Abstract
Background:
Returning to community walking remains a major challenge for persons with incomplete spinal cord injury (iSCI) due, in part, to impaired interlimb coordination. Here, we examined spatial and temporal features of interlimb coordination during walking and their associations to gait deficits in persons with chronic iSCI.
Research Question:
Do deficits in spatial and temporal interlimb coordination correspond differentially to clinical indicators of walking performance in persons with iSCI?
Methods:
Sixteen persons with chronic iSCI and eleven able-bodied individuals participated in this study. Participants walked at self-selected gait speeds along an instrumented walkway that recorded left and right step lengths and times. We quantified interlimb coordination in terms of normalized differences between left and right step lengths (spatial asymmetry index) and step times (temporal asymmetry index), as well as, gap and phase coordination indices. We then assessed the extent to which these indices independently associated with clinical measures of walking performance.
Results:
Participants with iSCI demonstrated greater spatial and temporal asymmetry, as well as, reduced gap and phase interlimb coordination as compared to age-matched controls (p<0.001). We found no linear relationships between spatial and temporal asymmetry indices (p>0.05) or between gap and phase coordination indices (p>0.05). Spatial and temporal asymmetry indices weakly correlated with SCI-FAI composite scores (r2=0.26; p=0.04). However, only spatial asymmetry indices strongly correlated with slower walking speed (r2=0.51; p<0.002). We also found participants who used a hand-held assistive device (walker) demonstrated great spatial asymmetry as compared to those who did not (p<0.03).
Significance:
Differential impairments in spatial and temporal interlimb coordination correspond to overground walking deficits in persons with chronic iSCI. Spatial asymmetry associated with decreased walking speed and increased reliance on hand-held assistive devices. Gait training methods that target well-defined space and time domains of interlimb coordination may enhance overground gait training in persons with iSCI.
Keywords: Spinal cord injury, gait kinematics, spatiotemporal asymmetry, walking, interlimb coordination
1. Introduction
Restoring community walking is a high priority for persons with incomplete spinal cord injury (iSCI) [1]. While many preserve some walking ability, most have life-long challenges with regaining functional walking without the need for hand-held assistive devices (AD; crutches, canes, walkers). Unfortunately, treatment strategies to overcome this priority remains frustratingly insufficient due, in part, to limited understanding of how injury-specific changes in motor coordination impact walking performance. Although routine clinical assessments that focus on walking quality, speed, and distance [2, 3] are functionally meaningful, they provide little insight into important spatiotemporal components that contribute to these coarse walking performance metrics. Establishing assessment tools to characterize injury-specific impairments may translate into greater walking improvements in persons with iSCI.
Restoring coordinated leg movements often remains compromised in persons with chronic iSCI [4]. Endogenous mechanisms of plasticity contribute to partial return of limb movements, but often result in abnormal muscle co-activity (e.g., spasms) during walking. Hayes et al. found persons who regained walking ability after iSCI have a limited repertoire of muscle co-activity patterns, which were relatively invariant across a range of overground cadence conditions as compared to able-bodied (AB) individuals [5]; participants who demonstrated less flexibility to recruit muscle patterns walked slower [5]. In several iSCI participants, abnormal muscle co-activity persisted for extended time during stance and swing phases of walking. Similar impairments in temporal coordination, depicted by Grasso and colleagues, showed co-activity of muscles spanning the hip, knee, and ankle coincided with inconsistent single-limb step placement during transitions between stance and swing phases of walking [4]. Similarly, Sohn and colleagues found iSCI-induced intralimb deficits in spatial (step placement) coordination corresponded to inconsistent step-by-step foot placement and interjoint coordination, which corresponded to limited walking speed and more restrictive assistive device (AD) use (i.e., walker); those who walked slower, demonstrated more kinematic variability and more reliance on AD [6].
Collectively, these studies nicely characterize iSCI-induced constraints on spatial and temporal intralimb coordination and subsequently deficits in walking ability. Since iSCI impacts bilateral locomotor control circuitry, differential changes in spatial and temporal coordination likely extend beyond a single limb. Thus, quantifying spatial and temporal contributions to interlimb coordination, as well as, to injury-specific walking deficits may uncover valuable treatment targets for gait rehabilitation.
Several studies show supraspinal structures contribute to sensorimotor adaptation during treadmill walking, with adaptation distinctly modulated for spatial and temporal aspects of interlimb coordination [7–9]. While spatiotemporal modulation is often compromised after cortical stroke [10], studies show restoring spatial (step length) and temporal (step time) components of interlimb coordination contribute to improved walking recovery [11, 12]. Thus, characterizing patterns of interlimb coordination in persons with iSCI may provide similar insight into spatial and temporal gait asymmetry parameters that 1) contribute to reduced walking performance (e.g., speed, AD use) and 2) serve as targets for new treatment methods.
Contributions of spatial and temporal interlimb coordination on walking performance after iSCI are important but remain poorly understood. Two recent studies showed abnormal step length ratios contribute to reduced walking speed and balance in persons with iSCI [13, 14]. However, they did not consider the relationship between and relative contributions of space and time components of interlimb coordination (i.e., step length and time) during walking. Incomplete SCI likely disrupts a disproportionate number of spared pathways important for coordinating interlimb movements in space and time. In this study, we assessed spatial and temporal interlimb coordination and their relative contributions to overground walking performance in persons with iSCI. We hypothesized that persons with iSCI demonstrate dissociated interlimb coordination impairments in space and time that uniquely correlate with functional walking deficits and assistive-device dependence. Results from this study support our hypothesis and provide rational for establishing injury-specific gait training that precisely targets spatial and/or temporal interlimb coordination impairments.
