Abstract
Mesenchymal stem cells (MSCs) represent an invaluable asset for the field of cell therapy. Human Bone marrow-derived MSCs (hBM-MSCs) are one of the most commonly used cell types in clinical trials. They are currently being studied and tested for the treatment of a wide range of diseases and conditions. The future availability of MSCs therapies to the public will require a robust and reliable delivery process. Cryopreservation represents the gold standard in cell storage and transportation, but its effect on BM-MSCs is still not well established. A systematic review was conducted to evaluate the impact of cryopreservation on BM-MSCs and to attempt to uncover the reasons behind some of the controversial results reported in the literature. Forty-one in vitro studies were analysed, and their results organised according to the cell attributes they assess. It was concluded that cryopreservation does not affect BM-MSCs morphology, surface marker expression, differentiation or proliferation potential. However, mixed results exist regarding the effect on colony forming ability and the effects on viability, attachment and migration, genomic stability and paracrine function are undefined mainly due to the huge variabilities governing the cryopreservation process as a whole and to the lack of standardised assays.
Keywords: Bone-marrow derived mesenchymal stem cells, Cell therapy, Cryopreservation, Mesenchymal stem cells, Tissue culture, Systematic review
Background
Bone marrow non-hematopoietic stem cells represent a fraction of the bone marrow cell population. They may arise from the constituents of the bone marrow structure and they can differentiate into mesenchymal tissues such as adipose, cartilage and bone. Bone marrow non-hematopoietic stem cells were first mentioned by Julius Cohnheim in 1867 and later cultured and characterized by Freidenstein et al. in the 1970s [1–4]. Friedenstein demonstrated that bone marrow non-hematopoietic stem can be selected by adherence to culture flask and exhibit the following characteristics: fibroblast morphology, colony-forming ability and in vitro proliferation and differentiation potentials [5]; all of which were indicative of ‘stemness’ properties [6]. With that said, it must be noted that within the scientific community, there is still an ongoing discussion about the true nature of these cells. Two names propagated for these cells “Stromal Stem Cells” [7] and “Mesenchymal Stem Cells” [8, 9].
The then newly discovered source of stem cells has attracted great interest in medical research. In addition to the characteristics listed above, isolating mesenchymal stem cells from bone marrow was surrounded with minimal ethical issues and could substitute embryonic stem cells [6]. Therefore, hBM-MSCs became the subject of intense research and in 1995 the first autologous intravenous infusion of these cells in cancer patients was performed [10]. Later, MSCs have been shown to have widespread immunomodulatory effects [11] as well as an angiogenic induction ability [12]. Taken together these characteristics enlarged the scope of application of hMSC-based therapies. As of April 2019, a search on the U.S. National Library of Medicine (ClinicalTrials.gov) using the term ‘bone marrow mesenchymal stem cells’ retrieved 368 clinical trials aiming to treat conditions like stroke, graft versus host disease, osteoarthritis, crohn’s disease, ischemic heart disease and multiple sclerosis.
The future availability of cell therapies to the public will be dependent on easy and quick logistics as well as robust and reliable delivery process. Abazari et al. [13] suggested that if cell therapies “cannot be delivered clinically and logistically then their benefit is irrelevant”. Cryopreservation remains the cell therapy industry “standard” for biopreservation [14] as well as the primary option of storage for hMSC-based products [15]. In fact, cryostorage has evolved from being a marginal process in the cell therapy manufacturing process to become a tool widening the availability of stem cell therapy in particular and regenerative medicine in general. However, despite its evolving role, cryobiology is lagging behind the speed at which the cell therapy industry is growing.
Cryopreservation is particularly crucial for a successful cell therapy for various reasons. It facilitates cell transport, it enables the generation of cell banks with indefinite shelf-life thus ensuring off-the-shelf steady supply, access and availability and it gives time for quality control testing and in vitro assays [14, 16, 17]. In addition, cryostoring therapeutic doses of cells in hospitals and clinics could make cell therapy a treatment choice for many diseases and conditions including acute conditions [18]. Furthermore, cryopreserved cells are ideal for sequential treatments such as the case of chronic heart failure or ischemic heart disease to ensure the consistency of the treatment [19]. Banking cells is also an appropriate option from an economical and a regulatory aspect [20]. The logistics of administration of MSC in many immunotherapy trials were simply described as cryopreserving cells, thawing them when needed and administering them within a couple of hours. This scenario would only be feasible if thawed cells preserved their viability, safety and potency [20].
Cryopreservation of cells is associated with several injuries; physical and molecular. A controversy still exists about the efficacy of fresh cells versus cryopreserved and whether viability implies functionality [21]. In early MSC-based clinical trials, using cryopreserved cells was hypothesised to be the source of failure [21]. In addition, the variability in the outcome of MSC-based clinical trials was proposed to mainly be due to the functional alterations that the freeze–thaw process provokes in MSCs rather than the freezing method itself [17].
Human Bone marrow-derived MSCs (hBM-MSCs) are the most commonly used source of MSCs in clinical trials [22] and have been deployed across 17 European centres manufacturing MSCs [23]. The effects of cryopreservation on this type of cells are not well defined. The aim of this review is to assess whether rigorous data exist regarding the impact of the freeze–thawing process on BM-MSCs phenotypic and functional traits. To our knowledge, this is the first review to factor numerous aspects of the freezing process (freezing solution composition, the freezing protocol, the duration of storage, the concentration of cells at freezing, the passage number at freezing as well as the thawing method) in one analysis, for studies conducted over about 20 years. Such detailed analysis may allow firm conclusions to be drawn regarding BM-MSCs performance after the freeze-thawing process as well as help uncover possible reasons behind some of the controversial existing results and highlight areas which require further investigation.
Methods
The inclusion criteria for this review were: Articles or conference papers assessing the impact of cryopreservation by slow freezing on BM-MSCs in suspension. There was no restriction on the species from which cells were derived. Studies where bone marrow itself was frozen, where freezing of BM-MSCs was done by vitrification or using a 3D structure and where cryopreservation impact was only assessed in vivo, were excluded. A systematic literature search was conducted using PubMed, Science direct and Google Scholar (last search performed April 2019). Two combinations of search terms were used ‘cryopreservation mesenchymal’ and ‘freezing mesenchymal’. The output of each search was first scanned for the relevance of title. Articles were excluded if the topic is unrelated or when an eligibility criterion is not met. The retained articles were then screened for the relevance of abstracts (and in few cases materials and methods) and retained when meeting all the eligibility criteria (Fig. 1). From the 41 retained studies, information regarding the freezing solution composition, the freezing protocol, the duration of storage, the concentration of cells at freezing, the passage number at freezing as well as the thawing method was extracted and tabulated. Next, studies were grouped in tables according to the “hMSC checklist” proposed in [24]. Cell surface marker expression, differentiation potential, proliferation and growth, attachment and migration potential, genomic stability and paracrine function were examined. In addition, post-thaw viability and morphology information was also collated because they are primary evaluators of cryopreservation.
