Abstract
Sialorrhoea is a frequent symptom of neurological diseases (e.g. Parkinson’s disease, motor neuron disease, cerebral palsy, and stroke) and is defined as excessive saliva accumulation leading to unintentional loss of saliva from the mouth. Sialorrhoea increases the overall burden on the patient and their caregivers, the impact of which can be both physical and psychosocial. Treatments for sialorrhoea range from lifestyle and behavioural guidance, to medications, surgery or radiation. Nonpharmacological interventions include advice on posture, swallowing control, cough management, dietary changes, eating and drinking techniques, and behavioural modification; however, these conservative measures may be ineffective for people with progressive neurological conditions. The pharmacological treatment of sialorrhoea is challenging because medications licensed for this purpose are limited, but treatments can include anticholinergic drugs and botulinum toxins. Surgical treatment of sialorrhoea is typically reserved as a last resort for patients. IncobotulinumtoxinA (Xeomin®) is the first botulinum toxin type A to receive US and UK marketing authorization for the symptomatic treatment of chronic sialorrhoea due to neurological disorders in adults. In this review, we discuss and compare the frequency and method of administration, location of treatment delivery, approximate annual costs and main side effects of botulinum toxin and different anticholinergic drugs. Management of patients with chronic neurological conditions requires input from multiple specialist teams and thus a multidisciplinary team (MDT) approach is considered fundamental to ensure that care is consistent and tailored to patients’ needs. To ensure that adult patients with neurological conditions receive the best care and sialorrhoea is well managed, we suggest a potential clinical care pathway for sialorrhoea with a MDT approach, which healthcare professionals could aspire to.
Keywords: sialorrhoea, botulinum toxin, incobotulinumtoxinA, Parkinson’s disease, Motor, neuron disease
Defining sialorrhoea
Saliva is the substance produced and secreted from the three paired major salivary glands (parotid, submandibular and sublingual) and comprises water, electrolytes, mucus, antimicrobial compounds and enzymes.1 Hypersalivation, sialorrhoea and drooling are often used interchangeably,2 but they refer to different aspects of salivary continence. Hypersalivation refers to the increased production of saliva, whereas sialorrhoea refers to excessive saliva accumulation and the unintentional loss of saliva from the mouth.3 Sialorrhoea can be caused by excessive production of saliva, or swallowing disturbances, which ultimately lead to an inability to retain saliva within the mouth.4 This is also known as drooling,5 defined as the presence of saliva beyond the margin of the lip.1 Sialorrhoea is considered pathologic after the age of 4 years, as salivary continence is typically established by the age of 15–36 months,2,6 and may be a frequent symptom of neurological diseases (Figure 1).3
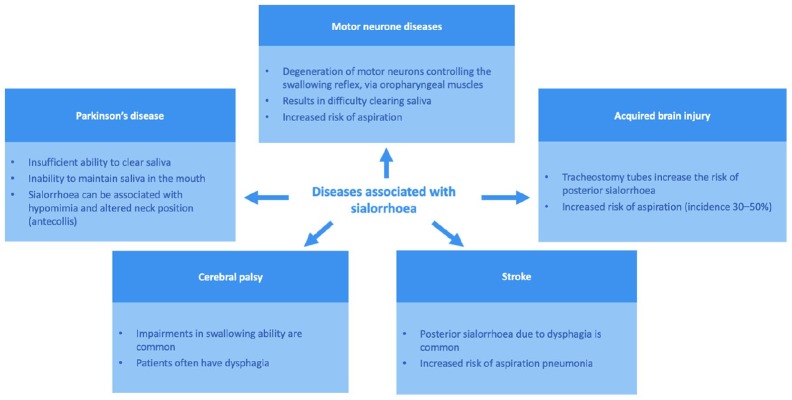
Figure 1.
Mechanism and consequences of sialorrhoea in adult neurological diseases.
This figure shows the adult neurological conditions often associated with sialorrhoea.
The swallowing process is divided into voluntary (oral preparation and propulsion) and involuntary (pharyngeal and oesophageal) stages.7 The involuntary stages of swallowing are under complex neural control, requiring coordinated contraction and inhibition of muscles located around the mouth, larynx, pharynx and oesophagus.8,9 Lack of coordination of the orofacial and palate–lingual musculature can inhibit the swallowing reflex and cause pooling of saliva in the oral cavity.2 This, coupled with other symptoms of neurological conditions such as poor head control, constantly open mouth, poor lip control and impaired tongue mobility can result in an inability to retain saliva within the mouth.1
In this review, we discuss the burden of sialorrhoea in the context of adult patients with neurological conditions and their caregivers, and current treatment options, including recent evidence on treatment with incobotulinumtoxinA (Xeomin®, Merz Pharmaceutical GmbH, Frankfurt am Main, Germany).
Impact of sialorrhoea in adult neurological diseases
Anterior sialorrhoea (unintentional loss of saliva specifically from the mouth) can have distressing consequences, such as damaging books and computers, soiling of clothes, rejection by friends and caregivers, and perioral infections.9,10 Less frequently, the spillage of saliva over the tongue and into the pharynx can occur (posterior sialorrhoea), which can result in aspiration pneumonia.10,11
Sialorrhoea is usually managed alongside other challenging symptoms caused by chronic neurological conditions and increases the overall burden on patients and caregivers. The physical and psychosocial burden for patients can result in a substantial impact on quality of life (QoL).2,3,12 Caregivers’ physical and mental health is also affected by sialorrhoea, since it can make assistance with feeding even more challenging and can lead to feelings of isolation, especially if sialorrhoea makes communication with the patient difficult.13 We summarize the impact of sialorrhoea by specific disease below.
Motor neuron disease
Degeneration of bulbar neurons in motor neuron disease (MND) causes weakness in orofacial and lingual muscles which can result in difficulties in clearing oral secretions, leading to perioral ulcerations and risk of aspiration pneumonia.14 The latter of these is the most harmful complication of MND, and has been associated with increased rates of hospitalization and reduced duration of survival.15,16 Non-invasive ventilation (NIV) can prolong survival in patients with amyotrophic lateral sclerosis (ALS),17 particularly in patients with spinal-onset ALS.18 However, bulbar impairment and the presence of secretions in the airways can reduce patients’ tolerance to NIV.19
Evidence from current clinical practice has shown that sialorrhoea is suboptimally treated in patients with MND. An observational study conducted among UK neurologists reported sialorrhoea in 42% of patients (n = 193) attending their last MND clinic; almost half of the treating physicians considered sialorrhoea to be poorly controlled.20 Despite sialorrhoea being a common symptom for MND patients, studies assessing its burden on patients and their caregivers, and potential contribution to speech difficulties, are lacking.21 Consensus guidelines from the European Federation of Neurological Societies (EFNS) recognized that loss of communication skills by ALS patients can cause caregivers to become emotionally and intellectually isolated.13 Additional responsibilities of caregivers, such as suctioning a patient’s airway and management of mechanical ventilators,13 and potentially, tracheostomies, may contribute to anxiety and reduce time for socializing.
Parkinson’s disease
Nonmotor symptoms of Parkinson’s disease (PD) are common and disabling,22 and associated with poorer QoL.23 Sialorrhoea or ‘dribbling saliva’ is a frequent complaint in early,24 and advanced, PD.23 Prevalence rates of drooling vary from 32% to 74%, depending on the definition used, with a pooled prevalence rate of 56%.5 In an early PD population [disease duration 3.5 years in patients with drooling (n = 320)], male sex, motor severity, excess daytime sleepiness, impaired sleep quality, constipation, and nontremor-dominant subtype were among the clinical factors associated with worsened drooling.25 With advanced PD [disease duration 5 years in patients with drooling (n = 273)], drooling was associated with higher frequencies of speech disturbances and dysphagia, and increased severity of motor and nonmotor symptoms, depression and anxiety.26 The mechanism of drooling in PD is multifactorial and includes reduced salivary swallowing and facial hypomimia, that, when severe, can lead to dribbling of accumulated saliva.27 A small pilot study using videofluoroscopy has suggested that an underestimated consequence of sialorrhoea in PD is silent aspiration, which, along with diurnal sialorrhoea, may increase the risk of respiratory infection (3/19 patients).28 However, such findings have not yet been replicated in larger cohorts.
In patients with PD, PD Questionnaire-39 (PDQ-39) subscores for stigma and difficulties with communication, mobility and activities of daily living, indicated greater impairment in QoL for patients experiencing sialorrhoea compared with patients without sialorrhoea.26 In a small case–control study [PD patients: n = 58 (mean disease duration 11.0 ± 8.7 years); healthy participants: n = 51], drooling was present in 59% of patients with PD, the majority of whom were bothered by drooling in social situations.29 Among PD patients who had sialorrhoea, one third reported that they had to swallow frequently to avoid difficulties in speaking, compared with <5% of PD patients without sialorrhoea.29
A semistructured interview of 37 patients with PD in a community setting revealed that some patients with sialorrhoea felt guilty for the added burden of additional meal preparation time required by carergivers.30 More than a third of Turkish primary caregivers (n = 50) reported symptoms of anxiety, and sialorrhoea was shown to negatively impact caregiver QoL scores (environment domain of the abbreviated version of the World Health Organization QoL assessment), during face-to-face interviews with a neurologist.31
Cerebral palsy
Cerebral palsy (CP) manifests in early childhood and persists throughout life.32 The worldwide prevalence of CP is estimated to be 2.0–2.5 per 1000 live births.9 The majority of patients with CP in the UK can be expected to survive to 30 years of age if they have no severe impairments; however, survival is substantially impaired in patients with at least two disabilities.33 Despite this, there is limited information available about the development of CP into adulthood and the accompanying needs of the adult patient. The majority of studies recruit only children and young adults with CP.
