Abstract
Objectives
Hepatitis C is one of the main causes of chronic liver diseases worldwide. One of the major barriers to effecting EU- and WHO-mandated HCV elimination by 2030 is underdiagnosis. Community-based screening strategies have been identified as important components of HCV models of care. HepCheck Europe is a large-scale intensified screening initiative aimed at enhancing identification of HCV infection among vulnerable populations and linkage to care.
Methods
Research teams across four European countries were engaged in the study and rolled out screening to high-risk populations in community addiction, homeless and prison services. Screening was offered to 2822 individuals and included a self-administered questionnaire, HCV antibody and RNA testing, liver fibrosis assessment and referral to specialist services.
Results
There was a 74% (n=2079) uptake of screening. The majority (85.8%, n=1783) were male. In total 44.6% (n=927) of the sample reported ever injecting drugs, 38.4% (n=799) reported ever being homeless and 27.9% (n=581) were prisoners. In total 397 (19%) active HCV infections were identified and 136 (7% of total sample and 34% of identified active infections) were new cases. Of those identified with active HCV infection, 80% were linked to care, which included liver fibrosis assessment and referral to specialist services.
Conclusions
HepCheck’s screening and linkage to care is a clear strategy for reaching high-risk populations, including those at highest risk of transmission who are not accessing any type of care in the community. Elimination of HCV in the EU will only be achieved by such innovative, patient-centred approaches.
Introduction
HCV is one of the main causes of chronic liver disease worldwide.1 The number of chronically infected persons is estimated to be approximately 71 million worldwide.2 In the EU and European Economic Area (EEA), approximately 5.6 million people have been infected with HCV (1.1% of the general population). However, national estimates of seroprevalence vary widely, from 0.1% in Belgium, Ireland and the Netherlands to 5.9% in Italy.3 Approximately 50%–80% of individuals infected with HCV will develop chronic infection, which is associated with liver cirrhosis and hepatocellular carcinoma (HCC).4 The long-term impact of HCV infection is highly variable, from minimal changes to extensive fibrosis and cirrhosis with or without HCC.5,6 Acute infection is asymptomatic in 60%–70% of cases, meaning that many do not become aware that they are HCV positive until decades after initial infection, after progression of the disease and emergence of sequelae.7 In its 2017 Global Hepatitis report, the WHO highlighted underdiagnosis as a major barrier to effecting HCV elimination by 2030.2
Effective diagnosis and follow-up care are heavily reliant on the screening of at-risk individuals.8 People who inject drugs (PWID) and ex-PWID bear the greatest burden of HCV infection in Europe and account for the majority of new infections. Estimates suggest 1.2 million PWID in Europe have been infected with HCV, with 500000 chronically infected.9 Prevalence varies substantially between countries: according to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), HCV antibody (anti-HCV) prevalence among national samples of PWID in 2014–15 ranged from 16% to 84%.10–14 In the EU, the prevalence of drug dependence among prisoners varies from country to country; a systematic review of the literature found the prevalence to range from 10% to 48% for male prisoners and 30% to 60% for female prisoners at the point of incarceration.15 Moreover, studies of infectious diseases in homeless people have found that the highest absolute rate is for hepatitis C.16,17 A study has estimated the prevalence among the three key risk groups for HCV (people in prison, MSM and PWID) throughout the EU; the highest prevalence of anti-HCV was found among people in prison (4.3%–86.3%) and PWID (13.8%–84.3%) followed by MSM (0.0%–4.7%).18
The traditional model of care of diagnosing and treating HCV patients in hospital settings is ill-adapted to the needs and life circumstances of vulnerable populations.15,19 A pilot study in the homeless population in Dublin, Ireland, has demonstrated the inadequacy of this model in addressing the specific needs of the homeless, reflected in only 2 of the 199 individuals testing positive going on to access treatment and cure.20 This reflects findings from other studies, which emphasize the importance of community-based approaches to testing and follow-up care.21,22 At the same time, the limited infrastructure and HCV knowledge in opiate substitution therapy (OST) clinics and primary care centres restrict their ability to provide HCV assessment and treatment without external support.23 Thus, for the foreseeable future, a multidisciplinary partnership approach is important. Various integrated care models have been demonstrated to successfully enhance HCV screening and IFN-based treatment of PWID, including telemedicine clinics between specialists and primary care providers,24 and on-site HCV nursing and specialist support within OST clinics and community health centres.25,26
The implementation of new testing technologies has been highlighted as important in the expansion of access to testing in health settings serving populations where there is a higher prevalence of HCV.27 A meta-analysis comparing HCV point-of-care tests (POCTs) with reference tests found that, on pooled analysis, POCTs were highly accurate for diagnosing HCV, although the authors cautioned care in the choice of test as the sensitivity and specificity of individual tests varied widely.28 POCTs provide results rapidly, thus identifying potential HCV patients and facilitating post-test counselling and referral to care at the time of testing.25 Dried blood spot (DBS) testing, while not providing an on-the-spot HCV antibody result, is a less invasive form of sampling and more convenient for transport to laboratories for testing.29 In community settings such as drug and alcohol treatment centres, homeless services and prisons, POCTs can be used alongside traditional venous and DBS testing and can be employed where they are available and the more suitable option. For those living with HCV, testing is the gateway to care and new HCV therapies.27
Objectives
HepCare Europe is an EU-supported project involving collaboration between five institutions across four member states: Ireland, UK, Spain and Romania. The project aims to develop, implement and evaluate interventions to improve HCV diagnosis, evaluation and treatment among vulnerable populations. The HepCheck component of HepCare focuses on screening and identifying new and previously known HCV-positive cases and linking them to care. In this article we aim to describe this outreach screening intervention.
