAC nor metformin influenced the oxidation status of CoQ in these H9c2 cardiomyocytes (Fig. 3C, D).
Fig. 3.
The impact of N-acetyl cysteine (NAC) treatment on endogenous levels and oxidation status of Coenzyme Q9/10 (CoQ9/10) in cultured H9c2 cardiomyocytes. Briefly, H9c2 cardiomyocytes were exposed to 33 mM high glucose (HG) for 24 h before treatment with NAC or metformin (MET) at a dose 1 μM for 6 h. Graphs depict CoQ9 (A) and CoQ10 (B) content, as well as oxidation status of CoQ9 (C) and CoQ10 (D). Results are expressed as the mean of at least two independent experiments. *p < 0.05 depicts statistical significance versus normal glucose (NG) control (5.5 mM). ##p ≤ 0.01 statistical significance versus HG control.
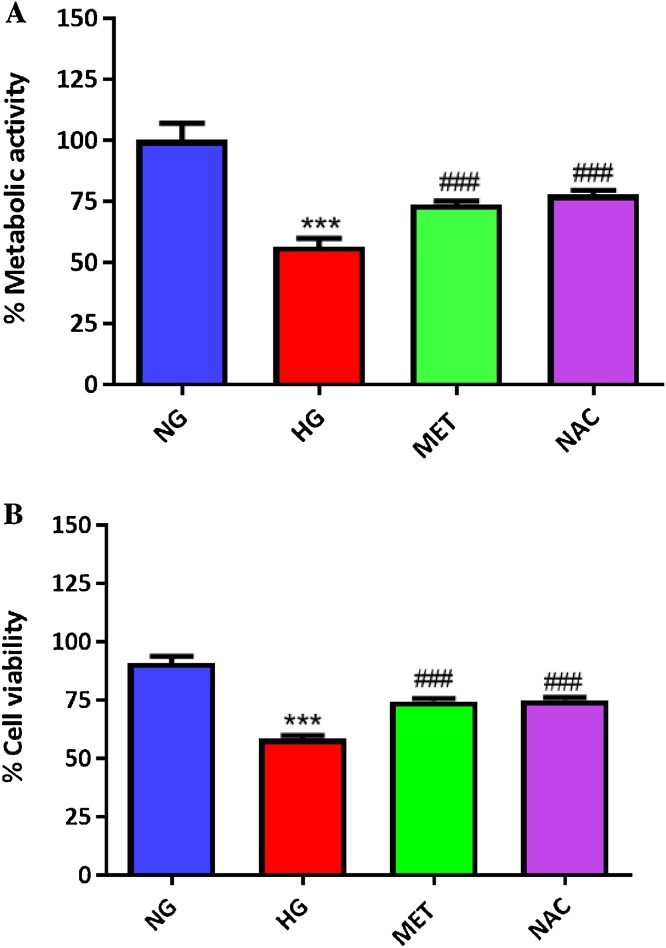
3.4. NAC improves metabolic activity and cell viability in high glucose-exposed cultured cardiomyocytes
Enhanced cardiomyocyte damage due to excess ROS production is a well-known consequence in a diabetic state. Exposure of H9c2 cardiomyocytes to high glucose significantly reduced both metabolic activity (p ≤ 0.001) and cell viability (p ≤ 0.001), when compared to normal glucose treated cells (Fig. 4A, B). Treatment with NAC and metformin demonstrated similar effect (p ≤ 0.001) in improving metabolic activity and cell viability of high glucose-exposed cardiomyocytes (Fig. 4A, B).
Fig. 4.
The impact of N-acetyl cysteine (NAC) treatment on metabolic activity and cell viability of cultured cardiomyocytes. Briefly, H9c2 cardiomyocytes were exposed to 33 mM high glucose (HG) for 24 h before treatment with NAC or metformin (MET) at a dose 1 μM for 6 h. Graphs depict metabolic activity (A) and cell viability (B) assays. Results are expressed as the mean of at least two independent experiments. ***p ≤ 0.001 depicts statistical significance versus normal glucose (NG) control (5.5 mM). ###p ≤ 0.001 statistical significance versus HG control.