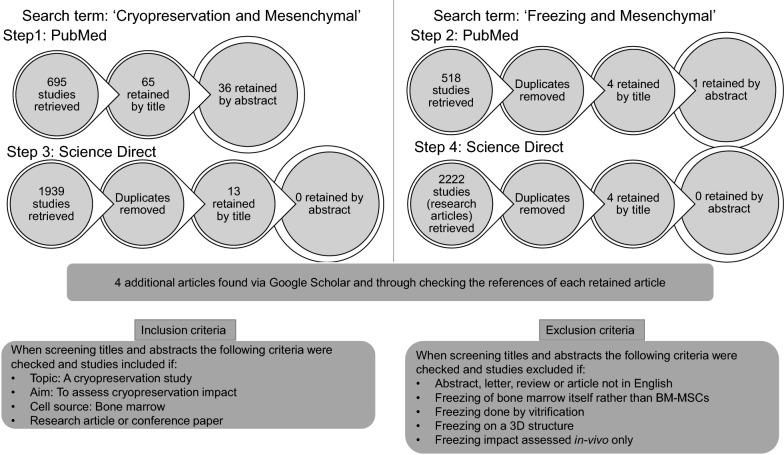
Fig. 1.
Schematic representation of the Bone-marrow derived mesenchymal stem cell cryopreservation search strategy. Diagram of the current systematic search analysis. Studies of bone-marrow derived mesenchymal stem cells aligned to cryopreservation and/or freezing were identified using a combination of two search terms ‘cryopreservation mesenchymal’ and ‘freezing mesenchymal’ using PubMed, Science direct and Google scholar. The output of each search was scanned at the title level, then at the abstract level and articles were retained when meeting eligibility criteria, both inclusion and exclusion (see boxes titled inclusion criteria and exclusion criteria in this figure). In specific, for the term ‘cryopreservation and mesenchymal’ in PubMed, 695 studies were retrieved. By checking the titles against the eligibility criteria, only 65 studies were retained. The abstracts of these 65 articles were then read and checked against the eligibility criteria and only 36 of the 65 articles were retained. For the subsequent searches, these steps of retaining and eliminating articles were followed but preceded by eliminating duplicates i.e. articles which appeared in previous searches
Results
Species, freezing and thawing methods
As shown in Fig. 1 41 studies met the inclusion criteria. MSCs were isolated from the bone marrow of 10 different species which included human (26 studies), rat (5 studies), monkey (3 studies), dog (3 studies), horse (2 studies), pig (2 studies), minipig (1 study), mouse (1 study), calf (1 study) and sheep (1 study) (Fig. 2).
Fig. 2.
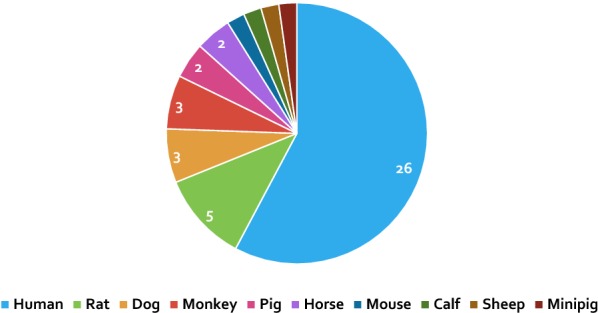
Pie chart showing the number of studies per species. The numbers of studies are presented on the diagram in Arabic numerals unless it is only a single study i.e. where no number is presented this species only represents a single included study. Of note, 3 of the 41 studies appear more than once because they have used more than one species, hence the total number of studies as it appears in this pie chart is 45
Across the retained studies, various freezing media formulations were used. 20% of studies (17% human) used commercially available freezing solution such as CELLBANKER and CRYOSTOR while the rest used “in-lab” homemade formulations. 66% of studies (41% human) used or tested various amounts of serum in the freezing media with the serum principally being animal-derived, 20% of studies (12% human) froze cells in serum-free media while 5% used freezing media containing plasma or human platelet lysates (all of which are human studies). For 17% of the studies, not enough information was included about FBS content and/or about the composition of the freezing medium (12% human). Of the serum-free studies, one study assessed the efficiency of Sericin as a substitute to FBS [25]. Across all of the studies, 13 assessed the freezing in xeno-free media. More than 90% of studies used dimethylsulfoxide (DMSO) at a concentration ranging from 1 to 20% with 10% being the most commonly used. Carboxylated poly-l-lysine (COOH-PLL) was investigated as a cryoprotectant to replace DMSO [26] and hydroxyethyl starch was added to freezing solution as a strategy to reduce the percentage of DMSO [27]. Two studies tested various freezing solutions containing polyethylene glycol (PEG), trehalose and 1,2 propanediol in order to develop a well-defined, serum-free and reduced-DMSO freezing solution [28, 29]. Only two studies utilized strategies to prevent post-thaw apoptosis through the addition of Rho-associated kinase inhibitor [30] and Caspase inhibitor z-VAD-fmk [31] in the freezing media.
Concerning freezing protocols, two procedures prevail. The first involves incubating the cells at a freezing rate of − 1 °C/min in a − 80 °C freezer for several hours (up to 24 h) then moving the cells to liquid nitrogen (LN2). The second is based on a two to seven-step sequential freezing process using a programmable freezing device to freeze the cells prior to − 150 °C freezer or LN2 storage. Four studies reported whether cells were stored in liquid phase [32] or vapour phase [30, 33, 34] of LN2. Five studies stored the cells at − 80 °C [26, 35–38] and 1 study stored the cells at − 70 °C [39].
