Abstract
True setae borne on the abdominal tergites of Ochrogaster lunifer Herrich-Schӓffer caterpillars are the agents of an irritating contact dermatitis, osteomyelitis, ophthalmia, and severe allergic reactions in humans, and are the cause of Equine Amnionitis and Fetal Loss in Australia. The setae are detached and readily dislodge from the integument whereby they disperse throughout the environment. To better understand the true setae of O. lunifer as agents of medical and veterinary concern, we studied their characteristics and distance dispersed. Whereas members of the European Thaumetopoeinae have been widely studied, their southern-hemisphere counterparts such as O. lunifer are not well known despite their harmfulness and known medical and veterinary importance. The caterpillar’s investment in true setae increased with age and size, and two distinct size classes co-occurred in setae fields. A previously undescribed morphological type of true seta was found on the first abdominal segment. All true setae were calculated to travel long distances in the air even under light breeze conditions. Our results show there is a high risk of exposure to airborne urticating setae within 100 m of elevated caterpillar activity, and a likely risk of exposure for some kilometers in the direction of the prevailing breeze. This information should be used to inform management strategies in areas where urticating processionary caterpillars are active, and especially during periods of an outbreak.
Keywords: integument, Lepidoptera, Notodontidae, setae, Thaumetopoeinae
Many caterpillars of the Thaumetopoeinae sub-family of moths are known for their gregarious herd-like behavior, processionary movement and itchy, urticating hairs (true setae). Ochrogaster lunifer Herrich-Schӓffer is one such species endemic to Australia (Common 1990, Mather et al. 2019) and whose larvae can cause serious problems for humans and livestock. Cohorts of the univoltine larvae co-habit producing a silk nest at the base of their host tree, on the trunk, or within the canopy depending on where the female moth has laid her egg mass (Perkins et al. 2016). During spring–summer, larvae develop synchronously until autumn–winter when fully grown larvae leave the nest in a procession crawling head-to-tail, hence the common name ‘processionary caterpillar’ (Steinbauer 2009). Larvae overwinter in the ground before pupating and emerging as non-feeding adults in spring.
True setae borne on the abdominal tergites of O. lunifer are the agents of an irritating contact dermatitis, osteomyelitis, ophthalmia, and more severe allergic reactions (Southcott 1978 as Ochrogaster contraria Walker, van Bockxmeer and Green 2013). The morphology of the seta allows it to penetrate the skin or mucous membranes of animals, and to travel through their tissues; setae can thus carry bacteria into internal organs (Todhunter et al. 2014a,b). In addition, these true setae are filled with fluid and contain proteins, at least one of which is a known allergen to humans (Berardi et al. 2015). This species is the cause of a serious condition in horses: when pregnant mares ingest larvae or exuviae, true setae penetrate the gut and migrate throughout the body, ultimately causing a range of outcomes from focal mucoid placentitis to abortion or perinatal death (Cawdell-Smith et al. 2012, 2013).
True setae are derived from typical arthropod hairs (Battisti et al. 2011) and occur in New World Theraphosidae (tarantulas) (Bertani and Guadanucci 2013), and in the adults and larvae of Lepidoptera (Delgado-Quiroz 1978). In the Thaumetopoeinae processionary caterpillars, true setae are a distinctive feature of the larval stage and characteristically occur on the integument of abdominal tergites in specialized structures or setae fields commonly called mirrors (Lamy 1990, Battisti et al. 2011, Petrucco-Toffolo et al. 2014, Perkins et al. 2016). The setae are small, approximately 50–800 µm long, detached, and barbed along the shaft (Battisti et al. 2011, Perkins et al. 2016). The true setae of O. lunifer are absent in the early instars, appearing from third instar onwards and increasing in abundance with successive molts; final instar larvae have approximately 2–2.5 million true setae per larva (Perkins et al. 2016).
Coinciding with the late instar investment in true setae by O. lunifer is the greater nutritional reward a protein- and fat-laden larger caterpillar presents to a predator. Setae and hairs on caterpillars would appear to provide a physical defense against predation. For example, most birds avoid hairy caterpillars (Kristin and Patocka 1997), although some species such as cuckoo birds are able to feed on urticating larvae (see Barbaro and Battisti 2011, for a review). Predatory invertebrates can be deterred by hairs also: Dyer and Floyd (1993) showed that hairy caterpillars were rejected more frequently than glabrous ones by ants, and long hairs protected caterpillars from predation by a carabid beetle (Sugiura and Yamazaki 2014), wasps and bugs (Castellanos et al. 2011). However, a recent study by Uemura et al. (2017) found that the rejection of O. lunifer larvae by predatory ants was due to a volatile chemical present in the larval integument, not the physical barrier of setae. The effectiveness of true setae as a defense against predators remains unclear (Battisti et al. 2011). Learning avoidance may be limited; symptoms such as dermatitis and necrosis of the mouth may take up to 12 h to develop (e.g., Lamy 1990), and therefore the predator may not associate the caterpillar with the adverse consequence.
True setae are readily dislodged from the integument by mechanical disturbance and then disperse further into the environment. Unlike other urticating arthropod hairs, a true seta is physically detached from the cuticle where its proximal, pointed end is inserted into a socket or pit, depending on the species, to hold it in place (see Battisti et al. 2011, for a review). Once dislodged from the insect, setae may be carried some distance in the wind (Werno and Lamy 1990, Fenk et al. 2007, Petrucco-Toffolo et al. 2014) and airborne setae have caused epidemics of irritant dermatitis, conjunctivitis, and upper respiratory distress in human populations (De-Long 1981, Maier et al. 2003, Gottschling et al. 2007, Vega et al. 2011). As might be expected, the size of setae affects their dispersal through the air and small true setae have been calculated to travel up to five times further than larger setae (Petrucco-Toffolo et al. 2014). Strategies to reduce human and other mammalian exposure to these harmful agents require knowing the distance setae may disperse to predict areas at risk.
