Abstract
This study investigated the ability to use feedback for decision-making in female college students who binge drink (BD) using the iowa gambling task (IGT) and event-related potentials (ERPs). Twenty-seven binge drinkers and 23 non-binge drinkers (non-BD) were identified based on scores on the Korean version of the Alcohol Use Disorder Test and the Alcohol Use Questionnaire. The IGT consists of four cards, including two cards that result in a net loss, with large immediate gains but greater losses in the long term, and two cards that result in a net gain, with small immediate gains but reduced losses in the long term. Participants were required to choose one card at a time to maximize profit until the end of the task while avoiding losses. The BD group showed a significantly lower total net score than the non-BD group, indicating that the BD group chose more disadvantageous cards. The BD group showed significantly smaller ΔFRN amplitudes [difference in amplitudes of feedback-related negativity (FRN) between gain and loss feedback] but not in P3 amplitudes. Additionally, ΔFRN amplitudes in the fronto-central area were positively correlated with the total net score and net scores for sectors 4 and 5. Thus, total net scores and later performance on the IGT increased as ΔFRN amplitudes from the fronto-central area increased. FRN is known to reflect early feedback evaluation employing a bottom-up mechanism, whereas P3 is known to reflect late feedback processing and allocation of attentional resources using a top-down mechanism. These results indicate that college students who binge drink have deficits in early evaluation of positive or negative feedback and that this deficit may be related to decision-making deficits.
Keywords: binge drinking, decision-making, feedback-related negativity, feedback utilization, event-related potentials, P3
Introduction
Binge drinking (BD) is defined as a repeated pattern of excessive alcohol consumption and abstinence over a short period of time (Wechsler and Nelson, 2001; Parada et al., 2012; Maurage et al., 2013). BD is most prevalent among young adults, especially college students (Wechsler and Nelson, 2001; Chun et al., 2003; Stephens and Duka, 2008), and is associated with various problems including assault, drunk driving, unguided or unsafe sexual behavior, and academic underachievement (Wechsler and Nelson, 2001; Chun, 2002; Naimi et al., 2003; Cha, 2005). Additionally, binge drinkers exhibit similar structural and functional brain abnormalities and neuropsychological deficits to patients with alcohol use disorder (AUD) (Crego et al., 2010; Lopez-Caneda et al., 2012; Campanella et al., 2013; Kanny et al., 2013; Maurage et al., 2013; Mota et al., 2013), and BD predicts the development of AUD in the future (O’Neill et al., 2001; Tucker et al., 2003; Jennison, 2004; Kanny et al., 2013).
Patients with AUD cannot stop drinking alcohol even though they suffer from its negative consequences [American Psychiatric Association (APA), 1994, 2013]. Such behaviors reflect inefficient decision-making among patients with AUD, as they continue to seek immediate rewards and ignore future consequences (Mazas et al., 2000; Bechara et al., 2001). In other words, they not only underestimate the negative consequences of alcohol consumption (Mallett et al., 2006) but also emphasize immediate rewards over long-term consequences (MacKillop et al., 2010; Amlung et al., 2014). Decision-making deficits have been observed in patients with AUD (Bechara et al., 2001; Bechara, 2003; Fein et al., 2004; Goudriaan et al., 2005; Mitchell et al., 2005; Noel et al., 2007) and in binge drinkers (Goudriaan et al., 2007; Johnson et al., 2008; Xiao et al., 2009, 2013; Yoo and Kim, 2016).
Decision-making is defined as a process of forming a preference for an option, making a choice based on the preference, executing the choice, and evaluating the consequences of the choice (Ernst and Paulus, 2005). Decision-making is a complex process including both cognitive and non-cognitive processes (i.e., emotions) (Bechara et al., 1999), and various brain areas, such as the orbitofrontal, ventromedial prefrontal, anterior cingulate cortices, and amygdala, are involved in decision-making (Bechara et al., 2000; Bush et al., 2002; Ernst et al., 2002; Kennerley et al., 2006; Wallis, 2007). The iowa gambling task (IGT) (Bechara et al., 1994; Bechara, 2004) is widely used to measure decision-making ability. Participants are asked to choose one of four cards on every trial to maximize profit while avoiding loss. The chosen card results in gains on every trial, but also results in intermittent losses. The cards differ in feedback magnitude and probability. Two cards (A and B) result in large immediate gains but greater losses, causing a net loss (disadvantageous cards), whereas the other two cards (C and D) lead to small immediate gains and smaller losses, resulting in a net gain (advantageous cards). Participants must evaluate feedback such as valence (gain or loss), magnitude (large or small), and the probability of encountering losses to learn the contingency between the card and its consequences (Dunn et al., 2006; Webb et al., 2014). Studies investigating decision-making ability in patients with AUD using the IGT found that patients with AUD performed poorly compared to normal controls, choosing significantly more disadvantageous cards and significantly fewer advantageous cards compared with the controls (Bechara et al., 2001; Fein et al., 2004; Goudriaan et al., 2005; Dom et al., 2006; Noel et al., 2007). Additionally, positive correlations were observed between IGT performance and gray matter volume in the dorsal and ventromedial prefrontal cortices, which are crucial for decision-making (Le Berre et al., 2014). Poor IGT performance has also been observed in individuals with BD (Goudriaan et al., 2007; Johnson et al., 2008; Moreno et al., 2012; Yoo and Kim, 2016). For example, adolescents (Moreno et al., 2012) and college students with BD (Yoo and Kim, 2016) performed significantly worse on the IGT than did non-BD groups.
Feedback utilization, a process of identifying whether an action induces positive or negative consequences and evaluating those consequences, is crucial to making efficient decisions (San Martin, 2012). Considerable improvement in our understanding of the neurological basis of feedback utilization has revealed that the orbitofrontal, ventromedial prefrontal, and anterior cingulate cortices as well as the ventral striatum are involved in feedback utilization (Delgado et al., 2000; Elliott et al., 2000; Knutson et al., 2000; O’Doherty et al., 2001; Rogers et al., 2004). The ventral striatum is involved in prediction errors, i.e., how actual feedback differs from personal expectations, whereas the orbitofrontal cortex is involved in evaluating feedback based on prediction errors (Elliott et al., 2000; Pagnoni et al., 2002; McClure et al., 2003; O’Doherty et al., 2003). Additionally, the anterior cingulate cortex evaluates rewards in situations where contingencies are uncertain and then relays the evaluation of the reward to motor areas for response execution (Bush et al., 2002).
Studies, which have used event-related potentials (ERPs) to investigate feedback utilization, reported two components, feedback-related negativity and P3, as the electrophysiological indices of feedback utilization (Gehring and Willoughby, 2002; San Martin, 2012). Gehring and Willoughby (2002) used a simple gambling task to observe a negative peak approximately 265 ms post feedback whose amplitude was larger in response to negative than to positive feedback. This peak is known as feedback-related negativity (FRN) or outcome-related negativity (ORN) (Kamarajan et al., 2009). FRN is sensitive to feedback valence (gain or loss) (Yeung and Sanfey, 2004) and is associated with activation of the midbrain dopaminergic system (Tobler et al., 2005). Additionally, reinforcement-learning theory suggests that FRN reflects prediction errors, i.e., the difference between actual feedback and personal expectation (Wu and Zhou, 2009; Bellebaum et al., 2010; San Martin, 2012). P3, another ERP component related to feedback utilization, is a positive peak observed in central-parietal areas at 275–700 ms post feedback (Kamarajan et al., 2009; San Martin, 2012). P3 is known to be sensitive not only to feedback valence but also to feedback magnitude and probability (Yeung and Sanfey, 2004; Hajcak et al., 2007; Wu and Zhou, 2009; Polezzi et al., 2010; Xu et al., 2011). It has been suggested that P3 reflects activation of the locus coeruleus-norepinephrine system and processing of task-relevant information to maximize decision-making efficiency (Nieuwenhuis et al., 2005). In other words, P3 reflects, unlike FRN, a top-down mechanism that processes and evaluates feedback-related information in detail (Wu and Zhou, 2009; San Martin, 2012).
The effects of alcohol consumption on feedback utilization are reflected on the FRN and P3 amplitudes. For example, a study that used a gambling task and measured ERPs found that the alcohol consumption group exhibited significantly lower FRN amplitudes in response to both gain and loss feedback, especially to loss feedback, than did a placebo group, indicating that alcohol consumption affects feedback utilization (Nelson et al., 2011). Deficits in feedback utilization are also observed in patients with AUD. For example, Fein and Chang (2008) using the Balloon Analogue Risk Task, observed that patients with AUD and a family history of AUD exhibited significantly smaller FRN amplitudes than did those without a family history. Kamarajan et al. (2010) used a gambling task and reported that patients with AUD exhibited lower P3 amplitudes in response to both gain and loss feedback and smaller FRN amplitudes to loss feedback than did normal controls. Additionally, they observed increased activation in primary sensory and motor areas during the FRN time window and decreased activation in the cingulate gyrus during the P3 window in patients with AUD relative to normal controls. These results indicate that the sensory and motor areas of patients with AUD are hyper-excited during early feedback evaluation, and areas involved in feedback evaluation are hypo-activated compared to normal controls (Kamarajan et al., 2010).
To our knowledge, only one study has investigated feedback utilization deficits in binge drinkers using ERPs. That study, which used the IGT, found that the BD group tended to exhibit smaller FRN amplitudes (p = 0.06) than the non-BD group (Wahlstrom, 2013). However, that study used the original computerized IGT (Bechara et al., 1994; Bechara, 2007), which had two limitations: first, the original IGT consisted of 100 trials, which is not suitable for an ERP study where a sufficient number of trials are needed (Schuermann et al., 2011). Second, the original IGT displays gains in every trial and subsequently displays losses according to each card’s probability. When multiple stimuli are displayed in succession, the ERPs to loss feedback might be contaminated by previous gain feedback.