4. Discussion
It is currently known that cardiovascular complications are the leading cause of death in diabetic patients [28]. Independently of atherosclerosis buildup, it has been found that hyperglycemia could greatly impact cardiac efficiency by accelerating oxidative stress-induced myocardial injury, leading to heart failure and reduced life expectancy [28,29]. For this reason, much research has focused on the exploration of compounds with antioxidant activity to attenuate oxidative stress and improve cardiac function in the diabetic state [10,30]. Due to its known antioxidant properties, NAC is increasingly explored for its cardioprotective effects against diabetes-associated complications, especially the targeting of oxidative stress-induced cellular injury [10]. Although some progress has been made in identifying therapeutic mechanisms associated with the cardioprotective effects of NAC in experimental models of diabetes [10], less is known regarding its influence on mitochondrial function under hyperglycemic conditions. Thus, acknowledging the contribution of impaired mitochondrial function to increased ROS production and oxidative stress, the current study explored the impact of NAC on maintaining cellular viability, and improving bioenergetics under hyperglycemic conditions in cultured H9c2 cardiomyocytes.
Over the years, high glucose-exposed H9c2 cardiomyocytes have become a reliable model to study hyperglycemia-associated pathological abnormalities [20,31]. This experimental model has become even more relevant for assessing the direct association between the hyperglycemic state and accelerated oxidative stress-induced injury, while excluding other cardiovascular complications such as atherosclerosis [20,31]. Certainly, exposing H9c2 exposed to elevated glucose concentrations for 24 h in the current study was associated with enhanced cytosolic and mitochondrial ROS production, that was accompanied by the impaired bioenergetics. The latter could be determined by measuring basal respiration, ATP production, maximal respiration, and spare respiratory capacity after cardiomyocytes were incubated with high glucose. Subsequently, a clear trend showing suppressed mitochondrial respiration could be identified by measuring these mitochondrial energetic factors, further suggesting that compromised integrity of the electron transport chain plays a significant role in the production of ROS and subsequent cellular injury. Supporting these findings are others showing that impaired oxidative phosphorylation capacity in experimental models of metabolic syndrome is directly implicated in oxidative stress-induced myocardial injury [2,16,32,33]. Nevertheless, although intracellular antioxidants were not measured in the current study, it was clear that exposure of cardiomyocytes to hyperglycemia prominently affected cellular adaptive responses against oxidative stress, as demonstrated by significant reduction in endogenous CoQ levels. In this case, supplementation drug agents that strengthen intracellular antioxidant responses, including increasing endogenous CoQ levels remains a viable option to protect against diabetes-induced cardiac injury.
Interestingly, administration of NAC, together with CoQ10 and superoxide dismutase mimetic has already been shown to prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes [34]. Relevant to the heart, NAC and CoQ10 have been demonstrated to be effective in attenuating impaired myocardial energy expenditure and oxidative stress, induced by carbon tetrachloride intoxication in rats [35]. Consistently with CoQ administration, NAC displays the potential to ameliorate oxidative stress-induced toxicity by improving mitochondrial function in the diabetic state. NAC has already been shown to enhance intracellular antioxidants such as GSH to protect against hyperglycemia-induced cardiomyocyte damage [5]. Thus, although intracellular antioxidants like GSH were not tested in the current study, it was clear that NAC could protect H9c2 cardiomyocytes against hyperglycemia-linked oxidative stress by improving endogenous CoQ levels and mitochondrial energetics. In fact, novel findings from this study suggest that enhancement of endogenous CoQ levels may be an important mechanism by which NAC attenuates hyperglycemia-induced cardiomyocyte toxicity. These results were concomitant to improved metabolic activity, cell viability and ATP production of cardiomyocytes under hyperglycemic conditions. Metformin, a well-known anti-diabetic agent, also showed cardioprotective effects similar to NAC, however it failed to significantly impact the endogenous CoQ levels, suggesting its limited efficacy to efficiently trigger other adaptive responses that are important to protect against hyperglycemia-associated complications. CoQ is known to to play a key role in the process of mitochondrial bioenergetics, actively participating in aerobic cellular respiration, thereby contributing to effective generation of energy in the form of ATP, and also maintenance of redox status of cells [19,23]. Overall, although did not impact the CoQ oxidative status, a phenomenon that has to be further explored, the current findings suggest that NAC administration could be an effective therapy to improve cardiac function under diabetic conditions by enhancing adaptive responses against oxidative stress, especially enhancement of endogenous CoQ levels. However, before such hypothesis is accepted, it is necessary to confirm these preliminary results in other experimental models, especially in vivo settings.