2. Methods
2.1. Study Population
Sixteen persons with chronic, motor-incomplete iSCI, as defined using International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [15], participated in the study (Table 1). We also enrolled eleven able-bodied (AB) individuals to serve as age-matched controls (average age: 39.1 ± 13.2 years). Inclusion criteria: d≥ 18 years of age, > 1-year post-iSCI, injury level between C2-T12, ability to reliably follow two-step simple verbal instructions, and ability to walk 10 meters without human assistance (with or without AD). Participants were excluded if they had a brain injury or cognitive impairment impacting their ability to follow simple instructions, progressive iSCI, other concurrent severe medical condition, severe weight-bearing pain that limited walking, and/or severe visual impairment. All participants reviewed and provided written informed consent approved by Institutional Review Boards prior to study participation.
Table 1.
Demographics of participants with iSCI
| Participant | Age (yrs) | Gender | Level of Injury | Years post - injury | Walking Aid | LEMS (R/L) | AIS |
|---|---|---|---|---|---|---|---|
| S01 | 27 | M | C5 | 8 | Loftstrands | 15/14 | D |
| S02 | 59 | M | T3 | 24 | Cane | 23/24 | D |
| S03 | 24 | M | C5 | 3 | Loftstrands | 12/24 | D |
| S04 | 61 | M | T8 | 6 | Loftstrands | 24/25 | D |
| S05 | 58 | F | C3 | 2 | Walker | 17/24 | D |
| S06 | 27 | M | C5 | 7 | Single Lofstrand | 19/22 | D |
| S07 | 28 | F | C6 | 5 | Walker | 22/13 | D |
| S08 | 42 | F | C5 | 6 | None | 23/25 | D |
| S09 | 30 | M | C5 | 2 | Walker | 23/21 | D |
| S10 | 35 | M | C5 | 5 | Walker | 21/13 | D |
| S11 | 54 | M | C4 | 2 | Walker | 19/19 | D |
| S12 | 32 | M | T9 | 9 | Lofstrands | NA | C |
| S13 | 56 | M | T11 | 3 | Cane | 22/12 | D |
| S14 | 69 | F | C4 | 1 | Walker | 23/23 | D |
| S15 | 25 | F | T11 | 5 | Cane | 11/11 | C |
| S16 | 29 | M | C5 | 11 | Loftstrands | 20/24 | D |
| Range | 24–61 | C3 - T11 | 1 – 24 |
Abbreviations: M, Male; F, Female; C, Cervical; T, Thoracic; LEMS, Lower Extremity Motor Score; AIS, American Spinal Injury Association Impairment Scale
2.2. Clinical assessments
Participants with iSCI received a physical evaluation by the study’s licensed physical therapist. ISNCSCI classified neurological injury level and completeness [15]; lower extremity motor scores (LEMS) from ISNCSCI measured leg strength. The 10-Meter Walk Test (10MWT) and 6-Minute Walk Test (6MWT) assessed walking speed and endurance, respectively [16]. Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI)[17] evaluated walking function. We required the same upper-limb AD for walking assessments and experiment protocols. We randomized the order of assessments.
2.3. Protocols
We recorded spatiotemporal gait parameters during self-selected walking speed using commercially available GAITRite® and CIRFace® Systems (CIR Systems, Inc.; USA) (Table 2). These gait assessment systems have high test-retest reliability, concurrent validity within and between systems, and have previously been used to assess overground walking in persons with iSCI [18, 19]. Participants completed approximately 30 steps (~3–10 consecutive walking trials) across the instrumented walkway. Rest breaks were provided between trials as needed to minimize fatigue effects. Participants started and stopped walking 2.0 m beyond the data collection area to reduce confounding effects of acceleration/deceleration on walking variability [20]. Participants with iSCI walked with the same upper-limb AD they use during community ambulation (Table 1). During each trial, digitized kinematic data corresponding to number of steps, swing time, stride time, step time for left and right legs, stride length, step length, and step width between left and right legs were recorded. Gait assessment data were stored on a desktop computer for further processing and analyses.
Table 2.
Spatiotemporal gait parameters
| Parameters | AB (Mean ± SE) | iSCI (Mean ± SE) | P-value |
|---|---|---|---|
| Velocity (m/s) | 1.39 ± 0.04 | 0.59 ± 0.09 | <0.001* |
| Stride time (s) | 2.63 ± 0.33 | 1.08 ± 0.17 | <0.001* |
| Stride length (cm) | 148.93 ± 3.44 | 98.50 ± 8.01 | <0.001* |
| Stride width (cm) | 7.98 ± 0.57 | 14.82 ± 1.57 | 0.001* |
Abbreviations: AB, Able-body; iSCI, Incomplete Spinal Cord Injury;
SD, Standard Deviation;
Significant difference between groups (p<0.005)
2.4. Data processing
Spatial and temporal interlimb coordination were quantified using asymmetry indices as defined previously [21]. To ensure consistent analyses across participants, the leg with the higher average swing time corresponded to the “reference” leg [22]. We calculated standardized step lengths (m) and step times (s) as distance and time between contact of reference leg and contact of opposite leg. We computed an asymmetry index (AI) for step length (spatial) as the absolute value of the mean difference between left and right step length over the sum of step lengths for left and right legs. Similarly, we computed an asymmetry index (AI) for step time (temporal) as the absolute value of the mean difference between left and right step time over the sum of step times for left and right legs. An AI=0 indicated perfect spatial and temporal symmetry.
We computed spatial and temporal coordination indices to determine accuracy and consistency of interlimb coordination [21]. Each index is a combined measure of symmetry ‘accuracy’ over several steps (interval) as well as large variations from step to step (coefficient of variation). Gap interval was defined as the ratio of step length to stride length and phase interval was defined as the ratio of step time to stride time [21]. These intervals were scaled to a unit circle and averaged to a maximum out-of-phase angle of 180 degrees [23]. Each interval therefore quantifies the accuracy of symmetry for each step of gait cycle where the difference from 180° represents the phase difference (degrees) from ideal gait symmetry. Coefficient of variation (CV) was computed for gap and phase intervals to assess step-by-step variability [21]. Gap coordination index (GCI) corresponded to spatial coordination as the sum of spatial coordination interval and CV. Similarly, phase coordination index (PCI) corresponded to temporal coordination as the sum of temporal coordination interval and CV.
