Highlights
-
•
Connectivity analyses complemented with a metric exploring switching in brain activity.
-
•
Lower insula-salience connectivity predicts insufficient antidepressant response.
-
•
This same insula region is activated less when switching from task to a rest.
-
•
This could be a potential biomarkers for predicting future antidepressant response.
Keywords: Depression, Resting state functional connectivity, Insula, Salience, fMRI, Insufficient antidepressant response
Abbreviations: MDD, major depressive disorder; RS-FC, Resting-state functional connectivity; DMN, default mode network; CCN, cognitive control network; TPN, task positive network; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; TRD, treatment resistant depression; NESDA, Netherlands Study on Depression and Anxiety; CIDI, Composite Interview Diagnostic Instrument; IDS, Inventory of Depressive Symptomatology; AD, antidepressants; BAI, Beck Anxiety Inventory; ICA, independent component analysis; SVC, small volume correction
Abstract
Insufficient response to treatment is the main cause of prolonged suffering from major depressive disorder (MDD). Early identification of insufficient response could result in faster and more targeted treatment strategies to reduce suffering. We therefore explored whether baseline alterations within and between resting state functional connectivity networks could serve as markers of insufficient response to antidepressant treatment in two years of follow-up. We selected MDD patients (N = 17) from the NEtherlands Study of Depression and Anxiety (NESDA), who received ≥ two antidepressants, indicative for insufficient response, during the two year follow-up, a group of MDD patients who received only one antidepressant (N = 32) and a healthy control group (N = 19) matched on clinical characteristics and demographics. An independent component analysis (ICA) of baseline resting-state scans was conducted after which functional connectivity within the components was compared between groups. We observed lower connectivity of the right insula within the salience network in the group with ≥ two antidepressants compared to the group with one antidepressant. No difference in connectivity was found between the patient groups and healthy control group. Given the suggested role of the right insula in switching between task-positive mode (activation during attention-demanding tasks) and task-negative mode (activation during the absence of any task), we explored whether right insula activation differed during switching between these two modes. We observed that in the ≥2 antidepressant group, the right insula was less active compared to the group with one antidepressant, when switching from task-positive to task-negative mode than the other way around. These findings imply that lower right insula connectivity within the salience network may serve as an indicator for prospective insufficient response to antidepressants. This result, supplemented by the diminished insula activation when switching between task and rest related networks, could indicate an underlying mechanism that, if not sufficiently targeted by current antidepressants, could lead to insufficient response. When replicated, these findings may contribute to the identification of biomarkers for early detection of insufficient response.
1. Introduction
Major depressive disorder (MDD) is a highly prevalent and disabling disease (Mathers and Loncar, 2006), however, its etiology and pathophysiology remain an enigma. The main indicator of prolonged suffering of MDD is an insufficient response to different (classes of) antidepressants (Ruhe et al., 2006) which is associated with chronic depression, long-term hospitalization(s), work absenteeism, suicide and high financial costs (Gibson et al., 2010). Early prediction of non-response to standard treatment will result in faster and more targeted treatment strategies and reduce suffering. Despite promising results in predicting antidepressant treatment outcomes based on demographic and clinical variables (Iniesta et al., 2016; Novick et al., 2015; Uher et al., 2012), early prediction of non-response with clinical data only has appeared, to some extent, to be unreliable (Fekadu et al., 2009). Therefore, biological pre-treatment markers are needed. Specific alterations in neurocircuitries indicating insufficient response could provide such markers.
Resting-state functional connectivity (RS-FC) provides a basis for understanding neurocircuitries involved in the pathophysiology of MDD (Greicius, 2008; Hamilton et al., 2015; Kaiser et al., 2015; Kuhn and Gallinat, 2013; Northoff et al., 2011; Wang et al., 2012). Abnormal functional connectivity in MDD has been found within the default mode network (DMN) (Hamilton et al., 2011; Manoliu et al., 2014; Sambataro et al., 2013), the salience network (Manoliu et al., 2014) and the cognitive control network (CCN) (Alexopoulos et al., 2012; Menon, 2011; Veer et al., 2010). The latter is also referred to as ‘task positive network’ (TPN). The DMN, also known as the task-negative network, consists of the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), precuneus and parietal cortex. Normally, the DMN is more active during rest and internal self-referential processing (Qin and Northoff, 2011), and is suppressed in the presence of an external task. Studies in MDD demonstrated an impaired de-activation during tasks (DMN-persistence), in association with rumination (an internally focused tendency to repetitively think about matters of distress) (Hamilton et al., 2011, 2015; Sambataro et al., 2013). The function of the salience network, encompassing the dorsal anterior cingulate cortex (dACC) and bilateral insula, appears to be important in the selection and segregation of relevant internal and external stimuli in order to guide behaviour (Menon and Uddin, 2010). Patients with MDD have shown aberrant RS-FC within the salience network and between the salience network and DMN. These aberrations are associated with a selection bias towards negative stimuli, characteristic for MDD (Manoliu et al., 2014). Finally, the CCN, involving the dorsolateral prefrontal (DLPFC) and posterior parietal cortex (PPC) (Seeley et al., 2007) is involved in attention, working memory and decision-making (i.e. important high-level cognitive processes) (Menon and Uddin, 2010). Decreased RS-FC within the CCN, associated with apathy and dysfunctional executive behavior, has been demonstrated in late-life MDD (Alexopoulos et al., 2012). Moreover, aberrant associations between the DMN and CCN have been related to severity of rumination (Hamilton et al., 2011; Manoliu et al., 2014).
Although substantial efforts to demonstrate alterations of resting state networks in MDD, RS-FC studies investigating insufficient treatment response and treatment resistant depression (TRD), defined as non-response to at least two antidepressants (Berlim and Turecki, 2007, Ruhe et al., 2012, Wijeratne and Sachdev, 2008), are scarce (Dichter et al., 2015). Using a seed-based approach, Lui et al. (2011) found reduced connectivity between prefrontal-limbic-thalamic areas in both TRD patients and non-TRD patients compared to healthy controls. This decrease was larger in the non-TRD patients (vs. TRD patients), especially between a left amygdala seed and the ACC and between a right insula seed and precuneus and ACC, indicating that (non-)response can be attributed to distinct functional deficits. Furthermore, Guo et al. (2013) demonstrated reduced RS-FC between the cerebellum and DMN in TRD vs. non-TRD. Moreover, decreased RS-FC between the DMN and CCN, and reduced RS-FC between the anterior and posterior DMN has been found in TRD (de Kwaasteniet et al., 2015). These observations show a wide range of regional alterations that can be associated to (insufficient) treatment response.
