Abstract
Background
Spinal cord injury induces inflammatory responses that include the release of cytokines and the recruitment and activation of macrophages and microglia. Neuroinflammation at the lesion site contributes to secondary tissue injury and permanent locomotor dysfunction. Dexmedetomidine (DEX), a highly selective α2-adrenergic receptor agonist, is anti-inflammatory and neuroprotective in both preclinical and clinical trials. We investigated the effect of DEX on the microglial response, and histological and neurological outcomes in a rat model of cervical spinal cord injury.
Methods
Anaesthetised rats underwent unilateral (right) C5 spinal cord contusion (75 kdyne) using an impactor device. The locomotor function, injury size, and inflammatory responses were assessed. The effect of DEX was also studied in a microglial cell culture model.
Results
DEX significantly improved the ipsilateral upper-limb motor dysfunction (grooming and paw placement; P<0.0001 and P=0.0012), decreased the injury size (P<0.05), spared white matter (P<0.05), and reduced the number of activated macrophages (P<0.05) at the injury site 4 weeks post-SCI. In DEX-treated rats after injury, tissue RNA expression indicated a significant downregulation of pro-inflammatory markers (e.g. interleukin [IL]-1β, tumour necrosis factor-α, interleukin (IL)-6, and CD11b) and an upregulation of anti-inflammatory and pro-resolving M2 responses (e.g. IL-4, arginase-1, and CD206) (P<0.05). In lipopolysaccharide-stimulated cultured microglia, DEX produced a similar inflammation-modulatory effect as was seen in spinal cord injury. The benefits of DEX on these outcomes were mostly reversed by an α2-adrenergic receptor antagonist.
Conclusions
DEX significantly improves neurological outcomes and decreases tissue damage after spinal cord injury, which is associated with modulation of neuroinflammation and is partially mediated via α2-adrenergic receptor signaling.
Keywords: dexmedetomidine, macrophage polarisation, microglia, neuroinflammation, spinal cord injury, α2-adrenergic receptor
Editor's key points.
-
•
Neuroinflammation contributes to secondary tissue injury and permanent locomotor dysfunction in spinal cord injury.
-
•
The effect of dexmedetomidine, a selective α2-adrenergic receptor agonist with anti-inflammatory and neuroprotective properties, was investigated in a rat model of cervical spinal cord injury.
-
•
Dexmedetomidine improved the neurological function and decreased the damage after spinal cord injury partially via α2-adrenergic receptor-mediated mechanisms.
-
•
These animal data show that α2-adrenergic receptor agonists may be a promising class of immunomodulatory agents for the treatment of spinal cord injury.
Spinal cord injury (SCI) induces an inflammatory response that can lead to cell death, tissue damage, and neurological dysfunction.1, 2 Post-SCI inflammation is a precursor to subsequent pathological changes, including cellular necrosis, apoptosis, gliosis, and demyelination.2, 3 Anti-inflammatory therapies are effective in reducing tissue damage and in promoting functional recovery.4, 5, 6 Depletion of haematogenous macrophages or deletion of resident microglia can improve axonal regeneration and outcome.7, 8 Nonetheless, ‘primed’ macrophages can also be pro-reparative and may improve outcome.9 This paradox may be attributable to the presence of several functional macrophage varieties ranging from the classically activated (M1) to the alternatively activated (M2) phenotypes; even though some have challenged this dynamic construct as being more evident in vitro than in vivo,10, 11 for the purposes of this report, we will use this concept. Enhancement of M1 vs M2 responses is responsible for persistent neuroinflammation and impaired recovery.12 Therefore, treatments that modulate the balance from pro- to anti-inflammatory/pro-resolving responses may be promising therapies for SCI.
Dexmedetomidine (DEX), a highly selective α2-adrenergic receptor agonist, is often used as an anaesthetic adjunct for patient sedation and general anaesthesia in the intensive care unit (ICU) and operating room settings, respectively. Accumulating evidence has shown that DEX is protective in ischaemia–reperfusion injury in a variety of organs, including the spinal cord, brain, and heart.13, 14, 15, 16, 17, 18 In a series of studies using a spinal compression injury model (by clipping the cord), intrathecal delivery of DEX was shown to preserve neurones, and reduce lipid peroxidation and inflammatory responses with equivalent effectiveness to methylprednisolone.19, 20, 21 More recent studies have shown that DEX is neuroprotective by reducing tissue oedema, inflammation, and apoptosis, and improves locomotor activity,22, 23, 24 but such effect has not been well studied in traumatic SCI using a more clinically relevant treatment regimen. We used a well-established cervical spinal injury model (the most common site of SCI in humans)25 and employed DEX intervention with repeated administrations post-SCI, which mimics the continuous treatment to patients with SCI beginning in the emergency room or OR, and continuing during the ICU stay. We tested the hypothesis that DEX improves outcomes and modulates neuroinflammatory responses in order to develop evidence to support the clinical use of α2-adrenergic receptor agonists, which may be a promising class of immunomodulatory agents for post-SCI care.
Methods
Animals
Long–Evans female rats (77–87 days old) were housed in pairs with ad libitum access to food and water. All experiments were performed in accordance with the National Institutes of Health (NIH) and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines, and were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. There were no adverse events related to the animals throughout the entire experiment.
Spinal cord injury and animal care
All surgical procedures were performed aseptically. The animals were administered cefazolin 50 mg kg−1 before surgery and for 1 day post-SCI. The animals were anaesthetised with isoflurane 2%, and a dorsal midline skin incision was made. Connective tissue and muscle layers were dissected, and a C5 laminectomy was performed.25, 26 Unilateral (right) cervical contusion injury (75 kdyne) was produced with an Infinite Horizon Impactor device (Precision Systems and Instrumentation Company, Lexington, KY, USA). The surgical control group had all procedures without contusion. After injury, muscle layers were sutured and skin incision closed with wound clips. The animals were placed and monitored in an incubator at 37°C for the first day post-SCI. The animals were then checked twice daily for bladder function and wound healing for 2 weeks after SCI.
Animal drug treatment
After SCI, 25 μg kg−1 of DEX (Henry Schein Inc., Dublin, OH, USA) or vehicle (saline) was injected i.p. at 0, 2, 4, and 6 h on Day 0 and once daily from Day 1 to Day 5 post-SCI. Yohimbine (Yoh; Sigma-Aldrich, St. Louis, MO, USA), an α2-adrenergic receptor antagonist, was given at a dose of 10 μg kg−1 i.p. 15 min before each DEX administration post-SCI for 5 days.
Behavioural analysis
Paw placement test
Paw placement assessments were performed as described.25, 27 Briefly, the rats were placed in a clear plastic cylinder and their behaviour recorded. The number of times a rat placed its left, right, or both forepaws against the cylinder during weight-supported movements was counted. The frequency of contralateral (left) forepaw placement was calculated and used as an indicator for functional recovery on the ipsilateral (right) side. The rats were tested before surgery, at 2 days after SCI, and then weekly thereafter for 4 weeks.