Seven studies (3 human and 4 animal) did not specify the passage number at which cells were frozen. Six studies (3 human and 3 animal) used cells at passage 1, one study (monkey) used cells at passage 9 and the rest (20 human and 6 animal) used cells at passages ranging from 1 to 6. The concentration of cells at freezing was very variable ranging from 1 * 105 to 1 * 107 cells/mL with 1 * 106 cells/mL being the most frequently used (17 studies; 9 human and 8 animal). There was only one study in which human-derived cells were frozen in cryopreservation bags at a concentration of 1.8 * 108 [40]. There was a huge variation regarding the duration of storage of cells in the frozen state; the shortest period being 1 h [30] and the longest 10 or more years [37].
Seven studies (human) did not include information about their thawing protocols. Two studies (1 human and 1 animal) just mentioned ‘quickly thawed’ [20, 39], one in α-MEM [41], one at room temperature [37] and one in a 37 °C incubator [42]. For all the rest (16 human and 13 animal), there was some consistency; cells were typically thawed at 37 °C, most likely in a water bath with or without gentle agitation for 1–4 min.
Post-thaw assessment
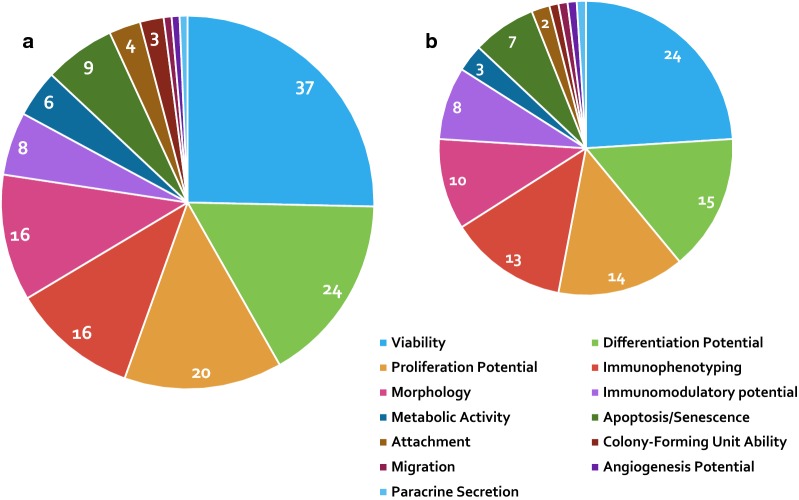
Presented in Fig. 3 are how many of the forty-one retained studies assessed different cellular attributes, it shows that viability and differentiation potential are the only attributes assessed in over half of the retained studies.
Fig. 3.
a Pie chart showing the proportion of the retained studies assessing different cellular attributes in all species. b Pie chart illustrating the proportion of human retained studies assessing different cellular attributes. It is supported by Additional file 1 which aggregates and delineates which studies undertook which analyses in a tabular form [arranged by species: human (chronologically and then alphabetically) and animals from most to least frequent species (chronologically and then alphabetically)]. Additional file 1 is a grid identifying which cell attributes each of the forty-one studies assessed. Of note each of the 41 studies may appear more than once depending on the attributes they assessed
Viability
Post-thaw viability was the most assessed cell attribute (37 studies). Table 1 lists the studies which assessed viability immediately post-thaw (37 studies) or after a period of post-thaw culture, which was monitored in five studies (post-thaw time point from 4 h to 3 passages). Three main methods for viability assessment were used; trypan blue exclusion, flow cytometry and fluorescent microscopy. The immediately post-thaw viability varied from about 50% to 100% which is noteworthy. Sixteen studies reported no change in viability immediately after thawing while 10 studies reported significantly lower viability. In some studies where a new freezing formulation was tested, viability was compared to 10% DMSO which is still considered the gold standard for freezing BM-MSCs. The timing of viability measure is crucial due to the induction of apoptotic events some time post-thaw [43]. As mentioned above only five studies assessed the long-term effect (up to 3 passages post-thaw of freezing) with two reporting lower viability and three reporting no effect. It was also noted that variability in the level of viable cells within the same study differed when using different methods of measurement such as trypan blue exclusion compared to flow cytometry [44, 45].
Table 1.
Experimental studies assessing viability immediately post-thaw or after a period of post-thaw culture
| Study | Species | Method of freezing | Concentration at freezing | Method of thawing | Passage number at freezing | Results post-thaw | Method of assessment |
|---|---|---|---|---|---|---|---|
| Human | |||||||
| Bruder et al. [91] | Human | FBS with 10% DMSO in LN2 (24 h) | NA | NA | NA | Cell recovery after thawing was above 95% | Trypan blue exclusion |
| Hirose et al. [41] | Human | Cell Banker storage medium, cells cryopreserved at − 150 °C (NA) | 5 * 105 cells/mL | Cells were thawed in MEM-α supplemented with 15% FBS | P1 | Immediately post-thaw viability was retained post-thaw at about 98% | Fluorescent microscopy: live/dead viability assay kit |
| Kotobuki et al. [35] | Human | Cell Banker medium, cryopreserved at − 80 °C (NA) | 5 * 105 cells/mL | NA | P1 | Immediately post-thaw viability was retained post-thaw at above 90% | NucleoCounter (ChemoMetec) |
| Kotobuki et al. [92] | Human | Cell Banker storage medium (ready-to-use storage medium), then cells stored sequentially: 10 min at 4 °C, 1 h at − 30 °C, 2–3 days at − 80 °C then long-term storage at − 152 °C (0.3–33.6 months) | 5 * 105 cells/mL | NA | P1 | Immediately post-thaw viability ranged from 71.9 to 100% with average viability about 90% | NucleoCounter (ChemoMetec) |
| Xiang et al. [93] | Human | 30% serum-containing α-MEM with 10% DMSO, 4 °C for 10 min then cooled to − 80 °C at 1 °C/min in a controlled-rate freezer then LN2 (12 months) | 1 * 105 cells/mL | Thawed in a 37 °C water bath by shaking lightly for 1 or 2 min | P3 | Immediately post-thaw viability ranged from 84.6 to 100% | Flow cytometry—fluorescein diacetate, PI |
| Zhao et al. [94] | Human (with chronic myeloid leukaemia) | IMDM with 40% FCS and 10% DMSO at 4 °C, beaker with methanol in − 70 °C freezer for 24 h then LN2 (3 or 6 months or 1 year) | 1 * 106 cells/mL | 37 °C water bath for 2–4 min | P2–3 | Immediately post-thaw viability was retained post-thaw at about 90% | Trypan blue exclusion |
| Heng [30] | Human | Culture medium with 10% DMSO and 0, 10 or 100 microM of Rho-associate kinase (ROCK) inhibitor Y-27632, cooling to − 80 °C for 2 h, then vapour phase of LN2 (1 h) | 1.17 * 105 cells/mL | Thawed in a 37 °C water bath | P5 | Immediately post-thaw viability dropped to a range about 91.3% to 89.4%; No effect of Y-27632 immediately post-thaw but there was an increase in viability at 24 h post-thaw | Trypan blue exclusion |