Methods
Ethics
Ethics approval was granted by the Institutional Review Boards at each of the sites, namely: Mater Misericordiae University Hospital (Dublin, Ireland); North-West Haydock Research Ethics Committee (London, UK); Hospital Universiario de Valme (Seville, Spain); and Victor Babes Clinical Hospital for Infectious and Tropical Diseases (Bucharest, Romania). Governance and oversight for the study were provided through the overall governance structure of the HepCare Europe Project.
Study design
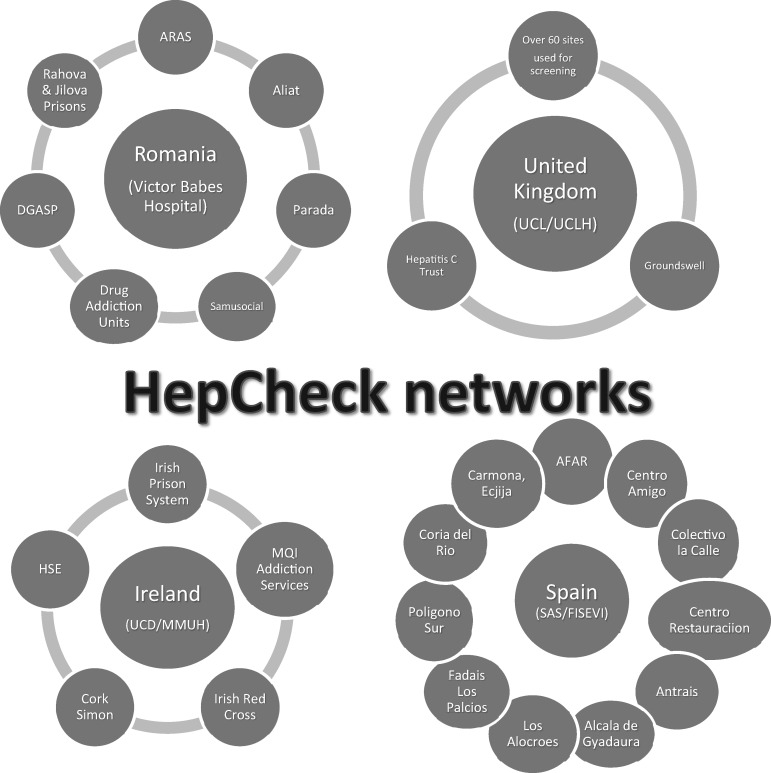
This was a multisite feasibility study of a hepatitis C screening intervention for vulnerable populations in which hepatitis C screening was carried out in community settings through outreach in community addiction, prison and homeless services. Each country targeted populations at sites with unmet needs, i.e. those not accessing routine clinical care. They were chosen in relation to available networks. The intensified screening was therefore applied depending on context. This initiative would not have been possible without the input of personnel across services and disciplines. The amount of networking involved at each site is shown in Figure 1.
Figure 1.
HepCheck networks.
Although the overarching principle of HCV identification through POCT was applied at each site, given the heterogeneity of settings the methodology varied slightly according to service structure and population needs at each site. The HepCheck intensified screening model was meant to be adaptable at each site and offered some flexibility regarding implementation to enable its application in a variety of settings in Europe for both high-income and low-resource settings.
Settings and recruitment
The study was conducted across four European sites: Ireland (Dublin, Cork), UK (London), Spain (Seville) and Romania (Bucharest). The study was carried out with PWID, homeless people and prisoners through their points of contact with services in the community. Table 1 shows the breakdown of types of services per site.
Table 1.
Service types across sites
| Service type | Ireland | UK | Romania | Spain | Total |
|---|---|---|---|---|---|
| Homeless | 2 (2%) | 41 (46%) | 3 (3%) | 1 (1%) | 47 (52%) |
| Addiction service | 1 (1%) | 17 (19%) | 3 (3%) | 8 (9%) | 29 (32%) |
| Prison | 1 (1%) | 0 (0%) | 2 (2%) | 0 (0%) | 3 (3%) |
| Other | 0 (0%) | 9 (10%) | 1 (1%) | 1 (1%) | 11 (12%) |
| Total | 4 (4%) | 67 (74%) | 9 (10%) | 10 (11%) | 90 (100%) |
Dublin, Ireland
Screening took place in a closed, medium-security prison for adult males with a population of over 500 prisoners on the north side of the city. Data were collected between April 2017 and July 2018. Prison authorities were contacted through the principal investigator’s network. A collaboration was established with a prison doctor who provides a methadone clinic on site for the prison population. Suitable dates for mass screening were arranged. Information regarding the screening was disseminated to the prisoners via a peer programme provided by a volunteer-led organization. On the day of screening, members of the research team approached prisoners to invite participation in the study. Those who wanted to take part in the screening were consented by a member of the research team. All prisoners able to provide consent were eligible for participation in the screening. In total 425 participants were recruited.
