Abstract
Background
Nail clipping histology is more sensitive than mycology for detecting nail fungi. However, in the absence of fungi, established diagnostic clues for this and other nail dystrophy causes are lacking, namely for nail psoriasis. Neutrophils have been reported in onychomycosis and nail psoriasis samples but have been insufficiently characterized.
Objectives
The aim of this paper is to differentiate neutrophil-containing nail clipping samples in nail psoriasis and onychomycosis regarding histology.
Methods
We performed a 3-year retrospective review of all nail clipping test results included in our department's database and re-analyzed samples containing neutrophils.
Results
In total,112 neutrophil-containing nail clipping samples were accounted. Onychomycosis was the commonest diagnosis (74.1%), followed by nail psoriasis (18.8%). Onychomycosis samples had more abundant neutrophils, more often arranged in collections (60.2%) (p = 0.002) and with smaller quantities of parakeratosis, in a lamellar distribution. In nail psoriasis, neutrophils were mostly aggregated (57.1%) with parakeratosis in all samples, in increased amounts, and showing no predominant pattern.
Conclusions
Neutrophils are present in both nail psoriasis and onychomycosis, warranting careful interpretation. However, less dense aggregates of neutrophils with more abundant parakeratosis are clues of nail psoriasis when all other fungal tests are negative.
Keywords: Nail clipping, Neutrophils, Histology, Psoriasis, Onychomycosis
Introduction
The differential diagnosis between onychomycosis and noninfectious onychodystrophy causes is often difficult on clinical grounds only [1, 2, 3]. Nail clipping is simple, painless, inexpensive, and noninvasive [4]. The histological examination of fragments subsequently obtained is useful in the diagnosis of onychomycosis and may help in other onychopathies, such as nail psoriasis.
Histological features of nail psoriasis are: nail bed intraepithelial neutrophils and in adherent parakeratotic fragments of the nail plate; hyperkeratosis with parakeratosis and serum-like proteinaceous exudates; focal hypogranulosis; and psoriasiform hyperplasia of the nail bed with dilated subepithelial blood vessels [5]. The first three of these features may be examined on clipping fragments.
Concerning onychomycosis, clipping histology with the Periodic Acid-Schiff (PAS) stain allows observation of fungi and the degree of invasion [6]. It has a higher sensitivity for the diagnosis of onychomycosis (87.8%) than for culture (46.3%) and direct microscopy (67.5%) [7], although it does not identify fungal species [7]. In onychomycosis, the histological pattern is often psoriasiform [6], with subungual hyperkeratosis, parakeratosis, neutrophils, and serum crusts [1, 8].
Similar histological features may be observed in nail clippings of nail psoriasis and onychomycosis. Observation of fungi may settle the difference, but psoriasis has been considered a risk factor for onychomycosis and both may coexist [3, 9]. In 1980, Sher and Ackerman [10] postulated that nail psoriasis and onychomycosis could be differentiated by examining the cornified cells of the nail bed. They proposed that psoriasis showed characteristic mounds of parakeratosis with neutrophils at their summits, interspersed foci of orthokeratosis, and no fungal elements. This has not been studied in detail so far. Few studies report the frequency of neutrophils or their arrangement in nail clippings. We aimed to characterize the presence of neutrophils and parakeratosis arrangement in both conditions.
Materials and Methods
We routinely perform nail clipping histology, as well as mycological examination (direct microscopy of a 40% KOH preparation and culture). A dual-action nipper is used to collect nail fragments of at least 5 mm longitudinally and 2 mm transversely and its adherent subungual hyperkeratosis. The fragment is processed using a softening solution of sodium hydroxide (10%), fixated in paraffin, and stained with hematoxylin and eosin (H&E) and PAS. Test results are inserted in a database together with the patient's age and sex, anatomical location, mycological results, and final diagnosis. The final diagnosis resulted from the correlation of clinical information and histological and mycological test findings. The presence of neutrophils in nail clipping has also been recorded for the past 3 years.