2.5. Statistical Analyses
We performed statistical analyses using SPSS 24® (IBM SPSS Inc., USA). Significant results are reported at the p ≤ 0.05 level and represented as mean±1 standard deviation. To test the primary hypothesis that iSCI impairs spatial and temporal interlimb coordination during overground walking, we performed an independent samples t-test with between subject groups (AB, iSCI) as the independent measure and spatial AI, temporal AI, GCI, and PCI as dependent measures. We additionally tested for differences in speed, stride length, width, and time between SCI and AB groups. Significant differences between AB and iSCI group comparisons are reported at a Bonferroni corrected α≤.006.
To determine the relationship between spatial and temporal coordination and functional walking deficits after iSCI, we performed linear regression analyses. Specifically, we tested for correlations between spatial and temporal AI, GCI, and PCI, as well as, AI and walking assessment scores (10MWT, 6MWT, and SCI-FAI) in iSCI subjects. Since a prior study showed persons with iSCI who required a “walker” for ambulation had greater intralimb coordination deficits as compared to those who used a less restrictive AD (i.e., cane, loftstrands) [6], we predicted a similar result with interlimb coordination. Thus, we stratified our iSCI subjects into those who used a “walker” (N = 7) for ambulation and those who did not (N = 9). Using an independent samples t-test, we compared spatial and temporal interlimb coordination between participants designated as “walker” and participants designated as “no-walker”. We used a parametric test based on a Levene Test that revealed no significance in variances between our comparison groups (p > 0.05) [24].
3. Results
Persons with iSCI demonstrated abnormal gait kinematics during overground walking. As compared to AB, participants with iSCI presented with decreased self-selected walking speed (iSCI:0.59 ± 0.09m/s, AB:1.39 ± 0.04m/s; t1,26 = 7.71, p < 0.001), stride time (iSCI: 2.63 ± 0.33s, AB: 1.08 ± 0.17s; t1,26=4.05, p<0.001), and stride length (iSCI: 98.50 ± 8.01cm, AB: 148.93 ± 3.4cm; t1,26 = 5.17, p < 0.001), but increased stride width (iSCI: 14.82 ± 1.57cm, AB: 7.98 ± 0.57cm; t1,26 = −3.63, p = 0.001). During self-selected walking, persons with iSCI exhibited different step times between legs (t1,15 = −2.70, p = 0.02), but not step lengths between legs (t1,15 = 0.72, p = 0.48). AB subjects exhibited consistent step times (t1,11 = 2.05, p = 0.07) and step lengths (t1,11 = 1.30, p = 0.22).
3.1. Spatiotemporal interlimb coordination
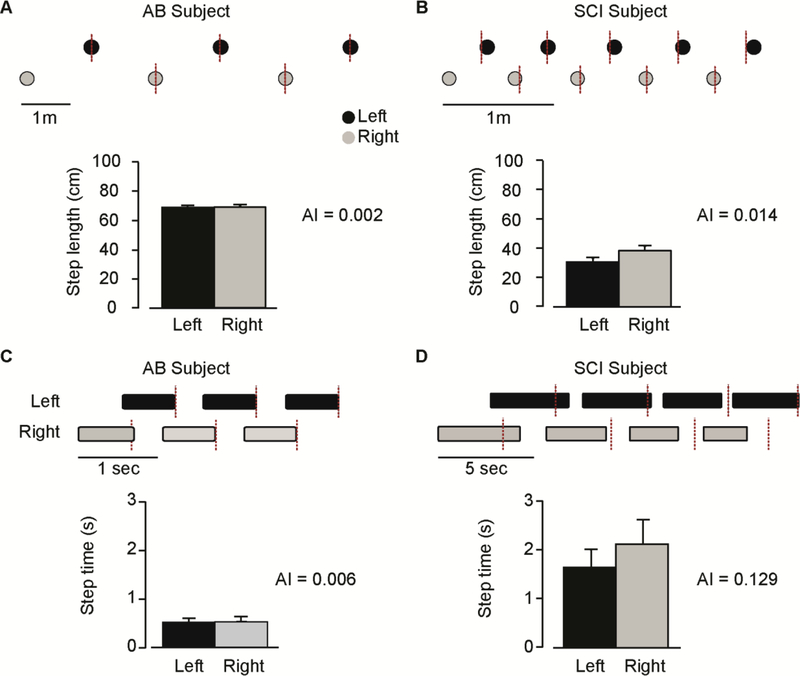
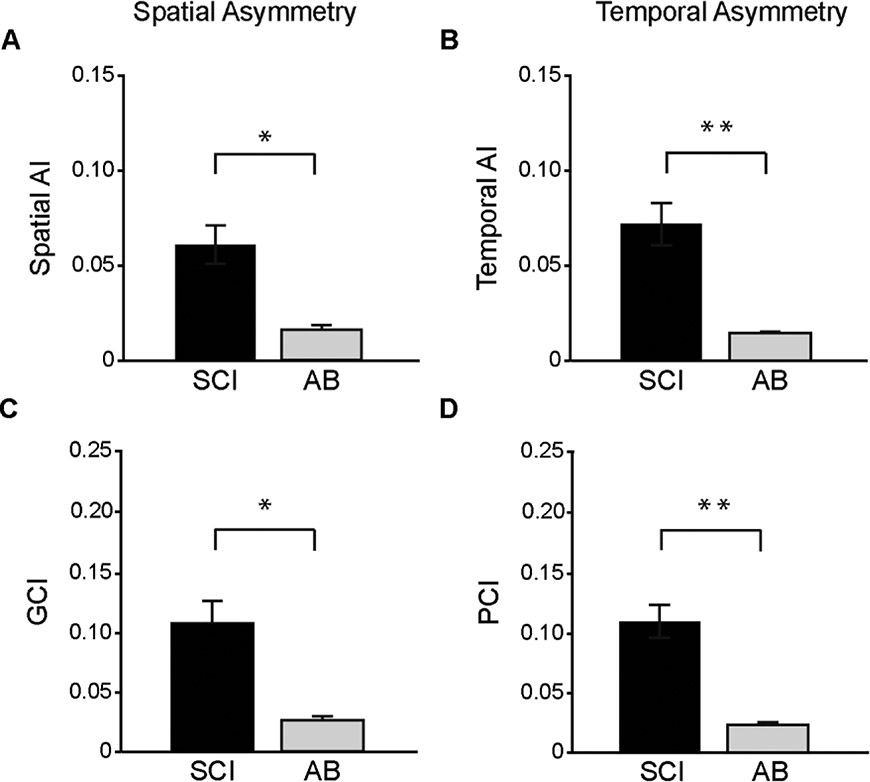
