Abstract
Background:
Aberrant frontal-plane hip and pelvis kinematics have been frequently observed in runners with patellofemoral pain (PFP). Gait retaining interventions have been shown to improve running kinematics and may therefore be beneficial in runners with PFP.
Purpose:
To investigate whether a 10% increase in the running step rate influences frontal-plane kinematics of the hip and pelvis as well as clinical outcomes in runners with PFP.
Study Design:
Case series; Level of evidence, 4.
Methods:
Runners with PFP underwent a 3-dimensional gait analysis to confirm the presence of aberrant frontal-plane hip and/or pelvis kinematics at baseline. A total of 12 participants with frontal-plane hip and/or pelvis kinematics 1 standard deviation above a reference database were invited to undergo the gait retraining intervention. Running kinematics along with clinical outcomes of pain and functional outcomes were recorded at baseline, 4 weeks after retraining, and 3 months. Gait retraining consisted of a single session where step rate was increased by 10% using an audible metronome. Participants were asked to continue their normal running while self-monitoring their step rate using a global positioning system smartwatch and audible metronome.
Results:
After gait retraining, significant improvements in running kinematics and clinical outcomes were observed at 4-week and 3-month follow-up. Repeated-measures analysis of variance with post hoc Bonferroni correction (P < .016) showed significant reductions in peak contralateral pelvic drop (mean difference [MD], 3.12° [95% CI, 1.88°-4.37°]), hip adduction (MD, 3.99° [95% CI, 2.01°-5.96°]), and knee flexion (MD, 4.09° [95% CI, 0.04°-8.15°]) as well as significant increases in self-reported weekly running volume (MD, 13.78 km [95% CI, 4.62-22.93 km]) and longest run pain-free (MD, 6.84 km [95% CI, 3.05-10.62 km]). Friedman test with a post hoc Wilcoxon signed-rank test showed significant improvements on a numerical rating scale for worst pain in the past week and the Lower Extremity Functional Scale.
Conclusion:
A single session of gait retraining using a 10% increase in step rate resulted in significant improvements in running kinematics, pain, and function in runners with PFP. These improvements were maintained at 3-month follow-up. It is important to assess for aberrant running kinematics at baseline to ensure that gait interventions are targeted appropriately.
Registration:
NCT03067545 (ClinicalTrials.gov identifier)
Keywords: patellofemoral pain, running, gait retraining, kinematics, step rate
Recreational running is an increasingly popular method of physical activity, with participation rates growing annually. Although running offers several health benefits, it also poses a considerable risk of injury to the musculoskeletal system. Overall injury incidence rates are reported to range between 19% and 78% among recreational runners,37 with recurrence rates in 20% to 70% of all cases.38 Of all running injuries, patellofemoral pain (PFP) is considered the most common running-related knee injury,36 with incidence and prevalence rates as high as 20.8% and 22.7%, respectively.34
PFP is known to have a multifactorial cause, with aberrant running mechanics identified as one risk factor.21,22,24 Runners with PFP have been reported to demonstrate increased hip adduction (HADD),24,25,41 hip internal rotation,25 and contralateral pelvic drop (CPD)41 when compared with injury-free controls. It is thought that aberrant frontal- and transverse-plane running kinematics may result in lateral tracking of the patella, leading to a rise in patellofemoral joint stress.30 When exposed to repeat loading cycles during running, the knee may experience damage to the underlying chondral surface, stress within the subchondral bone, and excitation of nociceptors, leading to pain and injury.2
Gait retraining is a clinical intervention that targets aberrant running kinematics within the rehabilitation of PFP. Current evidence has shown improvements in kinematics and clinical outcomes after mirror retraining,42 real-time feedback,26 and transitioning to a forefoot contact.32,33 However, the use of mirror retraining and real-time feedback is restricted to clinical and laboratory settings,limiting their practical applicability, while transitioning to a forefoot strike has been shown to increase Achilles tendon and ankle joint loading, which may increase the risk of lower limb injury.1 Furthermore, these studies often utilize a faded feedback design consisting of 8 sessions over a 2-week period, requiring closeclinical supervision. Therefore, there is a need for gait retraining methods that can be easily integrated outside of a laboratory setting while providing positive clinical and biomechanical outcomes.
Increasing step rate may be one method of gait retrainingthat could be integrated outside of a laboratory setting. Through the use of global positioning system (GPS) smartwatches and mobile metronome applications, runners may be able to self-retrain and monitor their step rate without the need for close clinical supervision.19,40 Currently, only 3 studies have investigated the effects of increasing the step rate among runners with PFP.11,12,20 Neal et al20 reported improved frontal-plane hip and pelvis kinematics along with reductions in pain but did not investigate whether improvements were maintained beyond the 6-week follow-up period. Esculier et al12 reported gait retraining to be no more effective than education on load management, and Dos Santos et al11 reported minimal changes in pain after a 2-week retraining period. Furthermore, both Esculier et al and Dos Santos et al did not report any change in frontal-plane hip and pelvis kinematics after the retraining period. Therefore, questions remain regarding the clinical effectiveness of increasing the step rate and whether step rate retraining results in long-term kinematic adaptations among runners with PFP.