5. Conclusion
Accelerated oxidative stress associated with impaired mitochondrial function is one of the prominent mechanisms implicated in hyperglycemia-induced cardiac injury. In fact, due to its contribution to excess ROS production, impaired mitochondrial energetics represents an important mechanism to be explored to understand the impact of hyperglycemia on the heart. This study provides important new information regarding the protective potential of NAC against hyperglycemia-induced cardiomyocyte injury, decreased mitochondrial energetics and endogenous CoQ levels. We propose that enhancement of endogenous levels of CoQ may be an important mechanism by which NAC improves mitochondrial function under hyperglycemic conditions, and thus ameliorate oxidative stress-linked myocardial toxicity. However, the missing link may be to understand the mechanism by which NAC could impact mitochondrial function by enhancing endogenous CoQ levels, and for this perhaps functional studies are required that target CoQ biosynthesis pathways. It also remains important to know how the combination of metformin and NAC could compare to the use of each compound as a monotherapy.
Funding statement
This work was supported in part by baseline funding from the Biomedical Research and Innovation Platform of the South African Medical Research Council (SAMRC) and the National Research Foundation (Grant number: 117829). PV Dludla was partially supported as a Post-Doctoral Fellow by funding from the SAMRC through its division of Research Capacity Development under the Intra-Mural Postdoctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and do not necessary represent the official views of the SAMRC or the funders.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Boudina S., Abel E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010;11(1):31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bugger H., Abel E.D. Mitochondria in the diabetic heart. Cardiovasc. Res. 2010;88(2):229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh K., Bernardo B.C., McMullen J.R., Ritchie R.H. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014;142(3):375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dludla P.V., Muller C.J., Louw J., Joubert E., Salie R., Opoku A.R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomedicine. 2014;21(5):595–601. doi: 10.1016/j.phymed.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong J.S., Steinauer K.K., Hornung B., Irish J.M., Lecane P., Birrell G.W. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9(3):252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 7.Lutchmansingh F.K., Hsu J.W., Bennett F.I., Badaloo A.V., McFarlane-Anderson N., Gordon-Strachan G.M. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mapanga R.F., Essop M.F. Damaging effects of hyperglycemia on cardiovascular function: spotlight on glucose metabolic pathways. Am. J. Physiol. Heart Circ. Physiol. 2016;310(2):H153–73. doi: 10.1152/ajpheart.00206.2015. [DOI] [PubMed] [Google Scholar]
- 9.Dludla P.V., Mazibuko-Mbeje S.E., Nyambuya T.M., Mxinwa V., Tiano L., Marcheggiani F. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: a systematic review of pre-clinical studies. Pharmacol. Res. 2019;146 doi: 10.1016/j.phrs.2019.104332. [DOI] [PubMed] [Google Scholar]
- 10.Dludla P.V., Dias S.C., Obonye N., Johnson R., Louw J., Nkambule B.B. A systematic review on the protective effect of N-acetyl cysteine against diabetes-associated cardiovascular complications. Am. J. Cardiovasc. Drugs. 2018;18(4):283–298. doi: 10.1007/s40256-018-0275-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun S.Y. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 2010;9(2):109–110. doi: 10.4161/cbt.9.2.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasupathy S., Tavella R., Grover S., Raman B., Procter N.E.K., Du Y.T. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in acute myocardial infarction]) Circulation. 2017;136(10):894–903. doi: 10.1161/CIRCULATIONAHA.117.027575. [DOI] [PubMed] [Google Scholar]
- 13.Kaga A.K., Barbanera P.O., do Carmo N.O.L., Rosa L.R.O., Fernandes A.A.H. Effect of N-acetylcysteine on dyslipidemia and carbohydrate metabolism in STZ-induced diabetic rats. Int. J. Vasc. Med. 2018;2018 doi: 10.1155/2018/6428630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes D.R.A., Gomes M.J., Rosa C.M., Pagan L.U., Damatto F.C., Damatto R.L. N-Acetylcysteine influence on oxidative stress and cardiac remodeling in rats during transition from compensated left ventricular hypertrophy to heart failure. Cell. Physiol. Biochem. 2017;44(6):2310–2321. doi: 10.1159/000486115. [DOI] [PubMed] [Google Scholar]