The true setae of O. lunifer larvae are similar to those from the northern hemisphere Thaumetopoeinae, Thaumetopoea pityocampa (Denis & Schiffermüller), T. pinivora (Treitschke), and Thaumetopoea processionea (Novak et al. 1987, Petrucco-Toffolo et al. 2014), that too cause dermatitis, conjunctivitis, oropharyngeal inflammation, and asthma-like symptoms in humans (Maier et al. 2003, Battisti et al. 2011, Vega et al. 2011). However, whereas members of the European Thaumetopoeinae have been widely studied, their southern-hemisphere counterparts such as O. lunifer are not well known despite their harmfulness and known medical and veterinary effects.
This study aimed to increase our understanding of the true setae of O. lunifer as agents of medical and veterinary concern, adding to the results already presented by Perkins et al. (2016). We examined changes in the morpho-metrics of true setae as O. lunifer larvae grow/ age, and recently discovered variation in the morphology of true setae within individual larvae. How true setae are released from larvae, and their dispersal potential once released into the environment were determined. These factors have implications for informing best-practise management of this species.
Materials and Methods
Insects
Larvae of O. lunifer were collected from nests at Gatton (−27.55, 152.34) and the Brisbane Valley (−27.52, 152.67), QLD, and various locations in the Hunter Valley (−32.27, 150.91), NSW, Australia. The samples included ground-, trunk- and canopy-nesting larvae.
Microscopy
Samples were examined using an Olympus SZX16 stereomicroscope with DP26 digital camera attachment and hair types were classified according to Battisti et al. (2011). The long hairs were trimmed off with micro-scissors so the true setae embedded in the integument in setae fields could be seen; this was carried out while the sample was immersed in propylene glycol to coat the sample and prevent dry setae from entering the atmosphere where they could have caused a health risk. Areas of interest were then washed in 70% ethanol and prepared as follows for scanning electron microscopy (SEM): dry samples were vapor-fixed in a closed vial using 2% aqueous osmium tetroxide overnight, then placed under vacuum for at least 48 h, mounted onto aluminum stubs using Araldite (www.Selleys.com.au, Sydney, Australia) and the adhesive polymerized for >3 h. Samples were then placed under vacuum until coated to 15–20 nm with iridium. Other samples were directly mounted onto Araldite on aluminum stubs and polymerized, placed under vacuum, and coated as above. SEM was performed using a JOEL JSM6460 at 12kV in secondary electron imaging (SEI) or backscattered electron (BSE) mode.
Individual setae were examined using a Zeiss Axioskop compound microscope with a Scion FW-1310C camera and Visicapture software. Setae were measured from photographs using Photoshop software. The Test for Trend of Proportions was performed to determine whether the increase in the proportion of small setae with instar age was statistically significant using R Version 3.2.0 (R Core Team 2015).
Histology of O. lunifer Larva
An 8th instar larva from a ground nest was preserved in 10% neutral-buffered formalin for at least 48 h. The dorsal integument was excised and stored in 70% ethanol. The sample was dehydrated by placing in ethanol at increments of 70, 90, 100% (two changes) and cleared in xylene (two changes) for 45 min each. The xylene was poured off and sample covered with paraffin wax at 60°C for 45 min; wax was poured off and fresh paraffin wax added and the sample held under a vacuum of 414 kPa at 60°C for 45 min. Samples were orientated and embedded in wax blocks and sectioned at 6 µm using a HM 325 rotary microtome and sections mounted on glass slides. Sections were rehydrated before staining with hematoxylin and eosin. Slides were photographed using an Aperio Slide Scanner at 40× magnification.
Theoretical Distance True Setae can Disperse in the Air
The method developed by Fenk et al. (2007) and Petrucco-Toffolo et al. (2014) was used to calculate the distance true setae could travel in the air. Calculations were made for common true setae of class sizes <100 μm and >100 μm, and for a new type of setae defined here as thin setae, under three wind speeds: light (2 m/s), moderate (7 m/s), strong (13 m/s), using Microsoft Excel. Separate calculations were made for setae traveling parallel and perpendicular to the direction of airflow. The first step was to measure the length and width of setae (n = 38, 112, 93, for each class of seta, respectively) to calculate the aerodynamic diameter, which is the hypothetical diameter that a water droplet would have in order to settle with the same velocity as the seta, using the equations:
where: df is the average seta diameter in μm; ρ f is the density of the seta, 1100 kg/ m3; ρ water is the density of water, 1000 kg/m3; β is the average aspect ratio of the setae (length/ width).
Then the Settling velocity of a seta is calculated from the Aerodynamic diameter using the equation:
where: g is the gravitational constant, 9.8 m/s2; and η is the viscosity of air, 1.86 × 10–5 kg/m/s at sea level and 27°C.
Finally, the distance dispersed is calculated from the Settling velocity using the equation:
where: h is the height that setae are released at (5 m was used as a typical height for caterpillar activity), and c is wind velocity (m/s).
Modeling the Dispersal of Airborne Setae
To further understand the airborne dispersal of setae and their concentration at ground level, we performed simulations of setae belonging to the three morphological classes (see above) dispersing from feeding caterpillars under a realistic scenario: a single trunk or canopy nest 5 m above the ground in a tree 10 m tall and subjected to a constant light breeze of 2 m/s blowing in an eastern direction with stable atmospheric conditions (i.e., Pasquill stability class F; Pasquill 1961). In this scenario, a procession of 100 caterpillars left the nest at 6:30 p.m. and spent 2.5 h maintaining the nest, then began to move to the top of the tree, with all individuals arriving at the treetop by 11 p.m. The caterpillars then remained at the top of the tree until 2:30 a.m. at which point they commenced their descent to the nest with all back in the nest by 6:30 a.m. This scenario is typical of the behavior of three trunk-nesting cohorts of O. lunifer observed from time-lapse camera recordings over several weeks. Each caterpillar was modeled as emitting setae at a fixed rate of 8000 setae p/h during the period outside the nest. Each caterpillar was considered a point source of setae at the location of the tree and at a height determined by the movement between the nest and tree top.