Grooming test
Grooming scores were used to evaluate the recovery of forelimb range of motion.25, 27 Cool water was gently sprayed and applied to a rat's head and back, and then the animal was placed in a clear plastic cylinder. Grooming activity was recorded and evaluated using a 6-point scoring scale as follows: 0, no contact with the forepaw to any part of the face or head; 1, contact of forepaw with the underside of the chin or mouth area; 2, contact of forepaw with the area between the nose and eyes, but not the eyes; 3, contact of forepaw with the eyes and the area between the eyes and the front of the ears, but not the ears; 4, contact of forepaw with the ears, but not the area of the head behind the ears; and 5, contact of forepaw with the area of the head behind the ears. The animals were tested at 2, 7, 14, 21, and 28 days after SCI.
Tissue processing
The animals were killed for histology under deep anaesthesia with ketamine by trans-cardiac perfusion with 0.9% saline followed by 4% paraformaldehyde.25 The lengths of the spinal cord (10 mm) centred around the lesion were removed and postfixed overnight in 4% paraformaldehyde. Tissue was cryoprotected in 30% sucrose for 2 days, and then embedded in optimal-cutting-temperature compound and sectioned 20 μm horizontally.
Histological analyses
Eriochrome cyanine staining
Slides were placed in ethanol:chloroform (1:1) for 1 h, washed in 100% ethanol (2×1 min), air-dried at room temperature (RT), and stained in eriochrome cyanine solution for 20–30 min, and then washed in running tap water.26 After differentiation with iron alum and borax ferricyanide solution, the slides were counterstained in 0.5% neutral red for 1–2 min at RT. Sections were then dehydrated in ethanol, cleared in xylene, and coverslipped with mounting medium.
Immunohistochemical staining
Fixed tissue sections were blocked and permeabilised for 1 h with 10% normal donkey serum and 0.3% Triton X-100. The sections were then incubated overnight at RT with mouse monoclonal antibody for CD11b (1:300; Bio-Rad Laboratories, Hercules, CA, USA).28 After washing with phosphate-buffered saline (PBS) 2 ml, the slides were incubated for 1 h at RT with fluorescent (Alexa 594®) donkey anti-mouse secondary antibody (1:1000; Life Technologies, Carlsbad, CA, USA). The slides were briefly rinsed with PBS 2 ml and coverslipped with VECTASHIELD® containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA). The stained spinal tissue sections were photographed using the BioRevo fluorescence microscope BZ-9000 Generation II (Keyence, Itasca, IL, USA). Fluorescence was measured using BZ-9000 Generation II analyser (Keyence) and analysed by NIH ImageJ (https://imagej.nih.gov/ij/).
RNA analysis
Total cellular RNA was isolated and purified from a 5 mm segment of injured or uninjured spinal cord using TRIzol® (Invitrogen, Carlsbad, CA, USA), followed by RNeasy® (QIAGEN, Germantown, MD, USA) binding and quantified by a NanoDrop™ Lite (ThermoFisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) was prepared from a total of RNA 2 μg by reverse transcription. Polymerase chain reactions (PCRs) were performed using cDNA 10 ng, 50 nmol of each primer (Table 1),29 and SYBR® Green Master Mix in 20 μl reactions. Levels of quantitative RT–PCR (Q-RT–PCR) product were measured using SYBR Green fluorescence (ThermoFisher Scientific) collected on an Agilent Mx3005P Real-Time PCR system (Agilent Technologies, Santa Clara, CA, USA). Standard curves were generated for each gene using a control cDNA dilution series. Melting-point analyses were performed for each reaction to confirm single amplified products.
Table 1.
List of selected genes and polymerase chain reaction (PCR) primers. GAPDH, glyceraldehyde-3 phosphate dehydrogenase; IL, interleukin; Q-RT–PCR, quantitative RT–PCR; TNF-α, tumor necrosis factor-α
| GenBank ID | Gene description | Q-RT–PCR primers (forward/reverse) |
|---|---|---|
| Rat | ||
| NM_031512.2 | IL-1β | 5′-TGCAGGCTTCGAGATGAACA-3′ |
| 5′-ACATGGGTCAGACAGCACGA-3′ | ||
| NM_012675.3 | TNF-α | 5′-GAACTCCAGGCGGTGTCTGT-3′ |
| 5′-GCCACGAGCAGGAATGAGAA-3′ | ||
| NM_012589.2 | IL-6 | 5′-ATTCTGTCTCGAGCCCACCA-3′ |
| 5′-CTGAAGGGCAGATGGAGTTGA-3′ | ||
| NM_201270.1 | IL-4 | 5′-CCA GAC GTC CTT ACG GCA AC-3′ |
| 5′-GCA GAT GAG CTC GTT CTC CG-3′ | ||
| NM_017134.3 | Arginase-1 | 5′-CAT TTG GGT GGA TGC TCA CA-3′ |
| 5′-GAG CTG GTT GTC AGC GGA GT-3′ | ||
| NM_001106123.2 | CD206 | 5′-TGT CGG AGT CGC AGA TCA TG-3′ |
| 5′-GCA CCC CCA AAC ACA ATT TG-3′ | ||
| NM_012711.1 | CD11b | 5′-CTG ATC AGA GCC CAG CCT GT-3′ |
| 5′-GCT GAA TTC CAT GGT TGC CT-3′ | ||
| NM_017008.4 | GAPDH | 5′-ACC CAG CCC AGC AAG GAT AC-3′ |
| 5′-TCA GCA ACT GAG GGC CTC TC-3′ | ||
| Mouse | ||
| NM_008361 | IL-1β | 5′-GCAACTGTTCCTGAACTCAACT-3′ |
| 5′-ATCTTTTGGGGTCCGTCAACT-3′ | ||
| NM_013693 | TNF-α | 5′-GTGATCGGTCCCCAAAGG-3′ |
| 5′-GGTGTGGGCCATAGAACTGATG-3′ | ||
| NM_031168 | IL-6 | 5′-GCCTCCTTGGGACTGATGCT-3′ |
| 5′-AGTCTCCTCTCCGGACTTGTG-3′ | ||
| NM_021283.2 | IL-4 | 5′-CTCGAATGTACCAGGAGCCA-3′ |
| 5′-TGTGGTGTTCTTCGTTGCTG-3′ | ||
| NM_007482.2 | Arginase-1 | 5′-GAACACGGCAGTGGCTTTAAC-3′ |
| 5′-TGCTTAGCTCTGTCTGCTTTGC-3′ | ||
| NM_008625.1 | CD206 | 5′-TCTTTGCCTTTCCCAGTCTCC-3′ |
| 5′-TGACACCCAGCGGAATTTC-3′ | ||
| NM_008084 | GAPDH | 5′-CCAGCTCGTCCTGTAGACAA-3′ |
| 5′-GCCTTGACTGTGCCGTTGA-3′ | ||
Lesion volume measurement
A camera lucida drawing of the section with the largest extent of lesion (the lesion epicentre) was made outlining intact tissue (grey and white matter) and the lesion.25 Pixel counts from digitised drawings in Adobe Photoshop 5.5 (Adobe Systems, Inc., San Jose, CA, USA) were used to determine the area of spared tissue for both hemi-cords at the lesion centre.25, 26 The percent sparing for the ipsilateral hemi-cord was determined by dividing the total spared ipsilateral tissue area, spared white-matter tissue area, or spared grey-matter tissue area, by the same measure from the contralateral hemi-cord. Lesion size from rostral-to-caudal direction at selected locations (–960, –720, –480, –240, 0, 240, 480, 720, and 960 μm) was calculated using the ratio of ipsilateral injured and contralateral hemi-cord area.