The aim of the current study was to investigate whether a 10% increase in the running step rate influences frontal-plane kinematics of the hip and pelvis as well as clinical outcomes in runners with PFP. Rather than use a clinically monitored faded feedback design, we sought to investigate whether runners can self-administer gait retraining interventions using an audible metronome and a GPS smartwatch. We hypothesized that a 10% increase in step rate would result in significant reductions in frontal-plane hipand pelvis kinematics and improvements in clinical outcomes and that these changes would be present at short- and long-term follow-up.
Methods
Participants
Participants were recruited through advertisements at local sports injury clinics, running clubs, and a university-based running clinic. Ethical approval for the study was obtained via the local ethics committee, and all participants provided written informed consent before participation. This study was registered as a clinical trial (ClinicalTrials.gov registration No. NCT03067545) with enrollment for the trial between March 2017 and December 2018. An a priori sample size calculation was conducted using data from a previous gait retraining study identifying a 2.3° reduction in CPD after retraining with an effect size of 1.09.26 Using G*Power software, we calculated that 12 participants would be required to detect an effect size of 1.25 with a power of 0.8 and an adjusted critical alpha of .016. This calculation was based on the use of paired tests to detect differences in peak CPD, which were similar to changes observed in previous studies after gait retraining (2.3°)26 and also of similar magnitude to differences observed between injured and healthy runners (2.7°).4
Inclusion/Exclusion Criteria
All participants were required to own a GPS smartwatch or running watch capable of monitoring step rate. Participants were included in the gait retraining intervention based on a 3-stage assessment process. First, a subjective assessment and clinical examination were used to confirm the presence of PFP. Once the diagnosis of PFP was confirmed, a 3-dimensional (3D) gait analysis was conducted to confirm the presence of aberrant hip and/or pelvis kinematics. To ensure that the injury diagnosis met the consensus definition of a running-related injury,43 participants had to report an insidious onset of anterior knee pain during running lasting for a minimum of 3 months, causing a self-reported restriction to either their running volume or duration. Participants were required to be running a minimum of twice per week, with their worst pain rated a minimum of 3 out of 10 on a numerical rating scale (NRS) for pain (0 = no pain, 10 = worst possible pain). Pain must also have been reproduced by ≥1 of the additional activities of squatting, kneeling, prolonged sitting, or ascending or descending stairs. Participants were excluded if they reported having any known medical condition, had undergone prior musculoskeletal surgery, had a neurological impairment, had diagnosed knee osteoarthritis, had a structural deformity of the knee, had an onset of knee pain caused by trauma or any other sporting activity, had ceased running, or were undergoing additional treatment outside of the study. To control for training errors as a potential underlying cause of injury, participants were also excluded if they reported the onset of symptoms to occur after an increase in their weekly training volume ≥30%.23
After the subjective assessment, participants were invited to undergo a clinical examination led by the lead clinician (C.B.) to confirm the diagnosis of PFP in accordance with previously published diagnostic criteria.9 Specifically, for inclusion in the study, pain must have been retropatellar or peripatellar in nature and reproduced on squatting with the exclusion of any patellar instability, ligamentous or meniscal injury.9 Pain on squatting has been shown to have a sensitivity of 91% and a negative predictive value of 74%, suggesting this examination to be the best available test for PFP.9,27 A combination of additional, but nonessential, clinical tests was used to further increase the diagnostic accuracy of PFP.8 Tests included patellar compression, patellar apprehension, pain on palpation of the lateral patellar facet, and pain on resisted quadriceps contraction in 30° of knee flexion.8,27 These tests are known to have low sensitivity and specificity when used in isolation and were not used as a sole diagnostic criterion for PFP.8,27
Once the diagnosis of PFP was confirmed, each participant underwent an initial 3D gait analysis as outlined below and completed clinical outcome measures to monitor pain and functional improvements. Clinical outcome measures included the Lower Extremity Functional Scale (LEFS), previously validated for use in PFP,3 as well as self-reported worst pain experienced in the past week using the NRS (0 = no pain, 10 = worst possible pain). Additional outcomes monitored were self-reported longest distance run pain-free and total weekly running volume.