We modeled the dispersion of setae using an advection-diffusion model built using the ‘ReacTran’ package (Soetaert and Meysman 2012) for R (R Core Team 2019). Under the atmospheric conditions described above, turbulent diffusivities were calculated from the Briggs parameterization for rural sites as:
where , , and are the diffusivities in the east-west direction, north-south direction and vertical orientation respectively; w is the wind speed (2 m/s) and c is the downstream calibration distance, which was taken to be 1000 m (as per table 3 of Griffiths 1994). These diffusion parameters were taken to be uniform across the modeling domain. Advection across the modeling domain was also taken to be uniform and was parameterized using 2 m/s wind to the east, no advection in the north-south orientation and a downward advection of setae due to gravity, taken as the settling velocity of the setae (see above). The aerodynamic diameter of the setae used was the geometric mean of setae traveling with their axis parallel and perpendicular to the flow of air. Importantly, we modeled the ground surface as being an absorbing barrier, so that once a seta had made contact with the ground, it remained there. In this way, setae would continue to accumulate on the ground with each foraging procession. The concentrations that would be expected for larger numbers of caterpillars and/or for multiple processions can be obtained by direct rescaling of these concentrations.
Results
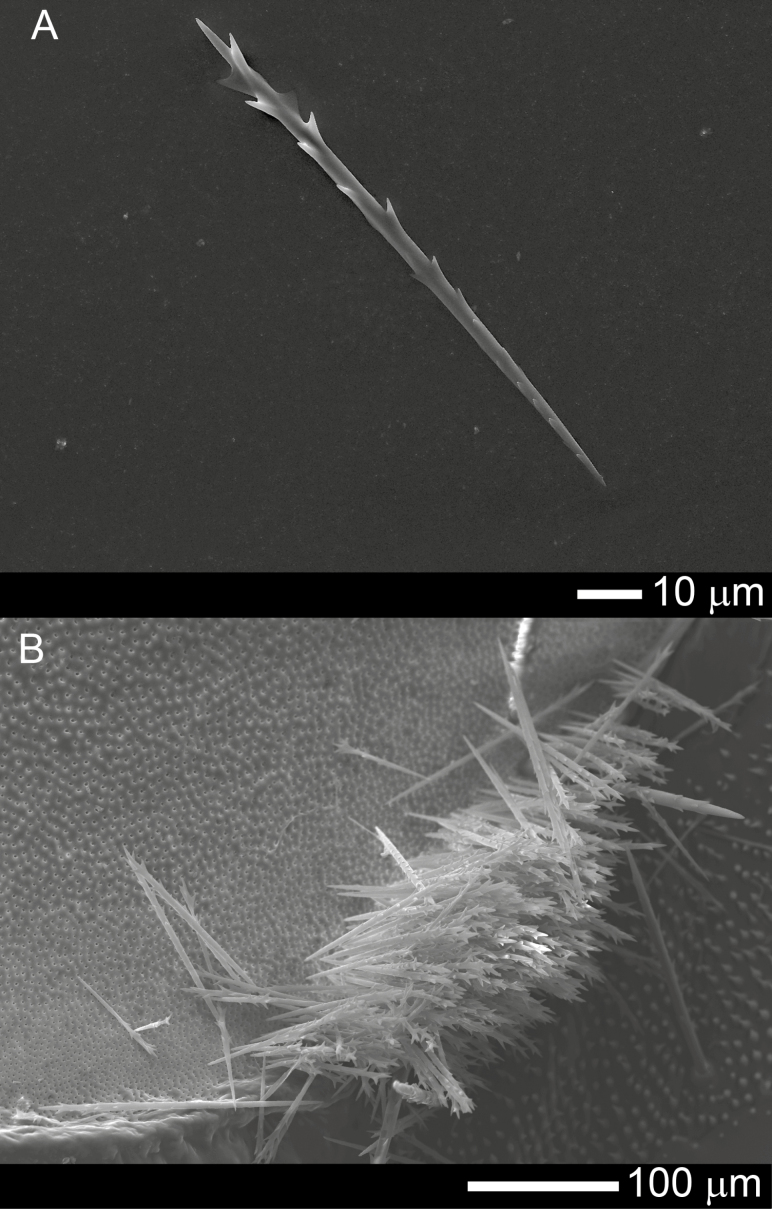
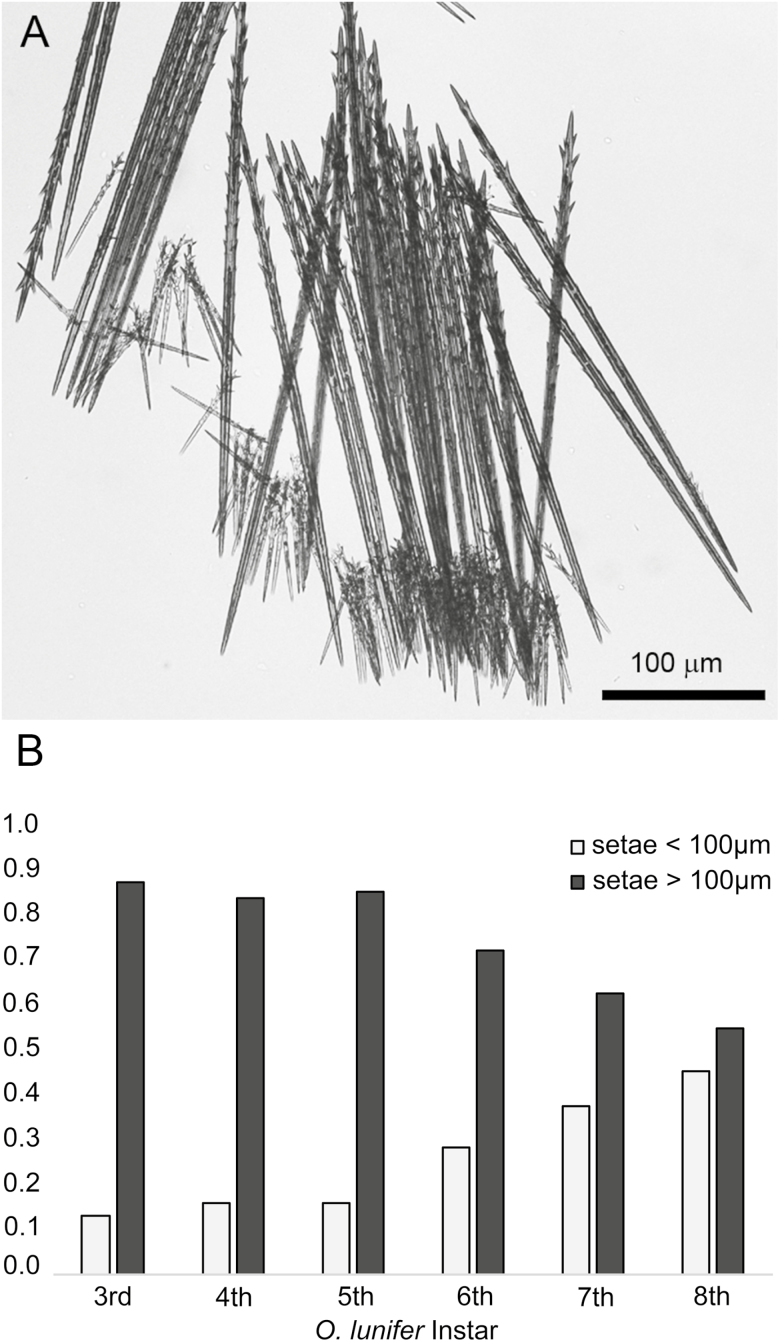
The true setae of O. lunifer were pointed and barbed (Fig. 1A). The proximal ends were inserted into pits in the integument within defined structures (setae fields) (Fig. 1B). The area of exoskeleton covered by true setae increased with the age of the larva, typically ranging from < 1 mm2 on 3rd instars to > 30 mm2 on 8th instars (Fig. 2). Within the setae fields distinctly sized setae are present. Small setae of <100 μm length are interspersed between longer setae (Fig. 3A) and the proportion of small setae increases with the age of the larvae (Test for trend of proportions, X-squared = 93.826, df = 5, P < 0.01, one-sided test) (Fig. 3B). There was no difference in the morphology of true setae from ground-, trunk- and canopy-nesting larvae.
