Abstract
Organizers of medical educational courses are often confronted with questions that are clinically relevant yet trespassing the frontiers of scientifically proven, evidence-based medicine at the point of care. Therefore, since 2007 organizers of breast teaching courses in German language met biannually to find a consensus in clinically relevant questions that have not been definitely answered by science. The questions were prepared during the 3 months before the meeting according to a structured process and finally agreed upon the day before the consensus meeting. At the consensus meeting, the open questions concerning 2D/3D mammography, breast ultrasound, MR mammography, interventions as well as risk-based imaging of the breast were presented first for electronic anonymized voting, and then the results of the audience were separately displayed from the expert votes. Thereafter, an introductory statement of the moderator was followed by pros/cons of two experts, and subsequently the final voting was performed. With ≥75% of votes of the expert panel, an answer qualified as a consensus statement. Seventeen consensus statements were gained, addressing for instance the use of 2D/3D mammography, breast ultrasound in screening, MR mammography in women with intermediate breast cancer risk, markers for localization of pathologic axillary lymph nodes, and standards in risk-based imaging of the breast. After the evaluation, comments from the experts on each field were gathered supplementarily. Methodology, transparency, and soundness of statements achieve a unique yield for all course organizers and provide solid pathways for decision making in breast imaging.
Keywords: Breast intervention, Imaging, Magnetic resonance imaging, Tomosynthesis, Tumor localization, Ultrasound
Introduction
The first German consensus meeting in breast imaging took place in Frankfurt/Main in 2007, with the main topic being microcalcifications [1]. It was organized by the German Roentgen Society and accredited by the German Society of Senology. The meeting addressed questions that could not be answered by scientific evidence but were still of clinical importance. Similar questions were also asked by the participants of breast imaging courses that were held by invited speakers as experts in their particular field. As the exact wording of the questions is crucial for the success of consensus meetings, the invited expert panel spent much effort on formulating the questions before the meeting. At the consensus meeting, these questions were answered by all participants of the meeting after an introductory presentation using an electronic voting system (the answers of the expert panel and the audience were displayed separately and evaluated). Since then, the consensus meetings were held biannually, and expert opinions were asked for on different subjects of breast imaging [2, 3, 4, 5].
Since 2017, after the 5th successful consensus meeting, the voting was done before and after pro and con presentations related to a selected issue. The answers after the presentations were used for the consensus evaluation [6]. In 2019, tomosynthesis, ultrasound, and MR mammography as adjunctive modalities for screening and assessment, axillary interventions, and risk-based imaging were the main topics of the meeting.
Data Acquisition
The expert panel of the consensus meeting 2019 consisted of 16 experienced organizers of breast imaging courses. To differentiate the opinions and experiences of panel and audience, the electronic votes were documented for panel (P) and audience (A) separately. All panelists (n = 16) as well as 75% of the audience (total: n = 189) had more than 10 years of experience in breast imaging.
Eighty-six percent of all participants were radiologists. Three of the 16 panel experts were gynecologists. Less than 20% of the participants had their workplace within a range of 50 km to the meeting venue. Half of the participants predominantly worked outside screening institutions (P: 50%; A: 53%), 14% in screening institutions (P = A) and one-third in similar proportions in- and outside screening institutions (P: 36%; A: 31%). All of the panelists use mammography, ultrasound, and MRI in their daily work (A: 51%).
The panel experts prepared the questions for electronic voting within their expert groups within 3 months before the meeting. The day before the consensus meeting, all experts worked finally on the questions to be presented during the meeting at the following day. Criteria for the appropriateness of a question were the clinical relevance, the insufficient scientific evidence, and the consensus among the panelists that the question is relevant for patient care. The questions concerning the particular chapter were asked before and after the presentations (short introduction by the moderator; pro and con presentations).
Only the voting results after the presentations were considered as “consensus vote,” as the more valid answers are to be expected after discussion of the issues and repetition of the questions. Only those answers were considered to represent a consensus opinion that received 75% or more of the votes. Changes between rounds 1 and 2 are reported only in case of significant changes.
2D/3D Mammography
The first part of the meeting dealt with mammography and tomosynthesis (DBT). Of all panel members, 92% have sound experience in tomosynthesis (A: 73%).