Kinematic Data Collection
Kinematic data were collected from all participants with confirmed PFP while running on a treadmill (F63; Sole Fitness) at 3.2 m/s wearing their own running shoes. After a 5-minute warm-up period, 30 seconds of kinematic data were collected using a 12-camera Oqus system (240 Hz; Qualisys). A total of 9 anatomic segments were tracked following a previously published protocol.17,31 Segments included the thorax; pelvis; and bilateral thigh, shank, and foot segments. Further details of the markers used to track each segment and the precise definition of the anatomic coordinate systems are provided in previous publications.13,17,31
Raw kinematic data were low pass filtered at 10 Hz. Intersegmental kinematics, along with motions of the pelvis and thorax with respect to the laboratory system, were calculated using a 6 degrees of freedom model with Visual3D software (C-Motion). Gait events were defined using a kinematic approach in which initial contact was defined as the first vertical acceleration peak of either the heel or metatarsal markers and toe-off defined as the vertical jerk peak of the second metatarsal marker.14 Gait events were subsequently used to segment each kinematic signal into a minimum of 10 consecutive gait cycles. An ensemble average for each signal was created and selected kinematic parameters derived from the ensemble average curves. This latter processing was carried out using a custom Matlab script (MathWorks).
Participants were invited to participate in the gait retraining study providing that they demonstrated aberrant hip and/or pelvis kinematics during the initial 3D gait analysis. Aberrant hip and pelvis kinematics were defined as peak HADD and CPD angles ≥1 standard deviation above the mean of a previously published database of healthy recreational runners running at the same speed4 (qualifying criteria = CPD ≥5.6° and/or HADD ≥13.2°). Runners who did not meet the kinematic inclusion criteria were not included in the study and were referred to a health care professional for further management.
Retraining Protocol
All participants included within the retraining protocol completed a single 10-minute retraining session conducted immediately after the initial 3D gait analysis. During the retraining session, participants were asked to run at the same speed with a 10% increase in their original step rate. Step rate was calculated as the number of foot contacts per minute. During the first 5 minutes of the retraining protocol, participants were instructed to match their footsteps to an audible metronome set to the new step rate. For the final 5 minutes of the retraining session, the audible metronome was removed, and participants were instructed to continue running at the increased step rate. Throughout this time, participants were monitored by the lead researcher to ensure that they were able to maintain the higher step rate and the metronome reintroduced if they failed to do so.
After the retraining session, participants were provided with instructions for self-administration and monitoring of the increased step rate. Specifically, during the first 2 weeks, participants were instructed to continue using a free downloadable metronome application set to the new step rate. During the third and fourth weeks, participants were instructed to continue running without the use of the metronome but were instructed to self-monitor their cadence using their GPS smartwatch. Participants were permitted to increase their running volume at any point in the retraining period provided that any knee pain experienced was rated below 3 of 10 on an NRS.
All participants were invited to follow-up 3D gait analysis sessions at 4 weeks and 3 months after the initial assessment. This follow-up period allowed us to investigate whether kinematic changes could be maintained across a time frame comparable with previous gait retraining studies.42 The follow-up sessions were completed following the same kinematic testing procedures as the first visit, recording the same clinical outcome measurements. After the 4-week follow-up assessment, participants were instructed to continue running without the use of the metronome. No restrictions to training parameters were provided; participants were instead instructed to increase their training volume or pace and change surfaces as they saw fit provided that any pain experienced was rated lower than 3 of 10 on the NRS.
Data Analysis
Several kinematic parameters were selected for data analysis. Kinematic parameters included peak CPD, HADD, hip internal rotation, and knee flexion. These parameters were selected based on previous research highlighting associations with these parameters and PFP.4,20,25,41 Peak angles at midstance were defined as the maximum joint angles between initial contact and toe-off. Stride rate was also included within the analysis, measured as steps per minute, along with clinical outcome measures of worst pain experienced in the past week using the NRS, longest distance run pain-free, total weekly running volume, and LEFS score.
Statistical Analysis
Repeated-measures analysis of variance was used to assess for differences in kinematic parameters between the initial assessment (baseline), 4-week follow-up, and 3-month follow-up, with a critical alpha of <.05. When significant differences were observed, post hoc Bonferroni testing, with an adjusted critical alpha of <.016, was used to identify differences between time points. Clinical outcomes of the NRS and LEFS were analyzed using the Friedman test for nonparametric data with a post hoc Wilcoxon signed-rank test. Effect sizes were calculated for pairwise comparisons using the Cohen d and interpreted as 0.2, 0.5, and 0.8 as small, medium, and large, respectively.6
Results
A total of 33 participants met the initial subjective inclusion criteria and were invited for a clinical examination. After the clinical examination, 18 were diagnosed as having PFP and invited to take part in the 3D gait analysis. After the 3D gait analysis, 12 participants met the inclusion criteria and were enrolled in the gait retraining study. There were 2 participants who dropped out of the study between the 4-week and 3-month follow-up points. The first participant failed to attend the 3-month follow-up and did not respond to contact, and the second developed a tibial stress fracture on the same limb and was unable to continue the study. Both participants were included in the final analysis using a last observation carried forward method35 (Table 1).
Table 1.
