Abstract
Background
HCV infection disproportionately affects underserved populations such as homeless individuals, people who inject drugs and prison populations. Peer advocacy can enable active engagement with healthcare services and increase the likelihood of favourable treatment outcomes.
Objectives
This observational study aims to assess the burden of disease in these underserved populations and describe the role of peer support in linking these individuals to specialist treatment services.
Methods
Services were identified if they had a high proportion of individuals with risk factors for HCV, such as injecting drug use or homelessness. Individuals were screened for HCV using point-of-care tests and a portable FibroScan. All positive cases received peer support for linkage to specialist care. Information was gathered on risk factors, demographics and follow-up information regarding linkage to care and treatment outcomes.
Results
A total of 461 individuals were screened, of which 197 (42.7%) were chronically infected with HCV. Referral was made to secondary care for 176 (89.3%) and all received peer support, with 104 (52.8%) individuals engaged with treatment centres. Of these, 89 (85.6%) started treatment and 76 (85.4%) had a favourable outcome. Factors associated with not being approved for treatment were recent homelessness, younger age and current crack cocaine injecting.
Conclusions
Highly trained peer support workers working as part of a specialist outreach clinical team help to identify a high proportion of individuals exposed to HCV, achieve high rates of engagement with treatment services and maintain high rates of treatment success amongst a population with complex needs.
Introduction
HCV infection is a major cause of chronic liver disease and death globally.1 In the UK, HCV infection occurs primarily through injecting drug use.2 Chronically infected people are at risk of progressive liver disease characterized by hepatocellular inflammation, hepatic fibrosis, cirrhosis and hepatocellular carcinoma.3
In the UK and many other countries HCV disproportionately affects underserved populations such as homeless individuals, people who inject drugs (PWID) and prison populations. New drug therapies, such as protease and polymerase inhibitors, called direct-acting antivirals (DAAs), have been shown to be well tolerated and highly effective, meaning that HCV elimination is being considered as a realistic possibility.4 However, underserved populations (whilst most at risk of HCV) encounter the biggest challenges in terms of testing, linkage to care and treatment.5
A high prevalence of 13% chronic HCV infection has been found previously among homeless people opportunistically screened at residential hostels and day centres across London.6 Data from the HALT Hepatitis Study showed that 35% of HCV antibody-positive recruits were homeless at enrolment and that over 50% of HCV-infected patients knew of their status but had disengaged from treatment services.7 This population therefore includes a high number of undiagnosed cases and previously known HCV-positive cases who are not accessing treatment services.
Strategies to improve HCV case detection and management can draw from emerging evidence from TB management. TB is a disease that affects a similar group of individuals in whom community models have been proposed and adopted for some time.8 These outreach models of care focus on active case finding and treatment support in vulnerable groups and have been shown to be cost-effective in high-income countries.9
Peer support has emerged as a potentially useful tool to improve patient outcomes and can be used as a mechanism to enable active engagement with healthcare among underserved populations. Peers, with their lived experience of a lifestyle or condition, can share similar experiences or characteristics with the target intervention group, giving them a connection that enables them to support others facing similar challenges.10
The use of peer support models in healthcare has been used particularly in mental health services, where peer support workers (PSWs) serve to improve engagement with healthcare and positive health outcomes among their clients.11,12 Peers will typically be recruited from within the client pool of a service and are given some simple training for the role of offering support to other individuals to aid their journey to recovery.13 This could entail helping people with attendance at appointments, medication adherence or healthy lifestyle changes, to achieve an optimal outcome that aids their recovery. Successful examples have been seen in chronic14,15 and infectious diseases16–18 and people who misuse substances.19
In HCV, peers have been used in a number of roles aiding individuals to engage with treatment and qualitative and observational studies have shown a breadth of strategies in the cascade of care with mixed results.19–27 One randomized controlled trial (RCT) has shown a positive effect of peer support in engagement with services compared with standard of care; however, this did not include treatment uptake or completion outcomes.7
HepCare is co-funded by the European Commission and aims to develop models of care that link primary, secondary, outreach and community care and treatment for at-risk populations across four EU sites (Ireland, UK, Spain and Romania). The London site leads on a work package that uses specialist outreach interventions based on peer support in the community to increase awareness of the risk of HCV and the importance of testing and to provide linkage to care and treatment support for vulnerable groups. This observational study assessed the burden of disease in an underserved population and describes the role of peer support in linking these individuals to specialist treatment services.