In a first stage, we retrospectively reviewed all nail clipping database records from the past 3 years. Second, there was a diagnosis-blinded histological re-observation of all neutrophil-containing clippings. Samples were analyzed focusing on the presence of neutrophils, parakeratosis, serous globules, fungi, bacteria, and hemorrhage (Table 1, Fig. 1 and 2). Neutrophils were described according to their pattern (scattered, aggregated, or in collections) and location (near globules, in/over parakeratosis, or in orthokeratosis). Parakeratosis was classified regarding presence (yes/no), pattern (scattered, lamellar, columnar, or confluent), quantity (in three levels), and in relation to serous globules (if present).
Table 1.
Classification of neutrophils and parakeratosis arrangement
| Neutrophil arrangement | |
| Scattered | Sparse and isolated neutrophils |
| Aggregated | Small group of neutrophils between corneocytes |
| Collections | Densely grouped neutrophils |
| Parakeratosis arrangement | |
| Scattered | Sparse parakeratotic cells |
| Lamellar | Multiple layers of parakeratosis disposed adjacently in a small area |
| Columnar | Multiple layers of parakeratosis vertically disposed |
| Confluent | Extensive parakeratosis, insignificant areas of orthokeratosis |
| Parakeratosis quantity | |
| + | Lengthy search is needed to detect parakeratosis |
| ++ | Easily detected but requiring classification in high-power view |
| +++ | Easily observable and classifiable in low-power view (×40) |
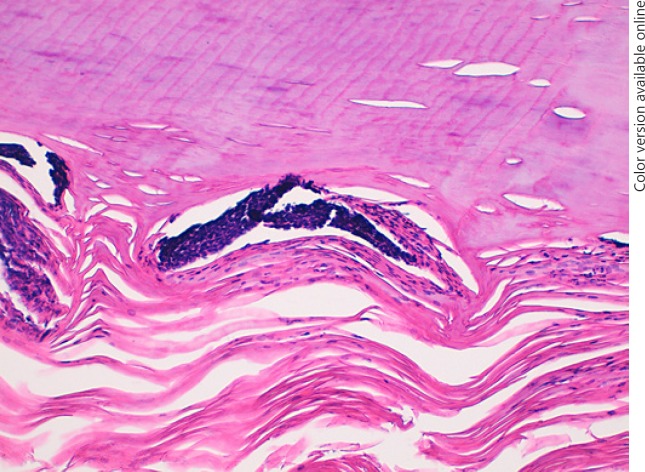
Fig. 1.
Histology of a nail clipping sample of nail psoriasis. Confluent parakeratosis, an aggregate of neutrophils (blue arrow), a serous lake (black arrow), and hemorrhage are observable (black star). H&E. ×100.
Fig. 2.
Histology of a nail clipping sample of onychomycosis. A dense collection of neutrophils over parakeratosis. H&E. ×200.
Analysis was performed using the software IBM SPSS Version 22 and Microsoft Excel 2007. Fischer's exact test was used to test the differences between 2 categorical variables at a significance level of 0.05, two-tailed.
Results
The 3-year database review identified 715 nail clippings, 112 (15.7%) of which contained neutrophils. Global clinical information of the studied group is presented in Table 2. The final diagnosis and presence of neutrophils are given in Table 3. In neutrophil-positive nail clippings, onychomycosis was the commonest diagnosis (n = 83; 74.1%), followed by nail psoriasis (n = 21; 18.8%). Only 22% of the onychomycosis diagnosed had neutrophil-containing clippings. All samples diagnosed as nail psoriasis had neutrophils.
Table 2.
Epidemiological data and anatomical localization of nail clippings
| With neutrophils (n = 112) | Without neutrophils (n = 603) | Total clippings (<I>N</I> = 715) | |
|---|---|---|---|
| Age, years | 60.2±18.8 | 59.4±19.1 | 59.6±19.1 |
| Female | 59 (52.7%) | 320 (53.1%) | 379 (53%) |
| Localization | |||
| Toenail | 88 (78.6%) | 503 (83.4%) | 591 (82.7%) |
| Fingernail | 15 (13.4%) | 80 (13.3%) | 95 (13.3%) |
| Non-specified | 9 (8%) | 20 (3.3%) | 29 (4.1%) |
Table 3.