With consensus majority of the panel votes (≥75%), the following questions were answered:
Adequate quality control provided, primary tomosynthesis with synthetic 2D visualization is superior to full-field digital mammography for 93% of the panel experts in asymptomatic women (A: 72%; all participants before/after presentations: 57/74%).
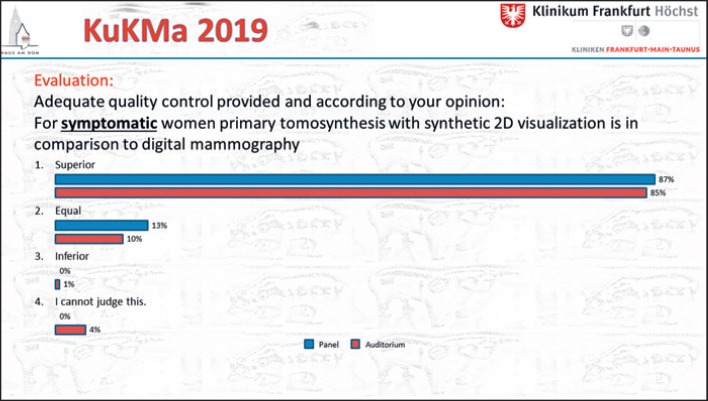
Adequate quality control provided, primary tomosynthesis with synthetic 2D visualization is superior to full-field digital mammography for 87% of the panel experts in symptomatic women (A: 85%; all participants before/after presentations: 76/85%; Fig. 1).
Adequate quality control provided, primary tomosynthesis with synthetic 2D visualization is superior to full-field digital mammography for 100% of the panel experts in women with mass lesions (A: 92%; all participants before/after presentations: 82/92%).
Adequate quality control provided, primary tomosynthesis with synthetic 2D visualization is superior to full-field digital mammography for 100% of the panel experts in women with architectural distortions (A: 96%; all participants before/after presentations: 90/96%).
Fig. 1.
Voting results for primary tomosynthesis in symptomatic women. Panel: n = 16; audience: n = 203.
With a large majority (almost consensus majority) of the panel votes, the following question was answered:
Adequate quality control provided, primary tomosynthesis with synthetic 2D visualization is superior or equal to full-field digital mammography for 73% of the panel experts in women with microcalcifications (A: 76%; all participants before/after presentations: 63/76%).
Comment from M. Bernathova
1 Although there is heterogeneity in study methodology as well as DBT technology, the presently available data show an improvement in sensitivity and, depending on technique, also a reduction in unnecessary assessment [7, 8, 9].
2 The present data show enough evidence for recommending DBT as a primary mammographic tool in symptomatic patients [10, 11, 12].
Comment from S. Weigel
1 As DBT increases the detection of spiculated masses and architectural distortions, the clinical relevance of increased cancer detection by DBT needs to be backed by the evaluation of the dynamics of subsequently occurring interval cancers or of tumor stages [13, 14].
2 Facing vendor differences that result in varying synthesized image qualities, randomized, controlled multivendor trial data are missing for screening purposes [13, 15].
Comment from P. Skaane
1 Regarding the assessment of findings (detected by screening, physicians, or self-reported), DBT should be recommended if available – the strong consensus in the meeting underlines this impressively.
2 Quality assurance is of importance; however, its delayed optimization should not hamper the introduction of a clinically relevant and advantageous technique.
Ultrasound
The next part of the consensus meeting addressed breast ultrasound.
A consensus majority of the panel votes (≥75%) answered the questions as follows:
In the German Mammography Screening Program, a supplemental ultrasound examination should be offered depending on the breast cancer risk of the patient (P: 86%; A: 77%; all participants before/after presentations: 69/77%).
In the German Mammography Screening Program,a supplemental ultrasoundexamination should be offered at least to women with a breast density of 4/detectability D (P: 100%; A: 91%); 53% of the panel members voted for a supplemental ultrasound exam at least in breast type 3/C.
Automated 3D breast ultrasound (e.g., ABVS, ABUS) is not used at the vast majority of workplaces (P: 87%; A: 98%); 50% of the panelists argue that the medical advantage is not convincing (A: 61%); 21% of the panelists, however, want to buy a system.