Methods
Ethics and governance
Research ethics approval was obtained from the North West – Haydock Research Ethics Committee (17/NW/0417). Governance and oversight for the study were provided through the overall governance structure of the HepCare Europe Project. An International Advisory Board (comprising clinicians, academics, researchers and representatives of EU regulatory bodies and service user organizations) provided external oversight for the project. A Project Steering Group comprising Work Package and Site leaders provided internal oversight. Finally, site-specific teams were established to execute the project at each site.
Study design and setting
The HepCare team recruited participants nested within the Find&Treat Mobile Health Unit (MHU), University College London Hospital (UCLH) NHS Trust, which provides health screening for homeless individuals across London using community interventions and specialist outreach workers. In collaboration with the homeless peer advocacy organization Groundswell, a peer-led community outreach service was developed to test individuals at risk of HCV, link them to specialist care and provide treatment support. Groundswell has developed a Homeless Health Peer Advocacy (HHPA) service that uses PSWs to accompany and support individuals to healthcare appointments. Peers were selected from an experienced pool of PSWs from Groundswell who expressed an interest in working with the project and who had a level of competency commensurate with the outreach role. They were given additional training in HCV awareness, testing and the use of a FibroScan for liver fibrosis by the outreach team and worked alongside the outreach nurses until they were assessed as having sufficient competency. Peers were also to take individuals to clinical appointments as well as monitor treatment adherence whilst working under the clinical supervision of a nurse specialist.
Sites and patient identification
Sites were identified if they were deemed to have a high proportion of individuals with risk factors for HCV such as injecting drug use, and included homeless hostels, day centres and drug treatment services. Inclusion criteria were being over 16 years of age, a willingness and ability to provide signed informed consent and being from an underserved population in the community. This was defined as groups whose social circumstances make it difficult to access services and could include people who are homeless, people who misuse substances and people exposed to the prison system. Prior to screening, sites were visited by a member of the team to speak to staff and service users. Posters and leaflets were also left with information about what activities were available on the day.
Patient recruitment
Individuals were approached by a member of the clinical team and, following provision of informed consent and a conversation regarding risk factors, were offered HCV testing. Initially individuals were tested using the OraQuick HCV rapid antibody point-of-care mouth swab, which gives a result in 20 min. Those positive for HCV antibody were then tested for chronic infection using either dried blood spot tests or venous sampling for HCV RNA. Results would take approximately 7 days. Those who reported previously having tested antibody or RNA positive were either re-tested for HCV RNA, or were confirmed as being chronically infected via healthcare records (retrospectively). Those testing positive, reporting a previous positive result or with risk factors for liver disease were offered a liver assessment using a portable FibroScan, which uses transient elastography to assess liver fibrosis.
Peer support and linkage to care
All those chronically infected were followed up by a PSW who would meet the patient after the test result to explain the care pathway. All were to be referred directly to specialist treatment services by a nurse specialist and clinical appointments were made. PSWs would accompany individuals to hepatology and related healthcare appointments and cover associated travel costs. Other facilitators provided would be a drink and food whilst waiting as well as mobile phone top-up credit. To support individuals through treatment, PSWs would keep in contact by regular phone calls or face-to-face meetings or supervising medication by directly observing therapy (DOT).
Data collection
Information was gathered on risk factors and demographic information at screening as part of routine patient care. Follow-up information regarding linkage to care and treatment outcomes was gathered by the contacting patients and support services by a member of the clinical team. All patient data were entered into a patient management system database and an anonymized extract of the data was analysed using STATA 15.1. Summary data were calculated and logistic regression was used to explore the associations with achieving linkage to care and a successful treatment outcome such as completing treatment with sustained virological response (SVR).