Participant Characteristics a
| Value | |
|---|---|
| Male/female sex, n | 4/8 |
| Age, y | 39.92 ± 6.50 |
| Weight, kg | 61.03 ± 6.48 |
| Height, cm | 170.33 ± 6.98 |
| Usual weekly running volume, km | 29.03 ± 8.11 |
Values are presented as mean ± SD unless otherwise specified.
Kinematics
Repeated-measures analysis of variance showed a significant effect of time for several kinematic parameters (Table 2). In particular, there were significant increases in step rate and reductions in peak CPD, HADD, and knee flexion after the step rate intervention (Table 2). The post hoc testrevealed that step rate significantly increased by an average of 11.2% at 4 weeks (mean difference [MD], 18.6steps/min [95% CI, 11.97-25.23 steps/min]) and9.2% at 3 months (MD, 15.1 steps/min [95% CI, 10.64-19.57 steps/min]) when compared with baseline. There was a significant 3.12° reduction in CPD (MD, 3.12° [95% CI, 1.88°-4.37°]) and 3.99° reduction in HADD (MD, 3.99° [95% CI, 2.01°-5.96°]) at 4 week follow-up, which was also significant at 3 months when compared with baseline for both CPD (MD, 2.7° [95% CI, 1.4°-4.1°]) and HADD (MD, 2.8° [95% CI, 0.4°-5.4°]). Similarly, there was a significant reduction in peak knee flexion during stance phase at 4 weeks (MD, 4.10° [95% CI, 0.04°-8.15°]) and 3 months (MD, 4.15° [95% CI, 0.81°-7.48°]). No significant differences were observed between the 4-week and 3-month follow-up time points for any of the kinematic parameters (Table 2).
Table 2.
Kinematic Parameters a
| Baseline | 4 wk | 3 mo | P Value (ANOVA) | Pairwise Effect Size (Cohen d) | ||
|---|---|---|---|---|---|---|
| Stride rate, steps/min | 165.93 ± 7.38 | 184.53 ± 10.10 b | 181.04 ± 7.78 b | <.01 | Baseline to 4 wk | 2.10 |
| Baseline to 3 mo | 1.99 | |||||
| 4 wk to 3 mo | 0.38 | |||||
| Peak CPD, deg | 7.46 ± 1.81 | 4.34 ± 2.47 b | 4.73 ± 2.95 b | <.01 | Baseline to 4 wk | 1.44 |
| Baseline to 3 mo | 1.11 | |||||
| 4 wk to 3 mo | 0.14 | |||||
| Peak HADD, deg | 15.95 ± 2.75 | 11.96 ± 1.77 b | 13.09 ± 3.20 b | <.01 | Baseline to 4 wk | 1.72 |
| Baseline to 3 mo | 0.95 | |||||
| 4 wk to 3 mo | 0.43 | |||||
| Peak hip internal rotation, deg | 4.06 ± 8.17 | 4.18 ± 9.35 | 4.42 ± 7.89 | .93 | Baseline to 4 wk | 0.01 |
| Baseline to 3 mo | 0.04 | |||||
| 4 wk to 3 mo | 0.02 | |||||
| Peak knee flexion, deg | 33.74 ± 5.25 | 29.64 ± 3.23 b | 29.59 ± 3.16 b | <.01 | Baseline to 4 wk | 0.94 |
| Baseline to 3 mo | 0.96 | |||||
| 4 wk to 3 mo | 0.02 | |||||
Values are presented as mean ± SD unless otherwise specified. Pairwise effect sizes are interpreted as 0.2 = small, 0.5 = medium, and 0.8 = large. ANOVA, analysis of variance; CPD, contralateral pelvic drop; HADD, hip adduction.
Significant difference when compared with baseline at P < .016 after Bonferroni correction.
Clinical and Functional Outcomes
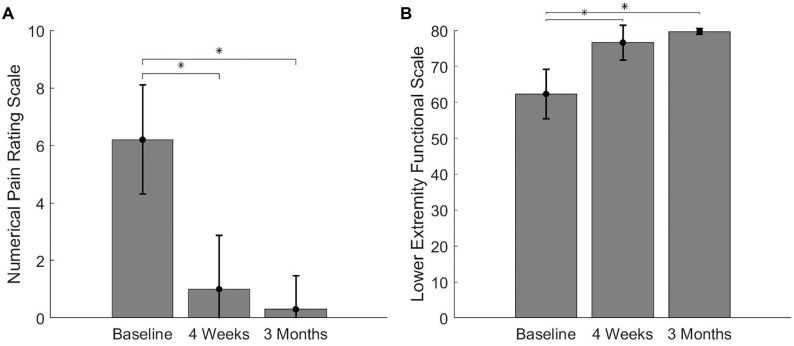
All clinical and functional outcomes demonstrated statistically significant improvements. Specifically, there was a significant reduction in pain scores on the NRS from an average of 6.2 out of 10 at baseline to 1.0 and 0.3 at 4 weeks and 3 months, respectively (χ2 = 21.38; P < .01) (Figure 1), which is above the minimal clinically important difference of 1.2 points.28 The LEFS demonstrated a statistically significant improvement from 62.3 at baseline to 76.6 at 4 weeks and 79.7 at 3 months (χ2 = 22.29; P≤ .01) (Figure 1). When compared with baseline, this was a 14.3- and 17.4-point improvement at 4 weeks and 3 months, respectively, which is above the minimal clinically important difference of 9 points.3 All participants demonstrated a significant increase in total weekly running volume (MD, 13.78 km [95% CI, 4.62-22.93 km]) and longest distance run pain-free (MD, 6.84 km [95% CI, 3.05-10.62 km]) from baseline to 4-week and 3-monthfollow-up (Table 3).