Data from the Northern Ireland Cerebral Palsy Register showed that 22% of children experienced excessive drooling, associated with more severe motor limitations (Gross Motor Function Classification System level IV or V) and intellectual impairment.34 Videofluoroscopic evaluations of swallowing in children have shown that silent aspiration can be a problem in these patients.35,36 Although these data cannot be directly applied to adult patients with CP, the burden of dysphagia is likely to extend into adulthood. Results from a small-scale interview-based study showed that adults with CP (n = 32) with confirmed dysphagia reported increased difficulty during mealtimes.37 Many patients reported a reluctance to change their diet to a softer consistency.37 Adult CP patients with dysphagia may therefore experience longer meal times, reduced overall food intake, and difficulty in achieving optimal nutrition.
Stroke
Stroke patients have an increased risk of posterior sialorrhoea and aspiration pneumonia due to swallowing difficulties, increasing the burden on patients, their caregivers and healthcare professionals. Patients need close monitoring and may require admission to a stroke care unit, which is associated with additional healthcare costs.38 The prevalence of swallowing disorders in stroke patients is 51–64%.39 Of particular concern are the observations during videofluoroscopy studies,39,40 in which over two thirds of stroke patients with dysphagia experienced silent aspiration.41
Acquired brain injury
Posterior drooling can delay weaning of patients from tracheostomies and rehabilitation following acquired brain injury,42,43 limiting the availability of optimal multidisciplinary care as access to the relevant departments and correct rehabilitation care is reduced. Tracheostomy formation can introduce significant physical and psychological barriers for patients in terms of functional outcomes. This, in turn, can substantially increase health and social care costs, since the admission of a patient with an acquired brain injury to specialist rehabilitation would cost £567/day (UK National Health Service [NHS]).44
Up to 44.4% of patients with acquired brain injuries and tracheostomies, admitted to a rehabilitation department between April and December 2011, experienced salivary aspiration, many of whom required frequent manual suctioning of saliva (>10 times per day).42 This requires an additional time commitment for nursing staff, equipment costs and long-term costs for a bed within the rehabilitation department.
Current treatment options for sialorrhoea
Current guidance for the clinical management of sialorrhoea
Initial treatment for sialorrhoea involves relevant lifestyle and behavioural changes that may be unfeasible for patients with progressive neurological conditions. If lifestyle changes are not possible or fail to adequately manage symptoms, options include medications, surgery or radiation.1,2 The majority of drug therapies used to treat sialorrhoea over the past decade are not licensed for this purpose, so must be prescribed off-label. The anticholinergic drug, glycopyrronium bromide, for example, is only licensed for the treatment of severe sialorrhoea in children and adolescents aged 3 years and older with chronic neurological disorders, in the UK.45
Current National Institute for Health and Care Excellence (NICE) guidance for the management of PD suggests that pharmacological management should only be considered if nonpharmacological management is unavailable or ineffective.46 For MND, current NICE guidance recommends that provision of advice on swallowing, diet, posture, positioning, oral care and suctioning should be considered first.47 After this, NICE guidance currently suggests that antimuscarinic medicines should be considered as a first-line treatment for patients with MND. At present, NICE guidance suggests that glycopyrrolate (glycopyrronium bromide) should be considered as the first-line treatment for sialorrhoea in both PD, and MND patients with cognitive impairment; if ineffective, patients can be referred to a specialist service for botulinum toxin type A (BoNT-A).46,47 On 30 May 2019, incobotulinumtoxinA received UK marketing authorization for the symptomatic treatment of chronic sialorrhoea due to neurological disorders in adults.
Evidence-based recommendations from neurological scientific societies are also available but may differ from the guidance provided by regulatory agencies. Most recently, the International Parkinson and Movement Disorder Society highlighted that there was insufficient evidence regarding the safety of ipratropium bromide spray and glycopyrrolate for the treatment of drooling in patients with PD, while BoNT-A and -B were considered clinically useful with an acceptable risk profile if used with specialized monitoring.48 The task force for ALS of the American Academy of Neurology acknowledged that anticholinergic drugs are generally tried first despite no proven effectiveness, and recommended that BoNT-B should be considered for patients with medically refractory sialorrhoea. Low-dose radiation therapy to the salivary glands may also be considered.49 It should be noted, however, that these recommendations were published in 2009, so do not consider the most recent evidence from randomized controlled trials (RCTs).
Treatment decision making should also consider the classification of sialorrhoea as either transitory or chronic. Since transitory sialorrhoea is likely to resolve over time, the use of irreversible interventions would be inappropriate, in patients with acquired brain injuries, for example.1 The choice of treatment could also have substantial downstream implications on healthcare resource utilization and costs. Treatment considerations for patients with sialorrhoea may be further complicated by the medications prescribed to control their underlying disease, or associated symptoms. This results in a high treatment burden for patients, with a range of the potential side effects that may require the prescription of further medications (polypharmacy). Indeed, the underlying primary disease requires treatment and clinical review by many different specialists (e.g. ear, nose and throat specialists, neurologists, specialist nurses, physical, occupational and speech therapists, pulmonologists, dietitians, psychologists, rehabilitation physicians, stroke consultants), as well as general practitioners (GPs) and social workers.50 Caregivers have a crucial role in facilitating communication between the patient and healthcare provider, providing emotional support, and coordinating attendance at medical appointments;51 all of which require a substantial time commitment and may require caregivers to take additional days off work or to arrange childcare. This is further complicated if patients have to attend separate appointments with each discipline on different days.51
Nonpharmacological treatment options to reduce or prevent sialorrhoea
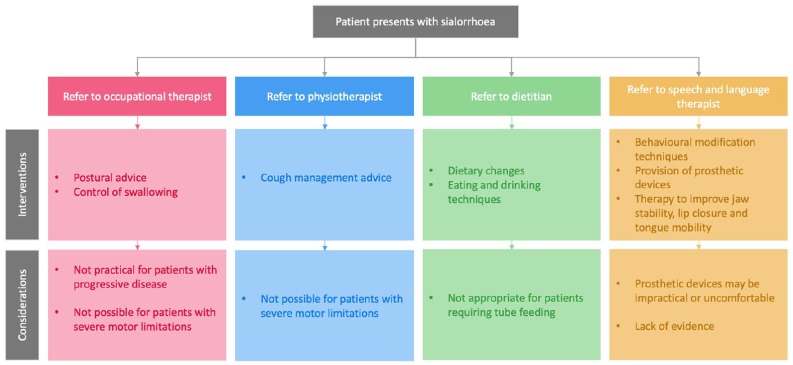
Nonpharmacological treatment options for sialorrhoea may require referral to specialists such as occupational therapists, physiotherapists, dietitians, and speech and language therapists who can provide advice on methods to control or manage salivary secretions (Figure 2).1,52
Figure 2.
Nonpharmacological interventions for the management of sialorrhoea.
Different specialists may suggest various nonpharmacological management techniques to patients presenting with sialorrhoea, to help patients to manage their symptoms. Key considerations are outlined for each specialist.
Speech and language therapists may encourage patients to make dietary changes (e.g. avoiding citrus fruits and alcohol) and introduce techniques to improve eating and drinking skills (lip closure, tongue movement and swallowing ).1 Suctioning of saliva may be used to assist with removing secretions from the mouth and throat,3 particularly in patients with an ineffective cough. The ability of patients to manage salivary secretions in their own home may be dependent on the loan of a suction unit from the patient’s GP or district nurse.52 However, evidence to support the use of nonpharmacological interventions for sialorrhoea management remains limited, and approaches may be unsuitable for patients with progressive, chronic conditions, where longer-term solutions may be more appropriate.
Pharmacological therapies to reduce or prevent sialorrhoea
If nonpharmacological approaches are ineffective, inappropriate or not accessible (e.g. the healthcare service may not be available in the local healthcare setting), drug therapies (Table 1) can be trialled to reduce the secretion of saliva, thereby improving the mood and QoL of patients with sialorrhoea, as well as reducing the associated care burden.
Table 1.
Comparison of pharmacological treatment classes commonly used in the management of sialorrhoea in MND, PD and stroke.
| Treatments | Licensed to treat sialorrhoea in adults? | Common or licensed dosage useda | Frequency of administration | Method of administration | Location of treatment delivery | Approximate annual cost of treatmentb | Main side effectsc | |
|---|---|---|---|---|---|---|---|---|
| Anticholinergic drugs | Hyoscine hydrobromide | No |
Hyoscine hydrobromide (1.5 mg patch):
1 patch every 72 h; if concerned about side effects start with ¼ or ½ patch; can be increased to 1½–2 patches if necessary Hyoscine hydrobromide (orally/via enteral feeding): 300 μg OD, increased every 2–3 days to 300 μg TDS Hyoscine butylbromide (subcutaneously): Subcutaneous injection: 20 mg every 4 h as required, increased as needed up to 20 mg every hour Subcutaneous infusion: 20–120 mg per 24 h |
2–3 times per day | Skin patches, oral tablets, or parenteral administration (subcutaneous injection or infusion)52 | Home | Skin patches: £195.20–£1561.56 [¼ patch (1.5 mg) –2 patches (1.5 mg) every 72 h] Not applicable Solution for injection: £637.73–£2550.91 (20 mg every 4 h to hourly) Not applicable |
Dry mouth, drowsiness, blurred vision, tachycardia, arrhythmias, flushing, constipation, urine retention, dizziness, skin reactions, confusion, hallucinations, irritation of the eyelids, dilated pupils, increased body temperature, increased fluid pressure inside the eye, and nausea53–57 |
| Atropine sulphate | No |
Atropine 1% eye drops (sublingual
administration):
1–2 drops OD; increased by 1 drop every 2 days, up to 2 drops QDS (as required and tolerated) |
3–6 times per day | Eye drops given sublingually52 | Home |
£480.08–£960.16
(1–2 mg OD) |
Dry mouth, drowsiness, blurred vision, tachycardia, arrhythmias, flushing, constipation, urine retention, hypotension, hallucinations, seizures, heart palpitations, difficulty swallowing and talking, nausea, vomiting, and confusion/delirium53,54,58,59 | |
| Glycopyrronium bromide | No | 200–500 μg TDS, increased in increments, as required and tolerated, up to 2 mg TDS | 2–3 times per day | Oral tablets or solution | Home | £986.44–£9864.40 (200 μg–2 mg TDS)d | Dry mouth, drowsiness, blurred vision, tachycardia, arrhythmias, flushing, constipation, urine retention vomiting, nasal congestion, headache, decreased sweating, dizziness, pupillary dilatation, and mental status changes53,54,60 | |
| Botulinum toxin injections | Serotype A | Yes, in the UK and the US (incobotulinumtoxinA only)e |
IncobotulinumtoxinA: 100 units (5 units/0.1 ml) per treatment session, given as 2 injections each into the parotid (30 units per side) and submandibular glands (20 units per side)61 | Every 16 weeks62 | Injection into the salivary glands | Clinical care setting |
£422.18
(100 units of incobotulinumtoxinA every 16 weeks) |
Pain at needle site, dry mouth, dysphagia, paraesthesia, viscous saliva, chewing weakness, tooth extraction, diarrhoea, hypertension, and respiratory infections61–63 |
| Serotype B | Yes, in the US | RimabotulinumtoxinB (NeuroBloc® or MyoBloc®, Sloan Pharma Sarl, Bertrange, Luxembourg): 1500–3500 units administered as 500–1500 units to per parotid gland and 250 units per submandibular gland64 | Every 12 weeks | Injection into the salivary glands | Clinical care setting |
£481.87
(1500 units every 12 weeks) £642.50 (3500 units every 12 weeks) |
Pain at needle site, dry mouth, viscous saliva, chewing weakness, dysphagia, dyspepsia, dental caries and respiratory infections64–66 | |
Dosages for licensed preparations within the treatment class are included for the treatment of sialorrhoea in adults, where no treatments are licensed within the treatment class, typical dosages (off label) used by clinicians are shown.