Microglia culture
The murine microglial BV-2 cell line30 were cultured in Dulbecco's modified Eagle's medium/F12 supplemented with 10% foetal bovine serum (Gemini Bio-Products, West Sacramento, CA, USA), penicillin 100 IU ml−1, streptomycin 100 μg ml−1, and 2 mM L-glutamine (ThermoFisher Scientific). Cells were grown in a 37°C incubator with humidified 95% air and 5% CO2, and were exposed to different reagents (e.g. lipopolysaccharide [LPS; 100 ng ml−1], DEX [1 μM], Yoh [100 μM], or vehicle in serum-containing medium). Typically, the cells were first treated with LPS for 1 h, followed by 24 h of incubation with vehicle only, DEX only, Yoh only, or Yoh 5 min before DEX (pretreatment for 5 min) plus DEX. The supernatant and the BV-2 cell lysate were collected at the end of the 24 h exposures.
Statistical analysis
GraphPad Prism 8 was used for statistical analyses and graphs (GraphPad, La Jolla, CA, USA). Data are expressed as means (standard error of the mean). Two-way analysis of variance (anova) with repeated measures was used for neurological and histological outcome analyses. One-way anova with post hoc test for three groups or unpaired t-tests for two group analyses were also applied as indicated in figure legends. Statistical significance was defined at P≤0.05, 0.01, 0.001, and 0.0001 levels.
Results
Dexmedetomidine improves long-term outcomes via α2-adrenoceptors
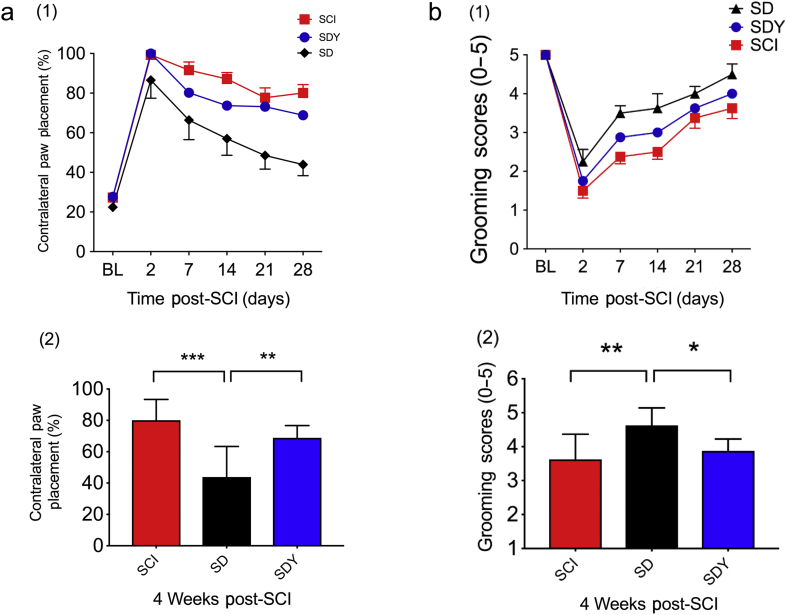
Animals with unilateral (right) C5 SCI were assessed for right-forelimb motor function using paw placement and grooming tests.25, 27 Both behavioural tests were improved in the DEX-treated SCI group (termed ‘SD’) compared with vehicle controls (SCI) over 4 weeks (Fig. 1a (1): P=0.0012; Fig. 1b (1): P<0.0001; two-way anova with repeated measures). At the end of 4 weeks after injury, the difference between the SCI and SD groups was also statistically significant (Fig. 1a (2): 80.1 [13.2]% vs 43.9[19.4]%, ***P<0.0001; Fig. 1b (2): 3.6 [0.7] vs 4.6 [0.5], **P<0.01; one-way anova with Tukey's test). To test whether the effect of DEX is mediated via α2-adrenergic receptor, Yoh, an α2 receptor antagonist, was given to the SCI rats 15 min before DEX (SDY). Yoh reversed the sedative effect of DEX (by observation). Both behavioural measures (paw placement and grooming tests) were attenuated (SD vs SDY in Fig. 1a (2): 43.9 [19.4]% vs 68.8 [7.8]%, **P<0.01; in Fig. 1b (2): 4.6 [0.5] vs 3.9 [0.4], *P<0.05; one-way anova with Tukey's test).
Fig 1.
Dexmedetomidine (DEX) improves neurological outcomes 4 weeks after spinal cord injury (SCI). SD, SCI+DEX; SDY, SCI+DEX+yohimbine (Yoh). (a) In paw placement test, rats with right C5 injury had less contralateral forepaw placement for weight support, which suggests better recovery in the ipsilateral forelimb after DEX treatment (SD) over a 4-week period compared with vehicle control (SCI) (1). Between SCI and SD groups, there are significant DEX treatment effects (F [1; 120]=40.8; P<0.0001) in percentage of contralateral paw placement using two-way analysis of variance (anova) with repeated measures. In SCI rats pretreated with the α2 antagonist Yoh before DEX administration (SDY), the functional benefit of DEX was partially reversed (SD vs SDY [F {1; 96}=13; P=0.0005], two-way anova with repeated measures). At the end of 4 weeks after SCI (2), there was a significant improvement in the SD group compared with SCI (***P<0.0001, one-way anova with Tukey's test). Such functional improvement was partially reversed by Yoh pretreatment (**P=0.0099; one-way anova with Tukey's test) (n=8–10 in each group). (b) In a grooming test, rats with right C5 SCI had better grooming scores in the right upper limb after DEX treatment (SD) over a 4-week period compared with the vehicle control (SCI) (1). Between the SCI and SD groups, there was a significant treatment effect (F [1; 14]=16.5; P=0.0012) by two way-anova with repeated measures. In a separate group, SCI rats were pretreated with the α2 antagonist Yoh before DEX administration, and the functional benefit of DEX was partially reversed (SD vs SDY [F {1; 14}=4.8; P=0.0459], two-way anova with repeated measures). At the end of 4 weeks after SCI (2), there was a significant improvement in the SD group compared with SCI (**P=0.005; one-way anova with Tukey's test). The functional improvement was partially reversed by Yoh pretreatment (*P=0.0366; one-way anova with Tukey's test) (n=8–10 in each group). BL, baseline.