Interlimb coordination was significantly impaired in persons with iSCI as compared to AB subjects. Figure 1 shows typical measurements of temporal and spatial interlimb coordination during a single trial from one AB subject (AB1) and one person with iSCI (SCI10). For the AB subject, contact of the right leg occurred approximately at mid-cycle duration of the left leg (Figure 1A) and stride length (Figure 1C), resulting in negligible spatial AI and temporal AI. In contrast, the participant with iSCI completed right foot contact prior to mid-stride duration of left foot (Figure 1B) and stride length (Figure 1D). We found greater spatial (t1,26 = 3.69, p = 0.001) and temporal (t1,26 = 4.48, p ≤ 0.001) AI in the iSCI group as compared to AB group (Figure 2A and 2B). Participants with iSCI also showed greater GCI (t26 = 5.40, p ≤ 0.001) and PCI (t1,26 = 5.34, p ≤ 0.001) as compared to AB subjects. We also found between-group differences in CV for gap (t1,26 = 3.12, p = 0.004, AB: 0.02 ± 0.001, iSCI: 0.06 ± 0.01) and phase (t1,26 = 4.54, p ≤ 0.001, AB: 0.01 ± 0.001, iSCI: 0.05 ± 0.01) coordination intervals.
Figure 1.
Spatial (A-B) and temporal (C-D) interlimb coordination for a person with incomplete spinal cord injury (right panel) and a healthy control (left panel). The four panels (A-D) are organized the same way. 1) Example of a few steps with stance (black bars) and swing (white spaces) phase durations for the spatial interlimb coordination panels (A-B) and distance between the left (black dot) and right (grey dot) contacts for the temporal interlimb coordination panels (C-D). The red dashed lines represent the value of right foot contact for a participant with perfect temporal and spatial symmetry. 2) Step length (A-B) and step time (C-D) mean values for the left (black bars) and right (grey bars) legs. An asymmetry index (AI) is also calculated to show the difference between the left and the right leg.
Figure 2.
Comparison of the spatial and temporal asymmetry indices of the iSCI and AB groups. Each bar graph represents the group mean (±1 SE) of each asymmetry index. Black bars for the iSCI group and light grey bars for the AB control group. Panels in the left column (A, C) depict spatial asymmetry indices while the right column panels (B, D) show temporal asymmetry indices. A: Spatial asymmetry index. B: Temporal asymmetry index. C: Gap coordination index. D: Phase coordination index. * indicates p=0.001. **indicates p<0.001
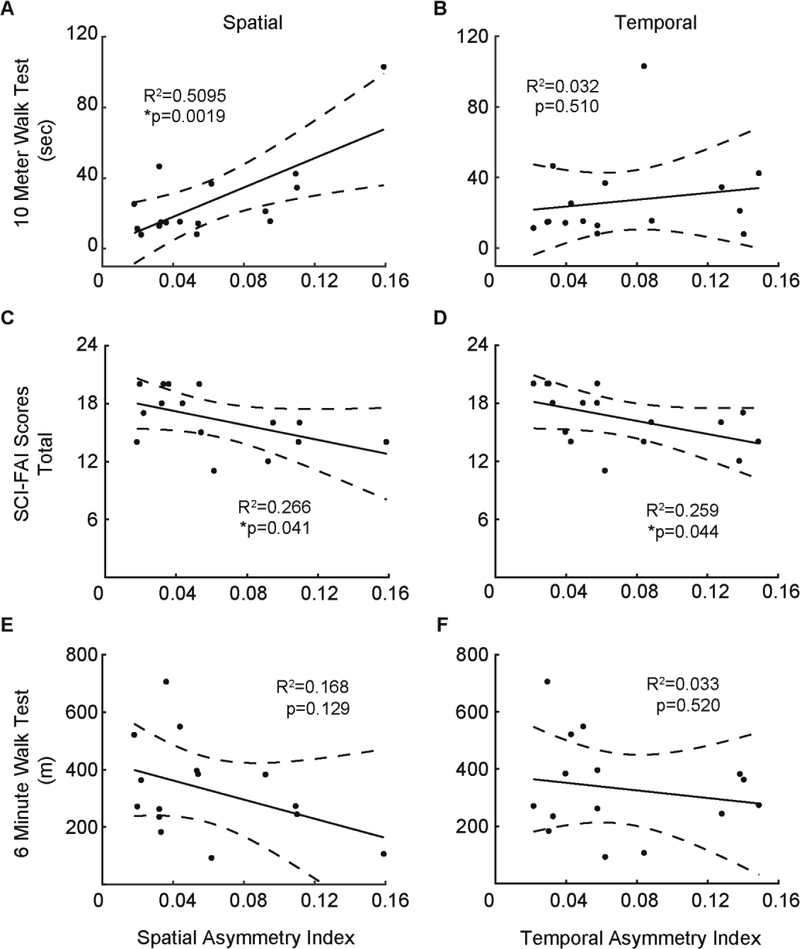
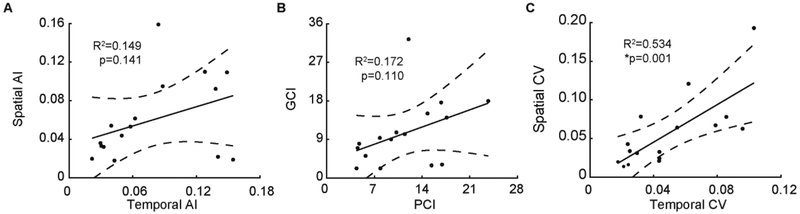
Using linear regression models, we examined the relationship between spatial and temporal interlimb coordination during overground walking in persons with iSCI. Surprisingly, we found no significant linear relationship between spatial and temporal AI (Figure 3A). Figure 3B shows no significant linear relationship between GCI and PCI; however, a linear relationship exists between their coefficient of variations (r2 = 0.53, p = 0.001, Figure 3C). These findings indicate co-variations in spatial and temporal interlimb coordination occurred apart from their dissimilar asymmetry indices.