Fig. 1.
The true setae and a setae field (mirror) of O. lunifer: (A) a seta is sharp at the proximal end facilitating penetration of the skin and mucous membranes of animals, and backward-pointing barbs allow forward only migration through the tissue as an animal moves; (B) the proximal ends of true setae are embedded into pits in the cuticle but not attached to the integument.
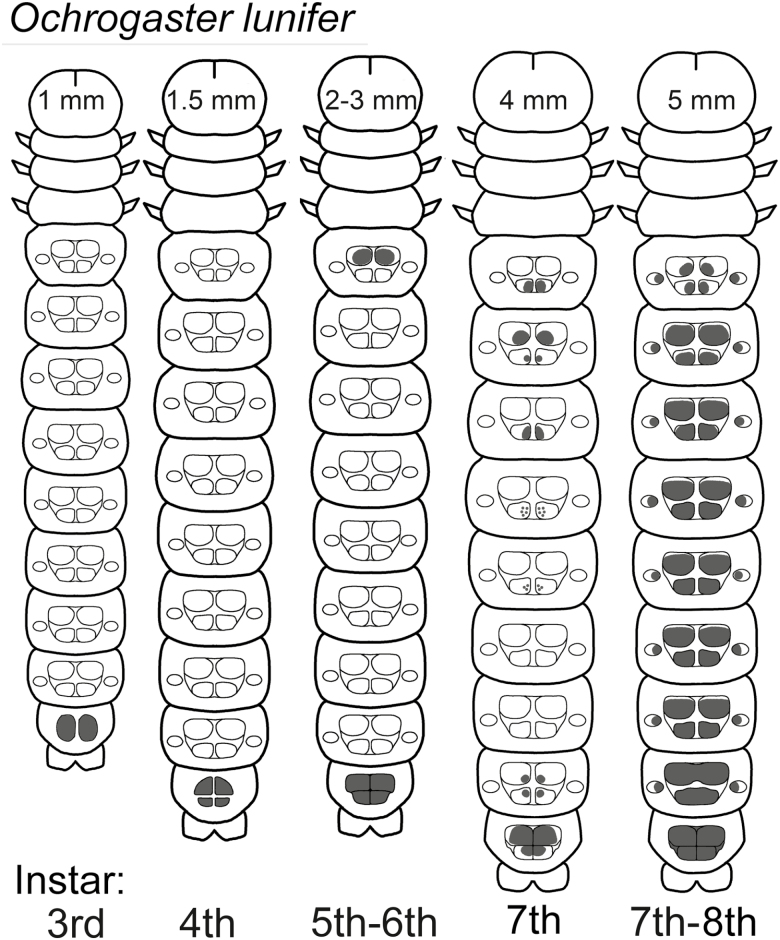
Fig. 2.
Schematic maps of setae fields on typical examples of 3rd–8th instars of O. lunifer caterpillars. The pattern on 7th instar larvae is variable. Head capsule widths are approximate and typical for the given instar.
Fig. 3.
Two size classes of the commonly-described true setae are found in each setae field of O. lunifer: A, compound micrograph of a clump of true setae removed from a setae field; (B) the proportion of short setae is greater on older instars.