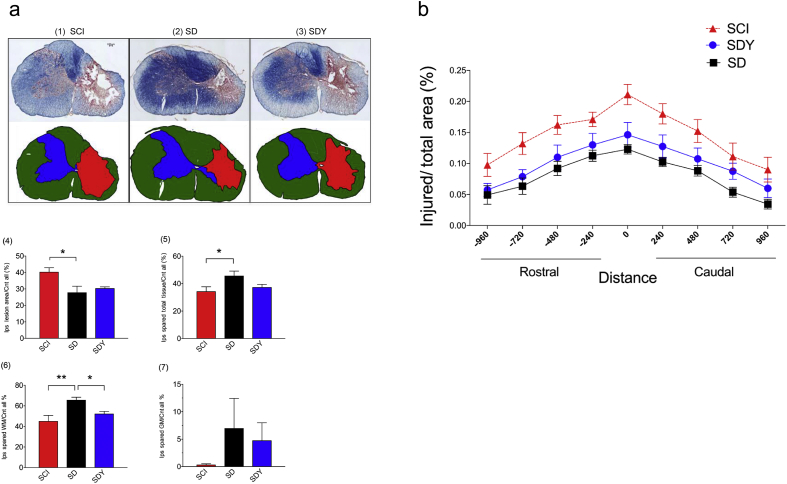
Dexmedetomidine reduces tissue damage and promotes white matter sparing
Four weeks post-SCI, tissue injury at the epicentre and equi-distant rostrocaudally (–960, –720, –480, –240, 0, 240, 480, 720, and 960 μm) was assessed using eriochrome cyanine staining (Fig. 2). At the maximal injury sections, DEX (SD) reduced lesion volume compared with the SCI group (Fig. 2a (1), (2), and (4); SCI vs SD: 40.4 [7.2] vs 27.9 [9.3]; *P<0.05; one-way anova with Tukey's test). The cavity area commonly seen in the centre of the damaged rat spinal cord was consistently decreased in the SD group (by observation). Tissue protection seen in the SD group was likely because of sparing of white matter (Fig. 2a (5): SCI vs SD: 34.4 [9.4]% vs 45.8 [8.8]%, *P<0.05; Fig. 2a (6): SCI vs SD: 45.2 [13.6]% vs 65.7 [7.0]%, **P<0.01; one-way anova with Tukey's test). There was no significant difference in grey matter sparing between the SCI and SD groups (Fig. 2a (7)). Yoh inhibited the effect of DEX and attenuated the amount of spared white matter (Fig. 2a (6): SD vs SDY: 65.7 [7.0]% vs 52.3 [5.4]%; *P<0.05; one-way anova with Tukey's test). Tissue injury area diminished away from the epicentre in the SCI group (Fig. 2b). However, DEX significantly reduced the injury area at all indicated locations (SCI vs SD; P<0.0001; two-way anova with multiple comparisons); this tissue protection was significantly attenuated by Yoh (SD vs SDY; P<0.0001; two-way anova with multiple comparisons).
Fig 2.
Dexmedetomidine reduces damage and protects spinal tissue at injury site after spinal cord injury (SCI). SD, SCI+dexmedetomidine (DEX); SDY, SCI+dexmedetomidine+yohimbine (Yoh). (a) DEX reduced maximal injury areas and spared white matter at the SCI epicentre. Eriochrome cyanine (EC) staining was used to assess the severity of spinal tissue injury at the epicentre. The size of the lesion volume and spared tissue was quantitated at the section with maximal injury. Microscopic images (4×) show representative sections in each treatment group (1–3). The lesion volume was significantly reduced (4), and there was more spared spinal tissue (5), especially white matter, in the SD group (6; **P<0.01; one-way analysis of variance (anova) with Tukey's test; n=8–10 in each group). Pretreatment with an α2 antagonist (SDY) partially reduced the spared white matter by DEX (6; *P<0.05; one-way anova with Tukey's test; n=8–10 in each group). Because of the large within-group variation, DEX had no significant effect on grey-matter sparing (7). (b) DEX decreased the lesion volume from rostral-to-caudal direction. Rostro-caudal sections were quantitated by EC staining for lesion size (–960, –720, –480, –240, 0, 240, 480, 720, and 960 μm). The sizes of injury areas at the indicated distances from the epicentre (distance=0 μm) were significantly reduced in the SD group compared with SCI (SCI vs SD; P<0.001; two-way anova with multiple comparisons; n=8–10 in each group). Pretreatment with an α2 antagonist (Yoh) led to partial reversal of the DEX effect on tissue protection (SD vs SDY; P<0.001; two-way anova with multiple comparisons; n=8–10 in each group). Ips, ipsilateral; Cnt, contralateral; WM, white matter; GM, grey matter.