Figure 3.
Relationship between spatial and temporal asymmetry indices of the iSCI group. Lines within each panel indicate linear regression and dashed lines delineate 95% confidence interval. Each marker represents one subject. A: spatial asymmetry index vs temporal asymmetry index. B: Gap coordination interval (GCI) versus phase coordination interval (PCI). GCI and PCI are quantified to reflect the global asymmetry as well as the step-to-step variability of this asymmetry. C: Coefficient of variation (CV) of the gap interval (spatial) versus coefficient of variation of the phase interval (temporal). Only the relationship between spatial and temporal CV was significant.
3.2. Interlimb coordination and walking performance
Persons with iSCI presented with spatial and temporal interlimb asymmetries that corresponded to overground walking deficits. Spatial AI and 10MWT significantly correlated with each other; iSCI subjects with slower 10MWT times demonstrated greater spatial asymmetry, while those with faster 10MWT times demonstrated lesser spatial asymmetry (Figure 4A). Spatial AI also correlated with composite SCI-FAI scores (Figure 4C). However, we found no linear relationship between spatial AI and 6MWT (Figures 4E). Temporal AI correlated with composite SCI-FAI score (Figure 4D) but did not correlate with 10MWT or 6MWT (Figures 4B and 4F).
Figure 4.
Relationship between clinical measures and spatial/temporal asymmetry measures for participants with iSCI. Line indicates linear regression while dashed lines delineates the 95% confidence intervals. Each marker represents one subject. Panels in left column (A, C, E) show the relationship between a single clinical measure against the spatial asymmetry index. Panels in the right column (B, D, F) show the regression between the same clinical measures and temporal asymmetry index. A, B: 10-meter walk test. C, D: Spinal cord injury functional ambulation inventory (SCI-FAI) score. E, F: 6-minute walk test. The relationship between 10MWT and spatial AI was significant. Only the SCI-FAI scores were significantly related to both spatial and temporal asymmetry indices.
Altered GCI and PCI coordination indices corresponded to overground walking deficits in persons with iSCI. Similar to spatial AI, we report linear relationships between GCI and overground walking speed (r2 = 0.26, p = 0.04) and 10MWT (r2 = 0.56, p = 0.001), but not between GCI and SCI-FAI (r2 = 0.21, p = 0.08) or 6MWT (r2 = 0.10, p = 0.18). Similar to temporal AI, we found no linear relationships between PCI and 10MWT (r2 = 0.05, p = 0.41), or 6MWT (r2 = 0.04, p = 0.47), but found a weak correlation between PCI and SCI-FAI score (r2 = 0.33, p = 0.02).
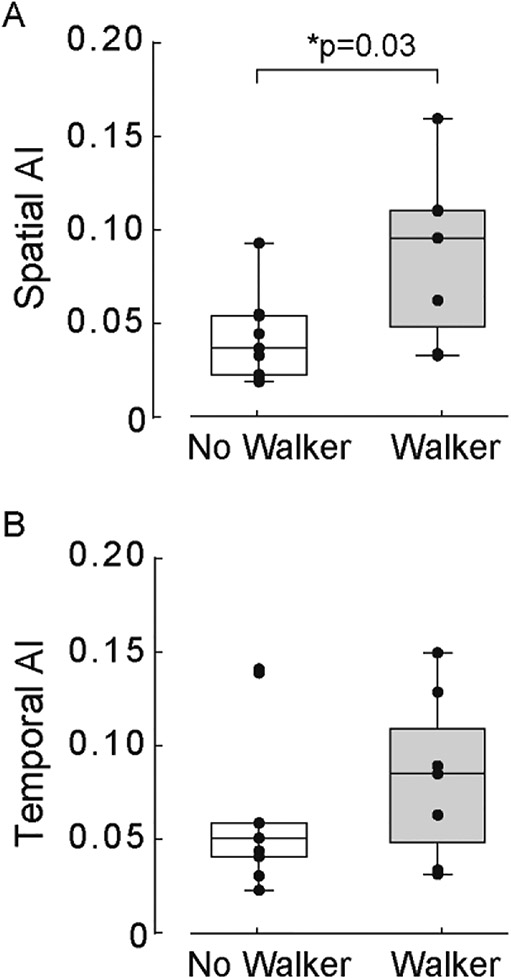
All participants with iSCI used upper-limb ADs for ambulation (Table 1). Here, we extend upon an earlier result that showed persons with iSCI who use “walker” ADs demonstrate the largest gait asymmetries [25]; we compared spatial and temporal components of interlimb coordination between those who used a “walker” AD (7 of 16) versus those who did not (no-walker). As shown in Figure 5A, we observed greater spatial AI (t1,16 = 2.37, p = 0.03); the “walker” group (0.09 ± 0.05) as compared to “no-walker” group (0.04 ± 0.02). However, similar comparisons for temporal AI, GCI, PCI revealed no difference between AD groups (all p>0.08, see Figure 5B). These findings suggest that iSCI-induced interlimb coordination impairments may differentially impact AD-use and functional walking ability in space and time.
Figure 5.
Box-and-whisker plot comparisons between “Walker” and “No-Walker” groups for (A) spatial asymmetry and (B) temporal asymmetry indices. Each black circle represents one participant.