Costs reflect the UK NHS indicative price in the online British National Formulary 2019 (prices correct at the time of publication); annual costs have been calculated using the licensed dosage or the typical dosages used by clinicians.
Reported side effects associated with these pharmacological treatments.
Costs are derived from the British National Formulary for Children 2019 as the oral solution of glycopyrronium is currently only licensed for use in children.
Alternative BoNT-A products are available but as they are currently unlicensed, there is no confirmed posology available at present.
BoNT-A, botulinum toxin type A; MND, motor neuron disease; NHS, National Health Serveice; OD: once a day; PD, Parkinson’s disease; QDS: four times a day; TDS: three times a day.
There is limited evidence to guide clinicians in their choice of drug across neurological disorders,20 apart from the recently published International Parkinson and Movement Disorder Society’s recommendations for PD treatments.48 Pharmacological options include local and systemic anticholinergic drugs and BoNT injections.
Anticholinergic drugs
Anticholinergic drugs inhibit the action of the neurotransmitter acetylcholine at muscarinic receptors, which reduces the production of saliva.3 Drugs such as atropine, hyoscine, glycopyrronium bromide, benzatropine and tropicamide can improve sialorrhoea in severely disabled patients and patients with PD.2,48,67–69
Glycopyrronium bromide response rates are between 12% and 81% (at a variety of doses; at least a 3-point improvement in drooling scores) in children,70,71 but limited evidence is available for use in adults. PD patients randomized to receive oral glycopyrrolate for 4 weeks, in a small-scale trial (n = 23), demonstrated improvements in the severity of sialorrhoea compared with placebo, using a self-reported sialorrhoea scoring scale.68 Transdermally applied hyoscine hydrobromide has been shown to reduce salivary secretions by 50–100% in patients with a wide range of disorders causing sialorrhoea, with patient-reported improvements in drooling frequency.67,72 One administration can remain stable within serum for 72 h; this is beneficial to patients, as frequent administrations are not required, which may also translate into healthcare cost savings.1
Botulinum toxins
BoNTs, when injected in the salivary glands, selectively bind to cholinergic nerve terminals and temporarily inhibit the release of acetylcholine, blocking salivary secretions.2 Until recently, injection of the salivary glands with BoNTs was suggested as a specialist (off-label) second-line treatment for sialorrhoea in MND and PD in the 2016/2017 NICE guidelines: Motor Neurone Disease: Assessment and Management and Parkinson’s Disease in Adults.46,47 Recently, the US Food and Drug Administration (FDA) approved the use of incobotulinumtoxinA and rimabotulinumtoxinB for the treatment of chronic sialorrhoea in adult patients in the US.62,64 In August 2019, NICE recommended incobotulinumtoxinA as an option for treating chronic sialorrhoea caused by neurological conditions in adults.73 BoNT injections may be administered every 16 weeks; however, there is limited information to inform recommendations of the optimal timing of subsequent injections.74 The reduced frequency of administration is a key advantage of BoNT treatment, in comparison with anticholinergic medications that are typically taken multiple times every day. Treatment with BoNT injection is typically well tolerated, and reported side effects appear to be less common than for anticholinergic treatments.53,54,63,75
Injection of BoNT-A into the salivary glands has been shown to improve the severity and frequency of sialorrhoea in patients with neurologic diseases including PD and ALS, with evidence from double-blind, placebo-controlled studies,75,76 and real-world evidence studies.77,78 Three formulations of BoNT-A and one formulation of BoNT-B are currently available, but only one of these formulations is currently licensed in the UK;79 both treatments exhibited similar effectiveness and safety in a prospective, double-blind, crossover RCT of ALS and PD patients.80
In a blinded RCT in patients with a range of neurologic disorders, greater improvement in sialorrhoea was observed upon injection of more salivary glands with BoNT.81 The choice of salivary gland to inject may also influence the efficacy of BoNT, but more evidence is required.63 The duration of effect of BoNT-A and -B ranged between 2 and 5 months in a retrospective study and an RCT.82,83 These findings suggest that substantial financial savings could be made since longer-term improvements in sialorrhoea could enable high-risk inpatients to be transferred from specialist to ward-based care (Box 1).
Box 1.
BoNT injections could reduce utilization of healthcare resources.
| An elderly patient (63 years) was admitted to ICU with a
posterior fossa haemorrhage, which required posterior
fossa decompression and evacuation of the haematoma. The
patient had a past medical history of
hypertension. The patient’s neurological recovery was slow, and the formation of a tracheostomy was required for slow respiratory wean. The patient required deep suctioning of saliva through the tracheostomy tube every 15 min by a trained member of nursing staff. The respiratory physiotherapist cleared the secretions from the patient’s chest twice per day. Consequently, it was not safe for the patient to be moved from the ICU to a ward-based setting. The patient consented to receive bilateral injections of BoNT to the parotid and submandibular salivary glands. Within 48 h of the injection, the patient experienced a reduction in sialorrhoea such that suctioning of saliva was required every 30 min, rather than every 15 min. Over the next 24 h, suctioning was only required every hour and the patient was considered safe to be moved to a ward for their ongoing care; this reduced use of hospital resources, thus had an economic benefit. The daily cost of a general, adult ICU bed is between £1136 (one organ supported) and £2075 (at least six organs supported), compared with an average of £254 per day for a stroke patient on a general hospital ward, depending on the complexity of care required.44 For those patients who need frequent suctioning to clear their airways, healthcare professionals should consider longer-acting treatments that improve QoL and provide overall cost and resource savings. Despite the initial investment of time, money, and expertise to provide BoNT injections, this approach could deliver long-term savings. |
BoNT, botulinum toxin; ICU, intensive care unit; QoL, quality of life.
Improvements in the severity and frequency of sialorrhoea were reported by patients with neurological disorders during an RCT,84 a dose comparison study,85 and a real-world evidence study.77 Patients with PD in Estonia reported thickening of saliva 1 month after injection with BoNT-A when interviewed, and a reduction in sialorrhoea severity from very intensive to moderate.86 Retrospective analysis of patients in a movement disorders clinic showed that all patients treated with BoNT-A experienced improvements; a minimum of 50% improvement in sialorrhoea was observed using the Drooling Severity and Frequency Scale (DSFS).87 The majority of PD outpatients completing an RCT reported satisfaction with BoNT treatment [moderate to dramatic improvement (77.7%; n = 18)] compared with placebo [no changes to mild improvement (94.4%; n = 18)].83 All patients receiving BoNT injections stated that they would receive repeated injections.83
Patient-reported improvements in quality of daily living have also been reported in an open-label, prospective study, with improved outcomes including how often clothes and pillows were stained by saliva 4 and 12 weeks after injection with BoNT-B.65 Similarly, a small-scale, open-label, prospective study reported improvements in Drooling Impact Scores in ALS patients treated with BoNT-A; patients scored factors that included how much sialorrhoea had influenced their speech, clothing, social activities and skin on their lower face.88 A 3-year retrospective analysis showed that the total number of patients with CP treated with BoNT-A who required hospitalization decreased by >50% up to 21 months after receiving treatment; however, it is not possible to conclude that BoNT-A treatment was the only reason for this observation.82 The administration of BoNT may improve the day-to-day QoL of patients, and their caregivers, which could substantially improve mood and independence (Box 2).
Box 2.
Improvements in quality of life following injection with BoNT.
| A patient (52 years) was admitted with a brainstem
stroke, following a vertebral artery dissection. Along
with other physical problems, the patient’s major
disability was swallowing and posterior drooling. The
patient was kept nil by mouth and commenced feeding
via PEG. The MDT noticed limited
progress in the patient’s tracheostomy weaning due to
posterior sialorrhoea. Nonpharmacological interventions to improve positioning and to assist with saliva secretion management and swallowing exercises were trialled. The patient also consented to bilateral injection of BoNT into the parotid and submandibular glands. In the next 2 days, saliva production started decreasing, allowing the patient to progress from a cuffed to an uncuffed tracheostomy. Subsequently, the patient was successfully weaned from the tracheostomy and was moved to a lower-dependency rehabilitation unit. The patient’s swallowing improved such that they were able to progress from sole PEG feeding to a texturized diet and thickened fluids, including their favourite food items. Following two further injections of BoNT, the patient regained mobility, was fully independent in activities of daily living, and had the PEG tube removed. The patient is now able to consume a fork-mashable dysphagia diet89 and vocalize normally, and has returned to work. Intervention with BoNT may improve the QoL of patients experiencing severe sialorrhoea by enabling removal of tracheostomy and PEG tubes, for example. These changes may substantially improve patients’ mood, level of discomfort, independence and ability to perform daily activities. These changes could also reduce burden on caregivers. |
BoNT, botulinum toxin; MDT, multidisciplinary team; PEG, percutaneous endoscopic gastrostomy; QoL, quality of life.