Results
Population characteristics
A total of 461 individuals were screened between September 2016 and May 2018 across 63 sites in London, such as drug and alcohol services, homeless day centres and homeless hostels, over 109 sessions. The majority (78.7%) were male, the median age was 45.7 years (IQR 39–52), they were mostly UK born (76.6%) and white ethnicity was the most common (88.8%). Over half (59.9%) had been recently homeless, defined as rough sleeping or in a hostel within the past 12 months. For full results see Table 1.
Table 1.
Population characteristics: overall and of those with and without anti-HCV antibodies
| Anti-HCV antibody status |
||||||
|---|---|---|---|---|---|---|
| Overall |
positive |
negative |
||||
| Characteristic | no. | % | no. | % | no. | % |
| Total population | 461 | – | 195 | 42.3 | 266 | 57.7 |
| male | 363 | 78.7 | 155 | 79.5 | 208 | 78.2 |
| female | 98 | 21.3 | 40 | 20.5 | 58 | 21.8 |
| Age category (years) | ||||||
| 16–25 | 13 | 2.8 | 10 | 5.1 | 3 | 1.1 |
| 26–35 | 61 | 13.2 | 29 | 14.9 | 32 | 12.0 |
| 36–45 | 160 | 34.7 | 63 | 32.3 | 97 | 36.5 |
| 46–55 | 157 | 34.1 | 61 | 31.3 | 96 | 36.1 |
| 56–65 | 64 | 13.9 | 28 | 14.4 | 36 | 13.5 |
| 66–75 | 3 | 0.7 | 1 | 0.5 | 2 | 0.8 |
| missing | 3 | 0.7 | 3 | 1.5 | 0 | 0.0 |
| UK born | ||||||
| no | 108 | 23.4 | 54 | 27.7 | 54 | 20.3 |
| yes | 353 | 76.6 | 141 | 72.3 | 212 | 79.7 |
| Homeless recently | ||||||
| no | 185 | 40.1 | 63 | 32.3 | 122 | 45.9 |
| yes | 276 | 59.9 | 132 | 67.7 | 144 | 54.1 |
| OST currently | ||||||
| no | 194 | 42.1 | 123 | 63.1 | 71 | 26.7 |
| yes | 267 | 57.9 | 72 | 36.9 | 195 | 73.3 |
| Ever injected drugs | ||||||
| no | 132 | 28.6 | 112 | 57.4 | 20 | 7.5 |
| yes | 329 | 71.4 | 83 | 42.6 | 246 | 92.5 |
| Currently injecting drugs | ||||||
| no | 336 | 72.9 | 160 | 82.1 | 176 | 66.2 |
| yes | 125 | 27.1 | 35 | 18.0 | 90 | 33.8 |
| Alcohol use | ||||||
| >50 units per week | ||||||
| no | 306 | 66.4 | 136 | 69.7 | 170 | 63.9 |
| yes | 155 | 33.6 | 59 | 30.3 | 96 | 36.1 |
| >100 units per week | ||||||
| no | 355 | 77.0 | 152 | 78.0 | 203 | 76.3 |
| yes | 106 | 23.0 | 43 | 22.1 | 63 | 23.7 |
| Previous HCV antibody test | ||||||
| no | 75 | 16.3 | 71 | 29.3 | 4 | 1.8 |
| yes | 356 | 77.2 | 146 | 60.3 | 210 | 95.9 |
| not sure | 4 | 0.8 | 4 | 1.65 | 0 | – |
| missing | 26 | 5.6 | 21 | 8.7 | 5 | 2.3 |
| FibroScan (kPa) | 295 | – | 66 | 6.8a | 229 | 11.0a |
| F1 | 184 | 62.4 | 53 | 27.2 | 131 | 49.3 |
| F2 | 44 | 14.9 | 7 | 3.59 | 37 | 13.91 |
| F3 | 22 | 7.5 | 1 | 0.5 | 21 | 17.9 |
| F4 | 45 | 15.3 | 5 | 2.6 | 40 | 15.0 |
These are mean FibroScan values rather than percentages.