Figure 1.
Clinical outcomes at baseline, 4 weeks, and 3 months. *Statistically significant difference when compared with baseline at P < .016. Error bars represent ±1 standard deviation.
Table 3.
Functional Outcomes a
| Parameter | Baseline | 4 wk | 3 mo | P Value | Pairwise Effect Size (Cohen d) | |
|---|---|---|---|---|---|---|
| Total distance per week, km | 13.34 ± 9.83 | 27.11 ± 11.25 b | 28.33 ± 12.96 b | <.01 | Baseline to 4 wk | 1.30 |
| Baseline to 3 mo | 1.30 | |||||
| 4 wk to 3 mo | 0.10 | |||||
| Longest run pain-free, km | 2.03 ± 1.19 | 8.87 ± 4.39 b | 11.33 ± 6.42 b | <.01 | Baseline to 4 wk | 2.10 |
| Baseline to 3 mo | 2.01 | |||||
| 4 wk to 3 mo | 0.44 | |||||
Values are presented as mean ± SD unless otherwise specified. Pairwise effect sizes are interpreted as 0.2 = small, 0.5 = medium, and 0.8 = large.
Statistically significant difference when compared with baseline at P < .016.
Discussion
The aim of this study was to investigate whether a 10% increase in the step rate would improve kinematics and clinical outcomes among runners with PFP who demonstrate aberrant frontal-plane pelvis and hip kinematics. In support of our hypothesis, we observed significant reductions in frontal-plane pelvis and hip kinematics as well as significant reductions in pain and improvements in function and running at 4 weeks, which appeared to be maintained at 3-month follow-up.
After the step rate increase, we observed a 3.12° and 3.99° reduction in CPD and HADD, respectively (Table 2), which may offer a mechanical explanation for the improved clinical outcomes seen in this study. These changes are greater than those observed in previous step rate studies,11,20 with this being the first study to highlight that kinematic adaptations are maintained at longer term follow-up. CPD and HADD have been cited as kinematic risk factors for PFP.4,21,24,25,41 It is thought that CPD will give rise to an increase in iliotibial band tension, resulting in lateral displacement of the patella,15,18 while HADD would cause the femur to shift medially under the patella.29 This would result in elevated contact pressure between the patella and lateral facet, leading to elevated joint stress and potential injury.30 Therefore, it is possible that the reductions in CPD and HADD after an increase in the step rate would contribute to reduced lateral displacement of the patella and a corresponding reduction in patellofemoral joint stress.
Similarly, the reduction in peak knee flexion during stance phase may also contribute to improvements in clinical outcomes. Peak knee flexion during stance phase has been shown to influence patellofemoral joint reaction force, explaining up to 64% of the variance in peak patellofemoral joint loading.16 Smaller knee flexion angles at midstance will likely reduce the external joint force as well as reduce the demand on the surrounding musculature.16 In the current study, we observed a 4.1° reduction in peak knee flexion (Table 2). Given the work of Lenhart et al,16 this magnitude of change is likely to contribute to reductions in peak patellofemoral joint force. These reductions, combined with the reductions in peak HADD and CPD, will likely lead to significant reductions in patellofemoral joint stress, which may explain the observed improvements in clinical outcomes within the present study.
We suggest that the improved frontal-plane hip and pelvis kinematics may be explained by alterations in neuromuscular activity of the hip. Willson et al39 found that runners with PFP demonstrate significantly delayed onset of the gluteus medius, which had a moderate correlation with HADD excursion. It is hypothesized that delayed muscle activation of the gluteus medius during the stance phase of running would result in a loss of neuromuscular stiffness about the hip and pelvis, leading to a loss of frontal-plane stability.39 Increasing step rate by 10% has been shown to directly influence the preactivation of gluteal muscles.5 Specifically, Chumanov et al5 reported significantly increased gluteus medius and maximus muscle activity in late swing phase, just before initial foot contact after a 10% increase in step rate. Considering the role that the gluteus medius plays in frontal-plane stability of the hip and pelvis, it is likely that the earlier onset of the glutealmuscles would result in increased neuromuscular stability during the stance phase of gait. This would likely explain the mechanical improvements of reduced CPD and HADD observed in the present study.