| Liu et al. [28] | Human | 13 different freezing media tested with various combinations of different concentrations of serum, DMSO, PEG, trehalose and 1.2-Propanediol, equilibration of cells with freezing media at 4 °C for 10 min, − 80 °C overnight then LN2 (min. 1 week) | 1 * 106 cells/mL | Thawed in a 37 °C water bath, shaking gently for 2 min | N/A | A freezing solution composed of 7.5% c(v/v) DMSO, 2.5% (w/v) PEG, 2% bovine serum albumin gave comparable viability (about 82.9%) to 10% DMSO (about 82.7%) | Flow cytometry–PI |
| Doan et al. [95] | Human | DMEM/F12 with 10% DMSO, incubation, 4 °C for 10 min, − 20 °C for 1 h, − 80 °C for 1 day then LN2 (1 year) | 1 * 106 cells/mL | In a water bath at 37 °C | P3 | Immediately post-thaw viability was retained post-thaw at 72.95% | Cell Viability Analyzer (Beckman Counter, USA) |
| François et al. [45] | Human | α-MEM with 30% FBS and 5% DMSO, − 80 °C for 24 h then LN2 (1 week) | NA | NA | Early passage | Immediately post-thaw viability dropped to ≤ 60% (Annexin V/PI) and > 80% (Trypan blue); At 4 h post-thaw viability was between 44 and 61%; viability increased after post-thaw culture | Trypan blue exclusion |
| Ginis et al. [50] | Human | CryoStor-2, CryoStor-5, CryoStor-10 containing 2%, 5% and 10% DMSO respectively or conventional freezing medium (90% growth medium with 10% FCS, 30% bovine serum albumin and 10% DMSO), pre-cooling on ice for 10 min, slowly cooled to − 5 °C, blast of chilling to − 25 °C, quick return to − 5 °C, cooling to − 60 °C at a rate of 1 °C/min, cooling to − 196 °C at a rate of − 25 °C using programmable cell freezer then LN2 (about 1 month or 5 months) | 1 * 106 cells/mL | Thawed fast in a 37 °C water bath with gentle agitation | P2–4 | Immediately post-thaw viability after 1-month freezing was about 91.7% and 95.6% and 95.4% for CryoStor-2, CryoStor-5, CryoStor-10 respectively; Immediately post-thaw viability after 5-month freezing was about 72% and 80% for CryoStor-5 and CryoStor-10 respectively | Fluorescence uptake: calcein-AM, ethidium homo-dimer-1 |
| Mamidi et al. [33] | Human | 90% FBS with 10% DMSO, programmable slow freezing unit then vapour phase of LN2 (long-term storage) | 3 * 106 cells/2 mL vial | Thawed in a 37 °C water bath, shaking gently for 1–2 min | P3 and then characterized at P4–6 (with another freezing at passage 4) | Viability was about 80% upon thawing then > 95% after subsequent plating (3 passages post-thaw) | Trypan blue exclusion; flow cytometry—7-AAD |
| Matsumura et al. [26] | Human | COOH-PLLs 7.5% (w/w) at pH of 7.4 OR 10% DMSO in DMEM without FBS, − 80 °C freezer (1 week or 24 months) | 1 * 106 cells/mL | Thawed in a 37 °C water bath with gentle shaking | P3–5 | Cryopreservation for one week with PLL (0.5–0.8) did not affect the viability at 0 h and 6 h post-thaw; Cryopreservation for 24 months with PLL (0.65) provides protection comparable to 10% DMSO | Trypan blue exclusion |
| Chinnadurai et al. [20] | Human | Freezing media, − 80 °C then LN2 (NA) | 5 * 106 cells/mL | Quickly thawed (1–2 min) | P3–5 | Immediately post-thaw viability dropped to about 87% (trypan blue) and 71.5% (flow cytometry) | Trypan blue exclusion; flow cytometry—Annexin V, PI |
| Holubova et al. [69] | Human | 60% α-MEM medium with 30% pHPL and 10% DMSO, programmable controlled rate freezer at rate 1 °C/min to − 80 °C then LN2 (1,3,6,7 and 8 months) | 1 * 106 cells/mL | NA | P3 | Immediately post-thaw viability is 70–90% | Flow cytometry—7-AAD staining |
| Moll et al. [38] | Human | 4 °C human blood type AB plasma containing 10% DMSO, frozen to − 80 °C using rate-controlled cell freezing device (NA) | 1–2 * 106 cells/mL | NA | P2–4 | Viability reduced twofold by cryopreservation when exposed to human serum (cell count and PI incorporation) | Cell counter and analyser system (CASY-TT); flow cytometry–Annexin V, PI |
| Verdanova et al. [25] | Human | 15 different freezing solutions containing various concentrations of DMSO (0, 1, 5, 10 and 100%) in the presence or absence of sericin at 1 or 5%, cooling to − 80 °C at a rate 1 °C/min in a CoolCell container then LN2 (72 h) | 1.4 * 105 cells/mL | In a 37 °C water bath as quickly as possible | P1–3 | Highest viability (24 h post-thaw) was obtained using standard freezing medium (10% DMSO and 25% FBS in culture medium); Viability of cells (24 h post-thaw) frozen in culture medium containing 10% DMSO and 1% sericin was not significantly different from standard freezing medium | Fluorescent microscopy—DAPI |
| Al-Saqi et al. [66] | Human | 10% DMSO in Mesencult-XF or STEM-CELLBANKER at 4 °C, cryovials on ice then moved to − 80 °C with a cooling rate − 1 °C/min for 24 h then then LN2 (NA) | 0.5–1 * 106 cells/mL | Thawed in a 37 °C water bath for 1 or 2 min | P3 | No difference in viability immediately post-thaw between two freezing media; CELLBANKER (85.6%) and 10% DMSO (86%); No significant difference in viability between non-cryopreserved and cryopreserved using both media | Fluorescence-based live/dead assay immediately post-thaw; flow cytometry—PI (two passages post-thaw) |
| Luetzkendorf et al. [40] | Human | 5% human albumin and 10% DMSO, automatized process in a programmable freezer then LN2 (21–51 days) | 1.8 * 108 in cryopreservation bags | Thawed at | P3–4 | Immediately post-thaw viability was retained at > 90% viability using both methods for 4 donors out of 5 | Trypan blue exclusion; flow cytometry: 7-AAD |