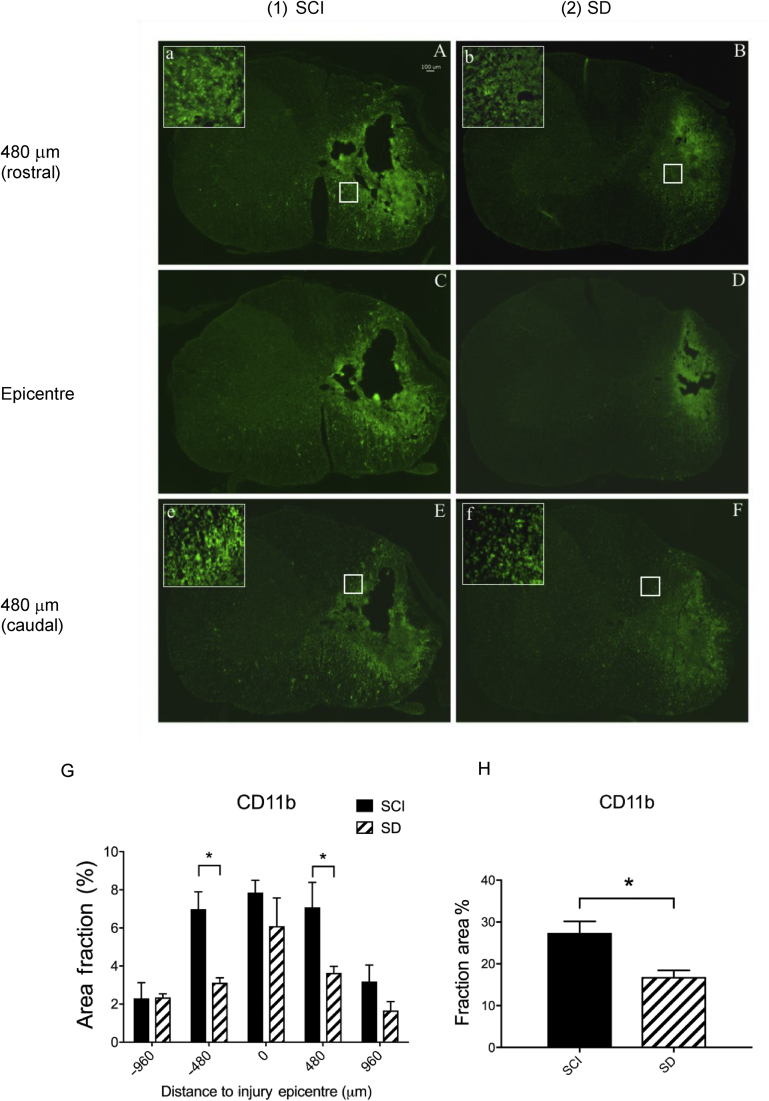
Dexmedetomidine modulates neuroinflammation at the injury site
We used anti-CD11b antibody to label the microglial population and to further assess the degree of intra-spinal neuroinflammatory response at the injury epicentre from rostral to caudal. As can be seen in Fig 3, the CD11b signal diminished rostro-caudally from the epicentre in the SCI rats. When all CD11b signals at different levels were aggregated, there was a significant difference between the SCI and SD groups (SCI vs SD: 27.4 [5.5]% vs 16.9 [3.2]%; *P<0.05; Student's t-test). At individual sections across the injury site, we found that the most significant differences were located in areas adjacent to the epicentre (+480 or –480 μm; *P<0.05; Student's t-test).
Fig 3.
Microglial activation is mitigated by dexmedetomidine (DEX) at the injury site 28 days after spinal cord injury (SCI). SD, SCI+DEX; SDY, SCI+DEX+yohimbine. CD11b-positive cells (microglia) are shown in spinal injured rats treated with saline (SCI: A, C, and E) or DEX (SD: B, D, and F). Images C and D are cross sections at the SCI epicentre, images A and B are sections 480 μm rostral to the epicentre, and images E and F are 480 μm caudal. Signals of CD11b-positive cells were decreased after DEX treatment (SD) compared with the vehicle control group (SCI). The decrease was statistically significant at 480 μm both rostrally (image A vs B) and caudally (image E vs F) to the injury epicentre (G: SCI vs SD; *P<0.05 by two-way ANOVA with multiple comparisons; n=6 in each group). The total count of CD11b-positive signals across the five different locations suggests a significant reduction of activated microglia in the SD group (H, *P<0.05; Student's t-test; n=6 in each group).
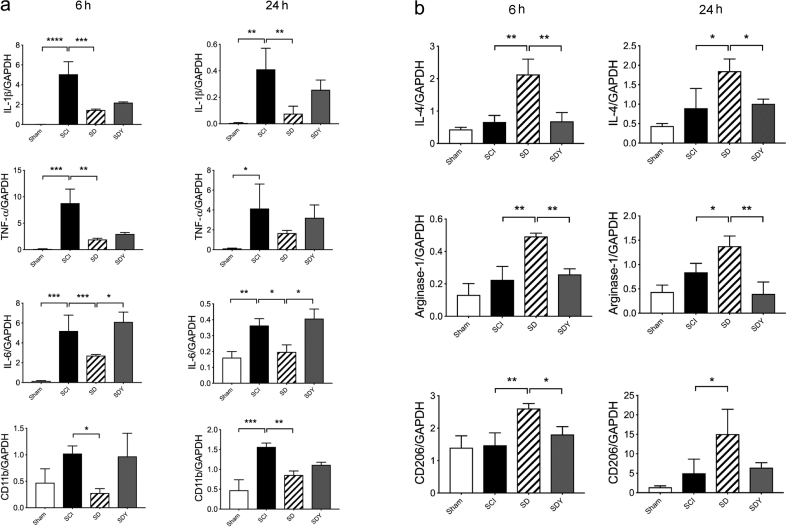
We further analysed the gene expression of multiple pro-inflammatory and anti-inflammatory cytokines and markers using Q-RT–PCR (Fig. 4). Elevation of classic pro-inflammatory cytokines post-SCI was reduced at 6 and 24 h in the DEX-treated groups (SD) after SCI (Fig. 4a: *P<0.05, **P<0.01, and ***P<0.001; one-way anova with Tukey's test). The elevated expression of CD11b (marker for microglia) RNA after injury was also decreased both at 6 and 24 h in SD groups (Fig. 4a: *P<0.05 and **P<0.01; one-way anova with Tukey's test). We also examined the expression of cytokines and markers that are pro-resolving and reparative (M2 responses) (Fig. 4b). Expression of interleukin (IL)-4, arginase-1, and CD206 mRNA was upregulated by DEX at both 6 and 24 h, compared with SCI only (Fig. 4b; SCI vs SD: *P<0.05 and **P<0.01; one-way anova with Tukey's test). Yoh reversed most of these gene responses, especially the M2 responses (Fig. 4b; SD vs SDY: *P<0.05 and **P<0.01; one-way anova with Tukey's test).
Fig 4.
Dexmedetomidine (DEX) modulates the neuroinflammatory response. SCI, spinal cord injury; SD, SCI+DEX; SDY, SCI+DEX+yohimbine (Yoh). (a) RNA expression of pro-inflammatory cytokines and markers was assessed at 6 and 24 h after SCI. At both 6 and 24 h, Interleukin (IL)-1β, tumour necrosis factor-α (TNF-α), and IL-6 were all elevated after injury (SCI), while their expression were reduced by DEX treatment (SD). The elevated expression of CD11b after SCI was also downregulated by DEX (SD). An α2-antagonist (Yoh) reversed the effect of DEX on IL-6, with a trend in others, including IL-1β, TNF-α, and CD11b (SDY). (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; one-way analysis of variance (anova) with Tukey's test; n=4 in each group). (b) Expression profile of anti-inflammatory and pro-resolving/reparative M2 genes at 6 and 24 h after SCI. At both 6 and 24 h, IL-4, arginase-1, and CD206 were enhanced by DEX (SD). Yoh significantly reversed DEX-induced upregulation (SDY) (*P<0.05, **P<0.01; one-way anova with Tukey's test; n=4 in each group). GAPDH, glyceraldehyde-3 phosphate dehydrogenase.