Reductions in peak knee flexion may also be explained by alterations in neuromuscular activity at the knee. Increasing the step rate has been shown to result in greater preactivation of the hamstrings, vastus lateralis, and rectus femoris muscles during late swing phase.5 It is thought that these changes in neuromuscular coordination contribute to a more extended knee throughout the stance phase, reducing peak knee flexion angles.5,16
In contrast to previous studies, we did not identify differences in peak hip internal rotation after gait retraining. Neal et al20 reported a 5.1° reduction in peak hip internal rotation after a 10% increase in step rate, whereas in the present study, we did not observe more than a 0.5° change. This may be explained by our baseline inclusion criteria of increased HADD and CPD rather than hip internal rotation. Participants within this study demonstrated 4.06° of hip internal rotation at baseline, which is less than the 9.1° reported in the study by Neal et al20 and similar to the 4.4° reported in the database of healthy runners used for baseline reference values.4 Therefore, it is possible that participants in the present study did not demonstrate increased hip internal rotation angles at baseline and thus would be unlikely to demonstrate any change.
An interesting finding was the magnitude of clinical improvements made by participants. Specifically, participants reported their worst pain to be, on average, 1.0 out of 10 at 4-week follow-up and 0.3 out of 10 at 3-month follow-up (Figure 1). This is greater than the minimal clinically important difference of 1.2 points28 and greater than improvements seen in previous step rate studies, which have reported average NRS scores of 3,11 3.8,12 and 2.920 out of 10 after retraining. We also observed significant improvements in function, with all runners reporting an increase in their weekly running volume and longest distance run pain-free as early as 4 weeks (Table 3), as well as a 17.4-point improvement on the LEFS at 3 months, exceeding the minimal clinically important difference of 9 points.3 This contrasts with previous step rate studies, with 1 study reporting participants to be running less than their preinjury status at 20-week follow-up12 and another study reporting less than a 9-point improvement on the LEFS.11
The reason for the magnitude of kinematic and clinical improvements in the present study compared with previous step rate studies may be because of the differences in inclusion criteria. In the present study, we specifically targeted participants who demonstrated aberrant kinematics at baseline. We did this to account for the multifactorial etiology of PFP and ensure that the appropriate underlying injury driver was targeted through the gait intervention. Failure to consider alternative causes of injury would likely result in the inclusion of biomechanical nonresponders in the retraining group. As such, these participants would be unlikely to demonstrate significant clinical improvements. Willy et al42 and Noehren et al26 are the only previous studies to use a similar inclusion criterion, with their results showing a similar magnitude of clinical improvement. Therefore, we suggest that future research should aim to establish the underlying pathological driver to appropriately target clinical interventions and that this be mirrored in clinical practice.
Clinical Relevance
In contrast to previous gait retraining studies, we opted to allow runners to self-administer and self-monitor their retraining using a metronome application and feedback from a GPS smartwatch. This proved successful, as all runners were able to maintain an increased cadence at 4-week and 3-month follow-up. Furthermore, at 4-week follow-up, all participants reported that they did not use the metronome beyond the first week and instead self-monitored their cadence using their GPS smartwatch. Previous studies have utilized a faded feedback design in which feedback is gradually removed over 8 sessions across a 2-week period. Although faded feedback designs have proven clinically effective, they require close clinical supervision and are restricted to clinical and laboratory settings. The present study demonstrates that simple step rate retraining can be applied outside of the laboratory and with minimal clinical contact. Importantly, 2-dimensional measures of CPD and HADD have been shown to be valid and reliable when compared with 3D measurements.10 Therefore, assessment of running kinematics and gait retraining can be easily integrated into clinical practice and a participant’s normal running routine.
Limitations
One limitation of the present study is the lack of a control group, making it difficult to ascertain whether the observed findings are true intervention effects. However, participants in the present study had an average NRS pain score of 6.2 out of 10 at baseline and a minimum symptom duration of 3 months, which has been reported to be predictive of poor prognosis at long-term follow-up.7 Therefore, it is unlikely that participants would have experienced the magnitude of symptom improvement without a clinical intervention. Furthermore, the kinematic differences observed between baseline and the 4-week and 3-month time points were statistically significant, with large effect sizes above the standard error of measurement previously reported for these parameters.17 These differences were of a similar magnitude to those observed previously between injured and healthy runners (2.7°)4 and after gait retraining interventions (2.3°).26 Therefore, we believe that the differences observed represent true intervention effects and could be considered biomechanically important.
A second limitation is the small participant numbers, which makes the statistical interpretation of small differences difficult. We observed no significant kinematic changes between 4-week and 3-month follow-up. We acknowledge that the small sample size limited the statistical power to detect small differences between these time points. However, the magnitude of the differences in biomechanical outcomes between these 2 follow-up points was typically small (Table 2) and lower than the standard error of measurement previously reported for these parameters.17 Therefore, we believe that subtle differences between these time points are unlikely to be clinically important. However, we acknowledge that future randomized controlled trials with larger participant numbers are necessary to further validate our findings and confirm that kinematic changes are maintained over longer time periods.