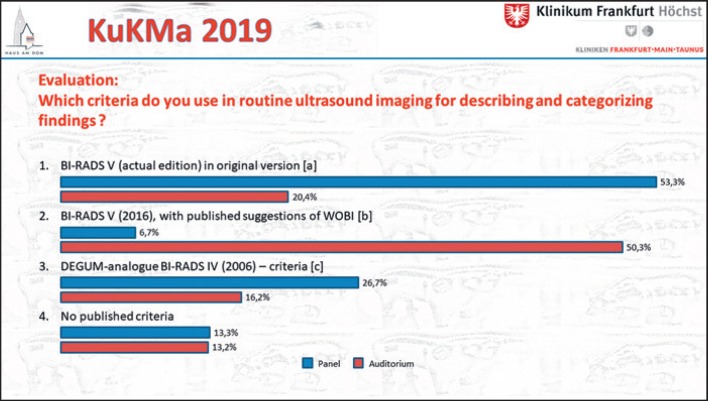
There was no consensus regarding the criteria for describing and categorizing findings in breast ultrasound in routine imaging (Fig. 2; P: n= 16; A: n = 186; Fig. 2a D'Orsi et al. [16]; Fig. 2b Müller-Schimpfle et al. [17]; Fig. 2c Madjar et al. [18]).
Fig. 2.
Voting results for criteria of ultrasound reporting. Panel: n = 16; audience: n = 186. a D'Orsi et al. [16]. bMüller-Schimpfle et al. [17]. cMadjar et al. [18].
Comment from A. Mundinger
1 The most important message to me is that more than 80% of the panel and more than 75% of the audience ascribe supplemental ultrasound a role within the German Screening Program.
2 The overwhelming majority follows published recommendations of a risk-based approach, particularly in women with dense breasts, where ultrasound reveals an additional cancer detection rate of 2–4 additional carcinomas in 1,000 screening participants [18].
Comment from K. Hellerhoff
1 Since supplemental breast ultrasound in breast cancer screening increases the recall and biopsy rate, it is not yet recommended for these reasons [19, 20].
2 Classification of breast density should be based on the use of automated software in order to reduce significant intra- and interobserver variability [21, 22].
Comment from W. Bader
1 The knowledge we have about genetic risk factors and the effects of mammographic breast density on early detection prompt individualized breast cancer screening.
2 Adequate quality control provided, ultrasound is able to increase breast cancer detection without increasing radiation dose or using contrast media; it is the ideal and feasible complementary method to mammography. As there are potential drawbacks like an increased biopsy rate of benign masses, breast ultrasound should only be performed after standardized patient information regarding the advantages and disadvantages of ultrasound.
MRI
The third part of the meeting focused on breast MRI.
A consensus majority of the panel votes (≥75%) suggested the following answers:
Both T2- and diffusion-weighted imaging (DWI) sequences are a valuable supplement to the clinical use of dynamic contrast imaging (P/A: 80%; before presentations: 67%).
In asymptomatic women with intermediate breast cancer risk (increased lifetime risk but not at high risk), MRI of the breast should be performed at least in distinct cases, transparent and explicable to external audits, e.g. ambiguous findings by mammography AND ultrasound (P: 87%; A: 91%).
In symptomatic cases difficult to assess by mammography and ultrasound (assessability C or D), the use of MRI is at least advisable if a percutaneous biopsy is not reasonably feasible (no definitive lesion) (P: 93%; A: 99%).
For the evaluation of breast MRI scans, knowledge of mammography and ultrasound reports is necessary (P: 93%; A: 96%).
Institutions that perform breast MRI should also be able to perform MR-guided interventional procedures or have at least a written cooperation contract with an unequivocal standard of practice (P: 93%; A: 89%).
Despite the consensus on the clinical usefulness of additive sequences to the dynamic contrast imaging, there was neither a consensus on the technical standard of breast MRI in asymptomatic nor in symptomatic women; a slight majority of the panel voted against DWI as a standard in asymptomatic/symptomatic women; the standard protocol should rather consist of 5–7 min of dynamic imaging plus T2-weighted imaging (P: 57/54%; A: 50/23%); in contrast to the panelists, a consensus majority of the audience voted for including DWI into the standard protocol for symptomatic women (A: 75%) but not for asymptomatic women (A: 33%).