Frequency of neutrophils in nail clippings according to the final diagnosis
| Diagnosis | With neutrophils | Without neutrophils | Total nail clippings | Frequency of neutrophils within diagnosis, % |
|---|---|---|---|---|
| No observable changes | 0 (0.0) | 47 (7.8) | 47 (6.6) | 0.0 |
| Unspecific changes | 0 (0.0) | 98 (16.2) | 98 (13.7) | 0.0 |
| OD | 5 (4.4) | 168 (27.9) | 173 (24.2) | 2.9 |
| OM | 83 (74.1) | 289 (47.9) | 372 (52.0) | 22.3 |
| NP | 21 (18.8) | 0 (0.0) | 21 (2.9) | 100.0 |
| Onychopapilloma | 0 (0.0) | 1 (0.2) | 1 (0.1) | 0.0 |
| Pionychia | 1 (0.9) | 0 (0.0) | 1 (0.1) | 100.0 |
| Lichen planus | 2 (1.8) | 0 (0.0) | 2 (0.3) | 100.0 |
| Total | 603 (100) | 112 (100) | 715 (100) | 15.7 |
Values are presented as n (%), unless otherwise indicated. OM, onychomycosis; OD, onychodystrophy; NP, nail psoriasis.
The histological features of neutrophil-containing nail clippings are presented in Table 4. In onychomycosis, neutrophils were more frequently in collections (60.2%) (p = 0.002). Parakeratosis was present in 89.2% of such samples, often in a small quantity (56.8%) and predominantly with a lamellar configuration (64.9%). Neutrophils in nail psoriasis were mostly aggregated (57.1%), and parakeratosis was found in all samples, in an increased amount when compared to onychomycosis (81%: ++/+++). These findings were statistically significant (p < 0.05).
Table 4.
Histological variables of patients with NP and OM
| OM (n = 83) | NP (n = 21) | p | |
|---|---|---|---|
| Neutrophil arrangement | |||
| Scattered | 16 (19.3) | 4 (19.0) | 0.002 |
| Aggregated (± scattered) | 17 (20.5) | 12 (57.1) | |
| Collections (± scattered ± aggregated) | 50 (60.2) | 5 (23.8) | |
| Location | |||
| Exclusively near serous lakes | 10 (12) | 2 (9.5) | 0.072 |
| In parakeratosis | 53 (63.9) | 19 (90.5) | |
| In orthokeratosis | 13 (15.7) | 0 | |
| In parakeratosis and orthokeratosis | 7 (8.4) | 0 | |
| Parakeratosis | |||
| Yes | 74 (89.2) | 21 (100) | 0.198 |
| Pattern | |||
| Scattered | 8 (10.8) | 1 (4.8) | 0.01 |
| Lamellar (± scattered) | 48 (64.9) | 8 (38.1) | |
| Columnar (± lamellar) | 11 (14.9) | 6 (28.6) | |
| Confluent (± lamellar ± columnar) | 7 (9.5) | 6 (28.6) | |
| Quantity | |||
| + | 42 (56.8) | 4 (19) | 0.03 |
| ++ | 20 (27) | 8 (38.1) | |
| +++ | 12 (16.2) | 9 (42.9) | |
| Serous lakes | |||
| Yes | 73 (88) | 18 (85.7) | 0.723 |
| Periglobular parakeratosis | 38 (52.1) | 7 (38.9) | 0.431 |
| Neutrophils next to serous lakes | 42 (57.5) | 14 (77.8) | 0.226 |
Values are presented as percentages. OM, onychomycosis; NP, nail psoriasis.