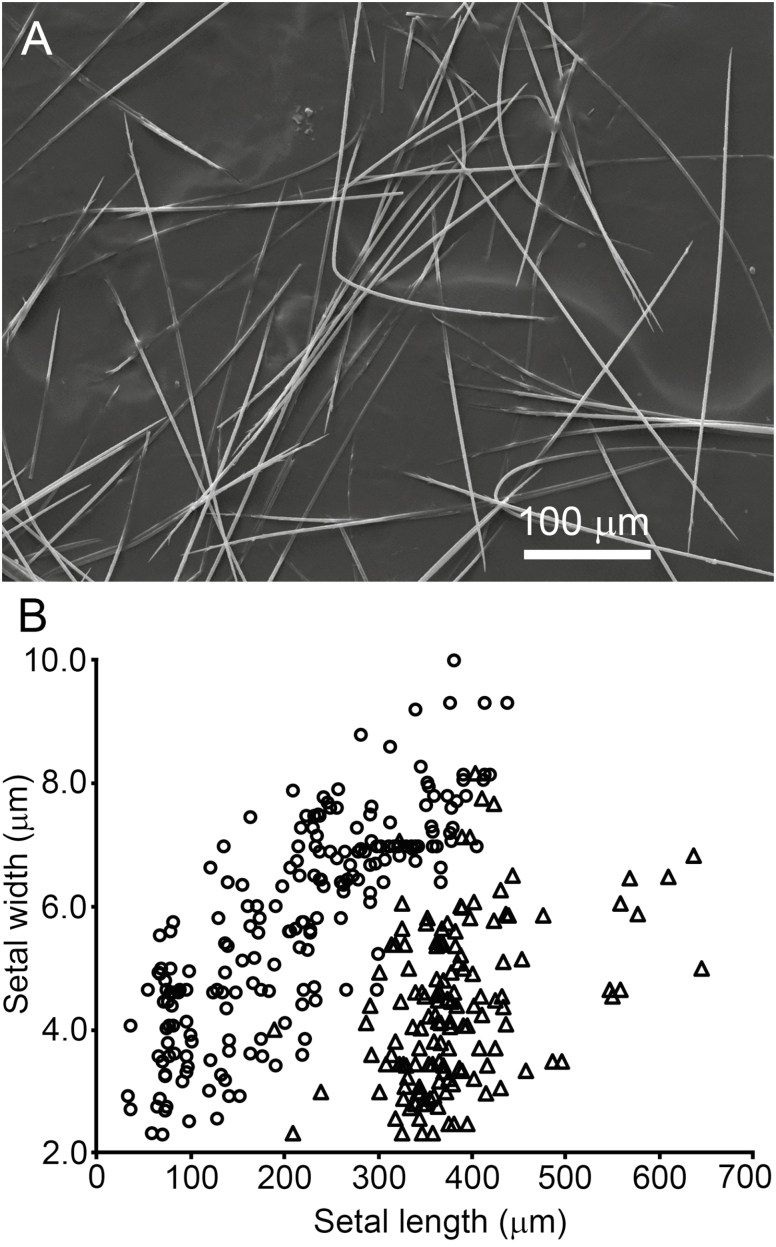
Within individual larvae, the two anterior dorsal setae fields on abdominal tergite 1 contain true setae that are significantly longer and thinner (Fig. 4A) than the true setae in all the other setae fields (see Fig.1A for comparison). Paired measurements of thin and common setae from the same individuals show three clusters of data points that correspond to the three morphological classes of true setae on each individual: common small setae of <100 μm length, common large setae of >100 μm length, and thin setae (Fig. 4B).
Fig. 4.
Thin true setae are found in the anterior setae fields of abdominal segment 1 on O. lunifer larva: (A) thin setae are long, narrow and somewhat bendable; (B) the length and width of thin (triangles) and previously-described true setae (circles) compared (paired data from the same larva).
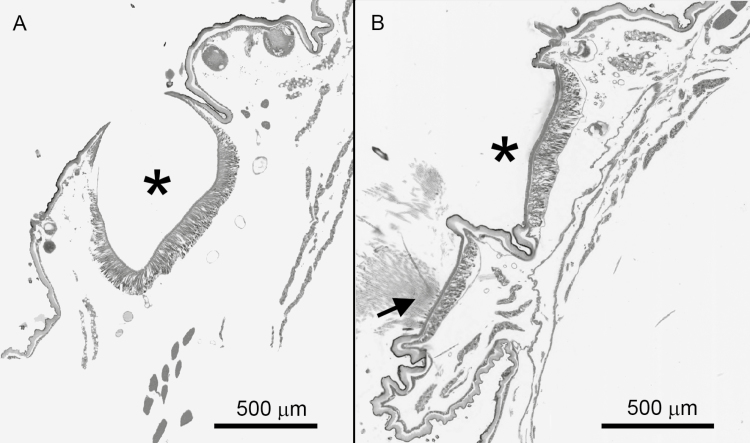
The two cuticular structures holding the thin setae on abdominal tergite 1 are deeper and narrower than the shallow basin-like structures comprising the posterior and lateral setae fields on abdominal tergite 1, and all other setae fields on abdominal tergites 2–9 (Fig. 5). The thin setae are held tightly within the deep, narrow structures and, unlike the common setae, are not dislodged by disturbance easily. Thin setae had to be removed with forceps to obtain samples for microscopy. The density of thin setae within the setae field was less than that of common setae from abdominal segment 1 of the same individual: 25,665 setae/ mm2 (area of 0.036 mm2 counted) for thin setae in anterior setae field compared to 78,660 setae/ mm2 (area of 0.02 mm2 counted) for common setae in posterior field. No connections of muscle to the integument of the setae fields were seen in the histological sections.
Fig. 5.
The shape of setae fields on the abdominal segments of an O. lunifer 8th instar caterpillar in longitudinal cross-section: (A) the deep cup-shaped anterior setae field (*) of abdominal segment 1 with setae removed; (B) the shallow basin-shaped anterior setae field (*) of segment 4 with true setae removed and the posterior field with true setae present (arrow).
All morphological classes of true setae are calculated to travel long distances in the air even under light breeze conditions (Table 1). Common short setae were calculated to travel the furthest with a range from approximately 4–36 km depending on the orientation of the seta in the air and the velocity of the breeze. The larger common setae were calculated to travel 1–11 km, and thin setae approximately 3–27 km.
Table 1.
The settling velocities and distance traveled in air under three airflow conditions calculated for true setae of the size found on O. lunifer when released from a height of 5 m
| Setal class | Settling velocity (cm/s) | Distance traveled (km) with breeze on Beaufort Scale | |||
|---|---|---|---|---|---|
| Axis Parallel | Axis perpendicular | Light (2 m/s) | Moderate (7 m/s) | Strong (13 m/s) | |
| Common < 100μm length | 0.27 | 0.18 | 3.7a–5.5b | 12.9–19.2 | 24.0–35.7 |
| Common > 100 μm length | 0.92 | 0.59 | 1.1–1.7 | 3.8–5.9 | 7.0–11.0 |
| Thin | 0.40 | 0.24 | 2.5–4.1 | 8.8–14.4 | 16.4–26.8 |
a,bRange is the distance dispersed by setae with their axis parallel to the airflow, to the distance dispersed by setae with their axis perpendicular to the airflow.