Comment from P. Baltzer
1 In line with the empirical evidence, the current consensus opinion is in favor of breast MRI as a problem-solving tool [23, 24, 25].
2 MRI is part of a multimodal approach requiring information from previous exams for improved interpretation [25].
Comment from S.H. Heywang-Köbrunner
1 Currently abbreviated MRI has not been generally accepted. This reflects the concern that possibly important information may be lost, and a repeat MRI may be required in a considerable number of patients.
2 The consensus reflects a very diligent and reasonable weighing of pros and cons concerning indications for MRI and its application in a multimodality setting [25].
Comment from T.H. Helbich
1 A multiparametric MRI concept is necessary to maximize breast lesion characterization by avoiding unnecessary biopsies [26].
2 If MRI of the breast is performed, information from mammography and ultrasound is deemed necessary. Excellent breast MRI includes MRI-guided breast biopsy as well [27].
Intervention
Part 4 of the meeting took a closer look at interventional procedures.
With consensus majority of the panel votes (≥75%), the following questions were answered:
If indicated, biopsied lymph nodes with positive histology should be marked with a permanent marker (clip, coil) before neoadjuvant systemic therapy (P: 79%; A: 46%); they are, however, not generally marked (P: 80%; A: 72%).
Before neoadjuvant systemic therapy, biopsied lymph nodes with positive histology should be marked with a larger (“macro”) marker (≥3 mm in diameter) (P: 80%; A: 79%; all participants before/after presentations: 58/80%).
A large simple majority of the panel votes (and a consensus majority of the audience) agreed that at least 2 specimens should be obtained using 14-G high-speed needle biopsy of lymph nodes (P: 73%; A: 87%).
Comment from M. Golatta
1 Morphologically suspicious lymph nodes should be biopsied to diagnose axillary involvement and plan the operative procedure [28].
2 Biopsied lymph nodes should be marked with a permanent, retrievable macromarker either during sampling or typically after histological confirmation and elucidation of the therapeutic implications.
Comment from C. Kurtz
1 If lymph nodes are biopsied with ≥2 samples using a larger ≤14-G needle taken from the cortex, the sensitivity for the detection of metastases can be increased [29].
2 Following neoadjuvant chemotherapy, initially positive, clipped lymph nodes are more difficult to detect by ultrasound [30, 31]. Apart from using larger markers (≥3 mm), newer clip technologies should be investigated to enable a better localization of clipped lymph nodes, e.g., iron-, radiofrequency-, or radiation-based markers [32].
Comment from M. Fuchsjäger
1 Morphologically suspicious lymph nodes should be biopsied to diagnose axillary involvement and plan the surgical procedure.
2 Marking of lymph nodes should be performed if needed for the therapeutic process, e.g., in targeted axillary dissection.
Risk-Based Imaging
The last part of the consensus meeting addressed mainly the imaging of women with familial breast cancer risk.
A consensus majority of the panel (≥75%) suggested the following answers:
In Germany, those providers who can give proof of their structural process and results of quality of care according to external audits (e.g., certified breast care or screening units) should be able to offer a reimbursed intensified surveillance for the early detection of breast cancer in high-risk patients (P: 77%; A: 82%; all participants before/after presentations: 69/81%).
The data from intensified surveillance for early detection should prospectively be included in a central registry with record linkage to the cancer registries (with evaluation and central certification) (P: 100%; A: 96%).
Women with a high risk of breast cancer (BRCA negative), who receive breast MRI examinations annually, should get a mammogram starting at the age of 40 years (P: 100%; A: 92%; all participants before/after presentations: 73/93%).
A simple majority of the panel declined the general need for an additional ultrasound examination in women with a high risk of breast cancer (BRCA negative), who receive breast MRI examinations annually (P: 57%; A: 56%; all participants before/after presentations: 29/56%).
Vice versa, a simple majority of the panel favored an additional ultrasound examination in women with a high risk of breast cancer (BRCA-1/2 mutation carriers), who receive breast MRI examinations annually (P: 60%; A: 57%; all participants before/after presentations: 79/56%).
Comment from M. Müller-Schimpfle
The intensified screening program in high-risk women should be performed by experienced health care providers who are able to furnish proof of their quality in externally transparent audits. In women with a high lifetime risk for breast cancer, mammography and ultrasound are becoming facultative adjuncts to breast MRI.