Discussion
Neutrophils were more frequent in nail clippings of nail psoriasis (100%) than in onychomycosis (22.3%). When present in onychomycosis, they were significantly more abundant than in nail psoriasis (p = 0.002). Parakeratosis was associated with both but more significant in nail psoriasis (p = 0.03). Furthermore, if neutrophils were densely organized in vast collections with less associated parakeratosis, it appeared more likely that the sample was of onychomycosis. Differently from what was described by Sher [10], we often saw mounds of parakeratosis with neutrophil-rich summits in onychomycosis samples (however, coexisting nail psoriasis could not confidently be excluded). There is little data about the presence or arrangement of neutrophils and parakeratosis in literature.
Werner and Antunes [11] studied nail clippings of normal nails and found no neutrophils in any of the 30 samples. Parakeratosis was variably present (86%), but the median number of parakeratotic layers was lower in normal nails versus nail psoriasis [12]. The presence of parakeratosis in nail psoriasis samples varies from 78–100% [13, 14], with extensive parakeratosis found in 87.5% in the study by Garbers et al. [15].
An increase of neutrophils in nail histology from nail psoriasis has been reported. In three studies of nail biopsies from patients with nail psoriasis, neutrophils were found in 63–75% of the cases [5, 13, 14]. In nail clippings of psoriatic patients with any degree of onychodystrophy, one study reported neutrophils in 12% (vs. 2% of patients with psoriasis without onychodystrophy) [12]. In a pediatric population with psoriasis, neutrophils were found in 7.6% [16]. When compared to our results, the frequency of neutrophils seems extremely low. However, these studies were performed in populations with psoriasis but not necessarily with evident nail psoriasis. Our population collected nail clippings for histology due to nail dystrophy requiring a differential diagnosis with onychomycosis. In case of nail psoriasis, this probably selected cases with subungual hyperkeratosis or total onychodystrophy. This bias, together with the histological features for nail psoriasis diagnosis, probably resulted in the presence of neutrophils in all of our nail psoriasis samples.
Concerning onychomycosis, a higher percentage of parakeratosis (63 vs. 40%) and neutrophils (11 vs. 8%) was found in nails positive for fungal elements [17].
Limitations
This was a retrospective study based on the nail clippings performed within the daily activity of our department. Sampling errors must be taken in account. The small number of cases of nail psoriasis is also an important limitation to withdraw significant results and conclusions. In addition, there may be psoriasis patients in the onychomycosis group.
Conclusions
Histological differences between nail psoriasis and onychomycosis in nail clipping are subtle, and their interpretation must be cautious. Neutrophils seem to be more frequent in nail psoriasis than in onychomycosis. However, when present in onychomycosis, they are more densely aggregated. Parakeratosis is associated with both diseases but more evident in nail psoriasis. Classically described mounds of parakeratosis with neutrophils at their summits were also observed in onychomycosis, so this is not distinctive of nail psoriasis. If neutrophils are present in nail clipping histology, then the PAS should be carefully screened for fungi. More studies of nail clipping histopathology are needed to enlarge the amount of evidence to establish firmer diagnostic correlations.
Statement of Ethics
The authors have no ethical conflicts to disclose. The study protocol followed our institute's guidelines and was approved by the ethics committee on human research. The authors declare that no patient data appear in this article.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding has been received for this work.
Author Contributions
All authors provided substantial contributions to the design of the work and to the analysis and interpretation of the data collected. All authors were involved in drafting and reviewing the manuscript. All authors approved the final version of the manuscript. All authors are accountable for all aspects of the work.