Many reported that they had had a previous HCV antibody test (356, 77.2%), with 255 (55.3%) individuals reporting a previous positive test. Most of individuals with a positive test (198, 77.6%) stated that they were disengaged from treatment services. In total 266 (57.7%) individuals were found to have been exposed to HCV. The vast majority (92.5%) had a history of injecting drug use with 33.8% currently injecting and 73% currently on opiate substitution therapy (OST). Problem alcohol use was high, with over a third reporting daily alcohol intake with >50 units each week and nearly a quarter (23.7%) with daily consumption and more than 100 units each week.
Cascade of care
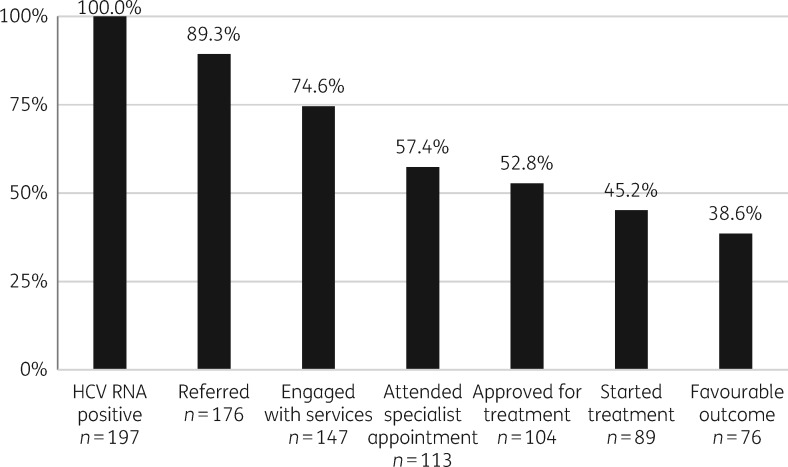
Following HCV RNA testing a total of 197 individuals (42.7% of the total population) were identified as being HCV RNA positive and disengaged from treatment services. Referral was made to secondary care for 176 (89.3%) individuals, 14 (7.1%) were lost to follow-up after RNA testing, 4 (2%) were in progress at the time of data collection and 3 (1.5%) were not required as they were already being treated. All received varying degrees of peer support, with 104 (52.8%) individuals sufficiently engaged with treatment centres to be approved for treatment. This would typically require at least two appointments and completion of pre-treatment investigations such as ultrasound scanning and baseline blood tests. Of those approved, 89 started treatment and of these 20 (22.5%) had completed treatment with SVR, 43 (41.3%) completed with SVR results pending and 13 (12.5%) were still on treatment (Table 2). This makes a total of 76 (85.4%) with a favourable outcome following treatment initiation, or 38.6% of all those identified with HCV (Figure 1).
Table 2.
The linkage to care cascade
| Characteristic | Number of patients | Percentage |
|---|---|---|
| Total number HCV RNA positive | 197 | 42.7 |
| Referred to specialist care | ||
| complete | 176 | 89.3 |
| in progress | 18 | 9.1 |
| not applicable | 3 | 1.5 |
| Outcomes post-referral | ||
| engaged with services | 147 | 74.6 |
| attended specialist appointment | 113 | 57.4 |
| Total approved for treatment | 104 | 52.8 |
| Started treatment | 89/104 | 85.6 |
| on treatment | 13 | 12.5 |
| ℞ complete with SVR | 20 | 19.2 |
| ℞ complete with SVR pending | 43 | 41.3 |
| Total positive outcome | 76/89 | 85.4 |
| ℞ completed no SVR | 2 | 1.9 |
| ℞ pause (medical) | 1 | 1.0 |
| ℞ pause (social) | 4 | 3.8 |
| ℞ started: died (other cause) | 2 | 1.9 |
| ℞ started: died (HCV) | 1 | 1.0 |
| treatment abandoned | 3 | 2.9 |
| Total negative outcome | 13/89 | 14.6 |
| Outcome of all chronic HCV | ||
| positive outcome | 76/197 | 38.6 |
| negative outcome | 121/197 | 61.4 |
Figure 1.