The present study investigated feedback utilization ability during decision-making in BD female college students using the IGT and ERP. Specifically, this study examined whether decision-making deficits in BD female students are related to feedback utilization deficits, and if so, how they are reflected in feedback-related ERP components, FRN, and P3. Based on previous findings, we hypothesized that the BD group would perform significantly worse than the non-BD group on the IGT; that the BD group would show significantly smaller FRN and P3 amplitudes than the non-BD group; and that IGT performance and feedback-related ERPs would be positively correlated. Gender differences are observed in BD (O’Malley and Johnston, 2002; Wechsler et al., 2002; Weitzman et al., 2003), decision-making (Bolla et al., 2004), and ERP amplitudes (Larson et al., 2011). For example, females tend to drink less (O’Malley and Johnston, 2002), perform poorer on the IGT (Bolla et al., 2004) than males, and exhibit different neural activities with regard to the N2 and P3 components (Larson et al., 2011). For these reasons, only female college students were included in this study.
Materials and Methods
Participants
The details of the participant screening procedures have been described in previous studies by our research group (Yoo and Kim, 2016; Park and Kim, 2018). The Korean version of the Alcohol Use Disorder Identification Test (AUDIT-K) (Barbor et al., 1992; Lee et al., 2000), Alcohol Use Questionnaire (AUQ) (Mehrabian and Russell, 1978), and a questionnaire inquiring about binge drinking episodes in the last 2 weeks were administered to 435 female college students. The BD and non-BD groups were defined based on (1) alcohol-related problems and drinking habits, (2) the number of BD episodes, and (3) drinking speed. The BD group included those who (1) scored at least 12 but less than 26 on the AUDIT-K, (2) had consumed four or more glasses at one sitting in the last 2 weeks, and (3) drank two or more glasses per hour. Although the World Health Organization (WHO) recommends using a score >8 as the cutoff point for problem drinking (Barbor et al., 1992), the cutoff score of 12 was applied because a cutoff point of 8 includes those who do not have apparent drinking problems but may display problem drinking in the future (Conigrave et al., 1995; Kim et al., 1999). In contrast, those who received scores >26 on the AUDIT-K were also excluded, as AUD was suspected. The non-BD group included those who (1) scored less than 8 on the AUDIT-K, (2) had not drunk four or more glasses in one sitting in the last 2 weeks, and (3) drank 1 glass or less per hour.
The Structured Clinical Interview of the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (SCID-NP) (First et al., 1995) was administered to ensure that no participants had a psychiatric disorder. Additionally, the Self-Rating Depression Scale (SDS) (Zung et al., 1965), the State-Trait Anxiety Inventory (STAI) (Speilberger et al., 1983), and Barratt Impulsivity Scale (BIS) (Patton et al., 1995) were administered to evaluate depression, anxiety, and impulsivity, respectively. To control for the influence of alcohol-related genes and family history, the Korean version of the Children of Alcoholics Screening Test (CAST-K) (Jones, 1983; Kim et al., 1995) was administered, and those who scored 6 or more were excluded. Last, those who were left-handed or ambidextrous were also excluded to control for the effect of brain lateralization.
In the end, 50 students participated in this study (27 in the BD group and 23 in the non-BD group). This study was approved by Sungshin Women’s University Institutional Review Board (SSWUIRB 2017-040). The participants provided written informed consent after receiving a description of the study, and they were paid for their participation.
The Korean Version of the Alcohol Use Disorder Identification Test (AUDIT-K)
The AUDIT (Barbor et al., 1992), a self-administered questionnaire designed to measure the presence of AUD and drinking problems, consists of 10 items. The total score ranges from 0 to 40. Three items inquire about frequency and quantity of alcohol consumption, three about symptoms related to alcohol dependence, and four about psychosocial problems related to alcohol consumption. The Korean version was administered in this study (Lee et al., 2000).
Alcohol Use Questionnaire
The AUQ (Mehrabian and Russell, 1978) is a self-administered questionnaire measuring dinking patterns. Items 10, 11, and 12 evaluate drinking speed, frequency of being drunk within the last 6 months, and the rate of being drunk when consuming alcohol, respectively. These three items were used to calculate a BD score (Townshend and Duka, 2002). The binge score was calculated using the following equation:
The Iowa Gambling Task
This study employed a modified version of the original computerized IGT (Bechara, 2007) to make the task suitable for measuring ERPs (Figure 1A). Four cards were displayed on a computer monitor, and participants were asked to maximize profits until the end of the game by choosing a card during each trial. Gain or loss feedback was displayed after each choice, with gain feedback consisting of a green smiling emoticon with points earned and loss feedback consisting of a red crying emoticon with the points lost (Figure 1B).
Figure 1.
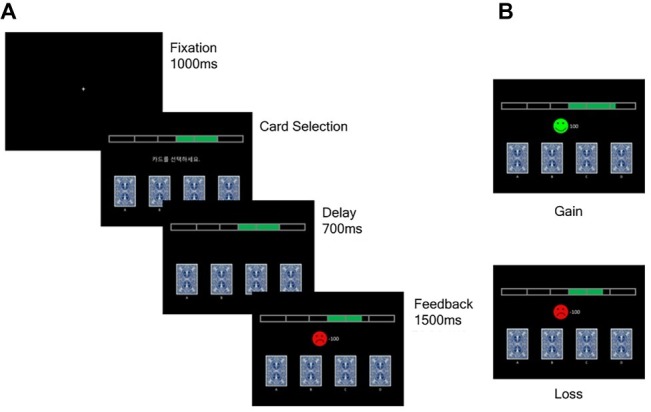
The modified IGT. (A) A fixation point will be displayed for 1,000 ms and then four cards will be displayed till the participants make their choice. At 700 ms after a card is chosen, feedbacks will be displayed for 1,000 ms. (B) The feedback stimuli consist of gain conditions and loss conditions. In gain conditions, green smiling emoticon and the earned points will be displayed whereas red crying emoticon and the lost points will be displayed in loss conditions.
The magnitude and probability of gain and loss for each card were set as for the original computerized IGT (Bechara, 2007). The cards consisted of two disadvantageous cards (A and B), which provided large gains and larger losses, resulting in a net loss, and two advantageous cards (C and D), which provided small gains but smaller losses, resulting in a net gain. Cards A and C each had a 50% chance of causing losses, whereas cards B and D had a 10% chance of causing losses.
The task consisted of three blocks; the locations of the cards were changed at the beginning of each block to keep participants motivated. Each block comprised 100 trials; a total of 320 trials, including 20 practice trials, were administered. Decision-making ability was measured by the net score, which was calculated by subtracting the frequency of choosing the disadvantageous cards (A and B) from the frequency of choosing the advantageous ones (C and D).
E-Prime software (version 2.0; Psychological Software Tools, Inc., Sharpsburg, PA, USA) was used to administer the modified IGT. A fixation point (+) was displayed for 1,000 ms, and the cards were then displayed until the participants made their choice by pressing a button. The feedback, either a gain or loss, was displayed for 1,000 ms at 700 ms after a card was chosen.
Electrophysiological Recording Procedure
Electroencephalography (EEG) was measured using a 64-channel Geodesic sensor net connected to a 64-channel, high-input impedance amplifier (Net Amp 300; Electrical Geodesics, Eugene, OR, USA) in a shielded and soundproofed room. All electrodes were referenced to Cz, and impedance was maintained at 50 kΩ or less (Tucker et al., 2003). EEG activity was recorded continuously using a 0.3–100 Hz bandpass filter at a sampling rate of 500 Hz. The recorded EEG data were digitally filtered using a 0.3–30 Hz bandpass and re-referenced to the average reference. The continuous EEG was then segmented into 800 ms epochs (from 100 ms pre- to 700 ms post-feedback). Additionally, epochs contaminated by artifacts such as eye blinks were removed based on the threshold of a peak-to-peak amplitude of ± 70 μV from the eye channels. The remaining data were averaged according to feedback valence, i.e., gain and loss feedback.
Statistical Analysis
Demographic variables were analyzed with independent t-tests. The total net scores on the modified IGT were analyzed with independent t-tests. Additionally, each block was subdivided into five sectors, and scores for each sector were averaged across the three blocks to calculate sector net scores to measure performance improvement across trials. The sector net scores were analyzed with mixed-design analysis of variance (ANOVA), where group (BD or non-BD) was a between-subjects factor, and sector (1–5) was a within-subject factor.
ERP components and time windows were determined based on grand averaged ERPs and individual ERP waveforms. FRN was defined as the most negative peak observed at 200–275 ms after feedback-onset, and P3 was defined as the most positive peak followed by FRN, i.e., observed 275–600 ms after feedback. Because the FRN and P3 time windows overlapped and because the FRN is a negative and P3 is a positive peak, it is possible that latent components representing FRN and P3 independently might be distorted on the ERP waveforms due to the overlapping windows where the amplitudes and latencies do not clearly represent the differences by feedback valence (Luck, 2014). To overcome this problem, it is necessary to isolate ERP components; difference waves have been recommended for this purpose (Luck, 2014). Therefore, ∆FRN (FRN effect) and ∆P3 (P3 effect) were defined as the amplitude difference between gain and loss feedback (Holroyd, 2004; Hajcak et al., 2007; Carlson et al., 2009; Holroyd et al., 2009; Walsh and Anderson, 2011; Xu et al., 2011).