Cork, Ireland
Through the professional network of the principal investigator in Dublin, a need for HCV screening in Cork City (south-west Ireland) was identified. Screening took place in Cork City in the largest drug and alcohol clinic in Ireland’s Health Services Executive’s Southern Region and two homeless services also in Cork City. Doctors and nurses from addiction and homeless services provide clinics, including methadone and in-reach clinics, at each of the sites. Data were collected between June 2017 and July 2018. At each of the three sites, the screening day was advertised by local staff and a poster and leaflet campaign was run at each site. Staff on the ground encouraged service users to attend the screening day. Patients were eligible to take part if they were over 18 years old, used any of the three services and were willing and able to provide informed consent. Consent was obtained at each site by the research team and the process was supported by staff on duty in the service on the day. In total 193 participants were recruited in Cork.
London, UK
Screening was rolled out across 61 sites in London. The HepCare team recruited participants within the Find & Treat Mobile Health Unit (MHU), UCLH NHS Trust, which provides health screening for homeless individuals across London using community interventions and specialist outreach workers. Sites were identified if they were deemed to have a high proportion of individuals with risk factors for HCV, such as injecting drug use, and included homeless hostels, day centres and drug treatment services. Data were collected between September 2016 and May 2018. Prior to screening, sites were visited by a member of the research team to speak to staff and service users. Posters and leaflets were left at services providing details of the screening day. On the day of screening, patients were approached by members of the Find & Treat team to discuss whether they were at risk of HCV. Those who were amenable to screening were then consented by a member of the team. Patients were eligible for participation if they were over 16 years of age; able to provide signed, informed consent; and part of an underserved population in the community. In total 461 were recruited at the UK site.
Bucharest, Romania
Nine sites were identified as suitable in Romania; they included three night shelters, three addiction centres, two prisons and a hospital outpatient clinic. In the case of prisons, screening took place on site at one prison, whereas prisoners from another prison attended a hospital setting for screening. Each of the services that participated in the study is involved in the management of high-risk behaviour and delivers both medical and social services to their service users. Data collection across sites took place between April 2016 and July 2018. Screening at each site was arranged between the GP in charge at the site and the research team. The GP informed patients of screening in advance. Information about screening was also disseminated in Bucharest through two well-known NGOs working with vulnerable populations. Participants were eligible to take part if they were 18 years or older; believed to be at high risk of HCV, i.e. an active or past injecting drug user; homeless; a prisoner; and able to provide consent. On the scheduled screening day, in collaboration with the GP and team on site, the research team assisted with providing information about the process to patients and obtaining informed consent from those willing to take part. In Bucharest 513 participants were recruited.
Seville, Spain
In total, 10 sites participated in Seville and its surrounding areas. Centres in which service users who were at high risk of HCV but were not accessing HCV testing or care were specifically identified through the professional networks of the research team. Data were collected between January 2017 and April 2018. Service users were informed verbally of the screening in advance. On the day they were provided with an information leaflet outlining the purpose of the study, procedures and how the findings would be utilized. Participants were eligible to participate if they were 18 years or older; a drug user; at risk of HCV; and were attending the service for any reason during the recruitment period. Those who were interested in participating were asked to sign a consent form. In total 490 participants were recruited.
Sampling
A non-probability, purposive sampling approach was taken to sampling.
Data collection
Screening
Before screening tests took place, participants were asked to complete a researcher-administered questionnaire. The questionnaire included demographic details, homeless status, history of drug use, healthcare service usage, HCV risk factors, previous history of HCV antibody-positive and/or HCV RNA-positive diagnoses and follow-up. Data where required and available were also collected through medical record review. Once the questionnaire was completed, participants underwent a POCT (oral swab or finger prick) or venous blood test for HCV antibody. The tests were chosen depending on available resources at each site and feasibility of use. In Dublin, resources were available to carry out a blood test on site at the time of screening and this was offered first. If a prisoner declined, a POCT was offered. The majority of prison tests were blood tests. In Cork, due to available resources, finger prick tests were used for all participants. In Seville and Bucharest oral swab tests were used but blood tests were also carried out when available and feasible. Testing in London was done using oral swabs. The POCTs provided an antibody result within 20 min of testing. The result indicated the next step for participants. If positive, they advanced to the next stage of screening (RNA testing). If antibody tests were negative, harm-reduction counselling was provided with advice regarding regular testing if engaging in risky behaviour.