4. Discussion
4.1. Summary
In the present study, we report significant interlimb coordination deficits during overground walking in persons with chronic iSCI. Despite observed deficits in where and when persons with iSCI place their limbs during overground walking, we found no association between space and time parameters. To our surprise, spatial and temporal interlimb coordination measures also correlated quite differently with our clinical indicators of walking performance (10MWT, 6MWT, and SCI-FAI) and AD use. These findings affirm the need for precision treatments that recalibrate spatial and temporal control of interlimb movements and subsequently improve functional walking after iSCI.
4.2. Interlimb coordination is impaired after iSCI
Identifying the contributions of spatial and temporal domains on interlimb coordination impairments during overground walking in humans with iSCI is important. Several studies have characterized spatial and temporal gait features during walking across a range of central nervous system pathologies such as stroke, Parkinson’s Disease, and multiple sclerosis [11, 26–28], but remain relatively understudied in persons with iSCI. A recent study reported an association between gait-related asymmetries and reduced walking speed and balance in persons with iSCI [13], but did not assess possible interdependence between spatial and temporal interlimb coordination and their association to walking performance. Here, we confirmed this prior study’s findings and expanded upon them by showing that interlimb coordination is differentially impaired in space and time following iSCI.
Participants with iSCI showed significantly greater step-to-step variability in interlimb coordination as compared to AB subjects as quantified by each coordination index. However, separating the coordination intervals into their accuracy (interval) and consistency (CV) components further reveals contrasts across each domain. SCI participants show greater mean interval deviations from symmetrical 180° in the temporal domain than the spatial domain (10.60° vs 8.17°). On the other hand, mean CV components between temporal and spatial domains were more similar (5.04° vs 5.79°). The CV components correlated with each other such that participants who demonstrated more spatial variability also demonstrated more temporal variability (Figure 4c). The non-significant association between the composite spatial and temporal asymmetry indices (Figure 4b) may therefore be driven by unequal deficits in phase accuracy between spatial and temporal domains rather than differences in step to step consistency. This suggests corrupt sensorimotor control underlying the reproducibility of consistent motor coordination during walking, supplementing prior reports of increased kinematic variability within a single limb [6]. Thus, even though accuracy maybe differentially impaired between space and time domains, consistency from step to step appears equally impaired. Reducing variability in anti-phase coordination rather than asymmetry magnitude alone could be a relevant clinical target to improve walking performance.
In several injury-specific cases, we found spatial asymmetry without temporal asymmetry and vice versa. This dissociation may reflect walking deficits involving distinct mechanisms and neural substrates that alter control priorities of limb position and time. Several studies in rodents and humans show cerebellar pathways are important for interlimb coordination [9, 29, 30] and other studies report the cerebellum tightly interacts with cortical structures to regulate spatial symmetry and with input from the brainstem and spinal cord, to regulate temporal symmetry [7, 9]. Earlier studies also report spinal interneurons are differentially encoded for timing and placement of limbs during locomotion [31, 32]. While our results appear in accordance with theoretical frameworks for dissociative [11, 21] and hierarchical [29, 33] control of interlimb coordination after iSCI, we provide no evidence for one control organization over the other nor for underlying pathways and mechanisms. Nevertheless, future research should focus on how SCI severity or treatment approaches may impact the interdependence, or lack thereof, between neural structures that provide spatial and/or temporal control of coordinated walking.
4.3. Clinical correlates of interlimb asymmetries
Clinical gait measures are useful tools to quickly assess walking ability in persons with iSCI [34]. However, they do not capture the underlying interlimb coordination strategies used by individuals [35], especially those more reliant on AD use [6]. SCI-FAI only modestly correlated with asymmetry parameters, suggesting that this qualitative clinical scale of leg coordination may not adequately identify interactions between spatial and temporal coordination deficits that are patient specific. An important qualification, however, is that these asymmetry indices captured sagittal plane coordination deficits whereas SCI-FAI more broadly encompasses other parameters such as weight shifts, step width, and step height. We observed a significant correlation between spatial asymmetry and 2 of 3 measures of functional walking ability (10MWT, SCI-FAI). This finding may imply that individuals shift spatial control strategies depending on tasks goals (i.e. speed vs. endurance) that optimize functions such as metabolic cost, balance [36, 37], and/or fatigue [38]. On the other hand, temporal asymmetries only weakly aligned with 1 of 3 measures of walking ability (SCI-FAI), which may reveal more spinally derived control strategies that poorly discriminate volitional-based strategies. Instead, temporal improvements may rely more on involuntary strategies and proprioceptive feedback. Such appears to be the case with operant conditioning of H-reflexes, a training paradigm that improved step time (temporal) symmetry during overground walking after 10 weeks of H-reflex matching in persons with iSCI [39]. Thus, differential impairments of spatial and temporal coordination may be injury-specific markers of and possibly contributors to overground walking deficits following iSCI.
4.4. Limitations
Despite our intriguing findings, there are some study limitations that should be noted. First, our small sample size restricted generalization of AD reliance on interlimb coordination. Our results linked AD use with spatial AI but were limited to comparing only “Walker” and “No Walker” sub-groups. Second, our higher functioning cohort of AIS D (14 of 16) participants did not enable us to examine the impact of more severe SCI on spatial and temporal interlimb coordination. While step length asymmetry is known to be significantly larger in persons with AIS C as compared to AIS D iSCI [14], this may not be the case for temporal asymmetry. Third, our iSCI participants walked ~58% slower than AB controls. Since interlimb asymmetry is greater at slower walking speed [40], we acknowledge this confound may contribute, in part, to observed between-group differences in spatial and temporal asymmetries. While matching gait speeds may yield some insight into this possibility, we contend that iSCI-matched walking speeds is unnatural for AB participants and adds task complexity. Last, this study did not assess the potential impact of frontal plane interlimb asymmetries on walking performance. Matsubara and colleagues found persons with iSCI modify their frontal plane mechanics in response to decreased lateral stabilization [34], but we do not yet know if these adaptations alter spatiotemporal symmetry. In sum, future work is needed to determine the extent to which iSCI disrupts neural representations of time and space and consequently limit sensorimotor control of coordinated leg movements during overground walking.