It is important to note that 1 participant dropped out of the intervention after suffering a tibial stress fracture. This participant reported that the injury onset occurred after a sudden increase in training volume in preparation for a half-marathon. As we did not control participant progression of training volume, it is possible that the injury could be the result of training behaviors rather than a response to the intervention. As such, we recommend that future clinical interventions provide participants with specific advice on the safe progression of running volume to reduce the risk of further injuries.
Conclusion
The results of this study highlight that a 10% increase in step rate improves running kinematics and clinical outcomes at 4 weeks, which are maintained at 3 months, among runners with PFP. Therefore, step rate retraining appears to be a clinically effective intervention in the rehabilitation of PFP and can easily be integrated into clinical practice. It is important to assess running kinematics at baseline to ensure that interventions are appropriately targeted.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This project was internally funded by the University of Salford. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Almonroeder T, Willson JD, Kernozek TW. The effect of foot strike pattern on Achilles tendon load during running. Ann Biomed Eng. 2013;41(8):1758-1766. [DOI] [PubMed] [Google Scholar]
- 2. Besier TF, Gold GE, Delp SL, Fredericson M, Beaupre GS. The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. J Orthop Res. 2008;26(12):1627-1635. [DOI] [PubMed] [Google Scholar]
- 3. Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79(4):371-383. [PubMed] [Google Scholar]
- 4. Bramah C, Preece SJ, Gill N, Herrington L. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46(12):3023-3031. [DOI] [PubMed] [Google Scholar]
- 5. Chumanov ES, Wille CM, Michalski MP, Heiderscheit BC. Changes in muscle activation patterns when running step rate is increased. Gait Posture. 2012;36(2):231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen J. Statistical Power Analysis for the Behavioral Sciences: Revised Edition. New York: Academic Press; 1977. [Google Scholar]
- 7. Collins NJ, Bierma-Zeinstra SM, Crossley KM, van Linschoten RL, Vicenzino B, van Middelkoop M. Prognostic factors for patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2013;47(4):227-233. [DOI] [PubMed] [Google Scholar]
- 8. Cook C, Hegedus E, Hawkins R, Scovell F, Wyland D. Diagnostic accuracy and association to disability of clinical test findings associated with patellofemoral pain syndrome. Physiother Can. 2010;62(1):17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crossley KM, Stefanik JJ, Selfe J, et al. 2016 patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester, part 1: terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50(14):839-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dingenen B, Staes FF, Santermans L, et al. Are two-dimensional measured frontal plane angles related to three-dimensional measured kinematic profiles during running? Phys Ther Sport. 2018;29:84-92. [DOI] [PubMed] [Google Scholar]
- 11. Dos Santos AF, Nakagawa TH, Lessi GC, et al. Effects of three gait retraining techniques in runners with patellofemoral pain. Phys Ther Sport. 2019;36:92-100. [DOI] [PubMed] [Google Scholar]
- 12. Esculier JF, Bouyer LJ, Dubois B, et al. Is combining gait retraining or an exercise programme with education better than education alone in treating runners with patellofemoral pain? A randomised clinical trial. Br J Sports Med. 2018;52(10):659-666. [DOI] [PubMed] [Google Scholar]
- 13. Foch E, Milner CE. Frontal plane running biomechanics in female runners with previous iliotibial band syndrome. J Appl Biomech. 2014;30(1):58-65. [DOI] [PubMed] [Google Scholar]
- 14. Handsaker JC, Forrester SE, Folland JP, Black MI, Allen SJ. A kinematic algorithm to identify gait events during running at different speeds and with different footstrike types. J Biomech. 2016;49(16):4128-4133. [DOI] [PubMed] [Google Scholar]
- 15. Herrington L, Law J. The effect of hip adduction angle on patellar position measured using real time ultrasound scanning. Knee. 2012;19(5):709-712. [DOI] [PubMed] [Google Scholar]
- 16. Lenhart RL, Thelen DG, Wille CM, Chumanov ES, Heiderscheit BC. Increasing running step rate reduces patellofemoral joint forces. Med Sci Sports Exerc. 2014;46(3):557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mason DL, Preece SJ, Bramah CA, Herrington LC. Reproducibility of kinematic measures of the thoracic spine, lumbar spine and pelvis during fast running. Gait Posture. 2016;43:96-100. [DOI] [PubMed] [Google Scholar]
- 18. Merican AM, Amis AA. Iliotibial band tension affects patellofemoral and tibiofemoral kinematics. J Biomech. 2009;42(10):1539-1546. [DOI] [PubMed] [Google Scholar]