Follow-up
Confirmatory tests were carried out for those testing HCV antibody positive. When a POCT had been used, a confirmatory DBS or venous sample was taken. Follow-up appointments were arranged to discuss results. Confirmatory DBS testing was also carried out on those whose HCV status was reported as positive and evidenced in medical records. During the HCV RNA testing as part of the HepCheck process, baseline bloods and testing for HIV and HBV were also offered and taken where the patient agreed and where there were no other barriers to testing, e.g. difficult venous access. HCV-positive patients were referred for a FibroScan (transient elastography), either in community sites or when not feasible in hospital, to ascertain stage of liver fibrosis, and to specialist hepatology/infectious diseases services to be assessed for treatment according to country guidelines. FibroScanning was carried out on screening days on patients who already knew their HCV-positive status. This was possible across sites due to the availability of a mobile FibroScan. A FibroScan was also available on follow-up result days for those newly diagnosed. In some instances in Romania when a FibroScan was not available, fibrosis was evaluated by FibroMax. The recommendation for the use of FibroMax is included in the Romanian National protocol for HCV treatment approved by the Ministry of Health.30 See also the technical manual for FibroMax, provided by Biopredictive (the manufacturer).31
Access to interferon-free therapies across sites
At the time HepCheck screening was being carried out, there were no restrictions to accessing direct-acting antiviral (DAA) treatment in Spain or the UK. However, some restrictions existed in Ireland and Romania. In Ireland, restrictions were related to the healthcare budget and the high cost of DAAs resulted in a 6 month treatment freeze by the government during the study. In Romania, there were restrictions due to social barriers and the ability to access national insurance and a healthcare card. Without these, patients could not be included in the national programme. The HepCheck team had to cooperate substantially with NGOs to overcome some of these barriers. There were also barriers according to stage of fibrosis, whether or not a patient was cirrhotic, and contraindications to IFN treatment. Additionally, patients coinfected with HIV were required to give a negative drug test in order to receive DAA treatment.32
Measures of feasibility
Feasibility was measured through numbers recruited and screened for HCV antibody and was followed up with HCV RNA testing. Feasibility was also measured through numbers of HCV-positive patients identified, both new and previously known, and subsequently linked to care.
Data analysis
We used descriptive summary statistics such as median with IQR and frequencies with percentages to describe continuous and categorical variables, respectively. Study population characteristics and outcomes were summarized by participating site (Ireland, UK, Romania and Spain). All analyses were conducted in Stata 13.1 (College Station, TX, USA)
Results
Sample characteristics
Of the 2825 people approached across the four European sites, 2079 (74%) were recruited to the study through 84 community and healthcare services. The uptake of participation in the study was 62% in Spain and the UK, 84% in Ireland and 99% in Romania. Table 2 provides information on baseline characteristics of the sample by site.
Table 2.
Baseline client characteristics by site
| Characteristic | Ireland | UK | Romania | Spain | Overall |
|---|---|---|---|---|---|
| (n=618) | (n=461) | (n=510) | (n=490) | (N=2079) | |
| Age, years, median (IQR) | 32 (27–39) | 46 (39–52) | 38 (32–49) | 48 (41–53) | 41 (32–50) |
| Gender, n (%) | |||||
| male | 565 (91.4) | 363 (78.7) | 421 (82.6) | 434 (88.6) | 1783 (85.8) |
| female | 53 (8.6) | 98 (21.3) | 89 (17.4) | 56 (11.4) | 296 (14.2) |
| Ethnicity, n (%) | |||||
| white | 605 (97.9) | 355 (77.0) | 308 (60.4) | 487 (99.4) | 1755 (84.4) |
| Roma | 0 (0) | 0 (0) | 165 (32.4) | 0 (0) | 165 (8.0) |
| other | 13 (2.1) | 106 (23.0) | 37 (7.2) | 3 (0.6) | 159 (7.6) |
| Homelessness, n (%) | |||||
| homelessness ever | 192 (31.1) | 363 (78.7) | 103 (20.2) | 141 (28.8) | 799 (38.4) |
| rough sleeping ever | 151 (24.4) | 297 (64.4) | 96 (18.8) | 140 (28.6) | 684 (32.9) |
| IDU ever, n (%) | 249 (40.3) | 324 (70.3) | 205 (40.2) | 149 (30.4) | 927 (44.6) |
| Prisoners | 425 (68.7) | 0 (0) | 156 (30.6) | 0 (0) | 581 (27.9) |
Of those screened, 85.8% (1783) were male. The median (IQR) age was 41.3 years (32–50). Ethnically the group were largely homogeneous, with 84.3% (n=1756) identifying as white. The largest minority were Roma [165 (8.0%)]. Of the sample, 581 (27.9%) were prisoners. Among participants, 38.4% (n=799) reported ever having experienced homelessness, whereas 32.9% (n=684) reported rough sleeping either currently or in the past. Ever injecting drugs was reported in 927 participants (44.6%).
Previous HCV testing and status
Just under two-thirds (n=1316) of the sample reported previously being tested for HCV antibody. Of those previously tested, 46% (n=607) received a positive result and of those reporting a positive HCV antibody result, 65% received a positive HCV diagnosis. In total, of the 393 who reported receiving a positive HCV diagnosis in the past, 71% (n=279) reported having been lost to follow-up. Table 3 provides information on previous HCV testing and status by study site.