For the development of more targeted treatment strategies, clinicians should ideally be able to distinguish a future responder to antidepressants from patients needing several switches of antidepressants early during treatment. In the present study, as a proxy for insufficient treatment response, we therefore aimed to investigate, with an explorative approach, whether baseline alterations in neural connectivity were an indicator for a switch of antidepressants during two years of naturalistic follow-up.
2. Materials and methods
2.1. Participants
Participants were recruited from the multi-center naturalistic, observational and longitudinal Netherlands Study on Depression and Anxiety (NESDA) (Penninx et al., 2008) conducted at the University Medical Center Groningen, VU Medical Center of Amsterdam and Leiden University Medical Center. Participants were recruited through general practitioners, primary care, and specialized mental health institutions. After approval by medical ethical committees of all centers and written informed consent, participant data was collected during a baseline measurement (including the MRI scan), and at one and two year follow-up measurements. Inclusion and exclusion criteria for the total NESDA sample have been described by Penninx et al. (2008)
For the current analysis, MDD patients and healthy controls were selected from the NESDA-MRI sample (N = 301). See appendix A, section A.1 for additional inclusion and exclusion criteria regarding this sample. Resting-state scans were available for 248 participants. We first selected MDD patients with a diagnosis of MDD (based on the Composite Interview Diagnostic Instrument [CIDI]) in the month prior to the baseline interview or a diagnosis of MDD in the 6 months prior to baseline plus a current moderate illness severity (Inventory of Depressive Symptomatology [IDS]) score ≥ 24; (Rush et al., 2008) yielding 112 patients. Second, in order to include patients with comparable treatment needs, only patients receiving antidepressant treatment between baseline and two year follow-up were selected, resulting into 55 patients. Of these, two groups were identified. The first patient group was treated with: (i) ≥2 adequate trials of antidepressants (AD) during one episode between baseline and 2 year follow-up. Adequate treatment was defined as daily use of medication, for ≥4 weeks, with an adequate dose according to the Multidisciplinary guidelines for depression (Spijker et al., 2013), and (ii) ≤1 adequate antidepressant step at baseline to exclude existing treatment-resistance. We thus selected 17 patients (≥2 AD group) (see appendix A, Fig. A.1). The second patient group had only 1 adequate AD treatment in the two years of follow-up (1 AD group). This selection resulted in 38 patients. We subsequently matched the 1 AD group to the ≥2 AD group by discarding participants with extremes on baseline demographic and/or clinical characteristics (IDS scores, Beck Anxiety Inventory (BAI) scores, age, sex, education and scan location) until p > .2 (representing a power >0.95 with an effect size of 0.5, determined through a post-hoc 2-tailed distribution calculation by G*Power 3.1 software (Faul et al., 2009)). Matching resulted in a sample of 32 patients in the 1 AD group. Appendix A, Table A.1 and Table A.2 display treatment characteristics of both patient groups and co-medication used in combination with the antidepressants (≥2 AD group). In order to also obtain optimal demographic matching on age, sex, education and scan location between the patient groups and the healthy controls, we discarded demographic extremes until p > .2, (representing a power >0.95 with an effect size of 0.5, determined through a post-hoc 2-tailed distribution calculation by G*Power 3.1 software (Faul et al., 2009)). This resulted in a group of 19 of 41 healthy controls from the NESDA resting-state MRI sample with no lifetime depression or anxiety diagnosis.
2.2. Data acquisition
Resting-state scans, as part of a fixed imaging protocol, were acquired on a Philips 3.0-T MR-scanner at all scanning sites. During the RS-fMRI scan, participants were asked to keep their eyes closed, lie as still as possible and to stay awake. Duration of the RS-fMRI scan was 7.51 min. See appendix A, section A.2 for details regarding the scan parameters.
2.3. Analysis
2.3.1. Data preprocessing
Resting state fMRI images were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm); see appendix A, section A.3 for details regarding all preprocessing steps (Ashburner, 2007).
2.3.2. Demographic data
Independent samples t-tests, analyses of variance (ANOVA), χ2-tests and non-parametric Mann-Whitney U test were used to compare demographic and clinical variables between both patient groups and healthy controls. Because NESDA does not measure depression severity at frequent intervals during follow-up, in this naturalistic cohort study we had to rely on two IDS-measurements which were obtained separate from the initiation and evaluation of the prescribed antidepressants. In order to quantify group differences in depression severity, differences in IDS-SR-scores at the 2 annual follow-up measurements (time) were examined in a linear mixed model with main effects of group and time. This model has the advantage that it can handle unbalanced or missing data. Because, despite matching, we observed a non-significant difference in baseline IDS-SR-scores, we corrected the 2 follow-up measurements for differences in baseline depression-severity by adding baseline IDS scores as covariate (Pocock et al., 2002). This adjustment for possible baseline imbalance between treatment groups improves precision of the estimated treatment differences. In this model, a significant main effect for group indicates a general difference between the groups regarding the overall depressive symptomatology over the entire follow-up period, while a significant main effect for time indicates a general effect during the follow-up. A group*time interaction during these follow-up measurements would not be of primary interest in this analysis, because this term would only indicate whether a possible difference between the groups (with IDS scores as dependent) could be attributed more to follow-up year one or year two in either group. Analyses were performed with SPSS v22.0 (SPSS Inc., USA); statistical threshold was set to p < .05. We judged model-fit by Akaike's Information Criteria (AIC).