Cascade of care from testing positive to treatment outcome.
Risk factors associated with approval for treatment
Variables thought to affect the likelihood of being approved for treatment were investigated using univariate logistic regression (Table 3). Increasing age was associated with being more likely to be approved for treatment (OR 1.04, CI 1.0–1.08, P=0.01). Those with a recent history of homelessness, defined as rough sleeping or living in a homeless hostel in the previous year, were less likely to be approved (OR 0.53, CI 0.3–0.93, P=0.03), as were those currently injecting crack cocaine (OR 0.49, CI 0.25–0.92, P=0.03).
Table 3.
Risk factors associated with approval for HCV treatment
| Not approved | Approved | Logistic regression |
||||
|---|---|---|---|---|---|---|
| (n=93) |
(n=104) |
|||||
| Characteristic | no. | % | no. | % | OR | P value |
| Homeless | 55 | 59.1 | 45 | 43.3 | 0.53 | 0.03 |
| Age (years) | 93 | 44.6a | 104 | 48.1a | 1.04 | 0.01 |
| Injecting crack cocaine (current) | 33 | 35.5 | 22 | 21.2 | 0.488 | 0.03 |
| Current injecting (all drugs) | 38 | 40.9 | 29 | 27.9 | 0.56 | 0.06 |
| Problem alcohol use (>100 units) | 27 | 29.0 | 18 | 17.3 | 0.512 | 0.05 |
| UK born | 77 | 82.8 | 76 | 73.1 | 0.564 | 0.10 |
| Gender | 73 | 78.5 | 86 | 82.7 | – | 0.46 |
| OST (current) | 65 | 69.9 | 79 | 76.0 | – | 0.34 |
| OST (disengaged) | 14 | 15.1 | 16 | 15.4 | – | 0.95 |
These are mean ages (in years) rather than percentages.
Discussion
This observational study in an underserved population at risk of HCV found a high level of peer-supported patient engagement, with over half (52.8%) of those screened and found to have chronic HCV being approved for treatment and 38.6% having a favourable treatment outcome. The burden of disease was high, with 42.7% of people screened identified as being infected with HCV, and amongst these liver disease was high, over a quarter having severe liver fibrosis or cirrhosis. The outcomes in this underserved population compare favourably with the standard of care reported in the UK, where the cascade of care typically treats 5% of those with HCV,28 albeit from data in the pre-DAA era. It also outperforms a previous RCT amongst a similar population, which showed that 18% of those referred directly into care without peer support achieved engagement with services against 36.5% who did have peer support.7
Whilst the outcomes achieved were encouraging, nearly half of the individuals were not approved for treatment and loss to follow-up was still significant. Consequently, the HepCare team has instituted a policy of continuous open-ended care whereby individuals are given multiple opportunities to engage. If individuals proved uncontactable, PSWs would contact keyworkers and visit hostels to try to maintain engagement with the patient. If treatment was declined at that time, the decision would be respected and they would be re-contacted at a later date, in case they changed their mind. Further qualitative work is under way amongst this patient group to explore some of the reasons why they feel that treatment is not right for them at that time.
The PSWs used a range of strategies depending on the individual needs of the patient and on the personal relationship developed. Some would simply require referral and a telephone reminder about an appointment, while others would need intensive support both to facilitate the patient to attend and reassure prescribing services that the patient is sufficiently motivated to commence therapy.
There have been many models of peer support, from using peer support groups24 to ‘buddy’-type interventions;29 however, often the peer has a limited role on the periphery of a service.30 Our model of having the peer central to the service, and using peers who are highly trained and can navigate a client from testing to treatment completion, may be a reason for the outcomes achieved here. Further qualitative work, which we have started, will explore these themes.