| Pollock et al. [67] | Human | 60% plasmalyte A, 20% of 25% HAS and 20% DMSO (final concentration of DMSO was 10% by volume), controlled rate freezer then LN2 (30–45 days) | 1–10 * 106 cells/mL | Thawed quickly in a 37 °C | P1–6 | Immediately post-thaw viability was retained at > 80% for almost all samples | Fluorescent microscopy—Acridine orange, PI |
| Chinnadurai et al. [68] | Human | IFNɣ, caspase inhibitor Z-VAD-FMK or 3-methyl adenine pre-licensing 48 h prior to cryopreservation, 5% human serum albumin, 5%, 20%, 40%, 90% hPL in aMEM with 10% DMSO OR CryoSOfree DMSO-free cryopreservation medium, cooling rate 1 °C/min then step-down freezing using a 7-step program in CryoMed controlled-rate freezer then LN2 (NA) | 5–10 * 106 cells/mL | In a 37 °C water bath for 1 min | P2–6 | The addition of various concentrations of human platelet lysate did not significantly enhance MSC recovery and viability; IFNɣ pre-licensing prior to cryopreservation enhances thawed MSC survival | Trypan blue exclusion; flow cytometry—7-AAD |
| Gramlich et al. [18] | Human | CryoStor CS5 media, − 80 °C for 90 min then vapour phase of LN2 (7–30 days) | 1 * 106 cells per mL | In a 37 °C water bath | P3–5 | Immediately post-thaw viability was retained at > 95% (viability only marginally reduced after thawing) | TUNEL staining; Fluorescent microscopy—Hoechst, PI |
| Lechanteur et al. [34] | Human | 40% PBS + 40% of HSA solution (20%) + 20% DMSO added under agitation at 4 °C, automated cryofreezer with a 9-step program to − 160 °C then vapour phase of LN2 (NA) | 2 * 106 cells/mL | Freezing bag is protected in sterile plastic bag and thawed in a 37 °C water bath for a few min | P3 | Immediately post-thaw viability ranged from about 50% to 90% with about 14% decrease in viability | Trypan blue exclusion |
| Yuan et al. [52] | Human (BM-MSC engineered to express TRAIL) | 5% DMSO, 30% FBS in alpha-MEM OR human albumin with 0.5–20% DMSO, isopropanol freezing box overnight in, − 80 °C freezer then LN2 (1–3 weeks) | 1 * 106 cells/mL or 5 * 106 cells/mL or 10 * 106 cells/mL | In a water bath at 37 °C with gentle shake for 2 min | P5 | Significantly reduced immediately post-thaw viability with 0% DMSO (5.16%); Immediately post-thaw viability increased with increased DMSO% in the freezing; 15% and 20% DMSO gave reduced viability (about 70.6% and 64.1% respectively) immediately post-thaw solution up to 10%; at 5% DMSO same viability obtained for different cell concentrations | Flow cytometry—Annexin V, DAPI |
| Other species | |||||||
| Carvalho et al. [44] | Rat | DMEM with 10% FBS and 5% DMSO, cells incubate at room temperature for 15 min then vials cooled at 3 °C/min, 5 °C/min, 10 °C/min during 15, 45, 10 min respectively until − 80 °C using programmable freezing device then LN2 (1 month) | 1 * 107 cells/mL | Thawed in a 37 °C water bath with constant gentle shaking | Frozen down after 4 weeks in culture | Immediately post-thaw viability dropped to about 90.58% (trypan blue) and 66.25% (flow cytometry) | Trypan blue; flow cytometry—Annexin V, 7-AAD |
| Liu et al. [29] | Rat, mouse and calf | 14 different freezing solutions tested with various combinations of different concentrations of serum, DMSO, PEG, trehalose and 1.2-Propanediol, equilibration for 15 min at 4 °C, − 80 °C overnight then LN2 (min. 1 week) | 1 * 106 cells/mL | Thawed in a 37 °C water bath with gentle shaking for 2 min | NA | There were variations between species with respect to cell viability—Mouse MSCs were more robust than rat and bovine MSCs; Reduced DMSO (5%) with 2% PEG, 3% trehalose and 2% albumin gave higher immediately post-thaw viability (91.5% [mouse]) to 10% DMSO (75.3% [mouse]) | Trypan blue exclusion |
| Naaldijk et al. [27] | Rat | Cryoprotectant consisted of hydroxyethyl starches of different mean molecular weights [MW = 109, 209, 309, 409, 509, 609 kDa] and/or DMSO, then cells were frozen according to one of seven different freezing protocols (NA) | 1 * 105 cells/0.5 mL | Thawed in a 37 °C water bath | P1–3 | Immediately post-thaw viability was approximately 85%; viability after 3 days of thawing was lower | Trypan blue exclusion |
| Davies et al. [42] | Rat | 10% DMSO in 90% FBS, then vials incubated for 1 h at 4 °C, 2 h at − 20 °C, overnight at − 80 °C then LN2 (NA) | 1 * 106 cells/mL | Thawing in a 37 °C RS Galaxy S + incubator for about 5 min | P1 | Immediately post-thaw viability was retained post-thaw at > 90%; But lower viability was obtained after in vitro expansion of cryopreserved cells | Trypan blue exclusion |
| Renzi et al. [31] | Sheep, horse and rat | 13 different freezing media tested with various combinations of different concentrations of FBS, DMSO, Trehalose, hydroxyethyl starch, bovine serum albumin and Caspase inhibitor z-VAD-fmk, 4 °C for 60 min, gradual reduction of temperature − 1 °C/min to − 40 °C, − 10 °C/min to − 70 °C in a controlled rate freezer then vapour phase of LN2 (5 days) | 1 * 106 cells/mL | Thawed in a 37 °C water bath | P4 | No DMSO or low DMSO gave very poor viability; The best viability was obtained when using FBS with 10% DMSO | Trypan blue exclusion (evaluated at 0, 24 and 48 h post-thaw) |
| Li et al. [96] | Dog | DMEM with 10% FBS and 10% DMSO, 4 °C for 1 h, − 20 °C for 2 h, − 80 °C for 10.5 h then LN2 (1 month) | 1 * 106 cells/mL | Thawed at 37 °C | P4 | Immediately post-thaw viability was retained post-thaw at 90.1% | Trypan blue exclusion |
| Zhu et al. [46] | Dog | DMEM containing 10% FBS and 10% DMSO, 4 °C for 1 h, − 20 °C for 2 h, − 80 °C for 10.5 h then LN2 (3 years) | 1 * 106 cells/mL | Thawed in at 37 °C | P4 | No significant difference in cell viability | Trypan blue exclusion |
| Edamura et al. [36] | Dog | Cryoprotectant solution with or without 10% DMSO and 10% FBS, biofreezing vessel at − 80 °C in a freezer (7 days) | 1 * 106 cells/mL | Thawed in a 37 °C water bath for 1 min | P1 | DMSO and FBS-free freezing gave higher viability (about 99%); DMSO and FBS containing freezing media gave lower viability (about 89.7%) | Trypan blue exclusion |