References
- 1.Arrese JE, Piérard-Franchimont C, Piérard GE. Facing up to the diagnostic uncertainty and management of onychomycoses. Int J Dermatol. 1999 Sep;38((S2 Suppl 2)):1–6. doi: 10.1046/j.1365-4362.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Bertanha L, Chiacchio ND. Nail clipping in onychomycosis. An Bras Dermatol. 2016 Sep-Oct;91((5)):688–90. doi: 10.1590/abd1806-4841.20164968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen S, Tosti A, Rubin AI. Diagnostic applications of nail clippings. Dermatol Clin. 2015 Apr;33((2)):289–301. doi: 10.1016/j.det.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Werner B, Fonseca GP, Seidel G. Microscopic nail clipping findings in patients with psoriasis. Am J Dermatopathol. 2015 Jun;37((6)):429–39. doi: 10.1097/DAD.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 5.Hanno R, Mathes BM, Krull EA. Longitudinal nail biopsy in evaluation of acquired nail dystrophies. J Am Acad Dermatol. 1986 May;14((5 Pt 1)):803–9. doi: 10.1016/s0190-9622(86)70097-2. [DOI] [PubMed] [Google Scholar]
- 6.André J, Sass U, Richert B, Theunis A. Nail pathology. Clin Dermatol. 2013 Sep-Oct;31((5)):526–39. doi: 10.1016/j.clindermatol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Cunha N, Lencastre A, João A. Comunicacao SPDV Neutrophils Clipping final n.d. [Google Scholar]
- 8.Lavorato FG, Guimarães DA, Premazzi MG, Bernardes-engemann JMPAR, Orofino-costa R. Performance of mycology and histopathology tests for the diagnosis of toenail onychomycosis due to filamentous fungi: Dermatophyte and non- dermatophyte moulds. 2017:1–7. doi: 10.1111/myc.12633. doi: [DOI] [PubMed] [Google Scholar]
- 9.Brasileiro A, Galhardar C, Fidalgo A, Apetato M. Onychomycosis in psoriatic patients - an underestimate finding? Rev Da Soc Port Dermatologia e Venereol. 2015;73:219–25. [Google Scholar]
- 10.Scher RK, Ackerman AB. Subtle clues to diagnosis from biopsies of nails. Histologic differential diagnosis of onychomycosis and psoriasis of the nail unit from cornified cells of the nail bed alone. Am J Dermatopathol. 1980;2((3)):255–6. doi: 10.1097/00000372-198000230-00014. [DOI] [PubMed] [Google Scholar]
- 11.Werner B, Antunes A. Microscopic examination of normal nail clippings. Dermatol Pract Concept. 2013 Jul;3((3)):9–14. doi: 10.5826/dpc.0303a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca GP, Werner B, Seidel G, Staub HL. Comparative microscopic analysis of nail clippings from patients with cutaneous psoriasis and psoriatic arthritis. An Bras Dermatol. 2017 Jan-Feb;92((1)):21–5. doi: 10.1590/abd1806-4841.20175056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover C, Reddy BS, Uma Chaturvedi K. Diagnosis of nail psoriasis: importance of biopsy and histopathology. Br J Dermatol. 2005 Dec;153((6)):1153–8. doi: 10.1111/j.1365-2133.2005.06862.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaul S, Singal A, Grover C, Sharma S. Clinical and histological spectrum of nail psoriasis: A cross-sectional study. J Cutan Pathol. 2018 Nov;45((11)):824–30. doi: 10.1111/cup.13334. [DOI] [PubMed] [Google Scholar]
- 15.Garbers LE, Slongo H, Fabricio LH, Schmitt JV, Bonalumi A. Incidence, clinical manifestations and clipping of nail psoriasis in the dermatology center of the Hospital Universitário Evangélico de Curitiba. An Bras Dermatol. 2016 May-Jun;91((3)):300–5. doi: 10.1590/abd1806-4841.20164296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uber M, Carvalho VO, Abagge KT, Robl Imoto R, Werner B. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018 Mar;35((2)):202–7. doi: 10.1111/pde.13402. [DOI] [PubMed] [Google Scholar]
- 17.Mayer E, Izhak OB, Bergman R. Histopathological periodic acid-schiff stains of nail clippings as a second-line diagnostic tool in onychomycosis. Am J Dermatopathol. 2012 May;34((3)):270–3. doi: 10.1097/DAD.0b013e318234cc49. [DOI] [PubMed] [Google Scholar]