The success of peer interventions relies in part on the interpersonal relationship between the peer and service user. Qualitative studies have highlighted the importance of trust between peer and service user born out of a shared experience.27 This is especially important with this client group as they are often stigmatized for their social situation and perceived behaviour, i.e. homelessness and substance use. Numerous studies have highlighted the difficulty for homeless people and PWID in accessing adequate healthcare, and so having someone with a similar lived experience to help them navigate through the system is a powerful tool in reducing health inequalities.31
The UK now has no restrictions on eligibility for HCV treatment based on fibrosis stage or injecting drug use; however, there are still many barriers to accessing care.32,33 There has been an increase in outreach activity in the community, such as in drug treatment centres,34 but these often rely on an individual being engaged with services, for example OST. Those not on OST and injecting drugs are at higher risk of transmission of HCV and are less likely to be in contact with harm reduction and drug treatment services. This particular group, whilst small in our study, was no less likely to be approved for treatment, indicating that engagement with services can be achieved. Future work should aim to explore this in more detail using a mixed-methods approach to inform future elimination strategies.
The majority of patients had been tested previously, suggesting that there is still a large pool of people who are disengaged from treatment services. This pool of ‘known positives’ highlights the need for an enhanced case management approach that is better tailored to the complex social needs of the individual, such as is common in TB control.35 Qualitative studies have shown a perceived lack of importance given to this disease by healthcare professionals, which is then taken on by the individual themselves and so acts as a disincentive to seek and adhere to treatment.36
Underserved populations such as the homeless, people with problematic substance and alcohol use and imprisoned individuals are known to suffer extreme health inequities.31 Univariate analysis in this population indicated that homeless individuals, injecting drug use and younger age were all associated with being more likely to be approved for treatment, supporting this concept. These risk factors are common among those infected with HCV and so addressing these complex social needs is important in efforts to reduce the burden and morbidity in this population. This is why HCV treatment strategies may be more effective, both in terms of impact and in cost-effectiveness, by being designed in a multidisciplinary manner. This could be a multidisciplinary community healthcare model, where multiple interventions can be simultaneously provided to an individual, who may have multiple risk factors, rather than a pathogen-specific model of disease elimination.
Peer support is considered a key priority in HCV elimination and it is perceived to be a low-cost intervention and to contribute to a more effective use of healthcare resources.27,37 A cost-effectiveness analysis of this intervention in comparison with current care pathways was carried out by the University of Bristol. Using the full list price for drug treatment it was found to be cost-effective at a willingness-to-pay threshold of £20000 per quality-adjusted life year (QALY) gained.38 Furthermore, Ward et al.38 found this intervention to be cost-saving at 45% of the UK list price for sofosbuvir and velpatasvir (£17539 per course). Future cost-effectiveness evaluation would be useful in assessing this peer model as part of a complex intervention for the multiple healthcare and social needs of underserved populations.
While it could be said that a limitation of this study is its observational study design and the lack of a comparator population, it was felt a comparator was no longer necessary as the aforementioned previous RCT already found peers to be beneficial in linkage to care.7 Rather, this study was intended to be a continuation of that work in exploring the care cascade of HCV and the peer-led model of care in this population. There were a number of patients who were not contactable following referral and it is possible that they were more likely to have a negative outcome. However, some of those who had approval or treatment dates pending may have likewise achieved a positive outcome following the end of data collection for this study. It is estimated that these two subgroups would balance each other out and would not significantly affect the results. The team relied on a small number of motivated, highly trained peers and so the rollout of this model may not achieve the same outcomes we found.
As part of knowledge and expertise sharing of the HepCare consortium, the development of peer networks has commenced in partner sites in Dublin, Seville and Bucharest. Peers have been trained in HCV awareness and are tasked with improving uptake in testing and improving linkage to care. It is hoped that future work will utilize the peer-led model described here: i.e. that peers are highly trained, central to the clinical team and involved with all aspects of the cascade of care; and that others can replicate our results in achieving good outcomes in terms of treatment uptake and completion.
Conclusions
Highly trained PSWs working as part of a specialist outreach clinical team help to identify a high proportion of individuals exposed to HCV, achieve high rates of engagement with treatment services and maintain high rates of treatment success amongst a population with complex needs. Peers have a unique role in engaging with underserved populations and we have shown they can be successfully integrated into community interventions to improve case finding and treatment outcomes. Peers can also be a powerful resource to empower patients to access treatment, which is vital if we are to eliminate HCV as a public health concern. The rollout of peer interventions across other sites could be a valuable tool in reducing HCV prevalence and its eventual elimination.