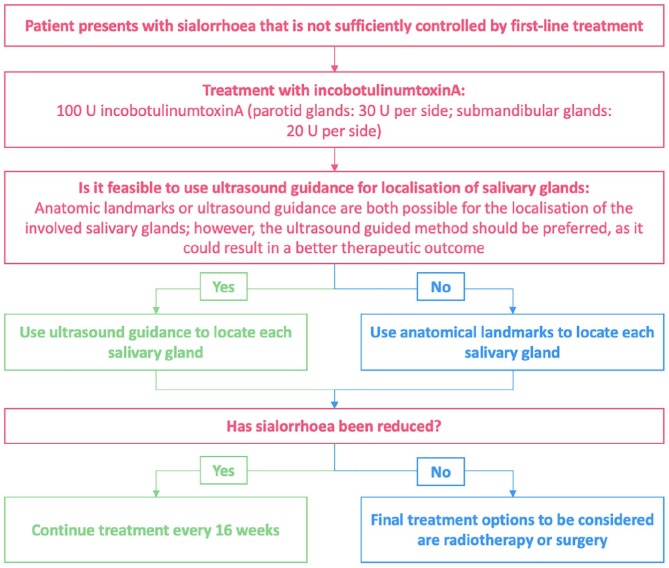
In patients who have not responded to nonpharmacological treatment or for whom these options are not feasible, BoNT injections may be an appropriate next step to manage sialorrhoea. We suggest a treatment algorithm (based on clinical practice in the UK) for the licensed BoNT, incobotulinumtoxinA, based on current information: a proposed algorithm for BoNT treatment of sialorrhoea in patients with ALS and the summary of product characteristics for incobotulinumtoxinA (Figure 3).63,61
Figure 3.
Proposed treatment algorithm for the treatment of sialorrhoea with incobotulinumtoxinA.
IncobotulinumtoxinA in the management of sialorrhoea
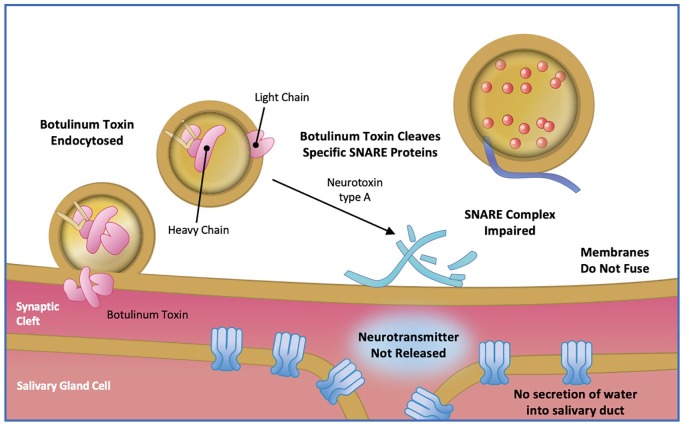
IncobotulinumtoxinA (Xeomin®, Merz Pharmaceutical GmbH, Frankfurt am Main, Germany) is a purified BoNT-A, free from complexing proteins, and administered by injection into the salivary glands. IncobotulinumtoxinA acts as a neuroglandular blocking agent by inhibiting the release of acetylcholine90 and consequently reducing the production of saliva, thus reducing sialorrhoea. The action of BoNT-A involves a four-step process:90 (a) the heavy chain of the BoNT binds to the presynaptic membrane of cholinergic nerve endings; (b) the toxin complex is internalized; (c) the light chain is released into the cytoplasm; and (d) the light chain cleaves selected proteins [N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins] that are critical for the release of acetylcholine into the synapse (Figure 4). A typical regimen for the administration of BoNT-A is two injections into the parotid gland and one injection into a single site of each submandibular gland.53
Figure 4.
Mechanism of action of botulinum toxin type A.
Adapted from Arnon SS, et al. 2001 and Kukreja & Singh 2015.79,91 SNARE, N-ethylmaleimide-sensitive factor attachment protein receptor protein.
The SIAXI trial was the largest prospective, randomized, double-blind, placebo-controlled multicentre study investigating the use of incobotulinumtoxinA (75 or 100 U) for the treatment of sialorrhoea in patients with neurologic disorders. Patients injected bilaterally (parotid and submandibular glands; 3:2 dose ratio) with 100 U incobotulinumtoxinA showed significant reductions in unstimulated salivary flow rate from baseline, compared with placebo by week 4, which was maintained to week 16.92 Patient-reported Global Impression of Change Scale (GICS) scores improved after 4 weeks of treatment with both dosages, compared with placebo.92 IncobotulinumtoxinA (75 and 100 U) was well tolerated by patients and no new safety concerns were reported.92 Overall, adverse events were reported in 32 (43.2%) and 34 (45.9%) patients treated with incobotulinumtoxinA, 75 U and 100 U, respectively, compared with 15 (41.7%) patients in the placebo group. The most frequent treatment-related AEs were dry mouth [incobotulinumtoxinA 75 U: 4 (5.4%) patients; incobotulinumtoxinA 100 U: 2 (2.8%) patients] and dysphagia [incobotulinumtoxinA 75 U: 2 (2.7%) patients; incobotulinumtoxinA 100 U: 0 patients].
A crossover RCT in the US (n = 10) found no significant difference in saliva weights 1 month after injection of BoNT compared with placebo; however, the small sample size is a limitation of this study.93 Placebo-controlled and real-world evidence studies of incobotulinumtoxinA have demonstrated improvements in sialorrhoea and chronic troublesome sialorrhoea in patients with neurological conditions.77,78,81 A small-scale (n = 20), real-world-evidence study showed that injection of incobotulinumtoxinA under ultrasound guidance [parotid gland 12.5 ± 3.9 U (PD patients), 14.25 ± 4.3 U (ALS patients); submandibular gland 12.2 ± 4.5 U (PD patients), 13.3 ± 4.1 U (ALS patients)] reduced sialorrhoea by approximately 50% in gauze, sugar lump and patient-reported measurements (visual analogue scale) after 30 days in patients with PD and ALS.78 Patients with sialorrhoea (n = 36) treated with incobotulinumtoxinA in community-based or tertiary hospital-based outpatient clinics, reported significant improvements in sialorrhoea using DSFS.77
IncobotulinumtoxinA is the only BoNT not requiring refrigerated storage prior to reconstitution, making this a practical option for use in community-based clinics.77,94 In some countries, such as the UK, the frequency of use of anticholinergic drug treatments (e.g. glycopyrronium bromide) may increase the overall annual cost of treatment, when compared with repeat injections with incobotulinumtoxinA every 16 weeks (Table 1). The cost effectiveness of incobotulinumtoxinA is currently being assessed.
IncobotulinumtoxinA first received marketing authorization in the UK for the symptomatic treatment of blepharospasm, cervical dystonia of a predominantly rotational form (spasmodic torticollis) in adults in 2007,61 and spasticity of the upper limbs in adults in 2009. IncobotulinumtoxinA received European Medicines Agency and UK approval for the treatment of chronic sialorrhoea due to neurological disorders in adults in May 2019.61 In August 2019, IncobotulinumtoxinA was recommended by NICE, within its marketing authorization, as an option for treating chronic sialorrhoea caused by neurological conditions in adults.73
Challenges of pharmacological therapies for sialorrhoea in neurological diseases
Pharmacological therapies for sialorrhoea can lead to significant improvements in patients’ symptoms, but the side effects and administration methods present some challenges. The additional burden may deter some patients from taking medications (and some carers from giving medication) to control their sialorrhoea.
Physicians and caregivers should monitor patients treated with anticholinergic drugs for associated adverse side effects95 (Box 3), which include urinary retention, blurred vision, hallucinations and confusion.96 Patients with PD who have cognitive impairments are more susceptible to developing these side effects,3 or increased Alzheimer’s disease pathology.97 Furthermore, care must be taken to avoid an excessively dry mouth in patients treated with anticholinergic drugs,96 as this could further impair patients’ QoL. Thus, use of an anticholinergic burden scale is important when making treatment choices, to highlight when medications with a lower cognitive burden should be considered (Box 4).98 Dryness of the mouth is a known side effect of both anticholinergic drugs and BoNTs,55,56,58,60,61,66 and should be carefully managed to avoid deterioration in patients’ dental health.99 Since the protective effects of saliva are reduced in patients with hyposalivation, this can lead to an increase in dental caries, halitosis, and oral infections such as candidosis and sialadenitis.99 Hyoscine hydrobromide patches can cause behavioural changes and visual disturbances (including blurred vision and accommodation problems). The observed behavioural changes were seen to resolve 12–24 h after removing the patch in a prospective RCT.67
Box 3.
Adverse events associated with anticholinergic drug treatment.95
| A patient with PD, reported that sialorrhoea was the
symptom of their disease causing the majority of their
concern, because they were unable to sit down to read
the newspaper without experiencing severe sialorrhoea.
After no response to nonpharmacological methods of
management, off-label oral administration of atropine
eye drops (10 mg/ml 0.5 ml per day) was
trialled. Over the next 24 h, the patient became delirious and experienced hallucinations that resulted in a car crash and hospitalization. Treatment with anticholinergic drugs, such as atropine, can cause delirium and inhibited cognitive performance by blocking muscarinic receptors in the brain. This is particularly apparent in PD patients who have dementia. Atropine treatment was stopped, and the patient returned to normal 48 h later. For patients with PD, the potential side effects of anticholinergic drugs should be considered, and a decision made as to whether this risk is acceptable. In order to avoid unnecessary, and potentially dangerous side effects, it may be more appropriate to avoid anticholinergic drugs in patients with cognitive impairment. |
PD, Parkinson’s disease.