Amplitudes and latencies of each component were analyzed by mixed ANOVA. Electrode site (FC3, FCz, FC4, C3, Cz, C4, P3, Pz, and P4) and valence (gain or loss) were within-subject factors, and group was a between-subjects factor. The electrode sites for ∆FRN and ∆P3 were a within-subject factor, and group was a between-subjects factor. Greenhouse-Geisser corrections were used in cases of violation of sphericity, and corrected p are reported when appropriate. The mean numbers of trials included in the FRN/P3 analysis for the BD and non-BD groups were 105.57 (gain = 161.89, loss = 51.26) and 111.33 (gain = 17.48, loss = 52.17), respectively. The two groups did not differ in terms of trials for averaging FRN/P3 in the gain feedback [F(1,48) = 0.62, p = 0.44], the loss feedback [F(1,48) = 0.04, p = 0.85] or both feedbacks [F(1,48) = 0.57, p = 0.46]. The relationships of the ∆FRN and ∆P3 amplitudes with performance on the IGT, i.e., total net scores and sector net scores, were analyzed using Pearson’s correlation coefficient analysis. A p < 0.05 was considered significant.
Results
Demographic Characteristics
The BD and non-BD groups did not differ in terms of age [t(48) = −1.08, p = 0.29], educational level [t(48) = −1.07, p = 0.29], SDS [t(48) = 0.80, p = 0.43], or trait anxiety on the STAI [t(48) = 1.05, p = 0.30]. However, the BD group exhibited significantly higher state anxiety on the STAI [t(48) = 5.49, p < 0.001], BIS [t(48) = 6.92, p < 0.001], AUDIT-K total score [t(48) = 16.81, p < 0.001], drinking speed [t(48) = 12.56, p < 0.001], frequency of being drunk within the last 6 months [t(48) = 5.63, p < 0.001], percentage of being drunk when consuming alcohol [t(48) = 3.73, p < 0.01], and AUQ binge score [t(48) = 9.94, p < 0.001] compared to the non-BD group. The demographic characteristics of the BD and non-BD groups are presented in Table 1.
Table 1.
Demographic characteristics of the non-BD and BD groups.
| Non-BD (n = 23) | BD (n = 27) | t | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 22.04 (1.92) | 21.44 (1.99) | −1.08 |
| Education (years) | 15.09 (1.08) | 14.74 (1.20) | −1.07 |
| SDS | 39.61 (5.39) | 41.15 (7.83) | 0.80*** |
| STAI state | 38.57 (8.10) | 56.93 (14.16) | 5.49** |
| STAI trait | 38.70 (7.56) | 41.26 (9.37) | 1.05 |
| BIS | 63.48 (10.95) | 83.26 (9.29) | 6.92** |
| AUDIT-K | 2.39 (1.80) | 17.37 (4.20) | 16.81*** |
| Speed of drinking (drinks/h) | 0.65 (0.57) | 4.22 (1.34) | 12.56*** |
| Times drunk in the last 6 months | 0.13 (0.34) | 5.07 (4.55) | 5.63*** |
| Percentage of times became drunk when drinking (%) | 11.87 (23.37) | 39.44 (28.83) | 3.73** |
| AUQ binge drinking score | 5.11 (5.17) | 29.85 (11.66) | 9.94*** |
p < 0.01;
p < 0.001.
SDS, self-rating dpression scale; STAI, Spieberger’s state-trait anxiety inventory; BIS, Barratt impulsivity scale; AUDIT-K, the Korean version of alcohol use disorder identify test; AUQ, alcohol use questionnaire.
As significant differences in state anxiety and impulsivity were detected, mixed analysis of covariance was performed with state anxiety and impulsivity as covariates to control their effect on the IGT and ERP components. However, the analysis revealed that state anxiety as a covariate was not significantly associated with the IGT (p = 0.086), FRN (p = 0.565), or P3 (p = 0.634) and that impulsivity as a covariate was not significantly associated with the IGT (p = 0.464), FRN (p = 0.295), or P3 (p = 0.631).
The Modified Iowa Gambling Task
The BD group exhibited a significantly lower total net score than the non-BD group [t(48) = −2.61, p < 0.05]. In terms of sector net scores, a main effect of sector was observed [F(4,192) = 2.45, p < 0.05]. A further post hoc analysis revealed a trend toward a lower net score for sector 2 than for sector 4 (p = 0.09). Additionally, a main effect of group was observed [F(1,48) = 7.28, p < 0.05], with the BD group exhibiting significantly lower sector net scores than the non-BD group. However, the sector × group interaction was not significant [F(4,192) = 1.23, p = 0.30]. Mean total and sector net scores of the BD and non-BD groups are presented in Table 2 and Figure 2.
Table 2.
Performance of the modified IGT in the non-BD and BD groups.
| Non-BD (n = 23) | BD (n = 27) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Sector 1 | −0.03 (4.16) | −1.73 (3.04) |
| Sector 2 | 0.72 (5.73) | −3.04 (4.14) |
| Sector 3 | 0.93 (5.53) | −1.63 (3.49) |
| Sector 4 | 2.23 (5.75) | −1.41 (4.35) |
| Sector 5 | 1.25 (5.14) | −1.93 (4.08) |
| Total | 5.10 (23.43) | −9.73 (15.11) |
Figure 2.
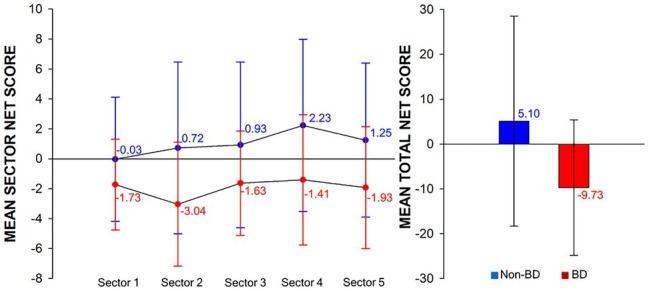
Performance of the modified IGT. Sector net scores (left) and total net scores (right) of the modified IGT in the non-binge drinking and binge drinking groups.
Electrophysiological Measures
The grand-averaged ERPs elicited by gain and loss feedback at fronto-central (FCz), central (Cz), and parietal midlines (Pz) for the BD and non-BD groups are displayed in Figure 3. The BD and non-BD groups exhibited the largest FRN and P3 amplitudes at Cz. The topographical distribution of FRN and P3 measured at all electrodes when the largest FRN and P3 amplitudes were observed are displayed in Figures 4, 5, respectively.
Figure 3.
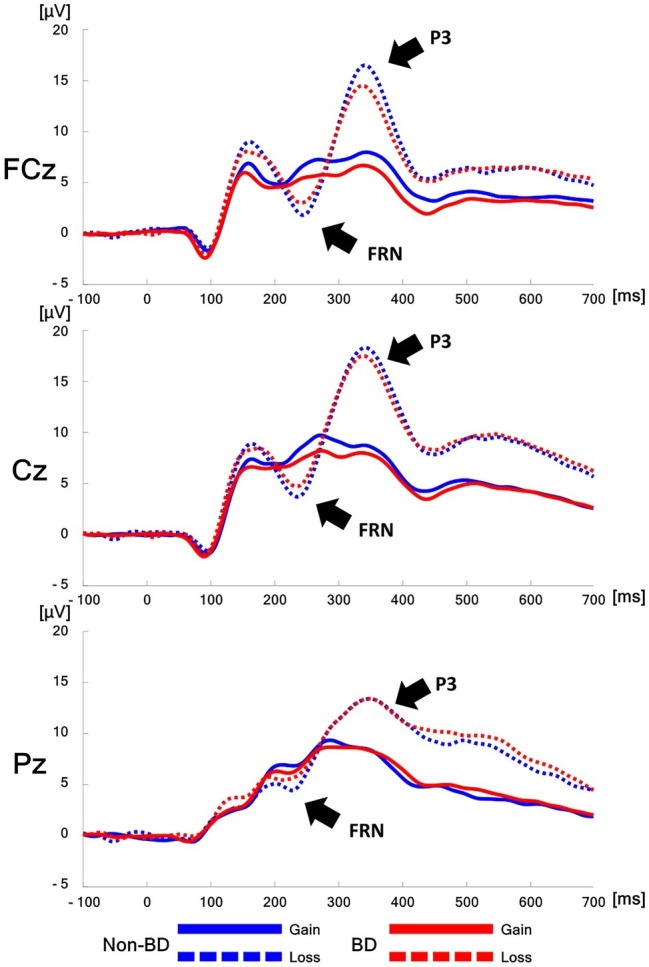
The grand-averaged ERPs. The grand-averaged ERPs elicited by gain (solid line) and loss (dotted line) feedbacks at FCz, Cz, and Pz for the non-binge drinking (blue) and binge drinking groups (red).
Figure 4.
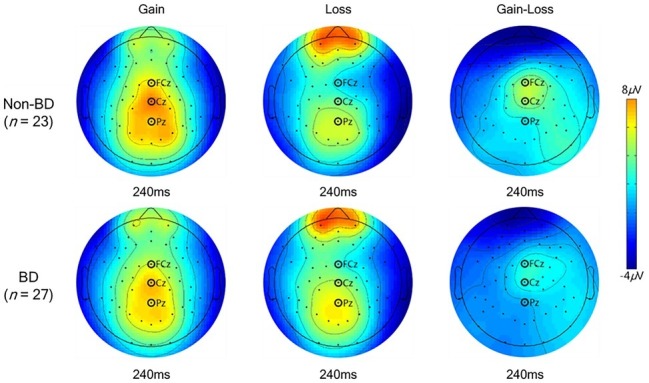
Topographical distribution of FRN. The topographical distribution of FRN measured at all electrodes when the maximum FRN amplitudes were observed.