Acknowledgements
We thank the following: our partner organizations Groundswell and the Hepatitis C Trust for their help in developing and undertaking this project; Jim Conneely for his advocacy on behalf of people living with hepatitis C; the Find&Treat team at UCLH NHS Trust; Vanessa Hack and Kay Musonda for project management support; Nazma Mahmud, Indra Macfarlane and Brigid Hamilton for their expertise in accessing patients; and The HepCare consortium.
Funding
This work is co-funded by the European Commission through its EU Third Health Programme (Grant Agreement Number 709844), University College London and University College London Hospitals NHS Trust.
Transparency declarations
John Gibbons and Ala Miah work for Groundswell, which has received financial support from the pharmaceutical company Gilead. The remaining authors have none to declare.
This article forms part of a Supplement sponsored by the HepCare Europe Project.
References
- 1.World Health Organization. Global Hepatitis Report, 2017. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
- 2.Public Health England. Hepatitis C in the UK 2018 Report https://webarchive.nationalarchives.gov.uk/20190221162859/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/732469/HCV_IN_THE_UK_2018_UK.pdf.
- 3. Chen SL, Morgan TR.. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 2006; 3: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grebely J, Genoway K, Khara M. et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy 2007; 18: 437–43. [DOI] [PubMed] [Google Scholar]
- 5. Dillon JF, Lazarus JV, Razavi HA.. Urgent action to fight hepatitis C in people who inject drugs in Europe. Hepatol Med Policy 2016; 1: 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aldridge RW, Hayward AC, Hemming S. et al. High prevalence of latent tuberculosis and bloodborne virus infection in a homeless population. Thorax 2018; 73: 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stagg HR, Surey J, Francis M. et al. Improving engagement with healthcare in hepatitis C: a randomised controlled trial of a peer support intervention. BMC Med 2019; 17: 71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farmer P, Kim JY.. Community based approaches to the control of multidrug resistant tuberculosis: introducing ‘DOTS-plus’. BMJ 1998; 317: 671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jit M, Stagg HR, Aldridge RW. et al. Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. BMJ 2011; 343: d5376.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacLellan J, Surey J, Abubakar I. et al. Peer support workers in health: a qualitative metasynthesis of their experiences. PLoS One 2015; 10: e0141122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouchard L, Montreuil M, Gros C.. Peer support among inpatients in an adult mental health setting. Issues Ment Health Nurs 2010; 31: 589–98. [DOI] [PubMed] [Google Scholar]
- 12. Gillard SG, Edwards C, Gibson SL. et al. Introducing peer worker roles into UK mental health service teams: a qualitative analysis of the organisational benefits and challenges. BMC Health Serv Res 2013; 13: 188.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mowbray CT, Moxley DP, Collins ME.. Consumers as mental health providers: first-person accounts of benefits and limitations. J Behav Health Serv Res 1998; 25: 397–411. [DOI] [PubMed] [Google Scholar]
- 14. Mosack KE, Patterson L, Brouwer AM. et al. Evaluation of a peer-led hypertension intervention for veterans: impact on peer leaders. Health Educ Res 2013; 28: 426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paul G, Keogh K, D'Eath M. et al. Implementing a peer-support intervention for people with type 2 diabetes: a qualitative study. Fam Pract 2013; 30: 593–603. [DOI] [PubMed] [Google Scholar]
- 16. Croft LA, Hayward AC, Story A.. Tuberculosis peer educators: personal experiences of working with socially excluded communities in London. Int J Tuberc Lung Dis 2013; 17: 36–40. [DOI] [PubMed] [Google Scholar]
- 17. Dutcher MV, Phicil SN, Goldenkranz SB. et al. ‘ Positive Examples’: a bottom-up approach to identifying best practices in HIV care and treatment based on the experiences of peer educators. AIDS Patient Care STDS 2011; 25: 403–11. [DOI] [PubMed] [Google Scholar]