2.3.4. fMRI analysis
The Group ICA FMRI Toolbox (GIFT) (Calhoun et al., 2001) was used to perform an independent component analysis (ICA). See appendix A, section A.4 for details regarding all ICA settings. The individual image maps of components functionally relevant to our objective were used as input for separate second-level analyses. ANOVAs were first used to test main effects of group. Thereafter, pairwise comparisons were used to investigate differences between individual groups. In order to lower the chance of type-I errors when testing for multiple components, we applied a stringent false discovery rate (FDR) cluster threshold of p < .01, with an initial threshold of p < .001 uncorrected, and spatially masked with a effects of interest F-contrast (Veer et al., 2010). A Bonferroni correction was applied to account for multiple testing across six second level components, adjusting the p-value threshold to 0.05/6 = 0.0083. Because differences in the use of baseline ADs were present between both patient groups, we compared groups, while adding a covariate for baseline AD use. In order to check whether psychotherapy during follow-up influenced the results we also conducted an analysis including receipt of psychotherapy during follow-up as a covariate. Furthermore, as an additional precaution, we addressed the possibility that findings were driven by baseline severity by investigating whether an association between baseline severity measures and connectivity findings was present.
2.4. Post-hoc ROI based analysis
Based on our results, we conducted a post-hoc analysis based on a metric proposed by Hamilton et al. (2011) to explore whether our finding in the insula could be attributed to dysfunctional DMN-TPN switching. To warrant a more independent approach, since both analyses are conducted on the same sample, we therefore applied a seed based correlation over time to identify DMN and TPN maps instead of the ICA components in our primary analysis (see supplementary material of Hamilton et al., 2011).
Here we will give a brief overview of the analysis method based on the Hamilton metric (Hamilton et al., 2011), see appendix A, section A.5 for more details regarding all steps. We used the preprocessed data as described above. First, we used the preprocessed data and performed additional steps to address the possibility of signal artifacts in voxel time courses. Second, we extracted time course data from MPFC-PCC seeds for each participant (Talairach coordinates MPFC: −1, 47, −4, PCC: −5, −49, 41). Third, we identified DMN and TPN maps by correlating seed-region time-course data against whole brain time series. Of note, the original threshold used by Hamilton et al. (2011) (p < .000001) resulted in empty DMN and TPN maps, possibly due to scanner and site variability. Therefore, we applied a less stringent threshold in order to create DMN and TPN maps and investigate switching between these networks. Fourth, we examined activation in the right insula during switching from the TPN to the DMN, defined at initiations of ascent of DMN activity, (a TPN peak) and from the DMN to the TPN, defined at initiations of ascent of TPN activity (a DMN peak).
After that, first-level general linear models were estimated that included these TPN and DMN onsets regressors and the same noise regressors as used in step 1 (see appendix A, section A.5.1). Contrast images were calculated with DMN and TPN onsets separated and combined to explore insula activity during switching in general, and for DMN onsets > TPN onsets, and TPN onsets > DMN onsets to look at insula functioning differences between TPN to DMN transition and DMN to TPN transitions. On second level, between group differences in right insula activation for these contrasts were explored with an ANOVA. Because of the specific hypothesis regarding the insula, a small volume correction (SVC) was applied for this region. For mask creation we used the Automated Anatomical Labeling (AAL) mask of the right insula created with the WFU PickAtlas toolbox (Maldjian et al., 2003, 2004; Tzourio-Mazoyer et al., 2002) to prevent bias selection driven by ICA.
3. Results
3.1. Demographic and clinical variables
The three groups did not differ significantly in age, sex, years of education and scan location. Severity of depression (IDS score), anxiety (BAI score), illness duration, no. of episodes and receipt of psychotherapy at baseline were not significantly different between the two patient groups (Table 1). Medication use at baseline was significantly higher in the ≥2 AD group (X2(1) = 9.54, p < .001) as well as receipt of psychotherapy during follow-up (X2(1) = 7.50, p = .01).
Table 1.
Demographic and clinical characteristics of the ≥2 AD group, AD group and healthy controls at baseline.
| Treated with ≥2 AD (N = 17) | Treated with 1 AD (N = 32) | HC (N = 19) | Test-statistic | p | ||
|---|---|---|---|---|---|---|
| Age (years) | Mean (SD) | 38.88 (10.13) | 38.81 (11.59) | 39.58 (9.66) | F(2,65) = 0.03 | .97 |
| Sex | Male/Female | 9/8 | 11/21 | 10/9 | X2(2) = 2.33 | .31 |
| Education (years) | Mean (SD) | 11.12 (2.71) | 12.16 (2.93) | 12.32 (2.49) | F(2,65) = 1.03 | .36 |
| Scan location | A/L/G | 5/7/5 | 11/9/12 | 6/7/6 | X2(4) = 0.97 | .91 |
| Illness severity (IDS) baselinea | Mean (SD) | 32.53 (8.26) | 29.42 (9.38) | 4.00 (3.30) | t(44) = 1.01b | .28 |
| Anxiety severity baseline (BAI)a | Mean (SD) | 18.13 (8.77) | 14.94 (8.20) | 1.74 (2.62) | t(44) = 1.21b | .23 |
| Anxiety comorbidity | N (%) | 11 (64.7) | 18 (56.3) | – | X2(1) = 0.33 | .57 |
| Months illness | ||||||
| (in past 5 years) | Mean (SD) | 22.76 (14.16) | 25.03 (16.86) | – | U(47) = 263 | .85 |
| No. of episodes | Mean (SD) | 9.53 (11.47) | 6.19 (8.39) | – | U(47) = 216 | .21 |
| Medication steps | N (1/2/3/4) | 0/14/2/1 | 32/0/0/0 | – | X2(3) = 49.00 | .00 |
| Med use baseline | N (%) | 16 (94.1) | 16 (50.0) | – | X2(1) = 9.54 | .00 |
| Psychotherapy at baseline | Yes/no | 12/5 | 15/17 | – | X2(1) = 2.52 | .11 |
| Psychotherapy during follow-up | Yes/unknown | 16/1 | 18/14 | – | X2(1) = 7.50 | .01 |
HC Healthy Controls, IDS = Inventory of Depressive Symptomatology, FU = Follow-up, BAI = Beck Anxiety Inventory, A = Amsterdam, L = Leiden, G = Groningen.
≥2 AD sample N = 15, 1 AD sample N = 31.
Test-statistic based on difference between the 1 AD group and the ≥2 AD group.