Table 3.
Self-reported previous HCV testing and status
| Reported prior status/tests | Ireland | UK | Romania | Spain | Total |
|---|---|---|---|---|---|
| HCV antibody test | 365/618 (59%) | 356/461 (77%) | 195/510 (38%) | 400/490 (82%) | 1316/2079 (63%) |
| HCV antibody positive | 72/365 (20%) | 257/356 (72%) | 116/195 (59%) | 162/400 (41%) | 607/1316 (46%) |
| HCV RNA-positive if HCV antibody positive | 50/72 (69%) | 228/257 (89%) | 15/116 (13%) | 100/162 (62%) | 393/607 (65%) |
| HCV RNA-positive and lost to follow- up | 22/50 (44%) | 200/228 (88%) | 9/15 (60%) | 48/100 (48%) | 279/393 (71%) |
Results of HepCheck intensified HCV screening
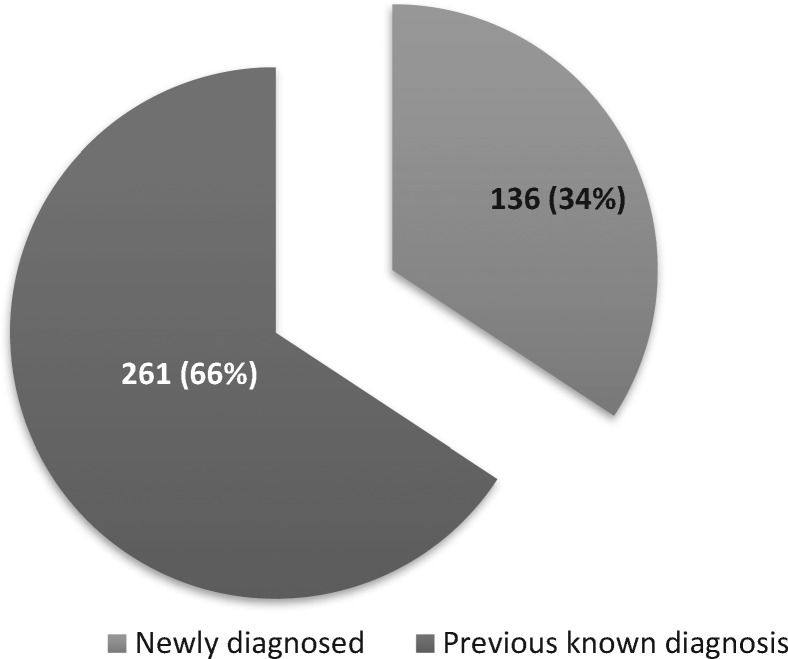
In total, 2079 individuals were screened. Of those screened, 37% (n=769) had an HCV antibody-positive result and 397 (19%) participants had an active HCV infection. Among the sample, 136 (7%) new cases of active HCV infection were found. Of all those who reported ever injecting drugs (n=927), 340 (37%) were HCV RNA positive. Those who ever injected drugs accounted for 86% of the total number of active HCV infections. Table 4 describes screening results by site. Figure 2 illustrates the proportion of new cases versus those previously known.
Table 4.
HCV screening results
| Characteristic | Ireland | UK | Romania | Spain | Total |
|---|---|---|---|---|---|
| Individuals screened | 618 (30%) | 461 (22%) | 510 (25%) | 490 (23%) | 2079 (100%) |
| Proportion of cases antibody positive | 121 (20%) | 266 (58%) | 211 (41%) | 171 (35%) | 769 (37%) |
| No. of RNApositive results | 62 (10%) | 197 (43%) | 47 (9.2%) | 91 (19%) | 397 (19%) |
| No. new cases of active HCV infection | 37 (6%) | 19 (4%) | 41 (8%) | 39 (8%) | 136 (7%) |
| No. of RNA positive cases among PWIDa | 49 (20%) | 179 (55%) | 44 (21%) | 68 (46%) | 340 (37%) |
Calculated based on total number of PWID per site: Ireland, 249; UK, 323; Romania, 205; Spain, 149.
Figure 2.
New versus previously known cases.
Linkage to care
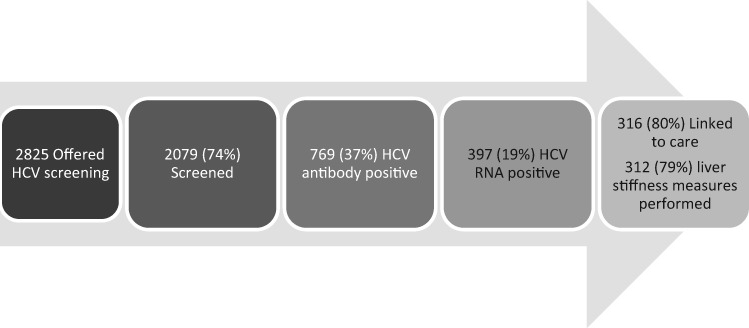
Linkage to the cascade of care for those who tested positive included FibroScanning, referral to specialist services and referral for treatment. Referral and treatment are currently ongoing, therefore data on FibroScanning and referral to specialist services only were amalgamated to provide an initial linkage to care estimate. Figure 3 shows the cascade of care to date. Data on the rest of the treatment cascade and treatment outcomes are being collated and will be fully analysed at the end of the project implementation. Of the 397 active HCV cases found through screening, 316 (80%) had been linked to care in May 2018. Table 5 shows linkage to care outcomes by site.