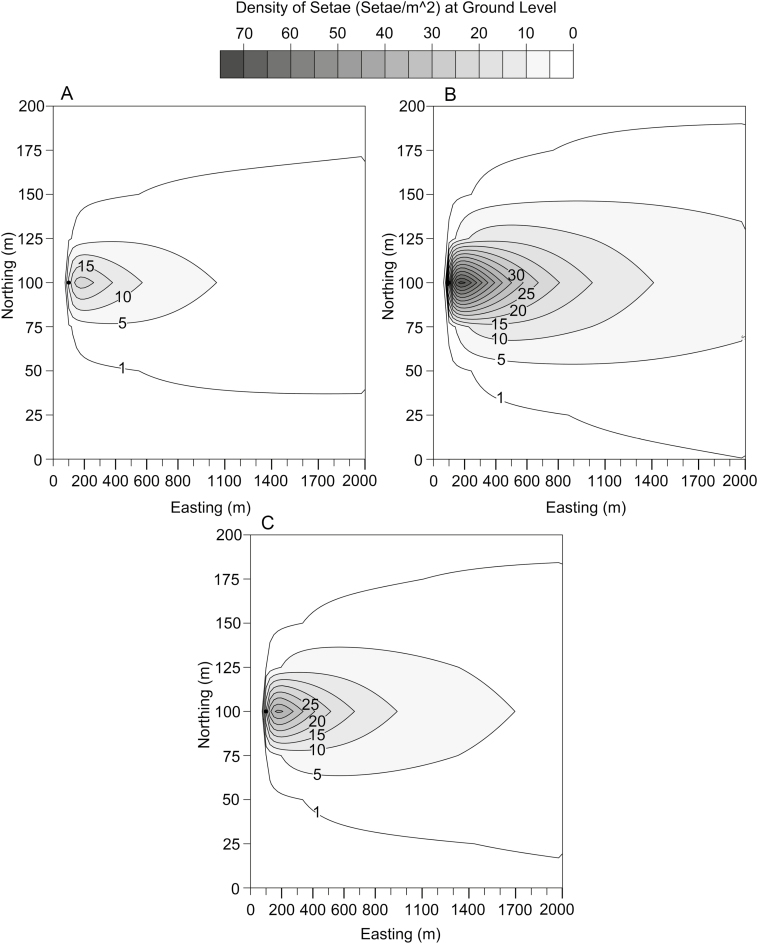
The three classes of setae were modeled in our simulations: small setae having a mean diameter of 3.7 μm and an aspect ratio of 21, large setae having a mean diameter of 6 μm and an aspect ratio of 39.9, and thin setae having a mean diameter of 4.1 μm and an aspect ratio of 103.5. Figure 6A–C shows the concentrations of setae at ground level that were obtained for each of the three classes of setae 96 h after a single foraging procession of 100 caterpillars. Our simulations showed that all setae that would be dispersed from the processionary caterpillars feeding at night were on the ground by 96 h post-release. The greatest concentration of setae on the ground occurred approximately 50–100 m from their source in the tree.
Fig. 6.
The density of setae on the ground for the three morphological classes of true setae dispersed from 100 caterpillars in a nest 5 m above ground in a tree 10 m tall, 96 h after a single foraging procession. (A) Setal class small: diameter of 3.7 μm and an aspect ratio of 21; (B) setal class large: diameter of 6 μm and an aspect ratio of 39.9; (C) setal class thin: diameter of 4.1 μm and an aspect ratio of 103.5. Easting and Northing correspond to Cartesian coordinates (in meters) in eastern and northern directions, respectively.
Discussion
A new morphological type of true seta has been described here for the first time. As for the known type, we found the processionary caterpillar of O. lunifer has two size classes of previously described true setae within the setae fields: distinct small setae of <100 μm interspersed with longer setae, as has been recorded for the northern hemisphere processionary caterpillars T. pityocampa, T. pinivora but not T. processionea (Petrucco-Toffolo et al. 2014). The new morphological type was found in the anterior pair of setae fields on the dorsal tergite of abdominal segment 1. These previously undescribed setae were longer, thinner, and bendable with less conspicuous barbs than the stouter commonly seen true setae. These thin setae are restricted to the first abdominal segment. This segment develops true setae early in the life-cycle (from 5th instar) after segment 9 (3rd instar). Thin setae were held tightly within the cuticular structures of the setae fields which were conical and deeper than the shallow basin-like structures of the other setae fields holding the common setae. Thin setae are not dislodged as readily as common setae, and their flexibility may hinder their penetration of animal skin and mucous membranes, therefore it is difficult to draw any conclusions about their defensive function in comparison to common setae. Often the thin setae are seen retained in the exuvia, therefore if retained in the pre-pupal (8th) instar and incorporated into the pupal case (Perkins et al. 2016), the thin setae may be seen as a last line of defense after the larva’s cache of common setae has been shed.
In addressing the function of urticating true setae generally, it is worth noting that they are present on the abdominal tergites of O. lunifer caterpillars from the 3rd to 8th instar only, with the increase in area and numbers of setae with age suggesting that more resources are invested in this defense as the caterpillar grows bigger. Lamy (1990) reported a similar increase in true setae with age of the northern hemisphere pine (T. pityocampa) and oak (T. processionea) processionary caterpillars. Urtication suggests that the setae have a defensive function and more resources are invested in this defense as the caterpillar grows and is more apparent to predators such as birds. The 1st and 2nd instar do not produce true setae, but these caterpillars remain within the egg mass, which is covered by urticating and thus protective scales from the abdominal tuft of the mother moth. The release of true setae from O. lunifer appears to be predominantly passive, as common true setae were easily dislodged from their shallow setae fields by the smallest mechanical disturbance, and, there was no musculature associated with the cuticle around the setae fields. The exception may be abdominal segment 9 where the setae field is sometimes observed folded beneath abdominal segment 8. This is in contrast to the northern hemisphere T. pityocampa, where the setae fields are kept folded under normal conditions and everted after disturbance (from Démolin 1963 in Battisti et al. 2011).