Box 4.
Anticholinergic burden scales.
| Anticholinergic burden scales have been developed,
typically based upon expert opinion, to categorize
medications in terms of anticholinergic activity.98 Possible anticholinergic drugs only have evidence
from in vitro studies reporting
antagonistic activity at the muscarinic receptor (score
of 1). Definite anticholinergic drugs have reported
anticholinergic adverse effects (score of 2) or delirium
(score of 3) from a more substantial evidence basis
(literature, prescriber’s information or expert opinion).100 The scales can be used to calculate a score that
reflects the patient’s cumulative exposure to
anticholinergic effects. This overall score can be used
to identify whether clinicians should consider
alternative medications, and can be applied to hospital
inpatients, patients within the community, or patients
within institutional care. However, it should be noted
that there is not a standard, validated rating scale for
the assessment of anticholinergic
burden. Examples include: (1) Anticholinergic Cognitive Burden Scale100,101 (2) Anticholinergic Drug Scale102 (3) Anticholinergic Risk Scale103 |
One of the key considerations of BoNT injection administration is whether the use of ultrasound guidance improves the effectiveness of sialorrhoea treatment; improvements have been observed in some studies.104,105 The availability of sufficiently trained staff to administer BoNT injections may also prevent patients from receiving this treatment.20 However, authorization of onabotulinumtoxinA (BOTOX®, Allergan plc, Dublin, Ireland) as a prophylactic treatment for chronic migraine in the UK, in 2012,106 may enable access to trained staff in established clinics. Improvements in sialorrhoea after administration of BoNT injections are temporary and repeat injections are necessary. The approved dosing guidance for incobotulinumtoxinA is every 16 weeks;62,61 repeat injections of BoNT within different timeframes may increase the chance of patients forming neutralizing antibodies against the BoNT.107 Some serotypes are associated with greater risk of forming antibodies than others, so alternative serotypes may be trialled if antibodies against BoNT develop.107 It is still unknown if injection intervals of less than 3 months can be safely used,74,107 but the risk of forming antibodies is considered to be relatively low in clinical practice. Clinicians may decide not to treat sialorrhoea with BoNT injections if they are concerned that dysphagia may worsen;20 despite a paucity of research in this area, some small-scale studies suggest that swallowing dynamics are unaffected.108,109 A potential limitation to the use of some BoNTs in community-based clinics is the absence of refrigerators to store the drug;77 however, the BoNT-A, incobotulinumtoxinA, does not require refrigerated storage prior to reconstitution.
Surgical treatment options
Due to the side effects and challenges associated with pharmacological treatments for sialorrhoea, some patients may prefer to take an alternative approach. Surgical treatment options are destructive and irreversible (Table 2), so are typically reserved as the last resort for patients.
Table 2.
Comparison of the surgical treatment options for the management of sialorrhoea.
| Surgical treatments for sialorrhoea | Duration of effect110–113 | Site of administration | Annual cost of treatment | Main side effectsc;6,111,114–116 |
|---|---|---|---|---|
| Ligation of salivary gland ducts | Up to 4.4 years | Parotid or submandibular salivary glands | £74.50a | Facial/glandular swelling, aspiration pneumonia, increased risk of dental caries, delayed re-initiation to oral feeding, risk of surgery |
| Radiation therapy | Up to 6 months | Bilateral submandibular gland and partial/bilateral parotid glands116 | £1239.38b | Risk of malignancy, severe mouth dryness, mild pain, erythema, mucositis |
Annual national average unit costs were derived from the NHS reference costs 2017/2018:
Costs were derived from ‘consultant-led general surgery’; one treatment every 2 years was assumed.
Costs were derived from ‘preparation for simple radiotherapy with imaging and dosimetry, with technical support’; two treatments per year were assumed.
Reported side effects associated with these surgical treatments.
NHS, National Health Service.
Current surgical options for patients with sialorrhoea include the removal of the salivary glands (typically the parotid and submandibular glands), surgery to ligate or reroute salivary gland ducts, or interrupt the parasympathetic nerve supply to the salivary glands.1,117,118 Due to their invasive nature, these options are unsuitable for patients with transitory sialorrhoea. Intraductal laser photocoagulation of the bilateral parotid ducts is a less invasive option with positive results from early reports.1,119 Patients may also require the formation of a tracheostomy for long-term assisted ventilation, due to loss of bulbar tone and difficulty in clearing salivary secretions that makes use of NIV ineffective.120 Reported outcomes following surgical management of sialorrhoea are mostly limited to studies in children, and evidence may not be relevant to adult patients.110,111,114 However, dental caries can occur following surgical treatment for sialorrhoea in children, so this risk should be considered when making treatment decisions.121,122
Radiation therapy of the salivary glands is a rarely used treatment option and is typically reserved for elderly patients who cannot undergo surgery or tolerate available drug therapies.2 Administration of palliative single-dose radiotherapy to the parotid salivary gland of patients with ALS significantly reduced salivary secretion, compared with salivation prior to treatment in a prospective study of 20 patients in Norway.123 Similar observations were made in a retrospective US case series study of 10 consecutive patients with ALS.124 Comparison between BoNT and salivary gland radiotherapy showed no differences in patient-reported burden of sialorrhoea.125
Models of care
As highlighted above, patients with chronic neurological conditions have complex symptoms that require input from multiple specialist teams. A multidisciplinary team (MDT) approach is considered fundamental to ensure that care is consistent and tailored to a patient’s needs.
The EFNS guidelines recommend multidisciplinary care for MND patients, including hospital- and community-based services.13 The provision of MDT clinics for patients with ALS in the Republic of Ireland, Sheffield and the Netherlands have demonstrated improvements in the prognosis and mental QoL of patients, when compared with those attending general neurology clinics.50,126,127 The study conducted in the Netherlands may provide useful insights for ALS care, but the researcheres were required to follow the Dutch ALS consensus guidelines, so may not be generalizable to other regions.127 The MDT approach is also important to ensure that all the patient’s concerns are addressed, including the needs of their family and primary caregivers.128
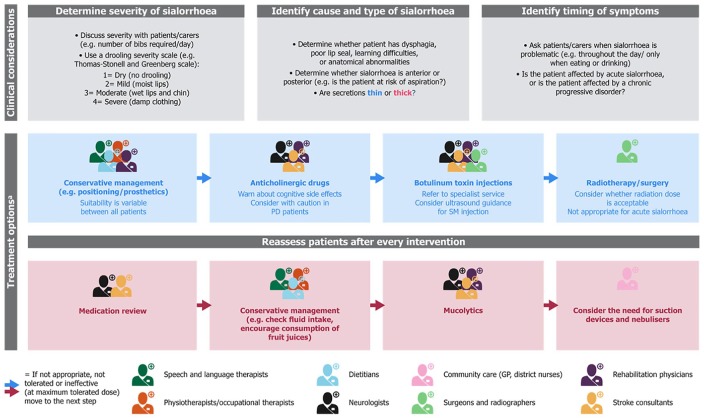
Mean monthly healthcare costs were similar for patients with ALS receiving MDT (€1336 per patient) or general care (€1271 per patient) in a cost-of-illness analysis conducted in the Netherlands.129 Early referral of ALS patients to a MDT could prevent unnecessary procedures and consultations, with an estimated saving of €2072 per patient receiving MDT care.130 Therefore, implementing an MDT approach in other complex conditions (e.g. PD and stroke) might be cost effective, especially if symptoms such as sialorrhoea could be addressed earlier. At present, patients do not follow a specified clinical route, as the services available to patients often vary by location. An example of a possible clinical pathway with an MDT approach including sialorrhoea management is provided in Figure 5.
Figure 5.
An example of a holistic model of care with an MDT approach in patients with neurological diseases and sialorrhoea.
This figure suggests the clinical considerations and treatment options that could be used when treating patients with neurological diseases and sialorrhoea. The potential opportunities for specialists to intervene within an MDT approach are highlighted. Adapted from McGeachan et al. 2017 and the Pan Mersey Area Prescribing Committee Prescribing Guidelines.3,131aTreatment options are shown in order of ideal implementation; however, in real clinical practice this may not be the case. Clinical judgement should be used, based upon the severity of symptoms and the burden of care.
GP, general practiotioner; MDT, multidisciplinary team; PD, Parkinson’s disease; SM, submandibular.
Expert opinions and recommendations
Sialorrhoea is a frequent and disabling symptom of adult neurological diseases, which often results in polypharmacy. Despite being associated with poorer QoL, speech difficulties, and harmful complications such as aspiration pneumonia, sialorrhoea is still an under-recognized and undertreated symptom. In addition, treatment pathways currently used for neurological conditions such as CP, stroke and acquired brain injury or neurodegenerative diseases such as ALS and PD, rarely include sialorrhoea management within the patient’s care plan. An example of a clinical pathway for sialorrhoea with an MDT approach that patients and clinicians could aspire to, is provided in Figure 5.
Here, we provide recommendations for management of sialorrhoea in adult neurological conditions:
(1) Increasing awareness of the burden of sialorrhoea on patients and their caregivers, among healthcare professionals, could lead to improved patient care.
(2) More research is needed to demonstrate the benefits, side effects, and cost effectiveness of treatment options in the various conditions causing sialorrhoea.
(3) Greater awareness among healthcare professionals of the treatment options available for sialorrhoea management could improve patient care. The options range from nonpharmacological to pharmacological treatments, which have varying levels of supporting evidence.
(4) The provision of training in the administration of BoNT injections to clinicians could increase the availability of this treatment and reduce delays in the referral of patients to this service.
(5) Additional funding (e.g. from the Clinical Commissioning Group in England) for sialorrhoea could help to reduce significant delays in treatment.