Comment from C. Solbach
Intensified surveillance for early detection of breast cancer in high-risk mutation and nonmutation carriers results in the detection of smaller tumors with negative lymph nodes [33]. Further prospective studies are needed to identify meaningful screening tools and intervals for high-risk mutation and nonmutation carriers. This can only be reached by data collection of specialized certified centers.
Comment from K.C. Siegmann-Luz
High-risk screening with breast MRI has been successfully implemented by the German consortium for hereditary breast and ovarian cancer. To further improve program efficacy, it is necessary to certify more high-risk screening centers and to adjust the screening intensity to the probability for breast cancer.
Conclusion
Primary DBT is already able to replace and improve digital mammography in symptomatic women; magnification views for the assessment of calcifications are still recommended. For screening purposes, open questions concern organization, quality control, and the extent of increasing effectiveness.
How to deal with breast density and risk-based imaging remains a challenge, there are pros and cons. Definitely, quality control and further standardization in reporting and interpretation of findings is needed, as taught in certified courses and supported by computer software that automatically calculates breast density. To improve the effectiveness of high-risk imaging programs, it is necessary to certify more high-risk centers and to adjust the screening modalities to the risk of developing breast cancer.
Similar to high-risk women, there is obviously a beneficial role of breast MRI also in the symptomatic patient in selected cases. If the indication is agreed upon in a tumor conference of a certified center of oncology, reimbursement should be guaranteed. The management of pathological axillary lymph nodes has to be clarified in the therapeutic dimension. If indicated, ultrasound-guided labeling of biopsy-proven, involved lymph nodes needs to be done with markers that can be retrieved consistently during operation, e.g., macromarkers.
Disclosure Statement
T.H. Helbich: research grants by Siemens Healthcare, Guerbet, and Bard; S.H. Heywang-Köbrunner (present or former research cooperation): Siemens Healthcare, Hologic Inc., iCAD Inc., and Screenpoint B.V.; M. Müller-Schimpfle: royalty from Cook Medical for the development of a tumor marker; honoraria for presentations at scientific meetings from Fujifilm and Devicor/Mammotome; A. Mundinger: research grant by General Electrics Healthcare. All other authors have no conflicts of interest to declare.
Funding Sources
The consensus meeting was supported by the following sponsors: Agfa, Fujifilm, General Electrics, Hologic, Mammotome, Philips, Samsung, and Siemens.
Author Contributions
Markus Müller-Schimpfle: conception; Markus Müller-Schimpfle, Werner Bader, Pascal Baltzer, Maria Bernathova, Michael Fuchsjäger, Michael Golatta, Thomas Helbich, Karin Hellerhoff, Sylvia H. Heywang-Köbrunner, Claudia Kurtz, Alexander Mundinger, Katja C. Siegmann-Luz, Christine Solbach, Per Skaane, and StefanieWeigel: acquisition, interpretation, drafting, revising, and approval.
Acknowledgment
The authors wish to acknowledge Prof. Dr. Axel Gossmann, Kliniken Köln, Germany, for chairing the industry session and contributing to the expert voting, as well as Birgit Jachmann, Director of the School of Medical Technical Assistants at Frankfurt-Höchst, for the immense logistic, organizational, and personal support, and Verena Hick, Steven Krawietz, and Soraya Lopez Simon for their extraordinary impact on the success of the consensus meeting.