5. Conclusion
This study reports on impaired interlimb coordination during overground walking in persons with iSCI. Commonly used clinical tools inadequately capture injury-specific spatiotemporal gait impairments between limbs during walking. Results from this study demonstrate that spatial and temporal interlimb coordination are impaired and appear dissociated after iSCI. Modification of step timing or step positioning between left and right legs may improve with gait training paradigms that use spatial and/or temporal feedback; however, this possibility remains unanswered. Thus, our findings motivate further inquiry into establishing targeted gait training approaches to recalibrate spatial and temporal control of interlimb coordination after iSCI.
Highlights.
Spatial and temporal interlimb coordination are differentially impaired after iSCI.
Spatial and temporal asymmetry correlate with walking deficits after iSCI.
Spatial asymmetry corresponds to increased reliance on walking aides after iSCI.
Treatments targeting spatial and temporal asymmetry may improve walking after iSCI.
Acknowledgements
The authors thank Heather Hayes for her assistance in data collection and Stella Barth for her assistance with manuscript editing. We especially thank our study participants.
Funding
This work was supported by NIH NICHD R01 HD081274.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
The authors declare no conflict of interest.
Contributor Information
Yann Thibaudier, College of Engineering, University of Florida.
Andrew Q Tan, Department of Physical Medicine & Rehabilitation, Harvard Medical School, Spaulding Rehabilitation Hospital.
Denise M. Peters, Department of Rehabilitation and Movement Science, University of Vermont
Randy D. Trumbower, Department of Physical Medicine and Rehabilitation, Harvard Medical School, Spaulding Rehabilitation Hospital
References
- [1].Ditunno PL, Patrick M, Stineman M, Ditunno JF, Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study, Spinal Cord 46(7) (2008) 500–6. http://www.ncbi.nlm.nih.gov/pubmed/18209742. [DOI] [PubMed] [Google Scholar]
- [2].Sturt RN, Holland AE, New PW, Walking ability at discharge from inpatient rehabilitation in a cohort of non-traumatic spinal cord injury patients, Spinal Cord 47(10) (2009) 763–8. https://www.ncbi.nlm.nih.gov/pubmed/19365395. [DOI] [PubMed] [Google Scholar]
- [3].Kim CM, Eng JJ, Whittaker MW, Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength, Spinal Cord 42(3) (2004) 156–62. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15001980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Castellano V, et al. , Distributed plasticity of locomotor pattern generators in spinal cord injured patients, Brain 127(Pt 5) (2004) 1019–34. https://www.ncbi.nlm.nih.gov/pubmed/14988161. [DOI] [PubMed] [Google Scholar]
- [5].Hayes HB, Chvatal SA, French MA, Ting LH, Trumbower RD, Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury, Clin Neurophysiol 125(10) (2014) 2024–35. http://www.ncbi.nlm.nih.gov/pubmed/24618214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sohn WJ, Tan AQ, Hayes HB, Pochiraju S, Deffeyes J, Trumbower RD, Variability of Leg Kinematics during Overground Walking in Persons with Chronic Incomplete Spinal Cord Injury, J Neurotrauma (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Malone LA, Bastian AJ, Torres-Oviedo G, How does the motor system correct for errors in time and space during locomotor adaptation?, J Neurophysiol 108(2) (2012) 672–83. http://www.ncbi.nlm.nih.gov/pubmed/22514294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Malone LA, Bastian AJ, Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation, J Neurophysiol 103(4) (2010) 1954–62. http://www.ncbi.nlm.nih.gov/pubmed/20147417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vasudevan EV, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ, Younger is not always better: development of locomotor adaptation from childhood to adulthood, J Neurosci 31(8) (2011) 3055–65. https://www.ncbi.nlm.nih.gov/pubmed/21414926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Finley JM, Long A, Bastian AJ, Torres-Oviedo G, Spatial and Temporal Control Contribute to Step Length Asymmetry During Split-Belt Adaptation and Hemiparetic Gait, Neurorehabil Neural Repair 29(8) (2015) 786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Krasovsky T, Levin MF, Review: toward a better understanding of coordination in healthy and poststroke gait, Neurorehabil Neural Repair 24(3) (2010) 213–24. http://www.ncbi.nlm.nih.gov/pubmed/19822722. [DOI] [PubMed] [Google Scholar]
- [12].Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS, Walking speed and step length asymmetry modify the energy cost of walking after stroke, Neurorehabil Neural Repair 29(5) (2015) 416–23. http://www.ncbi.nlm.nih.gov/pubmed/25288581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kumprou M, Amatachaya P, Sooknuan T, Thaweewannakij T, Mato L, Amatachaya S, Do ambulatory patients with spinal cord injury walk symmetrically?, Spinal Cord 55(2) (2017) 204–207. http://www.ncbi.nlm.nih.gov/pubmed/27824056. [DOI] [PubMed] [Google Scholar]
- [14].Kumprou M, Amatachaya P, Sooknuan T, Thaweewannakij T, Amatachaya S, Is walking symmetry important for ambulatory patients with spinal cord injury?, Disabil Rehabil (2017) 1–9. http://www.ncbi.nlm.nih.gov/pubmed/28094580. [DOI] [PubMed] [Google Scholar]
- [15].Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. , International standards for neurological classification of spinal cord injury (revised 2011), J Spinal Cord Med 34(6) (2011) 535–46. https://www.ncbi.nlm.nih.gov/pubmed/22330108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van Hedel HJ, Wirz M, Dietz V, Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests, Arch Phys Med Rehabil 86(2) (2005) 190–6. http://www.ncbi.nlm.nih.gov/pubmed/15706542. [DOI] [PubMed] [Google Scholar]