- 19. Napier C, Esculier JF, Hunt MA. Gait retraining: out of the lab and onto the streets with the benefit of wearables. Br J Sports Med. 2017;51(23):1642-1643. [DOI] [PubMed] [Google Scholar]
- 20. Neal BS, Barton CJ, Birn-Jeffrey A, Daley M, Morrissey D. The effects & mechanisms of increasing running step rate: a feasibility study in a mixed-sex group of runners with patellofemoral pain. Phys Ther Sport. 2018;32:244-251. [DOI] [PubMed] [Google Scholar]
- 21. Neal BS, Barton CJ, Gallie R, O’Halloran P, Morrissey D. Runners with patellofemoral pain have altered biomechanics which targeted interventions can modify: a systematic review and meta-analysis. Gait Posture. 2016;45:69-82. [DOI] [PubMed] [Google Scholar]
- 22. Neal BS, Lack SD, Lankhorst NE, Raye A, Morrissey D, van Middelkoop M. Risk factors for patellofemoral pain: a systematic review and meta-analysis. Br J Sports Med. 2019;53(5):270-281. [DOI] [PubMed] [Google Scholar]
- 23. Nielsen RO, Parner ET, Nohr EA, Sorensen H, Lind M, Rasmussen S. Excessive progression in weekly running distance and risk of running-related injuries: an association which varies according to type of injury. J Orthop Sports Phys Ther. 2014;44(10):739-747. [DOI] [PubMed] [Google Scholar]
- 24. Noehren B, Hamill J, Davis I. Prospective evidence for a hip etiology in patellofemoral pain. Med Sci Sports Exerc. 2013;45(6):1120-1124. [DOI] [PubMed] [Google Scholar]
- 25. Noehren B, Pohl MB, Sanchez Z, Cunningham T, Lattermann C. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech. 2012;27(4):366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med. 2011;45(9):691-696. [DOI] [PubMed] [Google Scholar]
- 27. Nunes GS, Stapait EL, Kirsten MH, de Noronha M, Santos GM. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14(1):54-59. [DOI] [PubMed] [Google Scholar]
- 28. Piva SR, Gil AB, Moore CG, Fitzgerald GK. Responsiveness of the activities of daily living scale of the knee outcome survey and numeric pain rating scale in patients with patellofemoral pain. J Rehabil Med. 2009;41(3):129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33(11):677-685. [DOI] [PubMed] [Google Scholar]
- 30. Powers CM, Witvrouw E, Davis IS, Crossley KM. Evidence-based framework for a pathomechanical model of patellofemoral pain: 2017 patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester, UK, part 3. Br J Sports Med. 2017;51(24):1713-1723. [DOI] [PubMed] [Google Scholar]
- 31. Preece SJ, Mason D, Bramah C. The coordinated movement of the spine and pelvis during running. Hum Mov Sci. 2016;45:110-118. [DOI] [PubMed] [Google Scholar]
- 32. Roper JL, Harding EM, Doerfler D, et al. The effects of gait retraining in runners with patellofemoral pain: a randomized trial. Clin Biomech (Bristol, Avon). 2016;35:14-22. [DOI] [PubMed] [Google Scholar]
- 33. Sinclair JK. Effects of a 10 week footstrike transition in habitual rearfoot runners with patellofemoral pain. Comp Exerc Physiol. 2016;12(3):141-150. [Google Scholar]
- 34. Smith BE, Selfe J, Thacker D, et al. Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Streiner D, Geddes J. Intention to treat analysis in clinical trials when there are missing data. Evid Based Ment Health. 2001;4(3):70-71. [DOI] [PubMed] [Google Scholar]
- 36. Taunton JE, Ryan MB, Clement D, McKenzie DC, Lloyd-Smith D, Zumbo B. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Gent RN, Siem D, van Middelkoop M, et al. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med. 2007;41(8):469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Mechelen W. Running injuries: a review of the epidemiological literature. Sports Med. 1992;14(5):320-335. [DOI] [PubMed] [Google Scholar]
- 39. Willson JD, Kernozek TW, Arndt RL, Reznichek DA, Scott Straker J. Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin Biomech (Bristol, Avon). 2011;26(7):735-740. [DOI] [PubMed] [Google Scholar]
- 40. Willy RW, Buchenic L, Rogacki K, Ackerman J, Schmidt A, Willson JD. In-field gait retraining and mobile monitoring to address running biomechanics associated with tibial stress fracture. Scand J Med Sci Sports. 2016;26(2):197-205. [DOI] [PubMed] [Google Scholar]
- 41. Willy RW, Manal KT, Witvrouw EE, Davis IS. Are mechanics different between male and female runners with patellofemoral pain? Med Sci Sports Exerc. 2012;44(11):2165-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Willy RW, Scholz JP, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech. 2012;27(10):1045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamato TP, Saragiotto BT, Lopes AD. A consensus definition of running-related injury in recreational runners: a modified Delphi approach. J Orthop Sports Phys Ther. 2015;45(5):375-380. [DOI] [PubMed] [Google Scholar]