Supplemental Material
Supplemental material, APPROVED_Xeomin_Prescribing_information_UKIE_combined_June_2019_v4 for The burden of sialorrhoea in chronic neurological conditions: current treatment options and the role of incobotulinumtoxinA (Xeomin®) by Francesca Morgante, Ganesh Bavikatte, Fahim Anwar and Biju Mohamed in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors would like to thank the patients who very kindly gave their permission for their case studies to be included within this publication. The authors also acknowledge Sarah Jayne Clements, PhD, from Costello Medical, Cambridge, UK, for medical writing and editorial assistance in preparing this publication, based on the authors’ input and direction.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication sponsorship, medical writing support and compensation for authors’ time were funded by Merz Pharma GmbH (Frankfurt).
Conflict of interest statement: FM: honoraria for advisory board/consultancy from Medtronic and Merz; speaker’s fees from Merz, BIAL, UCB Pharma, Medtronic, Chiesi, AbbVie and Zambon; editorial board member of Movement Disorders and Movement Disorders Clinical Practice; receipt of royalties from Springer for the book Disorders of Movement; Consultancy fees for expert review and guidance in the development of this supplement as an independent contractor for Merz Pharma GmbH; GB, FA: consultancy fees for providing expert review and guidance in the development of this supplement as an independent contractor for Merz Pharma GmbH; BM: grants from NeuroDerm, Bevan Commissions and Parkinson’s UK; honoraria for lectures from UCB Pharma and Profile Pharma; consultancy fees from AbbVie, Profile Pharma, Britannia and BIAL; consultancy fees for expert review and guidance in the development of this supplement as an independent contractor for Merz Pharma GmbH.
ORCID iD: F. Morgante  https://orcid.org/0000-0002-9834-3639
https://orcid.org/0000-0002-9834-3639
Contributor Information
Francesca Morgante, Neurosciences Research Centre, Molecular and Clinical Sciences Research Institute, St George’s University of London, London, United Kingdom; Department of Experimental and Clinical Medicine, University of Messina; Molecular and Clinical Sciences Research Institute, St George’s University of London, London, United Kingdom Cranmer Terrace, Jenner Wing, Ground Floor, Corridor 10, Room 0.135, London, SW17 0RE, UK.
Ganesh Bavikatte, Department of Rehabilitation Medicine, The Walton Centre NHS Foundation Trust, Liverpool, UK.
Fahim Anwar, Department of Rehabilitation Medicine, Cambridge University Hospital NHS Foundation Trust, Addenbrooke’s Hospital, Cambridge, UK.
Biju Mohamed, Department of Medicine and Gerontology, University Hospital of Wales, Cardiff, UK.
References
- 1. Bavikatte G, Sit PL, Hassoon A. Management of drooling of saliva. Br J Med Pract 2012; 5: 25–31. [Google Scholar]
- 2. Lakraj AA, Moghimi N, Jabbari B. Sialorrhea: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins 2013; 5: 1010–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGeachan AJ, McDermott CJ. Management of oral secretions in neurological disease. Pract Neurol 2017; 17: 96–103. [DOI] [PubMed] [Google Scholar]
- 4. Merello M. Sialorrhoea and drooling in patients with Parkinson’s disease: epidemiology and management. Drugs Aging 2008; 25: 1007–1019. [DOI] [PubMed] [Google Scholar]
- 5. Kalf JG, de Swart BJ, Borm GF, et al. Prevalence and definition of drooling in Parkinson’s disease: a systematic review. J Neurol 2009; 256: 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hockstein NG, Samadi DS, Gendron K, et al. Sialorrhea: a management challenge. Am Fam Physician 2004; 69: 2628–2634. [PubMed] [Google Scholar]
- 7. Gonzalez-Fernandez M, Daniels SK. Dysphagia in stroke and neurologic disease. Phys Med Rehabil Clin N Am 2008; 19: 867–888, x. [DOI] [PubMed] [Google Scholar]
- 8. Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol 2003; 114: 2226–2244. [DOI] [PubMed] [Google Scholar]
- 9. Erasmus CE, Van Hulst K, Rotteveel JJ, et al. Clinical practice: swallowing problems in cerebral palsy. Eur J Pediatr 2012; 171: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson J, Evatt ML, Chaudhuri KR. Sialorrhoea in Parkinson’s disease. In: Ray Chaudhuri K, Tolosa E, Schapira AHV, et al. (eds) Non-motor symptoms of Parkinson’s disease. 2nd ed. USA: Oxford University Press, 2014. [Google Scholar]
- 11. Raval TH, Elliott CA. Botulinum toxin injection to the salivary glands for the treatment of sialorrhea with chronic aspiration. Ann Otol Rhinol Laryngol 2008; 117: 118–122. [DOI] [PubMed] [Google Scholar]
- 12. Meningaud JP, Pitak-Arnnop P, Chikhani L, et al. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101: 48–57. [DOI] [PubMed] [Google Scholar]
- 13. Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS) – revised report of an EFNS task force. Eur J Neurol 2012; 19: 360–375. [DOI] [PubMed] [Google Scholar]
- 14. Simonds AK. Progress in respiratory management of bulbar complications of motor neuron disease/amyotrophic lateral sclerosis? Thorax 2017; 72: 199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pisa FE, Logroscino G, Giacomelli Battiston P, et al. Hospitalizations due to respiratory failure in patients with amyotrophic lateral sclerosis and their impact on survival: a population-based cohort study. BMC Pulm Med 2016; 16:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corcia P, Pradat PF, Salachas F, et al. Causes of death in a post-mortem series of ALS patients. Amyotroph Lateral Scler 2008; 9: 59–62. [DOI] [PubMed] [Google Scholar]
- 17. Mustfa N, Walsh E, Bryant V, et al. The effect of noninvasive ventilation on ALS patients and their caregivers. Neurology 2006; 66: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 18. Sancho J, Servera E, Morelot-Panzini C, et al. Non-invasive ventilation effectiveness and the effect of ventilatory mode on survival in ALS patients. Amyotroph Lat Scl Fr 2014; 15: 55–61. [DOI] [PubMed] [Google Scholar]
- 19. Vandenberghe N, Vallet AE, Petitjean T, et al. Absence of airway secretion accumulation predicts tolerance of noninvasive ventilation in subjects with amyotrophic lateral sclerosis. Respir Care 2013; 58: 1424–1432. [DOI] [PubMed] [Google Scholar]
- 20. Hobson EV, McGeachan A, Al-Chalabi A, et al. Management of sialorrhoea in motor neuron disease: a survey of current UK practice. Amyotroph Lat Scl Fr 2013; 14: 521–527. [DOI] [PubMed] [Google Scholar]
- 21. Chio A, Gauthier A, Vignola A, et al. Caregiver time use in ALS. Neurology 2006; 67: 902–904. [DOI] [PubMed] [Google Scholar]
- 22. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009; 24: 1641–1649. [DOI] [PubMed] [Google Scholar]
- 23. Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011; 26: 399–406. [DOI] [PubMed] [Google Scholar]
- 24. Malek N, Lawton MA, Grosset KA, et al. Autonomic dysfunction in early Parkinson’s disease: results from the United Kingdom tracking Parkinson’s study. Mov Disord Clin Pract 2016; 4: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mao CJ, Xiong YT, Wang F, et al. Motor subtypes and other risk factors associated with drooling in Parkinson’s disease patients. Acta Neurol Scand 2018; 137: 509–514. [DOI] [PubMed] [Google Scholar]
- 26. Ou R, Guo X, Wei Q, et al. Prevalence and clinical correlates of drooling in Parkinson disease: a study on 518 Chinese patients. Parkinsonism Relat Disord 2015; 21: 211–215. [DOI] [PubMed] [Google Scholar]
- 27. Kalf JG, Munneke M, van den Engel-Hoek L, et al. Pathophysiology of diurnal drooling in Parkinson’s disease. Mov Disord 2011; 26: 1670–1676. [DOI] [PubMed] [Google Scholar]
- 28. Nobrega AC, Rodrigues B, Melo A. Is silent aspiration a risk factor for respiratory infection in Parkinson’s disease patients? Parkinsonism Relat Disord 2008; 14: 646–648. [DOI] [PubMed] [Google Scholar]
- 29. Leibner J, Ramjit A, Sedig L, et al. The impact of and the factors associated with drooling in Parkinson’s disease. Parkinsonism Relat Disord 2010; 16: 475–477. [DOI] [PubMed] [Google Scholar]
- 30. Miller N, Noble E, Jones D, et al. Hard to swallow: dysphagia in Parkinson’s disease. Age Ageing 2006; 35: 614–618. [DOI] [PubMed] [Google Scholar]
- 31. Ozdilek B, Gunal DI. Motor and non-motor symptoms in Turkish patients with Parkinson’s disease affecting family caregiver burden and quality of life. J Neuropsychiatry Clin Neurosci 2012; 24: 478–483. [DOI] [PubMed] [Google Scholar]
- 32. Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol 2005; 47: 571–576. [DOI] [PubMed] [Google Scholar]
- 33. Haak P, Lenski M, Hidecker MJ, et al. Cerebral palsy and aging. Dev Med Child Neurol 2009; 51 Suppl 4: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parkes J, Hill N, Platt MJ, et al. Oromotor dysfunction and communication impairments in children with cerebral palsy: a register study. Dev Med Child Neurol 2010; 52: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 35. Wright RE, Wright FR, Carson CA. Videofluoroscopic assessment in children with severe cerebral palsy presenting with dysphagia. Pediatr Radiol 1996; 26: 720–722. [DOI] [PubMed] [Google Scholar]
- 36. Mirrett PL, Riski JE, Glascott J, et al. Videofluoroscopic assessment of dysphagia in children with severe spastic cerebral palsy. Dysphagia 1994; 9: 174–179. [DOI] [PubMed] [Google Scholar]
- 37. Balandin S, Hemsley B, Hanley L, et al. Understanding mealtime changes for adults with cerebral palsy and the implications for support services. J Intellect Dev Disabil 2009; 34: 197–206. [DOI] [PubMed] [Google Scholar]
- 38. Schwarz M, Coccetti A, Murdoch A, et al. The impact of aspiration pneumonia and nasogastric feeding on clinical outcomes in stroke patients: a retrospective cohort study. J Clin Nurs 2018; 27: e235–e241. [DOI] [PubMed] [Google Scholar]
- 39. Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis 2000; 10: 380–386. [DOI] [PubMed] [Google Scholar]
- 40. Kidd D, Lawson J, Nesbitt R, et al. Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med 1993; 86: 825–829. [PubMed] [Google Scholar]
- 41. Daniels SK, Brailey K, Priestly DH, et al. Aspiration in patients with acute stroke. Arch Phys Med Rehabil 1998; 79: 14–19. [DOI] [PubMed] [Google Scholar]
- 42. Kang Y, Chun MH, Lee SJ. Evaluation of salivary aspiration in brain-injured patients with tracheostomy. Ann Rehabil Med 2013; 37: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leder SB. Fiberoptic endoscopic evaluation of swallowing in patients with acute traumatic brain injury. J Head Trauma Rehabil 1999; 14: 448–453. [DOI] [PubMed] [Google Scholar]
- 44. National Health Service trusts and National Health Service foundation trusts. National schedule of reference costs: year 2017–2018, https://improvement.nhs.uk/resources/reference-costs/#rc1718 (2018, accessed 20 January 2019).