| Nitsch et al. [97] | Monkey | Freezing medium containing 0,1,5,10 or 15% DMSO (v/v), controlled rate freezer using an optimised freezing rate then − 150 °C freezer (1 week) | 1 * 106 cells/mL | In a 37 °C water bath | P9 | Immediately post-thaw viability was about 80% for the different DMSO concentrations; Highest viability 24 h post-thaw for cells frozen with 5 or 10% DMSO | Trypan blue exclusion |
| Lauterboeck et al. [49] | Monkey | Three different freezing solutions tested (2 of them xeno-free) containing different concentrations of DMEM, DMSO and/or FBS, methylcellulose, poloxamer-188, α-tocopherol, cell suspension equilibrated for 10, 30 or 60 min then placed in controlled rate freezer using one-step freezing protocol or two-step freezing protocol then − 150 °C (at least 24 h) | 1 * 106 cells/mL | In a 37 °C water bath for 90 s | NA | Viability maintained after thawing | Automatic cell counter |
| Ock and Rho [51] | Pig | ADMEM solution supplemented with 10% FBS and 1% penicillin–streptomycin with 40%, 20% or 10% DMSO, controlled rate programmable freezing device at − 1 °C/min from 25 °C to − 80 °C then then LN2 (< 1 month) | 2 * 106 cells/mL | In a 37 °C water bath for 1 min | P5 | There was a significant difference between fresh and cells cryopreserved with 10% (about 77.6%) or 20% DMSO (about 67%); No significant difference between fresh and cells cryopreserved with 5% DMSO (about 83.9%) | Trypan blue exclusion |
| Romanek et al. [98] | Pig (BM-MSC treated with a high hydrostatic pressure (HHP) before freezing) | 10% DMSO, 2 h at − 20 °C then LN2 (up to 4 weeks) | NA | 37 °C water bath with gentle shaking | NA | Significant difference between cells treated with HHP and control immediately post-thaw (about 75.2%–81.7%); No difference in viability at 8 days post-thaw (about 81.6%–82.1%) | Trypan blue exclusion |
| Mitchell et al. [32] | Horse | Six different freezing solutions tested (20% serum [autologous equine serum, commercial equine serum or FBS], 10% DMSO and 70% media OR 95% serum and 5% DMSO), − 80 °C freezer for 24 h then liquid phase of LN2 (2–5 days) | 10 * 106 cells/mL | In a 35 °C water bath with gentle agitation | P3–6 | Immediately post-thaw viability was retained at about 80–90% regardless of the cryopreservation formulation | Flow cytometry—Fluorescein diacetate, PI |
Details on the experimental cryopreservation processes taken by different research groups. This table aims to provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Morphology
Table 2 summarises the studies which assessed post-thaw cell morphology. This attribute was mainly assessed using microscopy. Irrespective of all the variables considered in this data analysis and the time post-thaw at which cell morphology was checked, 13 of the 16 studies agree that cryopreservation itself has no effect on post-thaw cell morphology. The addition of Rho-associate kinase (ROCK) inhibitor Y-27632 was reported to give BM-MSCs a web-like appearance which indicated some neuronal differentiation [30]. In addition, several cell shapes were observed at day 2 and day 5 post-thaw [46] and cell shrinkage was detected using flow cytometry [38].
Table 2.
Bone-marrow derived mesenchymal stem cell studies assessing post-thaw cell morphology
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Human | |||
| Kotobuki et al. [92] | Human | No effect on morphology | Microscopy (fluorescent/phase contrast) |
| Haack-Sorensen et al. [19] | Human | No effect on morphology | NA |
| Xiang et al. [93] | Human | No effect on morphology | Microscopy (light) at cell confluency post-thaw |
| Zhao et al. [94] | BM-MSC (human with chronic myeloid leukemia) | No effect on morphology | NA |
| Heng [30] | Human | The addition of Y-27632 altered the morphology of the cells (web-like appearance) | NA |
| Liu et al. [28] | Human | No effect on morphology | Microscopy (fluorescent) |
| Doan et al. [95] | Human | No effect on morphology | Microscopy (light) 7 days post |
| Mamidi et al. [33] | Human | No effect on morphology | NA |
| Moll et al. [38] | Human | Effect of cryopreservation seen on forward scatter but not side scatter when exposed to human serum | Microscopy; cell counter and analyser system (CASY-TT); Flow cytometry |
| Al-Saqi et al. [66] | Human | No effect on morphology | Microscopy (light) two passages post |
| Other species | |||
| Liu et al. [29] | Rat, mouse and calf | No effect on morphology | Microscopy (light) |
| Naaldijk et al. [27] | Rat | No effect on morphology | Microscopy (light) |
| Davies et al. [42] | Rat | No effect on morphology | Microscopy (phase contrast) |
| Zhu et al. [46] | Dog | Cells had several shapes such as long fusiform, polygon and astroid | Checked at days 2 and 5 after thawing NA |
| Edamura et al. [36] | Dog | No effect on morphology | Microscopy (light) |
| Mitchell et al. [32] | Horse | No effect on morphology | Microscopy (light) (24 and 72 h post) |
The key results on bone-marrow derived mesenchymal stem cell morphology are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Immunophenotyping
Marker expression is one of the International Society for Cellular Therapy (ISCT) criteria for defining MSCs [47] so it is of real importance to check BM-MSCs phenotype before freezing and/or after thawing. Table 3 lists the studies which assessed BM-MSCs marker expression post-thaw. Despite its importance, less than half of the 41 studies retained assessed post-thaw marker expression retention and despite all the variables taken into consideration in this investigation, there was a consensus regarding the methodology used (flow cytometry) and the results; cryopreservation does not affect BM-MSCs immunophenotype.