References
- 1.Müller-Schimpfle M, AG Mammadiagnostik der DRG Konsensustreffen der Kursleiter in der Mammadiagnostik am 5.5.2007 in Frankfurt am Main—Thema: mikrokalk. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2008 Jan;180((1)):66–8. doi: 10.1055/s-2007-963748. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Schimpfle MP, Heindel W, Kettritz U, Schulz-Wendtland R, Bick U. Konsensustreffen der Kursleiter in der Mammadiagnostik am 9.5.2009 in Frankfurt am Main – Thema Herdbefunde. Fortschr Röntgenstr. 2010;182((08)):1–5. doi: 10.1055/s-0029-1245474. [DOI] [PubMed] [Google Scholar]
- 3.Müller-Schimpfle MP, Heindel W, Kettritz U, Schulz-Wendtland R, Bick U. Konsensustreffen der Kursleiter in der Mammadiagnostik am 7.5.2011 in Frankfurt am Main – Magnet-Resonanz-Tomografie der Mamma. Fortschr Röntgenstr. 2012;184((10)):919–24. doi: 10.1055/s-0032-1313052. [DOI] [PubMed] [Google Scholar]
- 4.Müller-Schimpfle MP, Heindel W, Kettritz U, Schulz-Wendtland R, Bick U. für die AG Mammadiagnostik der DRG. Konsensustreffen der Kursleiter in der Mammadiagnostik am 04.05.2013 in Frankfurt am Main - Standards in Technik und Befundung. Fortschr Röntgenstr. 2014;186((04)):410–6. doi: 10.1055/s-0034-1368937. [DOI] [PubMed] [Google Scholar]
- 5.Müller-Schimpfle M, Bick U, Hahn M, Hegenscheid K, Helbich T, Kettritz U, Kurtz C, Mundinger A, Siegmann-Luz K, Skaane P, Schulz-Wendtland R. Diskurs - Konsensustreffen Mammadiagnostik – Was hat Bestand von den neuen Standards? Senologie - Zeitschrift für Mammadiagnostik und -therapie. 2017;14:78–81. [Google Scholar]
- 6.Müller-Schimpfle M, Bader W, Bernathova M, et al. Konsensustreffen Mammadiagnostik 2017: Neue Entwicklungen, neue Leitlinien. Senologie. 2018;15:1–4. [Google Scholar]
- 7.Sharma N, McMahon M, Haigh I, Chen Y, Dall BJ. The Potential Impact of Digital Breast Tomosynthesis on the Benign Biopsy Rate in Women Recalled within the UK Breast Screening Programme. Radiology. 2019 May;291((2)):310–7. doi: 10.1148/radiol.2019180809. [DOI] [PubMed] [Google Scholar]
- 8.Michell MJ, Batohi B. Role of tomosynthesis in breast imaging going forward. Clin Radiol. 2018 Apr;73((4)):358–71. doi: 10.1016/j.crad.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast Cancer Screening Using Tomosynthesis or Mammography: A Meta-analysis of Cancer Detection and Recall. J Natl Cancer Inst. 2018 Sep;110((9)):942–9. doi: 10.1093/jnci/djy121. [DOI] [PubMed] [Google Scholar]
- 10.Morel JC, Iqbal A, Wasan RK, Peacock C, Evans DR, Rahim R, et al. The accuracy of digital breast tomosynthesis compared with coned compression magnification mammography in the assessment of abnormalities found on mammography. Clin Radiol. 2014 Nov;69((11)):1112–6. doi: 10.1016/j.crad.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Kim WH, Chang JM, Moon HG, Yi A, Koo HR, Gweon HM, et al. Comparison of the diagnostic performance of digital breast tomosynthesis and magnetic resonance imaging added to digital mammography in women with known breast cancers. Eur Radiol. 2016 Jun;26((6)):1556–64. doi: 10.1007/s00330-015-3998-3. [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Hu FX, Zhu H, Wang QF, Gu YJ, Peng WJ. Digital breast tomosynthesis plus mammography, magnetic resonance imaging plus mammography and mammography alone: A comparison of diagnostic performance in symptomatic women. Clin Hemorheol Microcirc. 2017;66((2)):105–16. doi: 10.3233/CH-16242. [DOI] [PubMed] [Google Scholar]
- 13.Weigel S, Gerss J, Hense HW, Krischke M, Sommer A, Czwoydzinski J, et al. Digital breast tomosynthesis plus synthesised images versus standard full-field digital mammography in population-based screening (TOSYMA): protocol of a randomised controlled trial. BMJ Open. 2018 May;8((5)):e020475. doi: 10.1136/bmjopen-2017-020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lång K. The Coming of Age of Breast Tomosynthesis in Screening. Radiology. 2019 Apr;291((1)):31–3. doi: 10.1148/radiol.2019190181. [DOI] [PubMed] [Google Scholar]
- 15.Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019 Apr;29((4)):1762–77. doi: 10.1007/s00330-018-5668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston (VA): American College of Radiology; 2013. [Google Scholar]