- 45. Owen S. Hypersalivation – what drug treatment options are available? https://www.sps.nhs.uk/articles/hypersalivation-d-what-drug-treatment-options-are-available/ (2015, accessed 26 November 2018).
- 46. National Institute of Health and Clinical Excellence. Parkinson’s disease in adults, https://www.nice.org.uk/guidance/ng71 (2017, 26 November 2018).
- 47. National Institute of Health and Clinical Excellence. Motor neurone disease: assessment and management, https://www.nice.org.uk/guidance/NG42 (2016, 26 November 2018). [PubMed]
- 48. Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 2019; 34: 180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Neurology 2009; 73: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Traynor BJ, Alexander M, Corr B, et al. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J Neurol Neurosurg Psychiatry 2003; 74: 1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hogden A, Greenfield D, Nugus P, et al. What are the roles of carers in decision-making for amyotrophic lateral sclerosis multidisciplinary care? Patient Prefer Adherence 2013; 7: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hanratty A, Husbands E, Kelly F, et al. Managing saliva problems in motor neurone disease: information for health and social care professionals: MNDA, Motor Neurone Disease Association. Version 1.1. https://static.mndassociation.org/app/uploads/2012/04/19135720/information-sheet-p3-managing-saliva-problems.pdf (2017, accessed 09 November 2019).
- 53. Banfi P, Ticozzi N, Lax A, et al. A review of options for treating sialorrhea in amyotrophic lateral sclerosis. Respir Care 2015; 60: 446–454. [DOI] [PubMed] [Google Scholar]
- 54. Pellegrini A, Lunetta C, Ferrarese C, et al. Sialorrhoea: how to manage a frequent complication of motor neuron disease. EMJ Neurology 2015; 3: 107. [Google Scholar]
- 55. Bayer plc. Kwells 300 microgram tablets - hyoscine hydrobromide. https://www.medicines.org.uk/emc/files/pil.250.pdf (2015, accessed 01 May 2019).
- 56. Sanofi. Buscopan(R). Ampoules 20 mg/ml solution for injection. https://www.medicines.org.uk/emc/files/pil.890.pdf (2017, accessed 01 May 2019).
- 57. GlaxoSmithKline. Scopoderm 1.5 mg patches. https://www.medicines.org.uk/emc/files/pil.3276.pdf (2018, accessed 01 May 2019).
- 58. Martindale Pharma. Atropine eye drops 1.0% w/v. https://www.medicines.org.uk/emc/files/pil.3297.pdf (2016, accessed 01 May 2019).
- 59. De Simone GG, Eisenchlas JH, Junin M, et al. Atropine drops for drooling: a randomized controlled trial. Palliat Med 2006; 20: 665–671. [DOI] [PubMed] [Google Scholar]
- 60. Martindale Pharma. Glycopyrronium bromide 200 micrograms/ml solution for injection. https://www.medicines.org.uk/emc/files/pil.3389.pdf (2018, accessed 01 May 2019).
- 61. Merz Pharma. Xeomin summary of product characteristics (United Kingdom), 2019. https://www.medicines.org.uk/emc/product/4609/smpc (2019, accessed 10 June 2019).
- 62. Merz Pharma. Xeomin full prescribing information (United States). Raleigh, NC: Merz North America, Inc, 2018. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=ccdc3aae-6e2d-4cd0-a51c-8375bfee9458&type=display (2018, accessed 01 May 2019). [Google Scholar]
- 63. Stokholm MG, Bisgård C, Vilholm OJ. Safety and administration of treatment with botulinum neurotoxin for sialorrhoea in ALS patients: review of the literature and a proposal for tailored treatment. Amyotroph Lat Scl Fr 2013; 14: 516–520. [DOI] [PubMed] [Google Scholar]
- 64. US WorldMeds. Full Prescribing Information: MYOBLOC (rimabotulinumtoxinB) injection, for intramuscular or intraglandular use. US Food and Drug Administration, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103846s5190lbl.pdf#page=18 (2019, accessed 01 May 2019). [Google Scholar]
- 65. Costa J, Rocha ML, Ferreira J, et al. Botulinum toxin type-B improves sialorrhea and quality of life in bulbaronset amyotrophic lateral sclerosis. J Neurol 2008; 255: 545–550. [DOI] [PubMed] [Google Scholar]
- 66. Eisai Manufacturing Limited. Summary of product characteristics: NeuroBloc. https://www.ema.europa.eu/en/documents/product-information/neurobloc-epar-product-information_en.pdf. (2001, accessed 01 May 2019)
- 67. Mato A, Limeres J, Tomás I, et al. Management of drooling in disabled patients with scopolamine patches. Br J Clin Pharmacol 2010; 69: 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arbouw MEL, Movig KLL, Koopmann M, et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology. 2010; 74: 1203–1207. [DOI] [PubMed] [Google Scholar]
- 69. Hyson HC, Johnson AM, Jog MS. Sublingual atropine for sialorrhea secondary to parkinsonism: a pilot study. Mov Disord 2002; 17: 1318–1320. [DOI] [PubMed] [Google Scholar]
- 70. Zeller RS, Lee H-M, Cavanaugh PF, et al. Randomized phase III evaluation of the efficacy and safety of a novel glycopyrrolate oral solution for the management of chronic severe drooling in children with cerebral palsy or other neurologic conditions. Ther Clin Risk Manag 2012; 8: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mier RJ, Bachrach SJ, Lakin RC, et al. Treatment of sialorrhea with glycopyrrolate: a double-blind, dose-ranging study. Arch Pediatr Adolesc Med 2000; 154: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 72. Talmi YP, Finkelstein Y, Zohar Y. Reduction of salivary flow with transdermal scopolamine: a four-year experience. Otolaryngol Head Neck Surg 1990; 103: 615–618. [DOI] [PubMed] [Google Scholar]
- 73. National Institute of Health and Care Excellence. Final appraisal document: Xeomin (botulinum neurotxin type A) for treating chronic sialorrhoea, 2019. https://www.nice.org.uk/guidance/gid-ta10296/documents/final-appraisal-determination-document (2019, accessed 01 September 2019).
- 74. Reddihough D, Erasmus CE, Johnson H, et al. Botulinum toxin assessment, intervention and aftercare for paediatric and adult drooling: international consensus statement. Eur J Neurol 2010; 17 Suppl 2: 109–121. [DOI] [PubMed] [Google Scholar]
- 75. Ondo WG, Hunter C, Moore W. A double-blind placebo-controlled trial of botulinum toxin B for sialorrhea in Parkinson’s disease. Neurology 2004; 62: 37–40. [DOI] [PubMed] [Google Scholar]
- 76. Mancini F, Zangaglia R, Cristina S, et al. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type A in the treatment of drooling in parkinsonism. Mov Disord 2003; 18: 685–688. [DOI] [PubMed] [Google Scholar]
- 77. Martinez-Poles J, Nedkova-Hristova V, Escribano-Paredes JB, et al. Incobotulinumtoxin A for sialorrhea in neurological disorders: a real-life experience. Toxins 2018; 10: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marvulli R, Mastromauro L, De Venuto G, et al. Efficacy of incobotulinumtoxin type A (Xeomin®) in the management of sialorrhea in neurodegenerative diseases. J Alzheimers Dis Parkinsonism 2017; 7: 1–5. [Google Scholar]
- 79. Kukreja R, Singh BR. The botulinum toxin as a therapeutic agent: molecular and pharmacological insights. Res Rep Biochem 2015; 5: 173–183. [Google Scholar]
- 80. Guidubaldi A, Fasano A, Ialongo T, et al. Botulinum toxin A versus B in sialorrhea: a prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson’s disease. Mov Disord 2011; 26: 313–319. [DOI] [PubMed] [Google Scholar]
- 81. Restivo DA, Panebianco M, Casabona A, et al. Botulinum toxin A for sialorrhoea associated with neurological disorders: evaluation of the relationship between effect of treatment and the number of glands treated. Toxins 2018; 10: E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pena AH, Cahill AM, Gonzalez L, et al. Botulinum toxin A injection of salivary glands in children with drooling and chronic aspiration. J Vasc Interv Radiol 2009; 20: 368–373. [DOI] [PubMed] [Google Scholar]
- 83. Lagalla G, Millevolte M, Capecci M, et al. Long-lasting benefits of botulinum toxin type B in Parkinson’s disease-related drooling. J Neurol 2009; 256: 563–567. [DOI] [PubMed] [Google Scholar]
- 84. Jackson CE, Gronseth G, Rosenfeld J, et al. Randomized double-blind study of botulinum toxin type B for sialorrhea in ALS patients. Muscle Nerve 2009; 39: 137–143. [DOI] [PubMed] [Google Scholar]
- 85. Mazlan M, Rajasegaran S, Engkasan PJ, et al. A double-blind randomized controlled trial investigating the most efficacious dose of botulinum toxin-A for sialorrhea treatment in Asian adults with neurological diseases. Toxins 2015; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tiigimae-Saar J, Tamme T, Rosenthal M, et al. Saliva changes in Parkinson’s disease patients after injection of Botulinum neurotoxin type A. Neurol Sci 2018; 39: 871–877. [DOI] [PubMed] [Google Scholar]
- 87. Şen A, Arpaci B. Effects of repeated botulinum toxin treatment for sialorrhea in patients with Parkinson’s disease. Noro Psikiyatr Ars 2015; 52: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Verma A, Steele J. Botulinum toxin improves sialorrhea and quality of living in bulbar amyotrophic lateral sclerosis. Muscle Nerve 2006; 34: 235–237. [DOI] [PubMed] [Google Scholar]
- 89. National Patient Safety Agency, Royal College of Speech and Language Therapists, National Association of Care Catering, et al. Dysphagia diet food texture descriptors. http://www.hospitalcaterers.org/media/1160/dysphagia-descriptors.pdf (2011, accessed 18 January 2019).