Table 3.
Bone-marrow derived Mesenchymal Stem Cell studies evaluating surface marker expression post-thaw
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Human | |||
| Kotobuki et al. [92] | Human | No difference | Flow cytometry |
| Haack-Sorensen et al. [19] | Human | No difference | Flow cytometry |
| Xiang et al. [93] | Human | No difference | Fluorescent sorting at passage 1, 5, 10 and 15 post-thaw |
| Zhao et al. [94] | Human (with chronic myeloid leukaemia) | No difference | Flow cytometry |
| Doan et al. [95] | Human | No difference | Flow cytometry |
| Ginis et al. [50] | Human | No difference except lower expression of CD9 | Flow cytometry |
| Mamidi et al. [33] | Human | No difference | Flow cytometry |
| Matsumura et al. [26] | Human | No difference | Flow cytometry |
| Holubova et al. [69] | Human | No difference | Flow cytometry |
| Moll et al. [38] | Human | No difference | Flow cytometry |
| Al-Saqi et al. [66] | Human | No difference | Flow cytometry |
| Luetzkendorf et al. [40] | Human | No difference | Flow cytometry |
| Yuan et al. [52] | Human (BM-MSC engineered to express TRAIL) | No difference | Flow cytometry |
| Other species | |||
| Naaldijk et al. [27] | Rat | No difference | Flow cytometry |
| Davies et al. [42] | Rat | No change in the expression of CD29 and CD73; Increase in the expression of CD90, CD44 and CD105 | Flow cytometry for CD29 and CD90; RT-qPCR for CD44, CD105 and CD73 |
| Ock and Rho [51] | Pig | No difference | Flow cytometry |
The main results on bone-marrow derived mesenchymal stem cell surface marker expression are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Differentiation potential
Tri-lineage differentiation (adipogenic, osteogenic and chondrogenic) is another criterion listed by the ISCT guide for defining MSCs [47]. Hence, more than half of the studies [24] assessed BM-MSCs post-thaw differentiation potential and these are listed in Table 4. Osteogenesis is the most frequently assessed differentiation pathway (20 studies) qualitatively through Alizarin red staining and/or quantitatively through measurement of alkaline phosphatase activity. There was an agreement among 18 studies that cryopreservation did not affect BM-MSCs osteogeneic potential. One study reported lower osteogenesis [39] and one reported improved osteogenesis post-thaw [48]. Adipogenesis was next in terms of frequency of testing using Oil Red O staining as a qualitative assessment and no effect of cryopreservation was observed in 12 studies. Only one study provided a quantitative assessment of adipogenesis and it was the only one reporting lower differentiation level [49].
Table 4.
Published experimental studies detailing BM-MSCs post-thaw differentiation potential
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Human | |||
| Bruder et al. [91] | Human | No effect on osteogenic differentiation ability | Cell re-plated for one passage post-thaw then re-plated and incubated with osteogenic supplements; quantification of alkaline phosphatase activity |
| Hirose et al. [41] | Human | No effect on osteogenic differentiation ability | Incubation with osteogenic media for 25 days; quantitative fluorescence analysis of calcein uptake |
| Kotobuki et al. [35] | Human | No effect on osteogenic differentiation ability | Incubation with osteogenic medium for 2 weeks; calcium and alkaline phosphatase activity staining |
| Kotobuki et al. [92] | Human | No effect on osteogenic differentiation ability | Incubation in osteogenic media for 2 weeks; quantification of alkaline phosphatase activity and calcein uptake |
| Xiang et al. [93] | Human | No effect on adipogenic or neuro genic differentiation ability | Cells at P15 post-thaw incubated in adipogenesis medium for 12 days; Oil Red O staining |
| Cells at P15 post-thaw incubated in neurogenesis induction medium for 1 or 6 days; fluorescent staining and RT-qPCR | |||
| Zhao et al. [94] | BM-MSC (human with chronic myeloid leukaemia) | No effect on differentiation ability | Incubation in osteogenic medium for 21 days; Von Kossa staining and RT-qPCR |
| Incubation in adipogenic media for 14 days; Oil Red staining and RT-qPCR | |||
| Incubation in neurogenic medium; Immunocytochemistry and western blotting for NF, GFAP and GalC | |||
| Endothelial differentiation for 2 weeks; immunohistochemical and western blotting for CD31 and vWF | |||
| Liu et al. [28] | Human | Serum-free reduced-DMSO freezing solution gives comparable differentiation to 10% DMSO | Incubation in osteogenic or adipogenic media for 2 weeks, and chondrogenic media for 3 weeks |
| Doan et al. [95] | Human | No effect on adipogenic differentiation ability | Incubation in adipogenic medium for 2–3 weeks; Oil Red staining |
| Ginis et al. [50] | Human | No effect on osteogenic differentiation ability | Incubation with osteogenic media; quantification of alkaline phosphatase activity on days 7 and 14 after incubation as well as flow cytometry analysis at day 14 after incubation of alkaline phosphatase surface expression |
| Quantification of calcium deposition at day 21 after incubation | |||
| Mamidi et al. [33] | Human | No effect on tri-lineage differentiation ability | Incubation in osteogenic differentiation media for 3 weeks; Alizarin red staining |
| Incubation in adipogenic differentiation media for 3 weeks; Oil Red O staining | |||
| Incubation in chondrogenic differentiation media for 3 weeks; Alcian blue staining | |||
| Matsumura et al. [26] | Human | No effect on tri-lineage differentiation ability | Incubation in osteogenic differentiation media for 14 days; Alizarin red staining and alkaline phosphatase activity |
| Incubation in adipogenic differentiation media for 14 days; Oil Red O staining and GPDN activity | |||
| Incubation in chondrogenic differentiation media for 14 days; Alcian blue staining | |||
| Kumazawa et al. [37] | Human | No effect on adipogenic or osteogenic differentiation ability | Incubation in osteogenic medium for 1, 2, and 3 weeks then alkaline phosphatase activity, calcium levels, alizarin red staining and RT-qPCR |
| Incubation in adipogenic medium for 1, 2, and 3 weeks; Oil Red O staining | |||
| Luetzkendorf et al. [40] | Human | No effect on adipogenic and osteogenic differentiation ability | Incubation in diff media until morphological signs of differentiation were visible (10–15 days) |