Dexmedetomidine modulates microglial activity towards the M2 phenotype
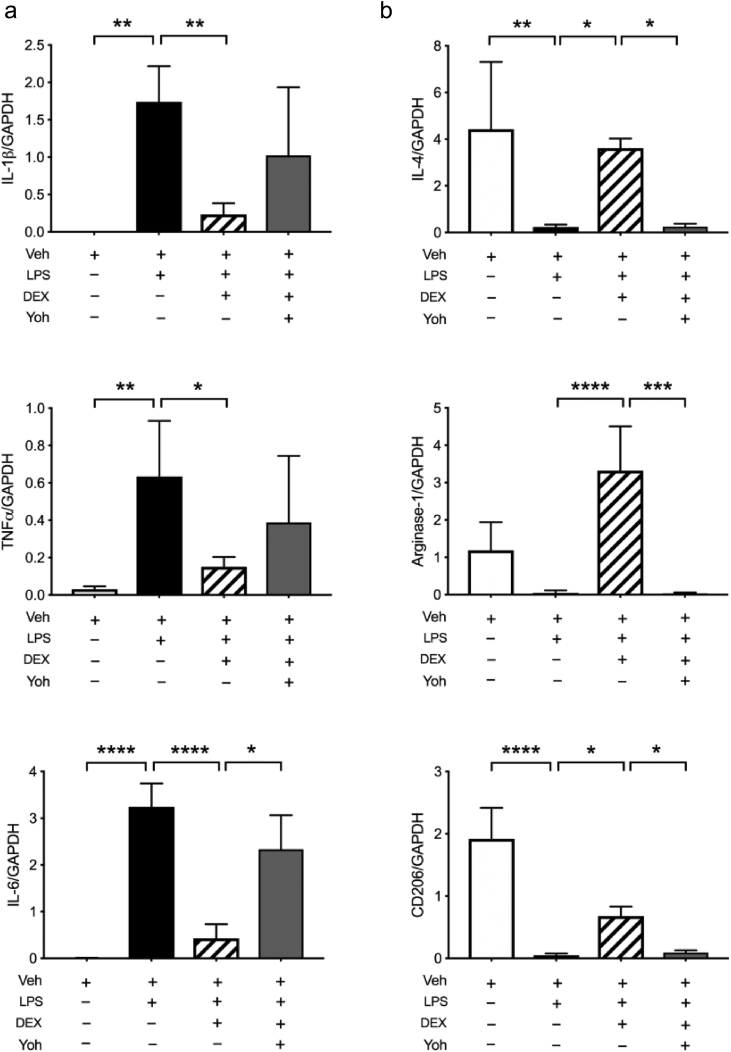
Pro-inflammatory cytokines, such as IL-1β, tumour necrosis factor-α (TNF-α), and IL-6, were all upregulated in LPS-treated microglia (Fig. 5a); these elevated cytokines were suppressed by DEX (Fig. 5a; LPS vs DEX: *P<0.05, **P<0.01, and ****P<0.0001; one-way anova with Tukey's test). In contrast, transcription of classic M2 response-associated genes, such as IL-4, arginase-1, and CD206, was enhanced in DEX-treated cells (Fig. 5b; LPS vs DEX; *P<0.05 and ****P<0.0001; one-way anova with Tukey's test); such DEX-induced effects were prevented by Yoh, especially in M2 responses (Fig. 5a and b; DEX vs Yoh: *P<0.05 and ***P<0.001; one-way anova with Tukey's test).
Fig 5.
Dexmedetomidine (DEX) polarises microglial responses. Gene expression profile of inflammatory cytokines and mediators in the cell lysate of cultured BV2 microglia. (a) Pro-inflammatory cytokines were upregulated after lipopolysaccharide (LPS) treatment for 1 h. DEX significantly reduced expression, whilst yohimbine (Yoh) reversed the suppression of interleukin (IL)-6 (*P<0.05, **P<0.01, ****P<0.0001; one-way analysis of variance (anova) with Tukey's test; n=6 wells in each group). (b) Expression of M2 response-associated genes (IL-4, arginase-1, and CD206) were assessed. Whilst all were decreased after LPS treatment for 1 h, the level of IL-4, arginase-1, or CD206 was significantly enhanced by DEX. Yoh inhibited the DEX effect on all (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; one-way anova with Tukey's test; n=6 wells in each group). Veh, vehicle; GAPDH, glyceraldehyde-3 phosphate dehydrogenase; TNF-α, tumour necrosis factor-α.
Discussion
We assessed the effect of DEX, a highly selective α2-adrenergic receptor agonist, on tissue injury and neurological outcome in a rat model of cervical SCI. DEX significantly improved functional recovery (paw placement and grooming tests), reduced lesion size, and spared spinal tissue, especially the white matter over 4 weeks after SCI. DEX inhibited activated microglia and promoted anti-inflammatory and pro-resolving gene expression at the injury site. In LPS-stimulated microglia, DEX produced a similar inflammation-modulatory effect, consistent with in vivo spinal injury. As a framework for considering how these findings can ultimately improve outcome in patients with SCI, we will address (i) how α2-adrenergic receptor signaling may be beneficial, (ii) how DEX may be changing the inflammatory milieu in the injured spinal cord, and (iii) whether DEX is the most appropriate pharmacon for this action.
Alpha2-adrenergic receptor signaling is beneficial and neuroprotective
Alpha-2 adrenergic receptor agonists, such as clonidine, have been used clinically in patients with SCI, especially in those with chronic injury, autonomic dysreflexia, and sexual dysfunction.31 Clonidine can also restore sensorimotor function, control autonomic dysfunction, and minimise spasticity in a cat SCI model, likely through its actions on the adrenergic system in spinal cord pathways.32 DEX, a more selective α2 agonist than clonidine, is anti-inflammatory in animal models of neurological disorders, including SCI.14, 22, 33, 34 However, its precise effects in the setting of traumatic SCI and the underlying mechanism for clinical benefit are not well understood. In this study, DEX was systemically delivered (i.p.) immediately after injury and every 2 h for 6 h on the day of SCI and once daily thereafter for 5 days. This regimen allowed acutely injured animals to remain in a sedated state for hours after injury, which reproduced the clinical scenario of acute post-SCI management in the OR and ICU. We observed a significant functional improvement as soon as 2 days post-SCI in the SD group, which was likely because of the multiple doses administered during Day 0, the day on which SCI occurred. At the injury epicentre, DEX reduced the lesion volume and spared white matter, although there was a trend of increased grey-matter preservation that was also observed with neuronal staining with anti-NeuN antibody (data not shown). Interestingly, the cavity area at the injury epicentre also appeared smaller in the SD group, most likely because of the spared white matter (observation only). These effects were largely negated by pretreatment with Yoh. Although Yoh blocks α2 adrenergic, dopaminergic and serotonergic receptors, it has extremely high affinity for α2-adrenergic receptors. Yoh may have off-target effects, but its dominant property at the dose used is α2-adrenergic receptor antagonism. The less-than-complete reversal by Yoh may be attributable to non-α2-adrenergic receptor-mediated actions of DEX that produce protection through imidazoline-receptor-mediated vagomimetic properties.35 As the mechanism for the beneficial effects of DEX is further elucidated, we can expect the development of selective probes for these targets that are devoid of the adverse cardiovascular effects (bradycardia and hypotension) of α2-adrenergic receptor agonists.