3.2. Depression severity during follow-up
For IDS-SR-scores at year 1 or 2 of follow-up, group differences were observed, when tested in the mixed model (F1,41.43 = 3.93; p = .054), Furthermore, the mixed model revealed no main effect for time (p = .18), nor for the group*time interaction (p = .50), while correcting for baseline IDS-SR scores (see appendix A, Fig. A.2).
3.3. Independent component analysis
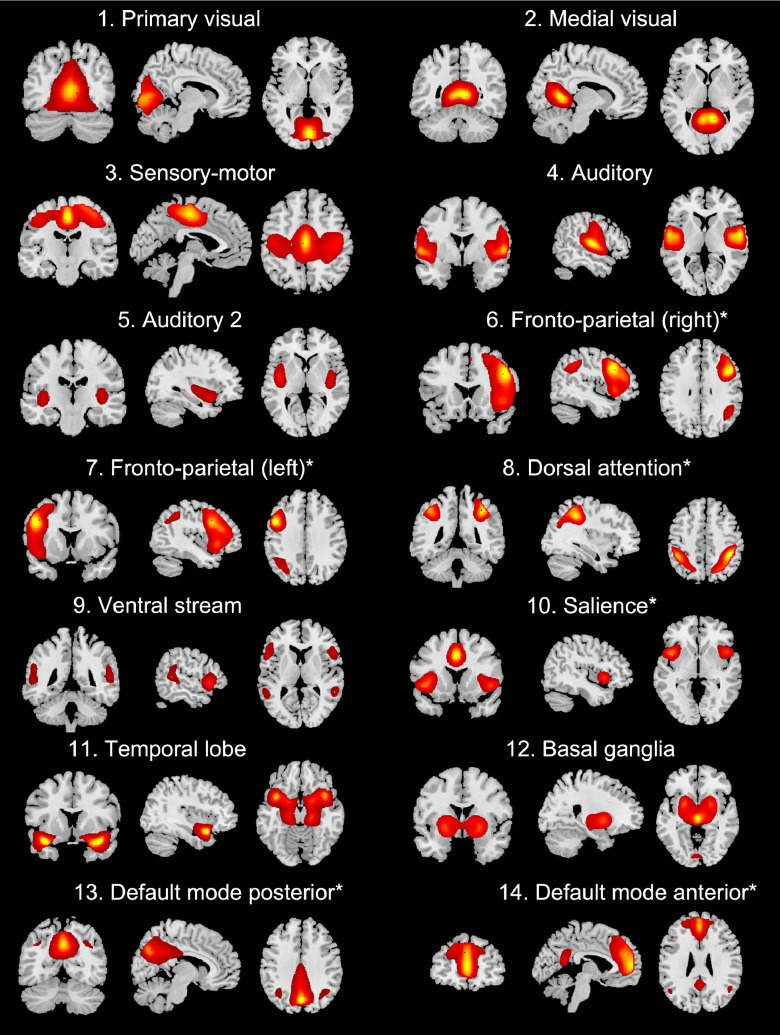
The Independent Component Analysis (GIFT) resulted in twenty-one temporally and spatially separated components. After discarding CSF and cerebellum components, fourteen components remained (Fig. 1), similar to previous reports (Allen et al., 2011; Damoiseaux et al., 2006; Veer et al., 2010). Six functionally relevant components were included as input for second level analyses: Fronto-parietal (right), Fronto-parietal (left), Dorsal attention, Salience, Default Mode posterior, Default Mode anterior. We discarded visual, sensory-motor, auditory components and other components of no interest.
Fig. 1.
Group ICA resting-state networks. The fourteen networks that were identified from the group ICA are shown. Images are z-statistics (ranging from 2 to 9) overlaid on a MNI-152 standard image. Asterisks (*) indicate components that were included in the second level analysis.
3.4. Group differences
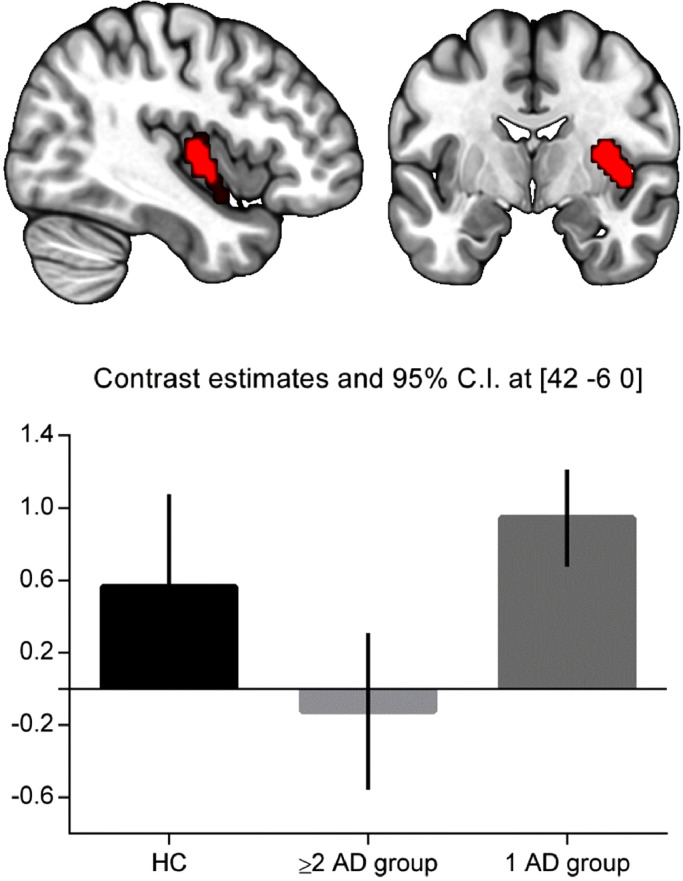
The one-way ANOVA revealed a main effect of group in the right insula within the salience component (F2,65 = 10.24, p = .003). No main effects of group were found for the other five components. Pairwise comparisons revealed lower connectivity within the salience network (right insula) in the ≥2 AD group compared to the 1 AD group (peak coordinates: x = 42, y = −6, z = 0; k = 97, Z = 3.86, pFDR = 0.007). No difference in connectivity was found when comparing the healthy controls with the 1 AD group or the ≥2 AD group. Visual inspection revealed that the right insula-salience connectivity in the healthy controls was intermediate between the ≥2 AD and 1 AD group (Fig. 2). The significant group difference remained after correcting for baseline AD use (Z = 4.89, pFDR = 0.005) and receipt of psychotherapy during follow-up (Z = 5.26, pFDR = 0.003). The analysis checking for a baseline association between severity measures and insula connectivity revealed no main effect of IDS (t46 = −0.56, p = .58), suggesting that our findings were not driven by severity (see appendix A, Fig. A.3).