The defensive mechanism of true setae remains untested against birds and mammals. An inflammatory reaction to true setae penetrating the skin or mucous membranes of the mouth or eyes could happen in a short enough time period and be severe enough for the animal to learn to avoid those caterpillars, especially after repeated exposure and sensitization. Other immunological reactions take longer, and again the severity of the reaction increases with exposure (Battisti et al. 2011). In addition, the cuticle of O. lunifer contains volatile chemical(s) that deter invertebrate predators (Uemura et al. 2017), and these may also have an effect on vertebrate predators especially since the caterpillars are gregarious and tend to occur in large cohorts. In tarantula spiders, Bertani and Guadanucci (2013) found five different morphologies and size of urticating setae and proposed that the different sized setae were effective against different types of predators: the longer setae were cast-off to actively defend against vertebrate predators, whereas smaller setae types were incorporated into molting webs and egg sacs to protect against invertebrate predators. Clearly, the role of true setae in defending processionary caterpillars is complex and needs further study.
Understanding the distribution of airborne urticating agents such as true setae is important for managing their impact on communities. Most documented reactions in humans have been caused by airborne setae (e.g., Maier et al. 2003, Vega et al. 2011) and children seem to suffer more often from general symptoms and airway affection than adults (Gottschling et al. 2007). The shape and size of setae impacts how they are carried by the wind (Petrucco-Toffolo et al. 2014) and our calculations for the three morphological classes of true setae show this. The mean settling velocity of true setae from T. processionea measured experimentally by Fenk et al. (2007) was 1.1 ± 0.1 cm/s and is close to our calculated settling velocity of 0.9 cm/s for larger common setae of O. lunifer moving with their axis parallel to the airflow. Petrucco-Toffolo et al. (2014) calculated a settling velocity of 0.5 cm/s for the true setae of T. processionea. Small true setae can travel further in the wind than larger setae as determined by the formula used where their velocity is inversely proportional to the square of their aerodynamic diameter (Petrucco-Toffolo et al. 2014). The wind speed and how high the caterpillar activity is in a host tree also greatly affect how far urticating setae can disperse in the wind. Stronger winds and higher activity both result in a larger area of the environment where exposure to hazardous setae is possible.
Our calculations and modeling show that the highest concentration of true setae dispersing from caterpillar activity five meters high in their host tree in a slight breeze (typical nighttime condition) occurs approximately 50–100 m from the source, with some setae traveling several kilometers. Fenk et al. (2007) used an Eulerian model to simulate the airborne dispersal of true setae from a source 20 m high in a moderate breeze and calculated maximum concentrations of setae were at a distance from the source between 174 m (day-time) and 562 m (nighttime), and found that several hundreds of setae traveled more than 1 km. Our results taken collectively with the previous studies of Fenk et al. (2007) and Petrucco-Toffolo et al. (2014) clearly show that there is a high risk of exposure to airborne urticating setae within 100 m of caterpillar activity, and a likely risk of exposure for some kilometers in the direction of the prevailing breeze. Boundaries for the likely dispersal of true setae can be determined for any given situation: size of nest, height of tree, wind direction, etc., using the equations presented here. This information should be used to inform management strategies in areas where urticating processionary caterpillars are active, and especially during periods of an outbreak.
Acknowledgments
We thank Assoc Prof Martin Steinbauer, School of Life Sciences, Department of Ecology, Environment and Evolution, La Trobe University, Melbourne, Australia, for generously providing us with specimens of Ochrogaster lunifer. Dr Darryl Whitehead, School of Biomedical Sciences, University of Queensland, performed the caterpillar histology. We acknowledge the facilities and scientific and technical assistance of the Histology Facility and the Australian Microscopy & Microanalysis Research Facility (AAMRF), now Microscopy Australia, at the University of Queensland. This research was supported by a University of Queensland Collaboration and Industry Engagement Fund (CIEF) grant, the Hunter Valley Equine Research Foundation (HVERF), and an Australian Research Council (ARC) Linkage Project grant (LP 140100687).
References Cited
- Barbaro L., and Battisti A.. . 2011. Birds as predators of the pine processionary moth (Lepidoptera: Notodontidae). Biol. Control 56: 107–114. [Google Scholar]
- Battisti A., Holm G., Fagrell B., and Larsson S.. . 2011. Urticating hairs in arthropods: their nature and medical significance. Annu. Rev. Entomol. 56: 203–220. [DOI] [PubMed] [Google Scholar]
- Berardi L., Battisti A., and Negrisolo E.. . 2015. The allergenic protein Tha p 2 of processionary moths of the genus Thaumetopoea (Thaumetopoeinae, Notodontidae, Lepidoptera): characterization and evolution. Gene. 574: 317–324. [DOI] [PubMed] [Google Scholar]
- Bertani R., and Guadanucci J. P. L.. . 2013. Morphology, evolution and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zool. 30: 403–418. [Google Scholar]
- van Bockxmeer J. J., and Green J.. . 2013. Paediatric osteomyelitis after exposure to toxic Ochrogaster lunifer moth. Med. J. Aust. 199: 331–332. [DOI] [PubMed] [Google Scholar]
- Castellanos I., Barbosa P., Zuria I., Tammaru T., and Christman M. C.. . 2011. Contact with caterpillar hairs triggers predator-specific defensive responses. Behav. Ecol. 1: 1020–1025. [Google Scholar]