Dexmedetomidine modulates neuroinflammatory responses after spinal cord injury
The post-SCI inflammatory response aggravated the initial tissue injury, and strategies to minimise the response were effective in reducing tissue damage. However, if macrophages, the major effector cell in innate immune response, are alternatively activated to the M2 phenotype, its function can become pro-resolving and reparative. After SCI, both types of macrophages were present at the injury site, but they were predominantly pro-inflammatory.
As microglia/macrophages are known to express α2-adrenergic receptors and play important roles in neural tissue damage and repair,36, 37 DEX may impact the resolution of injured spinal tissue by directly modulating microglial activity.38 We selected anti-CD11b antibody to label and examine the local microglial response. CD11b-positive cells were reduced by DEX, especially in the area adjacent to the injury epicentre. Blockade of α2-adrenergic receptor signaling did not change the effect of DEX on the number of CD11b-positive cells, which suggests that the α2-adrenergic receptor-mediated DEX effect may have had a greater impact on microglial function. By analysing RNA expression in the spinal homogenate, we sought to determine which microglial phenotype predominated in the microenvironment after DEX intervention. The SCI-mediated increased expression of each of the pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) was downregulated in the DEX-treated groups. CD11b, the marker for activated microglia, was also reduced by DEX. In contrast, the anti-inflammatory cytokine, IL-4, was promoted by DEX early after injury. DEX further enhanced the expression of M2 phenotype markers, such as arginase-1 and CD206. We speculate that this pro-resolving profile in the injured tissue after DEX may be responsible for the improved repair and recovery. In addition, we used microglial cells (BV-2 cell line) to show that DEX directly influenced the microglial phenotype. The upregulation of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) by LPS was all suppressed in DEX-treated cells. Furthermore, DEX promoted a beneficial M2 response, as reflected by the increased transcription of IL-4, arginase-1, and CD206. These reactions are mainly mediated via α2-adrenergic receptor signaling. The findings in cell culture are consistent with the transcriptional changes in the in vivo SCI studies. Our gene expression study corroborated findings from recent work,13, 24, 39 yet the underlying molecular mechanism via α2-adrenergic receptor signaling needs to be further elucidated.
Are α2-adrenergic receptor agonists promising as therapeutic immunomodulatory agents?
The autonomic nervous system plays a pivotal host defence response against injury and stressful stimuli.38 Stimulation of α2-adrenergic receptors on immune cells regulates their development, function, and recruitment,40, 41 and DEX has been shown to modulate macrophage/microglial activity.13, 23, 24, 39 We showed that the effect of DEX on neurological and histological outcomes is associated with a favourable neuroinflammatory transcriptional profile after SCI. The DEX-induced changes in gene expression in the injured tissue suggest skewing of microglia towards the M2 phenotype. DEX can act directly on microglial α2-adrenergic receptors, and may regulate downstream pathways involved in microglial polarisation.14, 24, 42 We observed prominent vagomimetic symptoms (e.g. increased micturition and salivary secretion) after DEX treatment in spinally injured rats compared with vehicle controls. Activation of vagal response in these animals could potentially regulate systemic immune response via cholinergic anti-inflammatory mechanisms.35, 43 By modulating both neuroinflammation and systemic immune response, DEX and other selective α2 agonists are promising therapies that can be used beyond the current clinical indication for neurotrauma (including SCI) to exploit their neuroprotective and immunomodulatory properties.
The interpretation of our findings must consider several limitations. Pattern recognition receptors are activated after SCI, likely through both damage-associated molecular patterns (DAMPs, e.g. acute sterile injury) and pathogen-associated molecular patterns (PAMPs, e.g. subacute and chronic systemic infection). In this experiment, we aimed to investigate the effect of DEX on neuroinflammation and chose LPS (PAMPs) as a stimulant for microglial activation. DAMPs (e.g. high-mobility group box 1 protein) can produce injury through different mechanisms, although these will likely converge on a similar downstream pathway. Therefore, LPS has limitations as a stimulant for model of neuroinflammation; and further studies are necessary. Most importantly, immune mechanisms in rodent may not mimic those in humans, so these results will require clinical confirmation.
Authors' contributions
Study concept/design: JCB, MM, MSB, JZP
Animal surgery, post-injury care, and behavioural tests: AL, JG, ZS, ZX, Y-WC, JZP
Cell culture: GW, XN, QD
Histology: AL, JG, ZS, Y-WC, ZX, QD
Polymerase chain reaction: JG, ZS, XN, YS, WS
Provided key reagents: JCB, MSB, JZP
Data analysis: all authors
Drafting of manuscript: JCB, MM, MSB, JZP
Revision and approval of the final version of the article: all authors
Declaration of interest
The authors declare they have no conflicts of interest.
Funding
University of California, San Francisco Anesthesia Department US National Institutes of Health T32 training grant, Foundation for Anesthesia Education and Research training grant, and University of California, San Francisco bridge funds to JZP; US National Institutes of Health (R01 NS038097) to JCB and MSB; National Natural Science Foundation of P.R. China (No. 81471373) to ZX.
Acknowledgements
The murine microglial BV-2 cell line, developed by E. Blasi (University of Perugia, Perugia, Italy), was generously provided by L. Van Eldik from the University of Kentucky. The authors also thank Xiaokui Ma (staff research associate, Department of Neurological Surgery, University of California, San Francisco) for her excellent technical support in histology staining.
Handling editor: H.C. Hemmings Jr
Editorial decision: 17 August 2019
Contributor Information
Michael S. Beattie, Email: Michael.Beattie@ucsf.edu.
Jonathan Z. Pan, Email: Jonathan.Pan@ucsf.edu.