Figure 3.
Cascade of care.
Table 5.
Linkage to care for those diagnosed with HCV (n=397)
| Linkage | Ireland | UK | Romania | Spain | Total |
|---|---|---|---|---|---|
| Linked | 45 (73%) | 176 (89%) | 37 (79%) | 58 (64%) | 316 (80%) |
| Not yet linked | 17 (27%) | 21 (11%) | 10 (21%) | 33 (36%) | 81 (20%) |
Feasibility
In total, 2822 service users were approached to take part in the study. Almost three-quarters (74%, n=2079) were screened. Of these, 769 (37%) were HCV antibody-positive cases and 397 (19%) tested positive for active HCV infection. Among these, 136 (34% of HCV RNA-positive patients and 7% of the entire sample) were newly identified cases. Data from self-reported outcomes for those previously diagnosed as HCV positive showed that 79% had been lost to follow-up. Among those identified as HCV positive through HepCheck screening, 80% were successfully linked to care.
Discussion
Key findings
Although there were 136 (7%) new cases of active HCV infection found across the four HepCheck sites, the majority of identified HCV-positive cases (66%, n=261) were previously known. This highlights the importance of screening not only to identify new cases but also to identify previously known cases and link them into the cascade of care. PWID accounted for 86% of all RNA-positive cases.
Our results indicate that the flexibility of testing methods was key in carrying out mass screening across such a range of sites. POCT technologies were convenient and user-friendly; however, they were used alongside traditional methods of testing depending on resources and feasibility at each site. For instance, the Research Ethics Committee in Ireland insisted on phlebotomy being offered first as the most accurate and reliable test. This led prisoners in particular to choose phlebotomy en masse as it was possible to use this screening method in this context. POCT would have been used in case phlebotomy was refused. Other sites did not encounter this barrier and would have used POCT directly for convenience because it was the most feasible option. However, many patients also had documented existing results and did not need re-testing.
The 74% uptake of screening among all of those approached indicates the importance of collaboration between secondary healthcare services and community health and social services, whose involvement facilitated the roll-out of screening in a variety of settings and encouraged service users to take part.
Comparison with existing literature
The WHO and EU have mandated the elimination of HCV by 2030.2,33 Underdiagnosis has been highlighted as an obstacle to achieving this goal.24 To address this issue, studies have highlighted the importance of implementing screening strategies appropriate for high-risk populations.25,34 The high uptake of screening (74%) among this study’s cohort and numbers of HCV RNA-positive patients linked to care indicates that an intensified screening strategy can be effective in vulnerable populations.
It has been estimated that 43% of PWID in the EU/EFTA region (member states plus Norway, Iceland, Liechtenstein and Switzerland) are HCV RNA positive.35 This is comparable to the HepCheck screening results, which showed 37% HCV RNA positivity among PWID in the four EU sites. In a recent publication on global, regional and national HCV estimates, the rate of HCV RNA among PWID in Ireland was estimated at 56%.36 This is almost three times the figure from our study (20%). The disparity may be due to PWID being defined in our study as anyone who has ever injected drugs, whereas Grebely et al.36 report on those with recent injecting drug use. There were also disparities between the Grebely et al.36 study results and our results from England, Romania and Spain. We found 55%, 21% and 45%, respectively, in these three countries whereas Grebely et al.36 reported 23% in England, 63% in Romania and 53% in Spain.
In their study on the control of HCV among PWID, Zeremski et al.37 advocate the co-localization of HCV management within drug services. Across sites, the rate of HCV infection among PWID was 37% and the proportion of drug users among all of those who tested positive for active HCV infection was 86%. Therefore our findings also suggest that the co-localization of HCV management within drug services could be beneficial.
Strengths and limitations
Although linkage to care results are reported in this study, data on whether or not this resulted in a patient successfully completing treatment are not yet available. Further analysis of these data is necessary in order to ascertain the full impact of the HepCheck intervention. Qualitative interviews regarding reasons for loss to follow-up and lack of linkage to care are also pending. Data on some variables were missing from some sites and therefore could not be reported on.
Whilst the intervention is aimed at intensified screening in the community, it would have missed those who access no services at all, who may be heavy users of injection drugs. In order to reach out to that population a different intervention involving peer workers would be necessary.
Implications for practice, policy and future research
The HepCheck model provides a template for intensified HCV screening that could be rolled out across European sites according to local healthcare systems and resources. It has the potential to increase numbers of HCV cases identified among vulnerable populations and ensure their linkage to care. Consortium members at participating sites have been working with regional and national bodies to develop services and structures of HCV care based on the HepCheck model. While social barriers to HCV care in Romania remain, some of the structural barriers have been removed as a result of recommendations by HepCare consortium members made to national bodies in charge of healthcare provision. Future research could continue to focus on vulnerable populations and in particular PWID who are at highest risk of transmission (i.e. those currently injecting and sharing equipment and not accessing any drug services or health care).