- Cawdell-Smith A. J., Todhunter K. H., Anderson S. T., Perkins N. R., and Bryden W. L.. . 2012. Equine amnionitis and fetal loss: mare abortion following experimental exposure to Processionary caterpillars (Ochrogaster lunifer). Equine Vet. J. 44: 282–288. [DOI] [PubMed] [Google Scholar]
- Cawdell-Smith A. J., Todhunter K. H., Perkins N. R., and Bryden W. L.. . 2013. Exposure of mares to processionary caterpillars (Ochrogaster lunifer) in early pregnancy: an additional dimension to equine amnionitis and fetal loss. Equine Vet. J. 45: 755–760. [DOI] [PubMed] [Google Scholar]
- Common I. F. B. 1990. Moths of Australia. CSIRO Publishing, Leiden, The Netherlands. [Google Scholar]
- Delgado Quiroz A. 1978. Venoms of Lepidoptera, pp. 555–611. InBettini S. (ed.), Arthropod venoms, handbook of experimental pharmacology, Vol. 48 Springer-Verlag, Berlin/ Heidelberg, Germany. [Google Scholar]
- De-Long S. 1981. Mulberry tussock moth dermatitis. A study of an epidemic of unknown origin. J. Epidemiol. Community Health 35: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démolin G. 1963. Les ‘miroirs’ urticants de la processionnaire du pin (Thaumetopoea pityocampa (Schiff.). Revue de Zoologie Agricole et Appliquee. 10–12: 107–14. [Google Scholar]
- Dyer L. A., and Floyd T.. . 1993. Determinants of predation on phytophagous insects: the importance of diet breadth. Oecologia. 96: 575–582. [DOI] [PubMed] [Google Scholar]
- Fenk L., Vogel B., and Horvath H.. . 2007. Dispersion of the bio-aerosol produced by the oak processionary moth. Aerobiologia 23: 79–87. [Google Scholar]
- Gottschling S., Meyer S., Dill-Mueller D., Wurm D., and Gortner L.. . 2007. Outbreak report of airborne caterpillar dermatitis in a kindergarten. Dermatology 215: 5–9. [DOI] [PubMed] [Google Scholar]
- Griffiths R. F. 1994. Errors in the use of the Briggs parameterzation for atmospheric dispersion coefficients. Atmos. Environ. 28: 2861–2865. [Google Scholar]
- Hossler E. W. 2009. Caterpillars and moths. Dermatol. Ther. 22: 353–366. [DOI] [PubMed] [Google Scholar]
- Kristin A., and Patocka J.. . 1997. Birds as predators of Lepidoptera: selected examples. Biologia. 52: 319–326. [Google Scholar]
- Lamy M. 1990. Contact dermatitis (erucism) produced by processionary caterpillars (Genus Thaumetopoeae). J. Appl. Entomol. 110: 425–437. [Google Scholar]
- Maier H., Spiegel W., Kinaciyan T., Krehan H., Cabaj A., Schopf A., and Hönigsmann H.. . 2003. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br. J. Dermatol. 149: 990–997. [DOI] [PubMed] [Google Scholar]
- Mather A., Zalucki M. P., Farrell J., Perkins L. E., and Cook L. G.. . 2019. Australian processionary caterpillars, Ochrogaster lunifer Herrich-Schäffer (Lepidoptera, Notodontidae), comprise cryptic species. Austral. Entomol. doi: 10.1111/aen.12410. [DOI] [Google Scholar]
- Novak F., Pelissou V., and Lamy M.. . 1987. Comparative morphological, anatomical and biochemical studies of the urticating apparatus and urticating hairs of some Lepidoptera: Thaumetopoea pityocampa Schiff. Th. Processionea L. (Lepidoptera, Thaumetopoeidae) and Hylesia metabus Cramer (Lepidoptera, Saturniidae). Comp. Biochem. Phys. A. 88: 141–146. [Google Scholar]
- Pasquill F. 1961. The estimation of the dispersion of windborne material. Meteorol. Mag. 90: 33–49. [Google Scholar]
- Perkins L. E., Zalucki M. P., Perkins N. R., Cawdell-Smith A. J., Todhunter K. H., Bryden W. L., and Cribb B. W.. . 2016. The urticating setae of Ochrogaster lunifer, an Australian processionary caterpillar of veterinary importance. Med. Vet. Entomol. 30: 241–245. [DOI] [PubMed] [Google Scholar]
- Petrucco Toffolo E., Zovi D., Perin C., Paolucci P., Roques A., Battisti A., and Horvath H.. . 2014. Size and dispersion of urticating setae in three species of processionary moths. Integr. Zool. 9: 320–327. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: Available from http://www.R-project.org/ [Google Scholar]
- R Core Team. 2019. R: A language and environment for statistical computing.R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/ [Google Scholar]
- Soetaert K., and Meysman F.. . 2012. Reactive transport in aquatic ecosystems: rapid model prototyping in the open source software R. Environ. Modell. Softw. 32: 49–60. [Google Scholar]
- Southcott R. V. 1978. Lepidopterism in the Australian Region. Records of the Adelaide Children’s Hospital. 2: 87–173. [Google Scholar]
- Steinbauer M. J. 2009. Thigmotaxis maintains processions of late instar caterpillars of Ochrogaster lunifer. Physiol. Entomol. 34: 345–349. [Google Scholar]
- Sugiura S., and Yamazaki K.. . 2014. Caterpillar hair as a physical barrier against invertebrate predators. Behav. Ecol. 1: 1–9. [Google Scholar]
- Todhunter K. H., Cawdell-Smith A. J., Bryden W. L., Perkins N. R., and Begg A. P.. . 2014a. Processionary caterpillar setae and equine fetal loss: 1. Histopathology of experimentally exposed pregnant mares. Vet. Pathol. 51: 1117–1130. [DOI] [PubMed] [Google Scholar]
- Todhunter K. H., Cawdell-Smith A. J., Bryden W. L., Perkins N. R., and Begg A. P.. . 2014b. Processionary caterpillar setae and equine fetal loss: 2. Histopathology of the fetal-placental unit from experimentally exposed mares. Vet. Pathol. 51: 1131–1142. [DOI] [PubMed] [Google Scholar]
- Uemura M., Perkins L. E., Zalucki M. P., and Cribb B. W.. . 2017. Predator-prey interaction between greenhead ants and processionary caterpillars is mediated by chemical defence. Anim. Behav. 129: 213–222. [Google Scholar]
- Vega J. M., Moneo I., Ortiz J. C., Palla P. S., Sanchís M. E., Vega J., Gonzalez-Muñoz M., Battisti A., and Roques A.. . 2011. Prevalence of cutaneous reactions to the pine processionary moth (Thaumetopoea pityocampa) in an adult population. Contact Dermatitis. 64: 220–228. [DOI] [PubMed] [Google Scholar]
- Werno J., and Lamy M.. . 1990. Animal atmospheric pollution: the urticating hairs of pine processionary caterpillar (Thaumetopoea pityocampa Schiff.) (Insect, Lepidoptera). Comptes rendus de l’Academie des Sciences Paris. Vol 310 Series III: 325–331. [PubMed] [Google Scholar]