Figure 5.
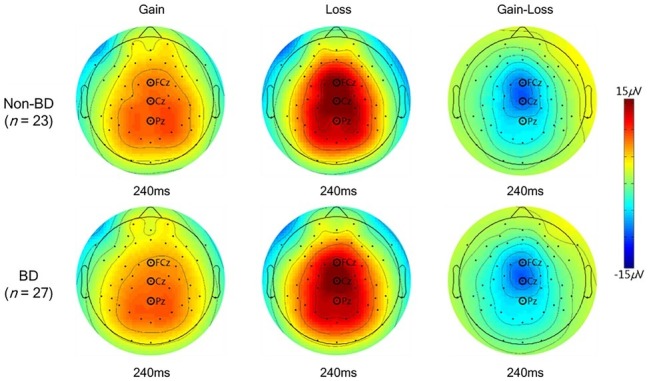
Topographical distribution of P3. The topographical distribution of P3 measured at all electrodes when the maximum P3 amplitudes were observed.
Main effects of valence [F(1,48) = 62.17, p < 0.001] and electrode site [F(8,384) = 18.52, p < 0.001] were observed in terms of FRN amplitudes. FRN amplitudes in response to loss feedback were significantly larger than those in response to gain feedback, and the largest and smallest FRN amplitudes were observed at Cz and FC4, respectively. Additionally, a valence × group interaction was observed [F(1,48) = 8.06, p < 0.01]. A simple effect analysis revealed that while the BD and non-BD groups exhibited comparable FRN amplitudes in response to both gain [F(1,48) = 0.84, p = 0.36] and loss feedback [F(1,48) = 1.81, p = 0.19], the magnitude of difference between the valences for each group was different. In other words, both groups exhibited larger FRN amplitudes in response to loss than to gain feedback, and the difference in the FRN amplitudes between the gain and loss feedback was larger in the non-BD group (mean difference = 2.32, p < 0.001) than in the BD group (mean difference: 1.09, p < 0.01). In addition, an electrode site × valence interaction was observed [F(8,384) = 12.32, p < 0.001] such that FRN amplitudes in response to the loss feedback were larger than those to the gain feedback in all electrodes except FC3. The main effect of group was not significant [F(1,48) = 0.08, p = 0.78]. The mean FRN amplitudes of the BD and non-BD groups are presented in Table 3.
Table 3.
Mean FRN amplitudes (μV) in the non-BD and BD groups.
| Non-BD (n = 23) | BD (n = 27) | |||
|---|---|---|---|---|
| Gain | Loss | Gain | Loss | |
| FC3 | 3.41 (2.27) | 2.79 (2.75) | 3.44 (2.02) | 3.43 (2.54) |
| FCz | 4.94 (3.50) | 1.53 (3.84) | 4.51 (2.59) | 3.13 (3.50) |
| FC4 | 3.32 (2.35) | 0.62 (2.56) | 3.38 (1.88) | 1.87 (2.68) |
| C3 | 3.98 (2.60) | 2.80 (2.73) | 3.73 (1.88) | 3.40 (1.97) |
| Cz | 7.11 (4.07) | 3.72 (4.24) | 6.88 (2.65) | 4.67 (3.37) |
| C4 | 4.03 (2.42) | 1.51 (2.48) | 3.48 (1.87) | 1.78 (2.46) |
| P3 | 4.49 (2.50) | 2.96 (2.68) | 3.39 (1.88) | 3.12 (1.82) |
| Pz | 7.04 (3.45) | 4.18 (3.73) | 6.04 (2.65) | 5.33 (2.90) |
| P4 | 4.30 (2.56) | 1.63 (3.11) | 3.50 (2.18) | 1.77 (3.08) |
() – Standard deviation.
Main effects of group [F(1,48) = 6.67, p < 0.05] and electrode site [F(8,384) = 12.32, p < 0.001] were observed for ∆FRN. The BD group exhibited a significantly smaller ∆FRN compared to the non-BD group. The greatest ∆FRN amplitude was observed at Cz, and the smallest was detected at FC3. The electrode site × group interaction was not significant [F(8,384) = 0.70, p = 0.70].
Main effects of valence [F(1,48) = 12.85, p < 0.01] and electrode site [F(8,384) = 3.46, p < 0.05] were observed in terms of FRN latencies. Thus, FRN latencies in response to gain feedback were shorter than those in response to loss feedback (p < 0.01). In addition, the shortest latency was observed at Pz, and the longest was observed at P4. The valence × electrode site interaction was also significant [F(8,384) = 10.25, p < 0.001]. The latencies in response to gain feedback were significantly shorter than those in response to the loss feedback at FCz, FC3, FC4, Cz, C3, and C4 but not at the other electrode sites. The valence × group interaction was not significant [F(1,48) = 2.75, p = 0.10]. Mean FRN latencies of the BD and non-BD groups are presented in Table 4.
Table 4.
Mean FRN latencies (ms) in the non-BD and BD groups.
| Non-BD (n = 23) | BD (n = 27) | |||
|---|---|---|---|---|
| Gain | Loss | Gain | Loss | |
| FC3 | 218.96 (22.23) | 244.17 (22.19) | 228.07 (28.63) | 235.93 (23.10) |
| FCz | 218.70 (23.68) | 244.17 (17.18) | 229.19 (28.19) | 240.07 (19.77) |
| FC4 | 226.61 (20.22) | 236.70 (19.58) | 228.07 (21.99) | 238.30 (8.53) |
| C3 | 216.96 (16.48) | 234.52 (23.45) | 227.85 (24.95) | 228.89 (23.09) |
| Cz | 218.35 (20.64) | 238.09 (17.71) | 225.41 (28.07) | 234.67 (17.59) |
| C4 | 228.96 (21.11) | 235.39 (17.95) | 230.52 (19.55) | 234.15 (17.51) |
| P3 | 230.26 (25.20) | 228.96 (24.55) | 221.85 (18.68) | 218.59 (20.49) |
| Pz | 228.26 (23.96) | 220.43 (17.44) | 224.15 (19.99) | 223.11 (21.48) |
| P4 | 236.17 (21.23) | 231.48 (18.67) | 236.22 (16.14) | 230.96 (15.77) |
() – Standard deviation.
Main effects of valence [F(1,384) = 180.72, p < 0.001] and electrode site [F(8,384) = 35.58, p < 0.001] were observed in the P3 amplitudes. The P3 amplitudes in response to loss feedback were larger than those in response to gain feedback, and the largest and smallest P3 amplitudes were observed at Cz and C3, respectively. A valence × electrode site interaction was also observed [F(8,384) = 84.46, p < 0.001], with the largest difference in P3 amplitudes between the gain and loss feedback at Cz and the smallest at P4. However, the main effect of group [F(1,48) = 0.64, p = 0.43], the interaction effect of valence × group [F(1,48) = 0.15, p = 0.70], and the electrode site × group interaction [F(8,384) = 0.59, p = 0.79] were not significant. The mean P3 amplitudes of the BD and non-BD groups are presented in Table 5.
Table 5.
Mean P3 amplitudes (μV) in the non-BD and BD groups.
| Non-BD (n = 23) | BD (n = 27) | |||
|---|---|---|---|---|
| Gain | Loss | Gain | Loss | |
| FC3 | 5.86 (2.90) | 10.49 (4.87) | 5.44 (2.40) | 10.11 (3.46) |
| FCz | 8.4 (4.49) | 16.65 (7.60) | 6.74 (2.90) | 14.63 (5.00) |
| FC4 | 7.19 (3.47) | 10.92 (5.53) | 6.42 (2.10) | 9.97 (4.16) |
| C3 | 6.02 (2.71) | 10.14 (4.43) | 5.95 (2.25) | 10.05 (3.27) |
| Cz | 9.08 (4.68) | 19.10 (7.69) | 8.18 (3.25) | 17.80 (5.43) |
| C4 | 7.95 (3.42) | 10.75 (4.58) | 7.14 (2.10) | 10.14 (3.58) |
| P3 | 6.80 (3.03) | 10.14 (3.65) | 6.71 (2.22) | 9.98 (3.13) |
| Pz | 8.71 (4.04) | 13.93 (4.22) | 8.96 (2.70) | 12.67 (4.67) |
| P4 | 8.42 (3.43) | 10.62 (4.17) | 7.62 (2.45) | 10.01 (4.04) |
() – Standard deviation.
A main effect of electrode site was observed in ∆P3 [F(8,384) = 73.33, p < 0.001]. The largest ∆P3 amplitude was observed at Cz and the smallest at P4. No main effect of group [F(1,48) = 0.23, p = 0.63] or group × electrode site interaction [F(8,384) = 1.08, p = 0.38] was observed.
Main effects of valence [F(1,48) = 51.89, p < 0.001] and electrode site [F(8,384) = 11.65, p < 0.001] were observed for the P3 latencies. The P3 latencies in response to loss feedback were significantly shorter than those in response to gain feedback (p < 0.001); the shortest latency was observed at Pz and the longest at FC4. An interaction effect of valence × electrode site was also significant [F(8,384) = 7.49, p < 0.001]. P3 latencies elicited by loss feedback were shorter than those by gain feedback at all electrode sites except FCz and Cz. The group × valence interaction was not significant [F(1,48) = 2.93, p = 0.09]. The mean P3 latencies of the BD and non-BD groups are presented in Table 6.
Table 6.