Fig. 2.
Connectivity differences between groups. Top: right insula showing lower connectivity with the salience network in the ≥2 AD group compared to the 1 AD group (Z = 3.86, p = .007 FDR corrected on cluster-level). Figure displays cluster with initial threshold of p < .001 uncorrected. Bottom: Parameter estimates averaged across total insula cluster and 90% confidence intervals showing decreased connectivity of the insula within the salience network in the ≥2 AD group.
3.5. Post-hoc analysis
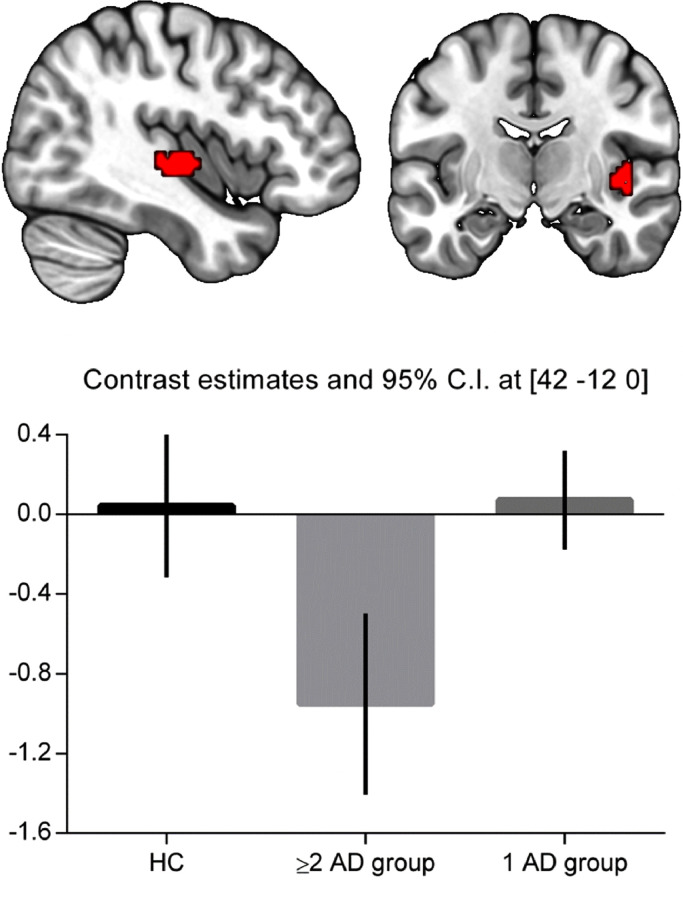
The ANOVA identified lower activation of the right insula (peak coordinates: x = 42, y = −12, z = 0; k = 36 voxels, Z = 4.00, pFDR = 0.008) in the ≥2 AD group compared to the 1 AD group at the moment of a switch to DMN compared to a switch to TPN (Fig. 3). No significant differences were observed in insula activity between the healthy controls and both patient groups on this contrast. Furthermore, no significant group differences were found for the contrasts DMN+TPN switch combined, DMN and TPN onsets separately, and TPN>DMN.
Fig. 3.
Post-hoc analysis: Between-group comparison during switching. Top: right insula showing decreased activity in the ≥2 AD group compared to the 1 AD group for the contrast DMN onsets > TPN onsets (Z = 4.00, p = .008 FDR corrected on cluster-level). Figure displays cluster with initial threshold of p < .001 uncorrected. Bottom: Parameter estimates and 90% confidence intervals showing decreased activity of the insula in the ≥2 AD group.
4. Discussion
The present study investigated whether distinct patterns of neural connectivity before treatment could serve as an indicator for the need of ≥2 antidepressants trials in MDD treatment. Our results revealed that decreased functional connectivity of the right insula with the salience network appears to be associated with prospective insufficient response. Post-hoc, in these patients requiring ≥2 antidepressant trials, this same right insula appeared to be activated less when switching to the DMN compared to switching to the TPN.
Previous neuroimaging studies investigated the association between insula functioning and depressive pathology (Sliz and Hayley, 2012), and showed that the insula plays an important role in MDD. Volume reductions of the insula have been observed in patients with current and remitted depression compared to healthy controls (Lee et al., 2011; Takahashi et al., 2010). Furthermore, the insula appeared to be hyperactive in MDD in response to negative stimuli (Hamilton et al., 2012; van Tol et al., 2012), and resting state studies have demonstrated decreased functional connectivity between the insula and the affective brain network (Hamilton et al., 2011; Veer et al., 2010) and decreased regional homogeneity (ReHo) in the insula (Liu et al., 2010; Yao et al., 2009) in MDD patients compared to healthy controls. Moreover decreased insula activation was related with symptom reduction in MDD (Opmeer et al., 2015). Our results contribute to these findings and suggest that altered insula functioning might be related to (prospective) non-response to antidepressants and potentially treatment resistance.
The insula is thought to mediate the ability to shift attention towards and away from emotional subjective feelings (empathy, happiness, love, anger, fear, sadness) through a joint activation with the ACC, which together form the salience network (Craig, 2009). Because dysfunctional emotion regulation has shown to play an important role in MDD (Rive et al., 2013), and reduced insula activation has been linked to a loss in the ability to experience emotions (Menon and Uddin, 2010), the observed altered salience network connectivity could also suggests that more persistent emotional dysregulation is associated with an insufficient response. These hypotheses need further empirical investigation in more rigorously controlled antidepressant trials in combination with fMRI.