References
- 1.Ahmed A., Patil A.A., Agrawal D.K. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol Cell Biochem. 2017;441:181–189. doi: 10.1007/s11010-017-3184-9. [DOI] [PubMed] [Google Scholar]
- 2.Beattie M.S., Hermann G.E., Rogers R.C., Bresnahan J.C. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly D.J., Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shultz R.B., Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. 2017;12:702–713. doi: 10.4103/1673-5374.206633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novrup H.G., Bracchi-Ricard V., Ellman D.G. Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. J Neuroinflammation. 2014;11:159. doi: 10.1186/s12974-014-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B., Bailey W.M., Kopper T.J., Orr M.B., Feola D.J., Gensel J.C. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popovich P.G., Guan Z., Wei P., Huitinga I., van Rooijen N., Stokes B.T. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly D.J., Longbrake E.E., Shawler T.M. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz M., Yoles E. Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J Neurotrauma. 2006;23:360–370. doi: 10.1089/neu.2006.23.360. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff R.M. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 11.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell M.T., Agoston V.A., Freeman K.A. Interruption of spinal cord microglial signaling by alpha-2 agonist dexmedetomidine in a murine model of delayed paraplegia. J Vasc Surg. 2014;59:1090–1097. doi: 10.1016/j.jvs.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Kim E., Kim H.C., Lee S. Dexmedetomidine confers neuroprotection against transient global cerebral ischemia/reperfusion injury in rats by inhibiting inflammation through inactivation of the TLR-4/NF-kappaB pathway. Neurosci Lett. 2017;649:20–27. doi: 10.1016/j.neulet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Bell M.T., Puskas F., Bennett D.T. Dexmedetomidine, an alpha-2a adrenergic agonist, promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion. J Thorac Cardiovasc Surg. 2014;147:500–506. doi: 10.1016/j.jtcvs.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Kimelberg H.K. Neuroprotection by alpha 2-adrenergic agonists in cerebral ischemia. Curr Neuropharmacol. 2005;3:317–323. doi: 10.2174/157015905774322534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibacache M., Sanchez G., Pedrozo Z. Dexmedetomidine preconditioning activates pro-survival kinases and attenuates regional ischemia/reperfusion injury in rat heart. Biochim Biophys Acta. 1822;2012:537–545. doi: 10.1016/j.bbadis.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z., Zhao T., Lv S., Gao Y., Masters J., Weng H. Dexmedetomidine attenuates spinal cord ischemia-reperfusion injury through both anti-inflammation and anti-apoptosis mechanisms in rabbits. J Transl Med. 2018;16:209. doi: 10.1186/s12967-018-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Can M., Gul S., Bektas S., Hanci V., Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesth Scand. 2009;53:1068–1072. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 20.Celik F., Gocmez C., Kamasak K. The comparison of neuroprotective effects of intrathecal dexmedetomidine and metilprednisolone in spinal cord injury. Int J Surg. 2013;11:414–418. doi: 10.1016/j.ijsu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Gul S., Hanci V., Bahadir B. The effectiveness of dexmedetomidine in experimental spinal cord injury compared to methylprednisolone in rats. J Clin Neurosci. 2010;17:490–494. doi: 10.1016/j.jocn.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Rong H., Zhao Z., Feng J. The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun. 2017;64:195–207. doi: 10.1016/j.bbi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Wang W.G., Wang L., Jiao Z.H., Xue B., Xu Z.W. Locomotor activity of rats with SCI is improved by dexmedetomidine by targeting the expression of inflammatory factors. Mol Med Rep. 2018;18:415–420. doi: 10.3892/mmr.2018.8930. [DOI] [PubMed] [Google Scholar]
- 24.He H., Zhou Y., Zhou Y. Dexmedetomidine mitigates microglia-mediated neuroinflammation through upregulation of programmed cell death protein 1 in a rat spinal cord injury model. J Neurotrauma. 2018;35:2591–2603. doi: 10.1089/neu.2017.5625. [DOI] [PubMed] [Google Scholar]
- 25.Gensel J.C., Tovar C.A., Hamers F.P., Deibert R.J., Beattie M.S., Bresnahan J.C. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- 26.Irvine K.A., Ferguson A.R., Mitchell K.D. The irvine, beatties, and bresnahan (IBB) forelimb recovery scale: an assessment of reliability and validity. Front Neurol. 2014;5:116. doi: 10.3389/fneur.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue T., Lin A., Ma X. Combined SCI and TBI: recovery of forelimb function after unilateral cervical spinal cord injury (SCI) is retarded by contralateral traumatic brain injury (TBI), and ipsilateral TBI balances the effects of SCI on paw placement. Exp Neurol. 2013;248:136–147. doi: 10.1016/j.expneurol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang Y.W., Waxman S.G. Minocycline attenuates mechanical allodynia and central sensitization following peripheral second-degree burn injury. J Pain. 2010;11:1146–1154. doi: 10.1016/j.jpain.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Pan J.Z., Jornsten R., Hart R.P. Screening anti-inflammatory compounds in injured spinal cord with microarrays: a comparison of bioinformatics analysis approaches. Physiol Genomics. 2004;17:201–214. doi: 10.1152/physiolgenomics.00177.2003. [DOI] [PubMed] [Google Scholar]
- 30.Blasi E., Barluzzi R., Bocchini V., Mazzolla R., Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 31.Courtois F., Rodrigue X., Cote I. Sexual function and autonomic dysreflexia in men with spinal cord injuries: how should we treat? Spinal cord. 2012;50:869–877. doi: 10.1038/sc.2012.83. [DOI] [PubMed] [Google Scholar]
- 32.Naftchi N.E. Functional restoration of the traumatically injured spinal cord in cats by clonidine. Science. 1982;217:1042–1044. doi: 10.1126/science.6126002. [DOI] [PubMed] [Google Scholar]
- 33.Ma D., Hossain M., Rajakumaraswamy N. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Goyagi T., Tobe Y. Dexmedetomidine improves the histological and neurological outcomes 48 h after transient spinal ischemia in rats. Brain Res. 2014;1566:24–30. doi: 10.1016/j.brainres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Hu J., Vacas S., Feng X. Dexmedetomidine prevents cognitive decline by enhancing resolution of high mobility group box 1 protein-induced inflammation through a vagomimetic action in mice. Anesthesiology. 2018;128:921–931. doi: 10.1097/ALN.0000000000002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H., Alam A., Chen Q. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth. 2017;118:504–516. doi: 10.1093/bja/aex006. [DOI] [PubMed] [Google Scholar]
- 37.Kroner A., Rosas Almanza J. Role of microglia in spinal cord injury. Neurosci Lett. 2019;709:134370. doi: 10.1016/j.neulet.2019.134370. [DOI] [PubMed] [Google Scholar]
- 38.Bellinger D.L., Lorton D. Autonomic regulation of cellular immune function. Auton Neurosci: Basic & Clin. 2014;182:15–41. doi: 10.1016/j.autneu.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P., Li Y., Han X., Xing Q., Zhao L. Dexmedetomidine Regulates 6-hydroxydopamine-Induced Microglial Polarization. Neurochem Res. 2017;42:1524–1532. doi: 10.1007/s11064-017-2209-9. [DOI] [PubMed] [Google Scholar]
- 40.Katayama Y., Battista M., Kao W.M. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Ajmo C.T., Jr., Collier L.A., Leonardo C.C. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao Y., Li L., Jiang B. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav Immun. 2016;58:118–129. doi: 10.1016/j.bbi.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Tracey K.J. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]