Mean P3 latencies (ms) in the non-BD and BD groups.
| Non-BD (n = 23) | BD (n = 27) | |||
|---|---|---|---|---|
| Gain | Loss | Gain | Loss | |
| FC3 | 336.70 (35.14) | 324.17 (22.20) | 334.89 (28.16) | 315.93 (23.10) |
| FCz | 329.57 (32.75) | 324.17 (17.18) | 328.00 (30.47) | 320.07 (19.77) |
| FC4 | 334.70 (31.24) | 316.70 (19.58) | 342.30 (25.59) | 318.30 (18.53) |
| C3 | 339.39 (35.19) | 314.52 (23.45) | 346.30 (30.74) | 308.89 (23.09) |
| Cz | 317.83 (32.79) | 318.09 (17.71) | 324.30 (36.81) | 314.67 (17.59) |
| C4 | 336.43 (29.55) | 315.39 (17.95) | 346.37 (24.51) | 314.15 (17.51) |
| P3 | 334.26 (33.27) | 308.96 (24.55) | 337.26 (33.62) | 298.59 (20.49) |
| Pz | 311.13 (29.54) | 300.43 (17.44) | 316.37 (35.65) | 303.11 (21.48) |
| P4 | 329.48 (29.67) | 311.48 (18.67) | 348.74 (27.94) | 310.96 (15.77) |
() – Standard deviation.
Correlations Between Performance on the Modified IGT and ∆FRN/∆P3
Positive correlations were observed between ∆FRN amplitudes at FCz and total net scores (r = 0.298, p < 0.05), sector 4 net scores (r = 0.333, p < 0.05) and sector 5 net scores (r = 0.357, p < 0.05) of the IGT. Thus, larger ∆FRN amplitudes at FCz were associated with better IGT performance, especially in the later sectors of the IGT. On the other hand, no significant association was detected between the ∆P3 amplitudes and IGT performance.
Discussion
This study investigated feedback utilization ability for decision-making in BD college students using the modified IGT and ERP data. The BD group exhibited significantly lower total net IGT scores and lower ∆FRN amplitudes than did the non-BD group. Additionally, the ∆FRN amplitude at the fronto-central area was positively correlated with the total net scores, sector 4 net scores and sector 5 net scores on the IGT.
The BD group exhibited significantly lower total net scores than the non-BD group did, and performance of the non-BD group tended to increase as the task progressed (mean sector 1 = −0.03; sector 2 = 0.73; sector 3 = 0.93; sector 4 = 2.23; sector 5 = 1.25), whereas the BD group persistently chose disadvantageous cards over advantageous ones (mean sector 1 = −1.73; sector 2 = −3.04; sector 3 = −1.63; sector 4 = −1.41; sector 5 = −1.93). These results are consistent with those of previous studies (Johnson et al., 2008; Xiao et al., 2009, 2013; Yoo and Kim, 2016) and suggest that individuals with BD have deficits in decision-making. To maximize gains on the IGT, one must choose more advantageous cards that provide small initial gains but result in a net gain over disadvantageous cards that provide a large initial gain but result in a net loss. Johnson et al. (2008) suggested that poor performance on the IGT in individuals with BD reflects their failure to consider consequences, i.e., tendency to pursue immediate rewards, disregarding the larger potential risk.
The statistical analyses of FRN, one of the ERP components elicited by feedback, revealed that the BD group exhibited significantly lower ∆FRN amplitudes than the non-BD group did. The non-BD group exhibited larger FRN amplitudes in response to loss feedback than to gain feedback, whereas the FRN amplitude differences in the BD group between gain and loss feedback were significantly smaller than those in the non-BD group. These results are consistent with previous studies on patients with AUD and male BD college students (Fein and Chang, 2008; Kamarajan et al., 2010; Wahlstrom, 2013). The present study also revealed that both groups exhibited larger FRN amplitudes in response to loss feedback than to gain feedback, which is consistent with many previous studies (Gehring and Willoughby, 2002; Holroyd and Coles, 2002; Goyer et al., 2008; Gu et al., 2011) and suggests that FRN is sensitive to feedback valence. FRN is known to reflect an early evaluation of feedback provided by the environment (Yeung and Sanfey, 2004; Gu et al., 2011; San Martin, 2012). For example, Yeung and Sanfey (2004) suggested that FRN and P3 reflect early and late stages of feedback processing, respectively. Gu et al. (2011) reported that FRN reflects early feedback evaluation based on the salience of the feedback information.
Insensitivity to future consequences (IFC) in patients with AUD and substance use disorder (SUD) has been consistently reported (Bechara and Damasio, 2002; Mallett et al., 2006; Cantrell et al., 2008). For example, Cantrell et al. (2008), using a modified version of the IGT, measured the preference for larger versus smaller rewards (PLvS), the difference between frequencies of choosing the cards that provide large gains and cards with small gains, and IFC, the difference between frequencies of choosing cards that result in a net loss and cards that result in a net gain in patients with AUD. The results showed that although patients with AUD did not exhibit significantly different PLvS scores, they exhibited significantly higher IFC scores than the control group. Additionally, a study of patients with SUD, including AUD, using the IGT and the prospect valence model analysis observed a consistent lack of sensitivity to losses in patients with SUD (Baitz, 2016). Therefore, significantly smaller ∆FRN amplitudes in the BD group compared to the non-BD group observed in the present study suggest that the BD group has deficits in early feedback evaluation and that they are less sensitive to loss feedback than are members of the non-BD group.
In this study, no significant difference in the P3 amplitudes was observed between the BD and non-BD groups, which was not consistent with previous studies reporting reduced P3 amplitudes in patients with AUD (Porjesz et al., 1987; Kamarajan et al., 2010). The generators of P3 are known to be located in the temporo-parietal junction or locus coeruleus-norepinephrine system (Nieuwenhuis et al., 2005). On the other hand, alcohol is known to affect frontal areas of the brain (Curtin and Fairchild, 2003; Nelson et al., 2011). For example, those who consume alcohol exhibit reduced N450 amplitudes in frontal areas, whereas P3 amplitudes in the parietal and occipital areas are not affected by alcohol consumption (Curtin and Fairchild, 2003). Nelson et al. (2011) also reported that alcohol consumption reduces both theta and delta band activities, which are known major components of FRN and P3, respectively, affecting theta band activity more severely. Whereas alcohol consumption affects the frontal area, overall gray and white matter volume reductions, including those in frontal areas, are observed in patients with AUD (Fein et al., 2002; Buhler and Mann, 2011; Le Berre et al., 2014). For example, one study observed reduced whole-brain network cluster coefficients in patients with AUD and reported that longer AUD duration was associated with a global decrease in the efficiency of the brain network (Sjoerds et al., 2017). These results suggest that alcohol consumption affects frontal areas first and then spreads over the whole area as drinking duration increases. Taking together, our results imply that BD of relatively short duration (the mean drinking duration in the BD group was 33.33 months) may affect later feedback evaluation and attentional resource allocation relatively less severely than does BD with a long drinking history.
Both groups exhibited larger P3 amplitudes with loss feedback than with gain feedback. Studies on feedback-related ERPs using tasks other than the IGT have reported larger P3 amplitudes in response to gain feedback than to loss feedback (Toyomaki and Murohashi, 2005; Hajcak et al., 2007; Bellebaum and Daum, 2008; Wu and Zhou, 2009), whereas studies using the IGT observed larger P3 amplitudes in response to loss feedback than to gain feedback (Carlson et al., 2009; Cui et al., 2013). Feedback-related P3 is known to be sensitive to different feedback information, not just to feedback valence but also to feedback magnitude and probabilities as well (Yeung and Sanfey, 2004; Goyer et al., 2008; Hajcak and Simons, 2008; Wu and Zhou, 2009; Polezzi et al., 2010; Gu et al., 2011; Xu et al., 2011). This suggests that P3 reflects feedback processing with a top-down mechanism that allocates attentional resources to the information relevant to the task at hand (Nieuwenhuis et al., 2005; Gu et al., 2011). The loss magnitudes of each card must be understood to maximize profit on the IGT. Thus, participants need to understand that disadvantageous cards (A and B) result in large gains, but losses will soon accumulate over gains, and thus shift their preference or attention progressively toward advantageous cards (C and D) (Webb et al., 2014). These results suggest that both groups allocated their attentional resources to feedback valence, especially to loss feedback, while taking the modified IGT.
Although the importance of feedback utilization for decision-making has been emphasized (Ernst and Paulus, 2005; San Martin, 2012), only one study has investigated the association between IGT performance and feedback-related ERPs (Carlson et al., 2009). Carlson et al. (2009) investigated how children responded to gain/loss feedback using P3 and evaluated how anticipation prior to the response was related to behavioral adjustment using stimulus-preceding negativity (SPN). That study found that the difference in children’s SPN amplitude between advantageous and disadvantageous decks was positively correlated with behavioral adjustment. In the present study, ∆FRN amplitudes at FCz were positively correlated with total net scores and sectors 4 and 5 net scores on the IGT. Thus, larger differences between FRN amplitudes with gain and loss feedback were associated with improved performance on the modified IGT. No previous study has reported an association between FRN and IGT performance; studies using the reversal learning task have reported associations between FRN and behavioral adjustments (Frank et al., 2005; Bellebaum and Daum, 2008). For example, Frank et al. (2005) compared negative learners, who learn stimulus-result contingencies by avoiding negative feedback, with positive learners, who learn these contingencies by pursuing positive feedback; they found significant positive correlations between the tendency to avoid negative feedback and error-related negativity (ERN) amplitudes, which is the ERP component known to share some neural sources with FRN (Holroyd, 2004). To perform successfully on the IGT, participants must learn the contingencies between the cards and their consequences implicitly during the task (e.g., gain or loss feedback) (Bechara, 2004). Therefore, these results suggest that early feedback evaluation in the fronto-central area is associated with the implicit learning process during decision-making.