- 18. Marino P, Simoni JM, Silverstein LB.. Peer support to promote medication adherence among people living with HIV/AIDS: the benefits to peers. Social Work Health Care 2007; 45: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norman J, Walsh NM, Mugavin J. et al. The acceptability and feasibility of peer worker support role in community based HCV treatment for injecting drug users. Harm Reduct J 2008; 5: 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crawford S, Bath N.. Peer support models for people with a history of injecting drug use undertaking assessment and treatment for hepatitis C virus infection. Clin Infect Dis 2013; 57: S75–S9. [DOI] [PubMed] [Google Scholar]
- 21. Grebely J, Knight E, Genoway KA. et al. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support. Eur J Gastroenterol Hepatol 2010; 22: 270–7. [DOI] [PubMed] [Google Scholar]
- 22. Grebely J, Robaeys G, Bruggmann P. et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015; 26: 1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grebely J, Tyndall MW.. Management of HCV and HIV infections among people who inject drugs. Curr Opin HIV AIDS 2011; 6: 501–7. [DOI] [PubMed] [Google Scholar]
- 24. Henderson C, Madden A, Kelsall J.. ‘Beyond the willing & the waiting’—the role of peer-based approaches in hepatitis C diagnosis & treatment. Int J Drug Policy 2017; 50: 111–5. [DOI] [PubMed] [Google Scholar]
- 25. Kresina TF, Sylvestre D, Seeff L. et al. Hepatitis infection in the treatment of opioid dependence and abuse. Subst Abuse 2008; 1: 15–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta SH, Genberg BL, Astemborski J. et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health 2008; 33: 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Treloar C, Rance J, Bath N. et al. Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis C virus infection in opioid substitution treatment clinics: the ETHOS study, Australia. Int J Drug Policy 2015; 26: 992–8. [DOI] [PubMed] [Google Scholar]
- 28. Simmons D, Cohn S, Bunn C. et al. Testing a peer support intervention for people with type 2 diabetes: a pilot for a randomised controlled trial. BMC Fam Pract 2013; 14: 5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keats J, Micallef M, Grebely J. et al. Assessment and delivery of treatment for hepatitis C virus infection in an opioid substitution treatment clinic with integrated peer-based support in Newcastle, Australia. Int J Drug Policy 2015; 26: 999–1006. [DOI] [PubMed] [Google Scholar]
- 30. Bonnington O, Harris M.. Tensions in relation: how peer support is experienced and received in a hepatitis C treatment intervention. Int J Drug Policy 2017; 47: 221–9. [DOI] [PubMed] [Google Scholar]
- 31. Aldridge RW, Story A, Hwang SW. et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet 2018; 391: 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marshall AD, Cunningham EB, Nielsen S. et al. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol 2018; 3: 125–33. [DOI] [PubMed] [Google Scholar]
- 33. Rich ZC, Chu C, Mao J. et al. Facilitators of HCV treatment adherence among people who inject drugs: a systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health 2016; 16: 994.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Norton BL, Akiyama MJ, Zamor PJ. et al. Treatment of chronic hepatitis C in patients receiving opioid agonist therapy: a review of best practice. Infect Dis Clin North Am 2018; 32: 347–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Story A, Cocksedge M.. Tuberculosis Case Management and Cohort Review. Royal College of Nursing, UK.
- 36. Harris M, Ward E, Gore C.. Finding the undiagnosed: a qualitative exploration of hepatitis C diagnosis delay in the United Kingdom. J Viral Hepat 2016; 23: 479–86. [DOI] [PubMed] [Google Scholar]
- 37. Ford N, Wiktor S, Kaplan K. et al. Ten priorities for expanding access to HCV treatment for people who inject drugs in low- and middle-income countries. Int J Drug Policy 2015; 26: 1088–93. [DOI] [PubMed] [Google Scholar]
- 38. Ward Z, Campbell L, Surey J. et al. The cost-effectiveness of an HCV outreach intervention for at-risk populations in London, UK. J Antimicrob Chemother 2019; 74 Suppl 5: v5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]