HepCheck results show a high number of previously known cases lost to follow up, which may have been due to the number of visits required to ascertain HCV status. Newer models being developed that provide HCV RNA results within 2 h38 used in conjunction with pangenotypic DAAs offer the possibility of diagnosis and commencement of treatment at a single visit39 in settings acceptable to this cohort. As efforts are being made to devolve DAA treatment to the community, the challenge of reaching numerous sites remains. HepCheck has screened in 90 sites. Such an outreach initiative will likely need to continue.
Conclusions
Our results show that HCV infection is common in vulnerable populations, in particular among PWID, and that many of these patients are not accessing care and treatment. Many are not yet diagnosed and many are previously diagnosed and lost to follow-up. New testing strategies, including point-of-care antibody testing, and point-of-care PCR testing, identifying not just exposure, but actual active infection, are important developments. To be able to go to the patient, diagnose them in the community, give them a timely diagnosis and immediately offer them treatment, eliminates the lost to follow-up problem encountered historically in these patients. Elimination of HCV in the EU will only be achieved by such innovative patient-centred approaches.
Acknowledgements
We thank the Third Health Programme of the European Union for co-funding this project. We also wish to express our gratitude to the participating community organizations that facilitated the research and the patients across the four European sites that took part in the study. Finally we wish to acknowledge all members of the HepCare Europe Consortium as follows: Juan A. Pineda (Hospital Universitario de Valme, Seville, Spain); Simin Florescu, Anca Luca, Silvia Suciu and Diana Sima (Victor Babes Hospital, Bucharest, Romania); Ioan Petre (Carusel, Romania); and Alina Dumitriu (ARAS, Romania). In total 82 people participated in this initiative, of whom 26 were from the HepCare Project and 56 from community stakeholders.
Funding
This research was co- funded by the Health Programme of the European Union (Grant Agreement 709844) and the Irish Health Service Executive.
Transparency declarations
J.S.L. has received non-restricted grants from Gilead, Abbvie and MSD for hepatitis C-related educational and research activities. J.S.L. has received honoraria for advisory board meetings on HIV and HCV, organized by Gilead, Abbvie, Glaxo Smith Kline, Viiv, and Merck. W.C. has been a principal investigator on research projects funded by the Health Research Board of Ireland, the European Commission Third Health Program and Ireland’s Health Services Executive. W.C. has also been a co-investigator on projects funded by Gilead and Abbvie. J.M. has served as an investigator in clinical trials supported by Bristol Myers-Squibb, Gilead and MSD. J.M. has also served as a paid lecturer for Gilead, Bristol-Myers-Squibb, and MSD, and has received consultancy fees from Bristol Myers-Squibb, Gilead and MSD. J.M. has received a grant from the Servicio Andaluz de Salud de la Junta de Andalucia. C.O. has served as a paid speaker for Janssen, BMS and Abbvie; has served as an advisory board member for Teva, ViiV and Gilead, and as a principal investigator on clinical trials supported by ViiV, and as a co-investigator on clinical trials supported by Abbvie and Tibot. All other authors: none to declare.
This article forms part of a Supplement sponsored by the HepCare Europe Project.
References
- 1. Perz JF, Armstrong GL, Farrington LA. et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45: 529–38. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Hepatitis Report, 2017 http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf; jsessionid=39DE8B60B231C47D1E3BD37BFE9CB595? sequence=1.
- 3.ECDC. Systematic review on hepatitis B and C prevalence in the EU/EEA Stockholm, 2016. https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/systematic-review-hepatitis-B-C-prevalence.pdf.
- 4. Chen E,, Morgan TR.. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 2006; 3: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee MH, Yang HI, Yuan Y. et al. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol 2014; 20: 9270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lingala S, Ghany MG.. Natural history of hepatitis C. Gastroenterol Clin North Am 2015; 44: 717–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westbrook RH, Dusheiko G.. Natural history of hepatitis C. J Hepatol 2014; 61: S58–68. [DOI] [PubMed] [Google Scholar]
- 8. Zuure FR, Urbanus AT, Langendam MW. et al. Outcomes of hepatitis C screening programs targeted at risk groups hidden in the general population: a systematic review. BMC Public Health 2014; 14: 66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hope VD, Eramova I, Capurro D. et al. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: a review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiol Infect 2014; 142: 270–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EMCDDA. European Drug Report 2017: Trends and Developments Luxembourg, 2017. http://www.emcdda.europa.eu/publications/edr/trends-developments/2017_en.
- 11.EMCDDA. Ireland, Country Drug Report 2017 Luxembourg, 2017. http://www.emcdda.europa.eu/system/files/publications/4520/TD0616149ENN.pdf.
- 12.EMCDDA. Romania, Country Drug Report 2017 Luxembourg, 2017. http://www.emcdda.europa.eu/system/files/publications/4507/TD0116919ENN.pdf.