No significant associations between ∆P3 amplitudes and IGT performance were observed in this study. Previous results for P3 amplitudes and behavioral adjustments using the reversal learning task are inconsistent (Frank et al., 2005; Chase et al., 2011). For example, Frank et al. (2005) reported that FRN amplitudes, not P3 amplitudes, predicted behavioral adjustment, whereas Chase et al. (2011) reported that P3 amplitudes, not FRN amplitudes, predicted behavioral adjustment. The differences between these two studies lay in the task instructions. Frank et al. (2005) did not provide any information regarding contingency shifts during the task and requested that participants make decisions based on their internal judgment, whereas Chase et al. (2011) told the participants that the contingency would shift during the task and requested that participants adjust their responses when they were certain that the contingency had shifted. Thus, the latter study reflected decision-making based more on a set of rules provided prior to the task than on the actual feedback during the task. San Martin (2012) suggested that the importance of FRN and P3 in behavioral adjustment varies depending on which information is more important when performing the given task. In our study, participants were only instructed regarding the goal and process of the task. Therefore, the rules of the task (probability of loss and magnitude of cards) must be learned solely through feedbacks. Such a task design is closer to the study by Frank et al. (2005). These results suggest that both the BD and non-BD groups relied more on early feedback evaluation of valence than on late evaluation with a top-down mechanism as they performed the modified IGT.
This study has several limitations. First, the feedback evaluation investigated here focused mainly on feedback valence. The magnitudes of the cards on the modified IGT increased, as was the case in the original IGT (Bechara, 2007). Although this may keep participants motivated, the increasing magnitude forbids examination of how feedback-related ERPs respond differently to feedback of small or large magnitude. Additionally, the probabilities of encountering losses from cards B and D were too low (10%) to secure enough trials to investigate how ERPs differ based on the probability of losses. However, as this study focused on how BD differs from non-BD in response to feedback during decision-making, the card properties of the original IGT were adopted in order to simulate everyday decision-making environment where various feedback information is combined. Second, this study measured feedback utilization using time-based ERPs. However, the time windows for FRN and P3 are close to each other, and they may distort each other in ERP waveforms. Difference waves were measured to isolate ERP components and prevent such distortion, but other techniques, such as time-frequency analysis (Zhu et al., 2019) or functional connectivity analysis, may reveal more detailed information, such as how different neural waves interact and communicate during feedback processing. Third, only female college students participated in this study to control gender differences. Future studies which include both male and female participants would provide more valuable information on how BD affects feedback utilization and decision-making ability depending on different genders.
In conclusion, the BD group exhibited significantly lower total net scores on the modified IGT and significantly lower ∆FRN amplitudes. On the other hand, no differences were observed in ∆P3 or P3 amplitudes between the groups. Additionally, positive correlations were observed between ∆FRN amplitudes in the fronto-central area and IGT performance. These results imply that the BD group had deficits in decision-making and early feedback evaluation, with a tendency to pursue immediate large gains even at greater potential risks, revealing deficits in early evaluation regarding feedback valence.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Sungshin Women’s University Institutional Review Board (SSWUIRB 2017-040). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
M-SK helped in conceptualization. EN and K-MJ helped in formal analysis. M-SK helped in funding acquisition. EN and K-MJ helped in methodology. M-SK helped in supervision. EN, K-MJ, and M-SK helped in writing, reviewing, and editing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2015S1A5A2A03047656).
References
- American Psychiatric Association (APA) (1994). Diagnostic and statistical manual of mental disorders (DSM-IV). Washington DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (APA) (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington DC: American Psychiatric Association. [Google Scholar]
- Amlung M., Sweet L. H., Acker J., Brown C. L., Mackillop J. (2014). Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addict. Biol. 19, 743–753. 10.1111/adb.12017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitz H. A. (2016). Component processes of decision making in persons with substance use disorders. dissertation. Burnaby, BC: Simon Fraser University
- Barbor T., La Fuente J., Saunders J., Grant M. (1992). AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care. Geneva: World Health Organization. [Google Scholar]
- Bechara A. (2003). Risky business: emotion, decision-making, and addiction. J. Gambl. Stud. 19, 23–51. 10.1023/A:1021223113233, PMID: [DOI] [PubMed] [Google Scholar]
- Bechara A. (2004). The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 55, 30–40. 10.1016/j.bandc.2003.04.001, PMID: [DOI] [PubMed] [Google Scholar]
- Bechara A. (2007). Iowa gambling task professional manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Bechara A., Damasio H. (2002). Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia 40, 1675–1689. 10.1016/S0028-3932(02)00015-5, PMID: [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 10, 295–307. 10.1093/cercor/10.3.295, PMID: [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A. R., Damasio H., Anderson S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15. 10.1016/0010-0277(94)90018-3, PMID: [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A. R., Lee G. P. (1999). Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 19, 5473–5481. 10.1523/JNEUROSCI.19-13-05473.1999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Dolan S., Denburg N., Hindes A., Anderson S. W., Nathan P. E. (2001). Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39, 376–389. 10.1016/S0028-3932(00)00136-6, PMID: [DOI] [PubMed] [Google Scholar]
- Bellebaum C., Daum I. (2008). Learning-related changes in reward expectancy are reflected in the feedback-related negativity. Eur. J. Neurosci. 27, 1823–1835. 10.1111/j.1460-9568.2008.06138.x, PMID: [DOI] [PubMed] [Google Scholar]
- Bellebaum C., Polezzi D., Daum I. (2010). It is less than you expected: the feedback-related negativity reflects violations of reward magnitude expectations. Neuropsychologia 48, 3343–3350. 10.1016/j.neuropsychologia.2010.07.023, PMID: [DOI] [PubMed] [Google Scholar]
- Bolla K. I., Eldreth D. A., Matochik J. A., Cadet J. L. (2004). Sex-related differences in a gambling task and its neurological correlates. Cereb. Cortex 14, 1226–1232. 10.1093/cercor/bhh083, PMID: [DOI] [PubMed] [Google Scholar]
- Buhler M., Mann K. (2011). Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol. Clin. Exp. Res. 35, 1771–1793. 10.1111/j.1530-0277.2011.01540.x, PMID: [DOI] [PubMed] [Google Scholar]
- Bush G., Vogt B. A., Holmes J., Dale A. M., Greve D., Jenike M. A., et al. (2002). Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. USA 99, 523–528. 10.1073/pnas.012470999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S., Peigneux P., Petit G., Lallemand F., Saeremans M., Noel X., et al. (2013). Increased cortical activity in binge drinkers during working memory task: a preliminary assessment through a functional magnetic resonance imaging study. PLoS One 8:e62260. 10.1371/journal.pone.0062260, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell H., Finn P. R., Rickert M. E., Lucas J. (2008). Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcohol. Clin. Exp. Res. 32, 1398–1407. 10.1111/j.1530-0277.2008.00714.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S. M., Zayas V., Guthormsen A. (2009). Neural correlates of decision making on a gambling task. Child Dev. 80, 1076–1096. 10.1111/j.1467-8624.2009.01318.x, PMID: [DOI] [PubMed] [Google Scholar]
- Cha D. (2005). Understanding binge-drinking: a test of the theory of planned behavior. Korean J. Journalism Commun. Stud. 49, 346–390. Retrieved from: http://journal.comm.or.kr/ [Google Scholar]
- Chase H. W., Swainson R., Durham L., Benham L., Cools R. (2011). Feedback-related negativity codes prediction error but not behavioral adjustment during probabilistic reversal learning. J. Cogn. Neurosci. 23, 936–946. 10.1162/jocn.2010.21456, PMID: [DOI] [PubMed] [Google Scholar]
- Chun S. (2002). Analysis of college student binge drinking and alcohol-related problems. J. Korean Alcohol Sci. 3, 221–233. Retrieved from: http://www.alcoholacademy.re.kr/html/sub3_01.html [Google Scholar]
- Chun S., Sohn A., Song C. H., Lee J. Y., Kim S. K. (2003). Health and behavioral consequences of binge drinking in college: a national survey of students at 60 campuses. J. Korean Alcohol Sci. 4, 119–135. Retrieved from: http://www.alcoholacademy.re.kr/html/sub3_01.html [Google Scholar]