- 90. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 2004; 75: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2001; 285: 1059–1070. [DOI] [PubMed] [Google Scholar]
- 92. Jost WH, Friedman A, Michel O, et al. SIAXI: placebo-controlled, randomized, double-blind study of incobotulinumtoxinA. Neurology 2019; 92: e1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Narayanaswami P, Geisbush T, Tarulli A, et al. Drooling in Parkinson’s disease: a randomized controlled trial of incobotulinum toxin A and meta-analysis of botulinum toxins. Parkinsonism Relat Disord 2016; 30: 73–77. [DOI] [PubMed] [Google Scholar]
- 94. Dressler D, Bigalke H. Long-term stability of reconstituted incobotulinumtoxinA: how can we reduce costs of botulinum toxin therapy? J Neural Transm 2017; 124: 1223–1225. [DOI] [PubMed] [Google Scholar]
- 95. Ferris A, Mohamed B, Thomas C. Eye drop psychosis in Parkinson’s disease: a cautionary tale. Prog Neurol Psychiatry 2018; 22: 11–14. [Google Scholar]
- 96. McGeachan AJ, Hobson EV, Al-Chalabi A, et al. A multicentre evaluation of oropharyngeal secretion management practices in amyotrophic lateral sclerosis. Amyotroph Lat Scl Fr 2017; 18: 1–9. [DOI] [PubMed] [Google Scholar]
- 97. Perry EK, Kilford L, Lees AJ, et al. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol 2003; 54: 235–238. [DOI] [PubMed] [Google Scholar]
- 98. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 2015; 15: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scully C, Felix DH. Oral medicine: update for the dental practitioner: dry mouth and disorders of salivation. Br Dent J 2005; 199: 423–427. [DOI] [PubMed] [Google Scholar]
- 100. Aging Brain Program. Anticholinergic cognitive burden scale: 2012 Update: Regenstrief Institute, Inc., 2008. https://psnc.org.uk/lancashire-lpc/wp-content/uploads/sites/97/2014/02/Anticholinergic-burden-scale-2012.pdf (2012, accessed 14 February 2019).
- 101. Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008; 4: 311–320. [Google Scholar]
- 102. Carnahan RM, Lund BC, Perry PJ, et al. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol 2006; 46: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 103. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168: 508–513. [DOI] [PubMed] [Google Scholar]
- 104. Dogu O, Apaydin D, Sevim S, et al. Ultrasound-guided versus ‘blind’ intraparotid injections of botulinum toxin-A for the treatment of sialorrhoea in patients with Parkinson’s disease. Clin Neurol Neurosurg 2004; 106: 93–96. [DOI] [PubMed] [Google Scholar]
- 105. Breheret R, Bizon A, Jeufroy C, et al. Ultrasound-guided botulinum toxin injections for treatment of drooling. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128: 224–229. [DOI] [PubMed] [Google Scholar]
- 106. National Institute of Health and Care Excellence. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine, 2012. https://www.nice.org.uk/guidance/ta260/resources/botulinum-toxin-typea-for-the-prevention-of-headaches-in-adults-with-chronic-migraine-pdf-82600545273541 (2012, accessed 01 May 2019).
- 107. Albrecht P, Jansen A, Lee JI, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019; 92: e48–e54. [DOI] [PubMed] [Google Scholar]
- 108. Nobrega AC, Rodrigues B, Melo A. Does botulinum toxin injection in parotid glands interfere with the swallowing dynamics of Parkinson’s disease patients? Clin Neurol Neurosurg 2009; 111: 430–432. [DOI] [PubMed] [Google Scholar]
- 109. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhoea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2000; 69: 121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Reed J, Mans CK, Brietzke SE. Surgical management of drooling: a meta-analysis. Arch Otolaryngol Head Neck Surg 2009; 135: 924–931. [DOI] [PubMed] [Google Scholar]
- 111. Khan WU, Islam A, Fu A, et al. Four-duct ligation for the treatment of sialorrhea in children. JAMA Otolaryngol Head Neck Surg 2016;142:278–83. [DOI] [PubMed] [Google Scholar]
- 112. Assouline A, Levy A, Abdelnour-Mallet M, et al. Radiation therapy for hypersalivation: a prospective study in 50 amyotrophic lateral sclerosis patients. Int J Radiat Oncol 2014; 88: 589–595. [DOI] [PubMed] [Google Scholar]
- 113. Guy N, Bourry N, Dallel R, et al. Comparison of radiotherapy types in the treatment of sialorrhea in amyotrophic lateral sclerosis. J Palliat Med 2011; 14: 391–395. [DOI] [PubMed] [Google Scholar]
- 114. Lawrence R, Bateman N. Surgical management of the drooling child. Curr Otorhinolaryngol Rep 2018; 6: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shirley WP, Hill JS, Woolley AL, et al. Success and complications of four-duct ligation for sialorrhea. Int J Pediatr Otorhinolaryngol 2003; 67: 1–6. [DOI] [PubMed] [Google Scholar]
- 116. Slade A, Stanic S. Managing excessive saliva with salivary gland irradiation in patients with amyotrophic lateral sclerosis. J Neurol Sci 2015; 352: 34–36. [DOI] [PubMed] [Google Scholar]
- 117. O’Dwyer TP, Conlon BJ. The surgical management of drooling—a 15 year follow-up. Clin Otolaryngol Allied Sci 1997; 22: 284–287. [DOI] [PubMed] [Google Scholar]
- 118. McAloney N, Kerawala CJ, Stassen LF. Management of drooling by transposition of the submandibular ducts and excision of the sublingual glands. J Ir Dent Assoc 2005; 51: 126–131. [PubMed] [Google Scholar]
- 119. Chang CJ, May-Kuen Wong aA. Intraductal laser photocoagulation of the bilateral parotid ducts for reduction of drooling in patients with cerebral palsy. Plast Reconstr Surg 2001; 107: 907–913. [DOI] [PubMed] [Google Scholar]
- 120. Andersen PM, Borasio G, Dengler R, et al. EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives: an evidence-based review with good practice points. Eur J Neurol 2005; 12: 921–938. [DOI] [PubMed] [Google Scholar]
- 121. Hallett KB, Lucas JO, Johnston T, et al. Dental health of children with cerebral palsy following sialodochoplasty. Spec Care Dent 1995; 15: 234–238. [DOI] [PubMed] [Google Scholar]
- 122. Stern Y, Feinmesser R, Collins M, et al. Bilateral submandibular gland excision with parotid duct ligation for treatment of sialorrhea in children: long-term results. JAMA Otolaryngol Head Neck Surg 2002;128:801–3. [DOI] [PubMed] [Google Scholar]
- 123. Neppelberg E, Haugen DF, Thorsen L, et al. Radiotherapy reduces sialorrhea in amyotrophic lateral sclerosis. Eur J Neurol 2007; 14: 1373–1377. [DOI] [PubMed] [Google Scholar]
- 124. Kasarskis EJ, Hodskins J, St Clair WH. Unilateral parotid electron beam radiotherapy as palliative treatment for sialorrhea in amyotrophic lateral sclerosis. J Neurol Sci 2011; 308: 155–157. [DOI] [PubMed] [Google Scholar]
- 125. Weikamp JG, Schinagl DA, Verstappen CC, et al. Botulinum toxin-A injections vs radiotherapy for drooling in ALS. Acta Neurol Scand 2016; 134: 224–231. [DOI] [PubMed] [Google Scholar]
- 126. Aridegbe T, Kandler R, Walters SJ, et al. The natural history of motor neuron disease: assessing the impact of specialist care. Amyotroph Lat Scl Fr 2013; 14: 13–19. [DOI] [PubMed] [Google Scholar]
- 127. Van den Berg JP, Kalmijn S, Lindeman E, et al. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology 2005; 65: 1264–1267. [DOI] [PubMed] [Google Scholar]
- 128. Cheng BHW, Chan KY, Judy Chung YK, et al. Supportive & palliative interventions in motor neurone disease: what we know from current literature? Ann Palliat Med 2017; 7: 320–331. [DOI] [PubMed] [Google Scholar]
- 129. Van der Steen I, Van den Berg JP, Buskens E, et al. The costs of amyotrophic lateral sclerosis, according to type of care. Amyotroph Lateral Scler 2009; 10: 27–34. [DOI] [PubMed] [Google Scholar]
- 130. Galvin M, Ryan P, Maguire S, et al. The path to specialist multidisciplinary care in amyotrophic lateral sclerosis: a population- based study of consultations, interventions and costs. PLoS One. 2017; 12: e0179796-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pan Mersey Area Prescribing Committee. Pharmacological management of hypersalivation in children and adults. Prescribing Guideline Version 1.2. https://www.panmerseyapc.nhs.uk/media/2066/hypersalivation.pdf (2017, accessed 30 November 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, APPROVED_Xeomin_Prescribing_information_UKIE_combined_June_2019_v4 for The burden of sialorrhoea in chronic neurological conditions: current treatment options and the role of incobotulinumtoxinA (Xeomin®) by Francesca Morgante, Ganesh Bavikatte, Fahim Anwar and Biju Mohamed in Therapeutic Advances in Neurological Disorders