Moreover, the right insular cortex plays an important role in switching between task negative DMN and TPN networks (Chang et al., 2013; Hamilton et al., 2011; Marchetti et al., 2012; Menon and Uddin, 2010; Sridharan et al., 2008). These networks have been proposed to be negatively correlated both in rest and during tasks (Marchetti et al., 2012). During rest people switch constantly between DMN and TPN activity, which is orchestrated by the right insula (Fox et al., 2005; Marchetti et al., 2012), with right insula activity preceding the DMN to TPN switch (Seeley et al., 2007). In MDD, it has been proposed that the DMN function is impaired in two ways: (i) in a rest-to-task transition, the DMN remains active when it should deactivate (DMN persistence/dominance), and (ii) the task positive network is deactivated when it should be active (TPN deficiency) (Marchetti et al., 2012). In our post-hoc analysis, supplementing our primary finding of decreased connectivity of the insula with the salience network in the ≥2 AD group, we found that in the ≥2 AD group the insula was less active relative to the 1 AD group especially when switching from TPN to DMN compared to switching from DMN to TPN activity. In the ≥2 AD group this lower activity suggests an easier switch to the DMN-mode, possibly resulting in DMN-persistence, which has been associated with treatment resistance before (Li et al., 2013). However, because insula activity was especially decreased when switching from TPN to DMN, this might also suggest that TPN-activity could not be maintained, resulting in more frequent deactivation of the TPN, which is indicative of TPN-deficiency, and has also been associated with treatment resistance (Groves et al., 2018). Like in previous reports (Figueroa et al., 2015; Hamilton et al., 2011,) patients and controls did not differ in percentages of activity of the DMN and TPN, for which we speculate that more advanced approaches (i.e. dynamic functional connectivity (Figueroa et al., 2019), investigating the durations/probabilities of more detailed FC-states) might be more sensitive.
Antidepressants are suggested to target DMN-persistence by reducing subgenual cingulate cortex, dorsal PCC and precuneus activation as well as to reduce TPN deficiency by increasing DLPFC and VLPFC function (Delaveau et al., 2011; Marchetti et al., 2012). However, we here speculate that if antidepressant treatment is targeting these DMN/TPN related regions only, in some subjects it might not interfere with the truly defective switching-hub, i.e. aberrant insula function. Consequently, in some depressed patients insula dysfunction is insufficiently targeted or influenced by the available treatments, resulting in prolonged DMN-persistence, persisting symptoms of depression and eventually treatment resistance. This hypothesis should be further investigated in future placebo-controlled neuro-imaging studies.
Previous resting state studies have already highlighted the involvement of the insula in non-responders and treatment resistant patients. Insula hypometabolism was associated with poor response to escitalopram in MDD patients (McGrath et al., 2013). Furthermore, Guo et al. (2011) demonstrated decreased ReHo in the left insula in TRD patients compared to non-TRD patients. However, Lui et al. (2011) who focused on (seed-based) RS-FC indicators for TRD, reported at first sight opposite findings: increased functional connectivity between the right insula and the cingulate cortex in TRD relative to non-TRD patients. The increased FC with the ACC (also part of the salience network) found by Lui et al. (2011) could either represent a compensatory increase in FC, potentially to assist the insula by the ACC, an increase in dysfunction in a more widespread part of the salience network or be confounded by the extreme difference in mean disease duration between the TRD/non-TRD groups in their study compared to the more balanced durations in our study (193 vs. 22 months, respectively). Other possible explanations of discrepancy in findings, apart from obvious differences in the analysis, patient selection, duration of MDD, and difference levels of non-response, might be the ethnicity of the sample (Chinese vs. European) (Serretti et al., 2007) or cultural differences (Li et al., 2018).
Visual inspection of our results revealed that the right insula-salience connectivity in the healthy controls was intermediate between both patient groups. This could be considered as surprising as one might expect both patient groups to show a difference with the healthy controls that points in the same direction. One possibility is that the higher RS-FC of the 1 AD group may predict response as it has been shown that changes in insula functioning occur with a variety of treatments for MDD, suggesting an involvement of this region in mediating treatment response (McGrath et al., 2013). Although McGrath et al., (2013) investigated insula functioning with FDG-PET, in light of these findings, intact functional connectivity of the 1 AD group (as in HC) could may represent a predictor of treatment response. However, this is in need of empirical resting.
4.1. Limitations
A first limitation is that, due to our selection process the remaining sample size of patients in need of ≥2 ADs is modest, and therefore smaller group differences may not have been detected. Second, only information on medication duration and daily dose was available. Unfortunately, specific information about when in a certain episode the medication was used exactly, was unspecified in NESDA, although a proxy for this information could be derived from the duration of use, especially when the duration of use summed up to most months of the year. Furthermore, an AD switch could also have been initiated because of side effects, however this occurs mostly early after initiation and we only selected treatments as ‘adequate’ when the antidepressant was prescribed for >4 weeks. We therefore expect, given our definition of an adequate trial, that the confounding of switching due to adverse effects is limited. Third, an AD switch could also have been initiated because the initial AD interacted with other medication. However, in clinical practice, at least in the Netherlands, interactions between antidepressants and new/additional medication are usually considered when a new drug is initiated. When checking for this, we did not identify co-medication that could have forced the switch from the initial antidepressant. We therefore assume that despite a technically possible misclassification as insufficient response forced by drug-drug interactions, this is not influencing the classification in these subjects. Fourth, in this naturalistic cohort design, illness severity (IDS) data was only collected at three visits (baseline, one year, and two years later). Therefore, it was difficult to determine whether and when a certain antidepressant led to symptom reduction, for which more stringent trials are preferable. Fifth, we included heterogeneous patients initially treated with two antidepressant classes (SSRIs/SNRIs). As such, we investigated AD-treatment more in general, suggesting that observed effects are independent of specific antidepressants, so this heterogeneity precludes translation to a specific AD. Therefore, the merits of this study do not lie in the specificity of the biomarker for a well described drug. However, we believe that our findings are important for the formulation of specific hypotheses in future (trial) studies. We would advocate that this biomarker of prospective insufficient response to antidepressants would be replicated and validated in independent, potentially more specific, controlled trials. This would be important before this work can be translated to clinical practice. Subsequently, randomized controlled trials investigating biomarker-based treatment versus treatment as usual are necessary to investigate the true advantage of implementation of potential biomarkers. Sixth, receipt of psychotherapy might be considered a confounder. Since the NESDA dataset does not allow to investigate the effects of antidepressant treatment without considering effects or timing of psychotherapy, clinical improvement in the groups cannot be attributed to a specific treatment modality. However, both patient groups did not differ significantly in receipt of psychotherapy at baseline, and receipt of psychotherapy during follow-up as a covariate did not influence the results. Furthermore, when exploring addition of psychotherapy during follow-up, i.e. patients who had no psychotherapy at baseline but received psychotherapy during follow-up (psychotherapy was added in 22% in the 1 AD group vs. 29% in the ≥2 AD group), we found that the addition of psychotherapy was not significantly different between groups (p = .56; X2-test). We therefore believe that our results, to the best of our notion, are not primarily confounded by psychotherapy and that the high number of patients that receive psychotherapy during follow-up in the ≥2 AD group (94% vs. 56% in the 1 AD group) substantiates the notion that the ≥2 AD group represents a clinically more difficult to treat group of patients. Overall, we believe our findings provide important information in the search for biomarkers for early identification of characteristics of non-response. Lastly, while sample matching across groups is helpful in detecting differences without the need to correct for additional confounding, resulting in more power to examine the brain measures of interest, this approach could result in reduced generalizability of our findings.