- Conigrave K. M., Hall W. D., Saunders J. B. (1995). The AUDIT questionnaire: choosing a cut-off score. Alcohol use disorder identification test. Addiction 90, 1349–1356. 10.1111/j.1360-0443.1995.tb03552.x, PMID: [DOI] [PubMed] [Google Scholar]
- Crego A., Rodriguez-Holguin S., Parada M., Mota N., Corral M., Cadaveira F. (2010). Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend. 109, 45–56. 10.1016/j.drugalcdep.2009.11.020, PMID: [DOI] [PubMed] [Google Scholar]
- Cui J. F., Chen Y. H., Wang Y., Shum D. H., Chan R. C. (2013). Neural correlates of uncertain decision making: ERP evidence from the Iowa gambling task. Front. Hum. Neurosci. 7:776. 10.3389/fnhum.2013.00776, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J. J., Fairchild B. A. (2003). Alcohol and cognitive control: implications for regulation of behavior during response conflict. J. Abnorm. Psychol. 112, 424–436. 10.1037/0021-843X.112.3.424, PMID: [DOI] [PubMed] [Google Scholar]
- Delgado M. R., Nystrom L. E., Fissell C., Noll D. C., Fiez J. A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 84, 3072–3077. 10.1152/jn.2000.84.6.3072, PMID: [DOI] [PubMed] [Google Scholar]
- Dom G., De Wilde B., Hulstijn W., Van Den Brink W., Sabbe B. (2006). Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol. Clin. Exp. Res. 30, 1670–1677. 10.1111/j.1530-0277.2006.00202.x, PMID: [DOI] [PubMed] [Google Scholar]
- Dunn B. D., Dalgleish T., Lawrence A. D. (2006). The somatic marker hypothesis: a critical evaluation. Neurosci. Biobehav. Rev. 30, 239–271. 10.1016/j.neubiorev.2005.07.001, PMID: [DOI] [PubMed] [Google Scholar]
- Elliott R., Dolan R. J., Frith C. D. (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb. Cortex 10, 308–317. 10.1093/cercor/10.3.308, PMID: [DOI] [PubMed] [Google Scholar]
- Ernst M., Bolla K., Mouratidis M., Contoreggi C., Matochik J. A., Kurian V., et al. (2002). Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology 26, 682–691. 10.1016/s0893-133x(01)00414-6, PMID: [DOI] [PubMed] [Google Scholar]
- Ernst M., Paulus M. P. (2005). Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol. Psychiatry 58, 597–604. 10.1016/j.biopsych.2005.06.004, PMID: [DOI] [PubMed] [Google Scholar]
- Fein G., Chang M. (2008). Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 92, 141–148. 10.1016/j.drugalcdep.2007.07.017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G., Di Sclafani V., Cardenas V. A., Goldmann H., Tolou-Shams M., Meyerhoff D. J. (2002). Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol. Clin. Exp. Res. 26, 558–564. 10.1111/j.1530-0277.2002.tb02574.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G., Klein L., Finn P. (2004). Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 28, 1487–1491. 10.1097/01.ALC.0000141642.39065.9B, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. (1995). Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Frank M. J., Woroch B. S., Curran T. (2005). Error-related negativity predicts reinforcement learning and conflict biases. Neuron 47, 495–501. 10.1016/j.neuron.2005.06.020, PMID: [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Willoughby A. R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295, 2279–2282. 10.1126/science.1066893, PMID: [DOI] [PubMed] [Google Scholar]
- Goudriaan A. E., Grekin E. R., Sher K. J. (2007). Decision making and binge drinking: a longitudinal study. Alcohol. Clin. Exp. Res. 31, 928–938. 10.1111/j.1530-0277.2007.00378.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A. E., Oosterlaan J., De Beurs E., Van Den Brink W. (2005). Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res. Cogn. Brain Res. 23, 137–151. 10.1016/j.cogbrainres.2005.01.017, PMID: [DOI] [PubMed] [Google Scholar]
- Goyer J. P., Woldorff M. G., Huettel S. A. (2008). Rapid electrophysiological brain responses are influenced by both valence and magnitude of monetary rewards. J. Cogn. Neurosci. 20, 2058–2069. 10.1162/jocn.2008.20134, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R., Lei Z., Broster L., Wu T., Jiang Y., Luo Y. J. (2011). Beyond valence and magnitude: a flexible evaluative coding system in the brain. Neuropsychologia 49, 3891–3897. 10.1016/j.neuropsychologia.2011.10.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Moser J. S., Holroyd C. B., Simons R. F. (2007). It's worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology 44, 905–912. 10.1111/j.1469-8986.2007.00567.x, PMID: [DOI] [PubMed] [Google Scholar]
- Hajcak G., Simons R. F. (2008). Oops!.. I did it again: an ERP and behavioral study of double-errors. Brain Cogn. 68, 15–21. 10.1016/j.bandc.2008.02.118, PMID: [DOI] [PubMed] [Google Scholar]
- Holroyd C. (2004). A note on the oddball N200 and the feedback ERN. Neurophysiology 78, 447–455. Retrieved from: https://www.apa.org/pubs/journals/neu/ [Google Scholar]
- Holroyd C. B., Coles M. G. H. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 109, 679–709. 10.1037/0033-295X.109.4.679, PMID: [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Krigolson O. E., Baker R., Lee S., Gibson J. (2009). When is an error not a prediction error? An electrophysiological investigation. Cogn. Affect. Behav. Neurosci. 9, 59–70. 10.3758/CABN.9.1.59, PMID: [DOI] [PubMed] [Google Scholar]
- Jennison K. M. (2004). The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am. J. Drug Alcohol Abuse 30, 659–684. 10.1081/ada-200032331, PMID: [DOI] [PubMed] [Google Scholar]
- Johnson C. A., Xiao L., Palmer P., Sun P., Wang Q., Wei Y., et al. (2008). Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia 46, 714–726. 10.1016/j.neuropsychologia.2007.09.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. (1983). The children of alcoholics screening test: Test manual (Chicago, Camelot unlimited). Chicago: Camelot Unlimited. [Google Scholar]
- Kamarajan C., Porjesz B., Rangaswamy M., Tang Y., Chorlian D. B., Padmanabhapillai A., et al. (2009). Brain signatures of monetary loss and gain: outcome-related potentials in a single outcome gambling task. Behav. Brain Res. 197, 62–76. 10.1016/j.bbr.2008.08.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C., Rangaswamy M., Tang Y., Chorlian D. B., Pandey A. K., Roopesh B. N., et al. (2010). Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J. Psychiatr. Res. 44, 576–590. 10.1016/j.jpsychires.2009.11.019, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanny D., Liu Y., Brewer R. D., Lu H. (2013). Binge drinking - United States, 2011. MMWR Suppl. 62, 77–80. Retrieved from: https://www.cdc.gov/mmwr/index.html. PMID: [PubMed] [Google Scholar]
- Kennerley S. W., Walton M. E., Behrens T. E., Buckley M. J., Rushworth M. F. (2006). Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 9, 940–947. 10.1038/nn1724, PMID: [DOI] [PubMed] [Google Scholar]
- Kim M., Chang H., Kim K. (1995). Development of the Korean version of the children of alcoholics screening test (CAST-K): a reliability and validity study. J. Korean Neuropsychiatr. Assoc. 34, 1182–1193. [Google Scholar]
- Kim J. S., Oh M. K., Park B. K., Lee M. K., Kim G. J. (1999). Screening criteria of alcoholism by alcohol use disorders identification test (AUDIT) in Korea. J. Korean Acad. Fam. Med. 20, 1152–1159. [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage 12, 20–27. 10.1006/nimg.2000.0593, PMID: [DOI] [PubMed] [Google Scholar]
- Larson M. J., South M., Clayson P. E. (2011). Sex differences in error-related performance monitoring. Neuroreport 22, 44–48. 10.1097/WNR.0b013e3283427403, PMID: [DOI] [PubMed] [Google Scholar]
- Le Berre A. P., Rauchs G., La Joie R., Mezenge F., Boudehent C., Vabret F., et al. (2014). Impaired decision-making and brain shrinkage in alcoholism. Eur. Psychiatry 29, 125–133. 10.1016/j.eurpsy.2012.10.002, PMID: [DOI] [PubMed] [Google Scholar]
- Lee B., Lee C., Lee P., Choi M., Namkoong K. (2000). Development of Korean version of alcohol use disorders identification test (AUDIT-K): its reliability and validity. J. Korean Acad. Addict. Psychiatry 4, 83–92. Retrieved from: https://www.addictionacademy.org/sub.php?menukey=18. PMID: 18640538 [Google Scholar]
- Lopez-Caneda E., Cadaveira F., Crego A., Gomez-Suarez A., Corral M., Parada M., et al. (2012). Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction 107, 1796–1808. 10.1111/j.1360-0443.2012.03908.x, PMID: [DOI] [PubMed] [Google Scholar]
- Luck S. J. (2014). An introduction to the event-related potential technique. Cambridge, MA: MIT Press. [Google Scholar]
- MacKillop J., Miranda R., Jr., Monti P. M., Ray L. A., Murphy J. G., Rohsenow D. J., et al. (2010). Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J. Abnorm. Psychol. 119, 106–114. 10.1037/a0017513, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallett K. A., Lee C. M., Neighbors C., Larimer M. E., Turrisi R. (2006). Do we learn from our mistakes? An examination of the impact of negative alcohol-related consequences on college students’ drinking patterns and perceptions. J. Stud. Alcohol 67, 269–276. 10.15288/jsa.2006.67.269, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P., Bestelmeyer P. E., Rouger J., Charest I., Belin P. (2013). Binge drinking influences the cerebral processing of vocal affective bursts in young adults. Neuroimage Clin. 3, 218–225. 10.1016/j.nicl.2013.08.010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazas C. A., Finn P. R., Steinmetz J. E. (2000). Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol. Clin. Exp. Res. 24, 1036–1040. 10.1111/j.1530-0277.2000.tb04647.x, PMID: [DOI] [PubMed] [Google Scholar]