- 17.Müller-Schimpfle M, Graf O, Madjar H, et al. Diskussionspapier - BI-RADS die 5. – eine Kurzmitteilung aus deutsch- / österreichischer Sicht. Röfo 2016;188: 346-352 und Röfo 2016; 188: E2; sowie auch in. Geburtshilfe Frauenheilkd. 2016;76:490–6. [Google Scholar]
- 18.Madjar H, Ohlinger R, Mundinger A, Watermann D, Frenz JP, Bader W, et al. [BI-RADS-analogue DEGUM criteria for findings in breast ultrasound—consensus of the DEGUM Committee on Breast Ultrasound] Ultraschall Med. 2006 Aug;27((4)):374–9. doi: 10.1055/s-2006-926943. [DOI] [PubMed] [Google Scholar]
- 19.Gartlehner G, Thaler K, Chapman A, Kaminski-Hartenthaler A, Berzaczy D, Van Noord MG, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013 Apr;((4)):CD009632. doi: 10.1002/14651858.CD009632.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnikov J, Fenton J, Whitlock E, Miglioretti DL, Weyrich MS, Thompson JH, Shah K. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Service Task Force. Center for Healthcare Policy and Research University of California, Davis Sacramento, CA. 2016 PMID: 26866210. [PubMed] [Google Scholar]
- 21.Moshina N, Sebuødegård S, Lee CI, Akslen LA, Tsuruda KM, Elmore JG, et al. Automated Volumetric Analysis of Mammographic Density in a Screening Setting: Worse Outcomes for Women with Dense Breasts. Radiology. 2018 Aug;288((2)):343–52. doi: 10.1148/radiol.2018172972. [DOI] [PubMed] [Google Scholar]
- 22.Nothacker M, Duda V, Hahn M, Warm M, Degenhardt F, Madjar H, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009 Sep;9((1)):335. doi: 10.1186/1471-2407-9-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spick C, Szolar DH, Preidler KW, Reittner P, Rauch K, Brader P, et al. 3 Tesla breast MR imaging as a problem-solving tool: diagnostic performance and incidental lesions. PLoS One. 2018 Jan;13((1)):e0190287. doi: 10.1371/journal.pone.0190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennani-Baiti B, Dietzel M, Baltzer PA. MRI for the assessment of malignancy in BI-RADS 4 mammographic microcalcifications. PLoS One. 2017 Nov 30;12((11)):e0188679. doi: 10.1371/journal.pone.0188679. doi: eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clauser P, Mann R, Athanasiou A, Prosch H, Pinker K, Dietzel M, et al. A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol. 2018 May;28((5)):1909–18. doi: 10.1007/s00330-017-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinker K, Helbich TH, Morris EA. The potential of multiparametric MRI of the breast. Br J Radiol. 2017 Jan;90((1069)):20160715. doi: 10.1259/bjr.20160715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, et al. European Society of Breast Imaging (EUSOBI), with language review by Europa Donna–The European Breast Cancer Coalition Breast MRI: EUSOBI recommendations for women's information. Eur Radiol. 2015 Dec;25((12)):3669–78. doi: 10.1007/s00330-015-3807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AGO Breast Committee Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Recommendations. 2019 www.ago-online.de. [Google Scholar]
- 29.Ganott MA, Zuley ML, Abrams GS, Lu AH, Kelly AE, Sumkin JH, et al. Ultrasound Guided Core Biopsy versus Fine Needle Aspiration for Evaluation of Axillary Lymphadenopathy in Patients with Breast Cancer. ISRN Oncol. 2014 Feb;2014:703160. doi: 10.1155/2014/703160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann Surg Oncol. 2017 Oct;24((10)):3011–6. doi: 10.1245/s10434-017-6023-z. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018 Sep;44((9)):1307–11. doi: 10.1016/j.ejso.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Hayes MK. Update on Preoperative Breast Localization. Radiol Clin North Am. 2017 May;55((3)):591–603. doi: 10.1016/j.rcl.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Bick U, Engel C, Krug B, Heindel W, Fallenberg EM, Rhiem K, et al. German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res Treat. 2019 May;175((1)):217–28. doi: 10.1007/s10549-019-05152-9. [DOI] [PubMed] [Google Scholar]