To summarize, we are aware that when considering our results in depth, various factors as switching due to adverse effects or drug-drug interactions might have influenced our classification and interpretation of insufficient response, however we do believe that these concerns are less relevant for the current sample and our finding therefore are valuable in providing starting points for research on long term effects of insufficient response. Especially because the long term burden of MDD is associated with multiple treatment steps due to insufficient response (Johnston et al. 2019). We therefore believe that this research is important because it contributes to long term effects of non-response and provides important naturalistic clinical information to finally aid to improve chances of response.
4.2. Conclusion and future directions
We identified decreased functional connectivity of the insula within the salience network as a potential biomarker for prospective insufficient response. With a post-hoc analysis, we linked this diminished connectivity of the insula to diminished insula activation when switching between task and rest related networks in patients in need of ≥2 ADs. We hypothesize that this insula dysfunction might not sufficiently be targeted by current antidepressants, which therefore can lead to treatment resistance. This should be investigated further with placebo-controlled neuroimaging studies in MDD.
Our findings suggest that altered insula function may be a potential neuroimaging biomarker for the prediction of prospective antidepressant non-response. More rigorously controlled studies are required to replicate aberrant insula functioning and insula-salience FC alterations as such a predicting biomarker for antidepressant non-response and treatment resistant depression.
Funding
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Centre, GGZ inGeest, Arkin, Leiden University Medical Centre, GGZ Rivierduinen, University Medical Centre Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Healthcare (IQ healthcare), Netherlands Institute for Health Services Research (NIVEL), and Netherlands Institute of Mental Health and Addiction (Trimbos Institute)).
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank all participants in NESDA study, all support staff and all collaborators for their cooperation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102064.
Contributor Information
H. Geugies, Email: hannekegeugies@gmail.com.
H.G. Ruhé, Email: h.g.ruhe@gmail.com.
Appendix. Supplementary materials
References
- Alexopoulos G.S., Hoptman M.J., Kanellopoulos D., Murphy C.F., Lim K.O., Gunning F.M. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A.M., Caprihan A., Turner J.A., Eichele T., Adelsheim S., Bryan A.D., Bustillo J., Clark V.P., Feldstein Ewing S.W, Filbey F., Ford C.C, Hutchison K, Jung R.E, Kiehl K.A, Kodituwakku P, Komesu Y.M, Mayer A.R, Pearlson G.D, Phillips J.P, Sadek J.R, Stevens M, Teuscher U, Thoma R.J, Calhoun V.D. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Berlim M.T., Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can. J. Psychiatry. 2007;52:46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kwaasteniet B.P., Rive M.M., Ruhe H.G., Schene A.H., Veltman D.J., Fellinger L., van Wingen G.A., Denys D. Decreased resting-state connectivity between neurocognitive networks in treatment resistant depression. Front. Psychiatry. 2015;6:28. doi: 10.3389/fpsyt.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P., Jabourian M., Lemogne C., Guionnet S., Bergouignan L., Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J. Affect. Disord. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Fekadu A., Wooderson S.C., Markopoulou K., Cleare A.J. The maudsley staging method for treatment-resistant depression: prediction of longer-term outcome and persistence of symptoms. J. Clin. Psychiatry. 2009;70:952–957. doi: 10.4088/JCP.08m04728. [DOI] [PubMed] [Google Scholar]
- Figueroa C.A., Ruhe H.G., Servaas M., Marsman J.B., Geugies H., Schene A.H. Default mode network dominance over the task positive network is not increased in remitted depressed patients at high risk for recurrence. Eur. Neuropsychopharmacol. 2015;25(suppl 2):S399. [Google Scholar]
- Figueroa C.A., Cabral J., Mocking R.J.T., Rapuano K.M., van Hartevelt T.J., Deco G., Expert P., Schene A.H., Kringelbach M.L., Ruhe H.G. Altered ability to access a clinically relevant control network in patients remitted from major depressive disorder. Hum. Brain Mapp. 2019;40:2771–2786. doi: 10.1002/hbm.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T.B., Jing Y., Smith Carls G, Kim E, Bagalman J.E, Burton W.N, Tran Q.V, Pikalov A, Goetzel R.Z. Cost burden of treatment resistance in patients with depression. Am. J. Manag. Care. 2010;16:370–377. [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Groves S.J., Douglas K.M., Porter R.J. A systematic review of cognitive predictors of treatment outcome in major depression. Front. Psychiatry. 2018;9:382. doi: 10.3389/fpsyt.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Liu F., Xue Z., Gao K., Liu Z., Xiao C., Chen H., Zhao J. Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;44:51–57. doi: 10.1016/j.pnpbp.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Guo W.B., Sun X.L., Liu L., Xu Q., Wu R.R., Liu Z.N., Tan C.L., Chen H.F., Zhao J.P. Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1297–1302. doi: 10.1016/j.pnpbp.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Iniesta R., Malki K., Maier W., Rietschel M., Mors O., Hauser J., Henigsberg N., Dernovsek M.Z., Souery D., Stahl D., Dobson R., Aitchison K.J., Farmer A., Lewis C.M., McGuffin P., Uher R. Combining clinical variables to optimize prediction of antidepressant treatment outcomes. J. Psychiatr. Res. 2016;78:94–102. doi: 10.1016/j.jpsychires.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Johnston K.M., Powell L.C., Anderson I.M., Szabo S., Cline S. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J. Affect. Disord. 2019;242:195–210. doi: 10.1016/j.jad.2018.06.045. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Tae W.S., Yoon H.K., Lee B.T., Paik J.W., Son K.R., Oh Y.W., Lee M.S., Ham B.J. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. J. Affect. Disord. 