- 15.He Y., Zhou L., Fan Z., Liu S., Fang W. Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by Nacetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress. Cell Death Dis. 2018;9(5):568. doi: 10.1038/s41419-018-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudina S., Sena S., Theobald H., Sheng X., Wright J.J., Hu X.X. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56(10):2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 17.Ansley D.M., Wang B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013;229(2):232–241. doi: 10.1002/path.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucharska J., Braunova Z., Ulicna O., Zlatos L., Gvozdjakova A. Deficit of coenzyme Q in heart and liver mitochondria of rats with streptozotocin-induced diabetes. Physiol. Res. 2000;49(4):411–418. [PubMed] [Google Scholar]
- 19.Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol. Biotechnol. 2007;37(1):31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R., Dludla P., Joubert E., February F., Mazibuko S., Ghoor S. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016;60(4):922–934. doi: 10.1002/mnfr.201500656. [DOI] [PubMed] [Google Scholar]
- 21.Bruge F., Damiani E., Marcheggiani F., Offerta A., Puglia C., Tiano L. A comparative study on the possible cytotoxic effects of different nanostructured lipid carrier (NLC) compositions in human dermal fibroblasts. Int. J. Pharm. 2015;495(2):879–885. doi: 10.1016/j.ijpharm.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Orlando P., Silvestri S., Ferlizza E., Andreani G., Carpene E., Falcioni G. Biochemical responses to cadmium exposure in Oncorhynchus mykiss erythrocytes. Ecotoxicol. Environ. Saf. 2017;145:476–482. doi: 10.1016/j.ecoenv.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Marcheggiani F., Cirilli I., Orlando P., Silvestri S., Vogelsang A., Knott A. Modulation of Coenzyme Q10 content and oxidative status in human dermal fibroblasts using HMG-CoA reductase inhibitor over a broad range of concentrations. From mitohormesis to mitochondrial dysfunction and accelerated aging. Aging. 2019;11(9):2565–2582. doi: 10.18632/aging.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazibuko-Mbeje S.E., Dludla P.V., Johnson R. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licitra F., Puccio H. An overview of current mouse models recapitulating coenzyme q10 deficiency syndrome. Mol. Syndromol. 2014;5(3–4):180–186. doi: 10.1159/000362942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvestri S., Orlando P., Armeni T., Padella L., Bruge F., Seddaiu G. Coenzyme Q10 and alpha-lipoic acid: antioxidant and pro-oxidant effects in plasma and peripheral blood lymphocytes of supplemented subjects. J. Clin. Biochem. Nutr. 2015;57(1):21–26. doi: 10.3164/jcbn.14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dludla P.V., Jack B., Viraragavan A., Pheiffer C., Johnson R., Louw J. A dose-dependent effect of dimethyl sulfoxide on lipid content, cell viability and oxidative stress in 3T3-L1 adipocytes. Toxicol. Rep. 2018;5:1014–1020. doi: 10.1016/j.toxrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Heart Association. Cardiovascular disease and diabetes. Available at https://www.heart.org/en/health-topics/diabetes/why-diabetes-matters/cardiovascular-disease--diabetes. (Accessed 20 July 2019).
- 29.Rubler S., Dlugash J., Yuceoglu Y.Z., Kumral T., Branwood A.W., Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972;30(6):595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 30.Ji L., Liu F., Jing Z., Huang Q., Zhao Y., Cao H. MICU1 alleviates diabetic cardiomyopathy through mitochondrial Ca(2+)-dependent antioxidant response. Diabetes. 2017;66(6):1586–1600. doi: 10.2337/db16-1237. [DOI] [PubMed] [Google Scholar]
- 31.Sun X., Chen R.C., Yang Z.H., Sun G.B., Wang M., Ma X.J. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Fillmore N., Mori J., Lopaschuk G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014;171(8):2080–2090. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heather L.C., Clarke K. Metabolism, hypoxia and the diabetic heart. J. Mol. Cell. Cardiol. 2011;50(4):598–605. doi: 10.1016/j.yjmcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez R., Ferrin G., Hidalgo A.B., Ranchal I., Lopez-Cillero P., Santos-Gonzalez M. N-Acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes. Chem. Biol. Interact. 2009;181(1):95–106. doi: 10.1016/j.cbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Elbaky N.A.A., El-Orabi N.F., Fadda L.M., Abd-Elkader O.H., Ali H.M. Role of N-acetylcysteine and coenzyme Q10 in the amelioration of myocardial energy expenditure and oxidative stress, snduced by carbon tetrachloride intoxication in rats. Dose-Response. 2018;16(3) doi: 10.1177/1559325818790158. [DOI] [PMC free article] [PubMed] [Google Scholar]