- 13.EMCDDA. Spain, Country Drug Report 2017 Luxembourg, 2017. http://www.emcdda.europa.eu/system/files/publications/4525/TD0116922ENN.pdf.
- 14.EMCDDA. United Kingdom, Country Drug Report 2017 Luxembourg, 2017. http://www.emcdda.europa.eu/system/files/publications/4529/TD0116925ENN.pdf.
- 15. Fazel S, Bains P, Doll H.. Substance abuse and dependence in prisoners: a systematic review. Addiction 2006; 101: 181–91. [DOI] [PubMed] [Google Scholar]
- 16. Fazel S, Geddes JR, Kushel M.. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014; 384: 1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beijer U, Wolf A, Fazel S.. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falla AM, Hofstraat SHI, Duffell E. et al. Hepatitis B/C in the countries of the EU/EEA: a systematic review of the prevalence among at-risk groups. BMC Infect Dis 2018; 18: 79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fourati S, Feld JJ, Chevaliez S. et al. Approaches for simplified HCV diagnostic algorithms. J Int AIDS Soc 2018; e25058.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lambert JS, Murtagh R, Menezes D. et al. HepCheck Dublin. An intensified hepatitis C screening programme in a homeless population demonstrates the need for alternative models of care. BMC Infect Dis 2019; 19: 128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morano JP, Zelenev A, Lombard A. et al. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. J Community Health 2014; 39: 922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruce DR, Eiserman J, Acosta A. et al. Developing a modified directly observed therapy intervention for hepatitis C treatment in a methadone maintenance program: implications for program replication. Am J Drug Alcohol Abus 2012; 38: 206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruggmann P, Grebely J.. Prevention, treatment and care of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015; 26 Suppl 1: S22–26. [DOI] [PubMed] [Google Scholar]
- 24. Arora STK, Murata G, Deming P. et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011; 364: 2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alavi M, Grebely M, Micallef AJ. et al. Enhancing treatment for hepatitis assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis 2013; 57 Suppl 2: S62–69. [DOI] [PubMed] [Google Scholar]
- 26. Grebely J, Alavi M, Micallef M. et al. Treatment for hepatitis C virus infection among people who inject drugs attending opioid substitution treatment and community health clinics: the ETHOS Study. Addiction 2016; 111: 311–9. [DOI] [PubMed] [Google Scholar]
- 27. Ward JW. Testing for HCV: the first step in preventing disease transmission and improving health outcomes for HCV-infected individuals. Antivir Ther 2012; 17: 1397–401. [DOI] [PubMed] [Google Scholar]
- 28. Khuroo MS, Khuroo NS, Khuroo MS.. Diagnostic accuracy of point-of-care tests for hepatitis C virus infection: a systematic review and meta-analysis. PLoS One 2015; 10: e0121450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kania D, Bekale AM, Nagot N. et al. Combining rapid diagnostic tests and dried blood spot assays for point-of-care testing of human immunodeficiency virus, hepatitis B and hepatitis C infections in Burkina Faso, West Africa. Clin Microbiol Infect 2013; 19: E533–41. [DOI] [PubMed] [Google Scholar]
- 30.Recommendations for fibrosis staging for patients with chronic HCV are with FibroScan or FibroMax (in Romanian; use of FibroMax, FibroScan mentioned at pages 215, 217, 220). http://www.ms.ro/wp-content/uploads/2018/08/Anexa_Ordin_protocoale_29_august_2018.pdf.
- 31.FibroMax. https://www.biopredictive.com/products/fibromax/.
- 32. Streinu-Cercel A. HCV Romanian Framework. In: Friends of the Liver – The Challenge of Hepatitis C in Central and South Eastern Europe, 22 March 2017. European Parliament.
- 33. Hatzakis A. Hepatitis C Elimination in Europe. European Policy Guidelines, November 2017. Hepatitis B and C Public Policy Association. http://www.hcvbrusselssummit.eu/images/documents/reports/HCV-Elimination-PolicyGuidelines.pdf.
- 34. Deerin JF, Mikre M, Castel AD. et al. Using HIV surveillance data for targeted, community-based hepatitis C virus testing among baby boomers in Washington, D.C. J Health Care Poor Underserved 2018; 29: 964–74. [DOI] [PubMed] [Google Scholar]
- 35. Negro F. Epidemiology of hepatitis C in Europe. Dig Liver Dis 2014; 46 Suppl 5: 158–64. [DOI] [PubMed] [Google Scholar]
- 36. Grebely J, Larney S, Peacock A. et al. Global, regional, and country‐level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2018; 114: 150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeremski M, Zibbell JE, Martinez AD. et al. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol 2013; 19: 7846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McHugh MP, Wu AHB, Chevaliez S. et al. Multicentre evaluation of the Cepheid Xpert® Hepatitis C virus (HCV) Viral 2 Load assay. J Clin Microbiol 2017; 55: 1550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Applegate TL, Fajardo E, Sacks JA.. Hepatitis C virus diagnosis and the Holy Grail. Infect Dis Clin North Am 2018; 32: 425–45. [DOI] [PubMed] [Google Scholar]