2011;133:128–136. doi: 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Li B., Liu L., Friston K.J., Shen H., Wang L., Zeng L.L., Hu D. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Li L.M.W., Luo S., Ma J., Lin Y., Fan L., Zhong S., Yang J., Huang Y., Gu L., Fan L., Dai Z., Wu X. Functional connectivity pattern underlies individual differences in independent self-construal. Soc. Cogn. Affect. Neurosci. 2018;13:269–280. doi: 10.1093/scan/nsy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xu C., Xu Y., Wang Y., Zhao B., Lv Y., Cao X., Zhang K., Du C. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res. 2010;182:211–215. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Lui S., Wu Q., Qiu L., Yang X., Kuang W., Chan R.C., Huang X., Kemp G.J., Mechelli A., Gong Q. Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthoffer D., Zimmer C., Forstl H., Bauml J., Riedl V., Wohlschlager A.M., Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2014;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H., Sonuga-Barke E.J., De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol. Rev. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath C.L., Kelley M.E., Holtzheimer P.E., Dunlop B.W., Craighead W.E., Franco A.R., Craddock R.C., Mayberg H.S. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Wiebking C., Feinberg T., Panksepp J. The 'resting-state hypothesis' of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neurosci. Biobehav. Rev. 2011;35:1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Novick D., Hong J., Montgomery W., Duenas H., Gado M., Haro J.M. Predictors of remission in the treatment of major depressive disorder: real-world evidence from a 6-month prospective observational study. Neuropsychiatr. Dis. Treat. 2015;11:197–205. doi: 10.2147/NDT.S75498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opmeer E.M., Kortekaas R., van Tol M.J., Renken R.J., Demenescu L.R., Woudstra S., Ter Horst G.J., van Buchem M.A., van der Wee N.J., Veltman D.J., Aleman A. Changes in regional brain activation related to depressive state: a 2-year longitudinal functional mri study. Depress. Anxiety. 2015 doi: 10.1002/da.22425. [DOI] [PubMed] [Google Scholar]
- Penninx B.W., Beekman A.T., Smit J.H., Zitman F.G., Nolen W.A., Spinhoven P., Cuijpers P., De Jong P.J., Van Marwijk H.W., Assendelft W.J., Van Der Meer K., Verhaak P., Wensing M., De Graaf R., Hoogendijk W.J., Ormel J., Van Dyck R., Research Consortium N.E.S.D.A. The netherlands study of depression and anxiety (NESDA): rationale, objectives and methods. Int. J. Methods Psychiatr. Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock S.J., Assmann S.E., Enos L.E., Kasten L.E. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat. Med. 2002;21:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhe H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Ruhe H.G., Huyser J., Swinkels J.A., Schene A.H. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J. Clin. Psychiatry. 2006;67:1836–1855. doi: 10.4088/jcp.v67n1203. [DOI] [PubMed] [Google Scholar]
- Ruhe H.G., van Rooijen G., Spijker J., Peeters F.P., Schene A.H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 2012;137:35–45. doi: 10.1016/j.jad.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Rush A.J., First M.B., Blacker D., Association American Psychiatric. American Psychiatric Publishing, Washington, DC; 2008. Handbook of Psychiatric Measures. [Google Scholar]
- Sambataro F., Wolf N.D., Pennuto M., Vasic N., Wolf R.C. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol. Med. 2013:1–11. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A., Kato M., De Ronchi D., Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol. Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Sliz D., Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front. Hum. Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker J., Bockting C.L.H, Meeuwissen J.A.C, van Vliet I.M, Emmelkamp P.M.G., Hermens M.L.M, van Balkom A.L.J.M, namens de, Werkgroep multidisciplinaire richtlijnontwikkeling angststoornissen/depressie . Guideline for diagnostics, treatment and guidance of adult clients with a depressive disorder]. Trimbos-instituut, Utrecht; 2013. Multidisciplinaire Richtlijn Depressie (derde revisie). Richtlijn Voor De diagnostiek, Behandeling En Begeleiding Van Volwassen Patiënten Met Een Depressieve Stoornis [multidisciplinary guideline Depression (Third Revision) [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Yucel M., Lorenzetti V., Tanino R., Whittle S., Suzuki M., Walterfang M., Pantelis C., Allen N.B. Volumetric mri study of the insular cortex in individuals with current and past major depression. J. Affect. Disord. 2010;121:231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uher R., Perlis R.H., Henigsberg N., Zobel A., Rietschel M., Mors O., Hauser J., Dernovsek M.Z., Souery D., Bajs M., Maier W., Aitchison K.J., Farmer A., McGuffin P. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol. Med. 2012;42:967–980. doi: 10.1017/S0033291711001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol M.J., Demenescu L.R., van der Wee N.J., Kortekaas R., Marjan M.A.N., Boer J.A., Renken R.J., van Buchem M.A., Zitman F.G., Aleman A., Veltman D.J. Functional magnetic resonance imaging correlates of emotional word encoding and recognition in depression and anxiety disorders. Biol. Psychiatry. 2012;71:593–602. doi: 10.1016/j.biopsych.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J., Ferrarini L., Milles J., Veltman D.J., Aleman A., van Buchem M.A., van der Wee N.J., Rombouts S.A. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. eCollection 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hermens D.F., Hickie I.B., Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J. Affect. Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Wijeratne C., Sachdev P. Treatment-resistant depression: critique of current approaches. Aust. N. Z. J. Psychiatry. 2008;42:751–762. doi: 10.1080/00048670802277206. [DOI] [PubMed] [Google Scholar]
- Yao Z., Wang L., Lu Q., Liu H., Teng G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J. Affect. Disord. 2009;115:430–438. doi: 10.1016/j.jad.2008.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.