- McClure S. M., Berns G. S., Montague P. R. (2003). Temporal prediction errors in a passive learning task activate human striatum. Neuron 38, 339–346. 10.1016/S0896-6273(03)00154-5, PMID: [DOI] [PubMed] [Google Scholar]
- Mehrabian A., Russell J. A. (1978). A questionnaire measure of habitual alcohol use. J. Psychol. Rep. 43, 803–806. PMID: [DOI] [PubMed] [Google Scholar]
- Mitchell J. M., Fields H. L., D'esposito M., Boettiger C. A. (2005). Impulsive responding in alcoholics. Alcohol. Clin. Exp. Res. 29, 2158–2169. 10.1097/01.alc.0000191755.63639.4a, PMID: [DOI] [PubMed] [Google Scholar]
- Moreno M., Estevez A. F., Zaldivar F., Montes J. M., Gutierrez-Ferre V. E., Esteban L., et al. (2012). Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol Depend. 124, 355–362. 10.1016/j.drugalcdep.2012.02.011, PMID: [DOI] [PubMed] [Google Scholar]
- Mota N., Parada M., Crego A., Doallo S., Caamano-Isorna F., Rodriguez Holguin S., et al. (2013). Binge drinking trajectory and neuropsychological functioning among university students: a longitudinal study. Drug Alcohol Depend. 133, 108–114. 10.1016/j.drugalcdep.2013.05.024, PMID: [DOI] [PubMed] [Google Scholar]
- Naimi T. S., Brewer R. D., Mokdad A., Denny C., Serdula M. K., Marks J. S. (2003). Binge drinking among US adults. JAMA 289, 70–75. 10.1001/jama.289.1.70, PMID: [DOI] [PubMed] [Google Scholar]
- Nelson L. D., Patrick C. J., Collins P., Lang A. R., Bernat E. M. (2011). Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology 218, 419–428. 10.1007/s00213-011-2323-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S., Aston-Jones G., Cohen J. D. (2005). Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol. Bull. 131, 510–532. 10.1037/0033-2909.131.4.510, PMID: [DOI] [PubMed] [Google Scholar]
- Noel X., Bechara A., Dan B., Hanak C., Verbanck P. (2007). Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology 21, 778–786. 10.1037/0894-4105.21.6.778, PMID: [DOI] [PubMed] [Google Scholar]
- O’Doherty J. P., Dayan P., Friston K., Critchley H., Dolan R. J. (2003). Temporal difference models and reward-related learning in the human brain. Neuron 38, 329–337. 10.1016/S0896-6273(03)00169-7, PMID: [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Kringelbach M. L., Rolls E. T., Hornak J., Andrews C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 4, 95–102. 10.1038/82959, PMID: [DOI] [PubMed] [Google Scholar]
- O’Malley P. M., Johnston L. D. (2002). Epidemiology of alcohol and other drug use among American college students. J. Stud. Alcohol Suppl. 14, 23–39. 10.15288/jsas.2002.s14.23, PMID: [DOI] [PubMed] [Google Scholar]
- O’Neill S. E., Parra G. R., Sher K. J. (2001). Clinical relevance of heavy drinking during the college years: cross-sectional and prospective perspectives. Psychol. Addict. Behav. 15, 350–359. 10.1037/0893-164X.15.4.350, PMID: [DOI] [PubMed] [Google Scholar]
- Pagnoni G., Zink C. F., Montague P. R., Berns G. S. (2002). Activity in human ventral striatum locked to errors of reward prediction. Nat. Neurosci. 5, 97–98. 10.1038/nn802, PMID: [DOI] [PubMed] [Google Scholar]
- Parada M., Corral M., Mota N., Crego A., Rodriguez Holguin S., Cadaveira F. (2012). Executive functioning and alcohol binge drinking in university students. Addict. Behav. 37, 167–172. 10.1016/j.addbeh.2011.09.015, PMID: [DOI] [PubMed] [Google Scholar]
- Park S., Kim M. S. (2018). An event-related potential study of spatial working memory in binge drinking college students. PLoS One 13:e0203696. 10.1371/journal.pone.0203696, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. H., Stanford M. S., Barratt E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. , PMID: [DOI] [PubMed] [Google Scholar]
- Polezzi D., Sartori G., Rumiati R., Vidotto G., Daum I. (2010). Brain correlates of risky decision-making. NeuroImage 49, 1886–1894. 10.1016/j.neuroimage.2009.08.068, PMID: [DOI] [PubMed] [Google Scholar]
- Porjesz B., Begleiter H., Bihari B., Kissin B. (1987). Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol 4, 283–287. 10.1016/0741-8329(87)90024-3, PMID: [DOI] [PubMed] [Google Scholar]
- Rogers R. D., Ramnani N., Mackay C., Wilson J. L., Jezzard P., Carter C. S., et al. (2004). Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry 55, 594–602. 10.1016/j.biopsych.2003.11.012, PMID: [DOI] [PubMed] [Google Scholar]
- San Martin R. (2012). Event-related potential studies of outcome processing and feedback-guided learning. Front. Hum. Neurosci. 6:304. 10.3389/fnhum.2012.00304, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann B., Kathmann N., Stiglmayr C., Renneberg B., Endrass T. (2011). Impaired decision making and feedback evaluation in borderline personality disorder. Psychol. Med. 41, 1917–1927. 10.1017/S003329171000262X, PMID: [DOI] [PubMed] [Google Scholar]
- Sjoerds Z., Stufflebeam S. M., Veltman D. J., Van Den Brink W., Penninx B. W., Douw L. (2017). Loss of brain graph network efficiency in alcohol dependence. Addict. Biol. 22, 523–534. 10.1111/adb.12346, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speilberger C., Gorsuch R. L., Lushene R., Vagg P., Jacobs G. (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists. [Google Scholar]
- Stephens D. N., Duka T. (2008). Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 363, 3169–3179. 10.1098/rstb.2008.0097, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler P. N., Fiorillo C. D., Schultz W. (2005). Adaptive coding of reward value by dopamine neurons. Science 307, 1642–1645. 10.1126/science.1105370, PMID: [DOI] [PubMed] [Google Scholar]
- Townshend J. M., Duka T. (2002). Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures. Alcohol Alcohol. 37, 187–192. 10.1093/alcalc/37.2.187, PMID: [DOI] [PubMed] [Google Scholar]
- Toyomaki A., Murohashi H. (2005). The ERPs to feedback indicating monetary loss and gain on the game of modified “rock–paper–scissors”. Int. Congr. Ser. 1278, 381–384. 10.1016/j.ics.2004.11.032 [DOI] [Google Scholar]
- Tucker J. S., Orlando M., Ellickson P. L. (2003). Patterns and correlates of binge drinking trajectories from early adolescence to young adulthood. Health Psychol. 22, 79–87. 10.1037/0278-6133.22.1.79, PMID: [DOI] [PubMed] [Google Scholar]
- Wahlstrom L.C. (2013). Feedback-related negativity, decision-making, and college binge drinking. dissertation. Lincoln, NE: The University of Nebraska-Lincoln.
- Wallis J. D. (2007). Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 30, 31–56. 10.1146/annurev.neuro.30.051606.094334, PMID: [DOI] [PubMed] [Google Scholar]
- Walsh M. M., Anderson J. R. (2011). Modulation of the feedback-related negativity by instruction and experience. Proc. Natl. Acad. Sci. USA 108, 19048–19053. 10.1073/pnas.1117189108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. A., Deldonno S., Killgore W. D. (2014). The role of cognitive versus emotional intelligence in Iowa gambling task performance: what’s emotion got to do with it? Intelligence 44, 112–119. 10.1016/j.intell.2014.03.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H., Lee J. E., Kuo M., Seibring M., Nelson T. F., Lee H. (2002). Trends in college binge drinking during a period of increased prevention efforts. Findings from 4 Harvard School of Public Health College alcohol study surveys: 1993-2001. J. Am. Coll. Heal. 50, 203–217. 10.1080/07448480209595713, PMID: [DOI] [PubMed] [Google Scholar]
- Wechsler H., Nelson T. F. (2001). Binge drinking and the American college student: what’s five drinks? Psychol. Addict. Behav. 15, 287–291. 10.1037/0893-164X.15.4.287, PMID: [DOI] [PubMed] [Google Scholar]
- Weitzman E. R., Nelson T. F., Wechsler H. (2003). Taking up binge drinking in college: the influences of person, social group, and environment. J. Adolesc. Health 32, 26–35. 10.1016/S1054-139X(02)00457-3, PMID: [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou X. (2009). The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Res. 1286, 114–122. 10.1016/j.brainres.2009.06.032, PMID: [DOI] [PubMed] [Google Scholar]
- Xiao L., Bechara A., Gong Q., Huang X., Li X., Xue G., et al. (2013). Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict. Behav. 27, 443–454. 10.1037/a0027892, PMID: [DOI] [PubMed] [Google Scholar]
- Xiao L., Bechara A., Grenard L. J., Stacy W. A., Palmer P., Wei Y., et al. (2009). Affective decision-making predictive of Chinese adolescent drinking behaviors. J. Int. Neuropsychol. Soc. 15, 547–557. 10.1017/S1355617709090808, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Shen Q., Chen P., Ma Q., Sun D., Pan Y. (2011). How an uncertain cue modulates subsequent monetary outcome evaluation: an ERP study. Neurosci. Lett. 505, 200–204. 10.1016/j.neulet.2011.10.024, PMID: [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A. G. (2004). Independent coding of reward magnitude and valence in the human brain. J. Neurosci. 24, 6258–6264. 10.1523/JNEUROSCI.4537-03.2004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J. Y., Kim M. S. (2016). Deficits in decision-making and reversal learning in college students who participate in binge drinking. J. Neuropsychiatr. 6, 321–330. 10.4172/neuropsychiatry.1000156 [DOI] [Google Scholar]
- Zhu R., Wu H., Xu Z., Tang H., Shen X., Mai X., et al. (2019). Early distinction between shame and guilt processing in an interpersonal context. Soc. Neurosci. 14, 53–66. 10.1080/17470919.2017.1391119, PMID: [DOI] [PubMed] [Google Scholar]
- Zung W. W., Richards C. B., Short M. J. (1965). Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch. Gen. Psychiatry 13, 508–515. 10.1001/archpsyc.1965.01730060026004, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.