- [17].Field-Fote EC, Fluet GG, Schafer SD, Schneider EM, Smith R, Downey PA, et al. , The Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI), J Rehabil Med 33(4) (2001) 177–81. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11506216 [DOI] [PubMed] [Google Scholar]
- [18].Perez-Sanpablo AI, Quinzanos-Fresnedo J, Loera-Cruz R, Quinones-Uriostegui I, Reyes GR, Perez-Zavala R, Validation of the instrumented evaluation of spatio-temporal gait parameters in patients with motor incomplete spinal cord injury, Spinal Cord (2017). http://www.ncbi.nlm.nih.gov/pubmed/28244503. [DOI] [PubMed] [Google Scholar]
- [19].Nair PM, Hornby TG, Behrman AL, Minimal detectable change for spatial and temporal measurements of gait after incomplete spinal cord injury, Top Spinal Cord Inj Rehabil 18(3) (2012) 273–81. https://www.ncbi.nlm.nih.gov/pubmed/23459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ, Assessing walking speed in clinical research: a systematic review, J Eval Clin Pract 14(4) (2008) 552–62. http://www.ncbi.nlm.nih.gov/pubmed/18462283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thibaudier Y, Frigon A, Spatiotemporal control of interlimb coordination during transverse split-belt locomotion with 1:1 or 2:1 coupling patterns in intact adult cats, J Neurophysiol 112(8) (2014) 2006–18. http://www.ncbi.nlm.nih.gov/pubmed/25057143. [DOI] [PubMed] [Google Scholar]
- [22].Plotnik M, Giladi N, Hausdorff JM, A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease, Exp Brain Res 181(4) (2007) 561–70. http://www.ncbi.nlm.nih.gov/pubmed/17503027. [DOI] [PubMed] [Google Scholar]
- [23].English AW, Lennard PR, Interlimb coordination during stepping in the cat: in-phase stepping and gait transitions, Brain Res 245(2) (1982) 353–64. http://www.ncbi.nlm.nih.gov/pubmed/7127076. [DOI] [PubMed] [Google Scholar]
- [24].Levene H, Robust tests for equality of variance, in: Olkin (Ed.), Contributions to probability and statistics: Essays in honor of Howard Hotelling, Stanford University Press, Palo Alto, 1960, pp. 278–292. [Google Scholar]
- [25].Kumprou M, Amatachaya P, Sooknuan T, Thaweewannakij T, Amatachaya S, Is walking symmetry important for ambulatory patients with spinal cord injury?, Disabil Rehabil 40(7) (2018) 836–841. https://www.ncbi.nlm.nih.gov/pubmed/28094580. [DOI] [PubMed] [Google Scholar]
- [26].Givon U, Zeilig G, Achiron A, Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system, Gait & posture 29(1) (2009) 138–42. https://www.ncbi.nlm.nih.gov/pubmed/18951800. [DOI] [PubMed] [Google Scholar]
- [27].Lauziere S, Betschart M, Aissaoui R, Nadeau S, Understanding spatial and temporal gait asymmetries in individuals post stroke, Int J Phys Med Rehabil 2 (2014) 201. [Google Scholar]
- [28].Plotnik M, Hausdorff JM, The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease, Mov Disord 23 Suppl 2 (2008) S444–50. http://www.ncbi.nlm.nih.gov/pubmed/18668626. [DOI] [PubMed] [Google Scholar]
- [29].Darmohray DM, Jacobs JR, Marques HG, Carey MR, Spatial and Temporal Locomotor Learning in Mouse Cerebellum, Neuron 102(1) (2019) 217–231 e4. https://www.ncbi.nlm.nih.gov/pubmed/30795901. [DOI] [PubMed] [Google Scholar]
- [30].Vinueza Veloz MF, Zhou K, Bosman LW, Potters JW, Negrello M, Seepers RM, et al. , Cerebellar control of gait and interlimb coordination, Brain Struct Funct 220(6) (2015) 3513–36. https://www.ncbi.nlm.nih.gov/pubmed/25139623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lafreniere-Roula M, McCrea DA, Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator, J Neurophysiol 94(2) (2005) 1120–32. https://www.ncbi.nlm.nih.gov/pubmed/15872066. [DOI] [PubMed] [Google Scholar]
- [32].Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA, Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion, J Physiol 577(Pt 2) (2006) 617–39. https://www.ncbi.nlm.nih.gov/pubmed/17008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gonzalez-Rubio M, Velasquez NF, Torres-Oviedo G, Explicit Control of Step Timing During Split-Belt Walking Reveals Interdependent Recalibration of Movements in Space and Time, Front Hum Neurosci 13 (2019) 207 https://www.ncbi.nlm.nih.gov/pubmed/31333429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lam T, Noonan VK, Eng JJ, Team SR, A systematic review of functional ambulation outcome measures in spinal cord injury, Spinal Cord 46(4) (2008) 246–54. http://www.ncbi.nlm.nih.gov/pubmed/17923844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Awai L, Curt A, Locomotor Recovery in Spinal Cord Injury: Insights Beyond Walking Speed and Distance, Journal of neurotrauma 33(15) (2016) 1428–35. http://www.ncbi.nlm.nih.gov/pubmed/26896097. [DOI] [PubMed] [Google Scholar]
- [36].Day KV, Kautz SA, Wu SS, Suter SP, Behrman AL, Foot placement variability as a walking balance mechanism post-spinal cord injury, Clin Biomech (Bristol, Avon) 27(2) (2012) 145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Matsubara JH, Wu M, Gordon KE, Metabolic cost of lateral stabilization during walking in people with incomplete spinal cord injury, Gait & posture 41(2) (2015) 646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Freixes O, Rivas ME, Agrati PE, Bochkezanian V, Waldman SV, Olmos LE, Fatigue level in spinal cord injury AIS D community ambulatory subjects, Spinal Cord 50(6) (2012) 422–5. [DOI] [PubMed] [Google Scholar]
- [39].Thompson AK, Pomerantz FR, Wolpaw JR, Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans, J Neurosci 33(6) (2013) 2365–75. https://www.ncbi.nlm.nih.gov/pubmed/23392666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Plotnik M, Bartsch RP, Zeev A, Giladi N, Hausdorff JM, Effects of walking speed on asymmetry and bilateral coordination of gait, Gait & posture 38(4) (2013) 864–9. http://www.ncbi.nlm.nih.gov/pubmed/23680424. [DOI] [PMC free article] [PubMed] [Google Scholar]