| Lechanteur et al. [34] | Human | No effect on differentiation ability | NA |
| Yuan et al. [52] | Human (BM-MSC engineered to express TRAIL) | No effect on tri-lineage differentiation ability | Differentiation procedures performed using StemPro differentiation kits according to manufacturer’s instructions |
| Other species | |||
| Liu et al. [29] | Rat, mouse and calf | No effect on adipogenic or osteogenic differentiation ability | Incubation with adipogenic media for 2 weeks; Oil Red O staining and alkaline phosphatase activity expression staining with BCIP/NBT |
| Incubation with adipogenic or osteogenic media for 2 weeks then Oil Red O staining and alkaline phosphatase activity expression staining with BCIP/NBT | |||
| Naaldijk et al. [27] | Rat | In general, no difference in differentiation was observed (qualitative observation); Quantification of ALP: ALP activity is lower at ‘high’ (> 5%) levels of DMSO compared to solutions with a higher HES 450 content | Incubation with osteogenesis, adipogenesis and chondrogenic media for 2 weeks |
| Quantification using alkaline phosphatase assay | |||
| Li et al. [96] | Dog | No effect on osteogenic differentiation ability | Incubation in osteogenic media for 5, 10 and 15 days; alkaline phosphatase activity measurement |
| Incubation in osteogenic media for 21 days; number of mineralized nodules determined | |||
| Zhu et al. [46] | Dog | No effect on osteogenic differentiation ability | Incubation with osteogenic media (21 days); measurement of alkaline phosphatase activity at 5, 10 and 15 days and Von Kossa staining and nodules counting at day 21 |
| Edamura et al. [36] | Dog | No effect on neurogenic differentiation ability | Incubation with neurogenic media for 6 h then RT-qPCR |
| Tokumoto et al. [48] | Monkey | Cryopreserved cells had a slightly higher ALP enzyme activity than non-cryopreserved cells | Incubation with osteogenic media for 4, 8 and 12 days; quantification of alkaline phosphatase enzyme activity |
| Nitsch et al. [97] | Monkey | No effect on adipogenic or osteogenic differentiation ability | Incubation with adipogenic media for 5 weeks; oil-red O staining |
| Incubation with osteogenic medium for 21 days; Von Kossa staining | |||
| Lauterboeck et al. [49] | Monkey | Significant decrease in oil droplet formation | Incubation with adipogenic medium for 20 days; Oil Red O staining |
| No difference in osteogenic differentiation ability | Incubation with osteogenic induction medium for 3 weeks; Alizarin red staining | ||
| Heino et al. [39] | Minipig | Cells lost their osteogenic differentiation potential | Cells incubated in osteogenic medium; stained for alkaline phosphatase activity (tested 9 days post-thaw) |
Results on bone-marrow derived mesenchymal stem cell tri-linage are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Chondrogenesis presented as the least studied tri-linage differentiation pathway. Only five studies differentiated thawed BM-MSCs into chondrocytes with a qualitative assessment made via Alcian Blue staining. It was concluded that cryopreserved cells did not lose the ability to chondrogenic differentiation (of note thawed BM-MSCs were also able to commit to neuronal and endothelial lineages).
Proliferation potential
Table 5 lists the 20 studies which examined post-thaw BM-MSCs proliferation potential. Various methods were used to determine proliferation rate such as population doublings and DNA quantification. The majority of results agree that cryopreservation does not affect post-thaw BM-MSCs proliferation potential, nonetheless lower proliferation rate was obtained by two studies [34, 39] and higher proliferation rate was obtained by one study [50]. Colony-forming unit ability, a traditional measure of BM-MSCs proliferation, was assessed by three studies with mixed results [25, 32, 51].
Table 5.
Experimental studies evaluating post-thaw BM-MSCs proliferation potential
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Human | |||
| Bruder et al. [91] | Human | No effect on proliferation | Cell re-plated for one passage post-thaw; crystal violet dye-binding method |
| Haack-Sorensen et al. [19] | Human | No effect on proliferation | PKH26-GL cell linker kit |
| Xiang et al. [93] | Human | No effect on proliferation | Growth curves |
| Zhao et al. [94] | Human (with chronic myeloid leukaemia) | No effect on proliferation | Cell count and cell-doubling time |
| Doan et al. [95] | Human | No effect on proliferation | NA |
| Ginis et al. [50] | Human | Proliferation of cryopreserved cells after 1 or 5 months of storage was higher than non-cryopreserved cells | Calcein-AM staining (at day 1, 4, 7 and 14 after post-thaw plating) |
| Mamidi et al. [33] | Human | No effect on proliferation | Population doublings, cumulative population doublings and population doubling time |
| Matsumura et al. [26] | Human | No effect on proliferation | Cell count; population doubling time (24 h, 48 h, 72 h and 96 h post-thaw) |
| Holubova et al. [69] | Human | No effect on proliferation | Cell count |
| Al-Saqi et al. [66] | Human | No significant difference in population doubling time but cells cryopreserved in DMSO had longer population doubling time compared to fresh | Population doubling (first and second passage post-thaw) |
| Luetzkendorf et al. [40] | Human | No effect on proliferation | Population doublings; Population doubling time |
| Pollock et al. [67] | Human | Population doublings decreased with increasing pre-freeze passage number | Population doublings |
| Lechanteur et al. [34] | Human | Very low recovery until day 4 then a slight increase indicating re-proliferation | Cell count (0–5 days after thawing) |
| Yuan et al. [52] | Human (BM-MSC engineered to express TRAIL) | No effect on proliferation | XTT assay |
| Other species | |||
| Edamura et al. [36] | Dog | DMSO and FBS-free freezing resulted in similar proliferative capacity as non-cryopreserved; DMSO and FBS containing freezing media gave lower proliferative capacity | Cell count (2, 4, 6,8, 10 and 12 days post-thaw) |
| Tokumoto et al. [48] | Monkey | No effect on proliferation | DNA quantification at 4, 8 and 12 days |
| Lauterboeck et al. [49] | Monkey | No effect on proliferation | Population doubling time |
| Heino et al. [39] | Minipig | Two to sixfolds decrease in the proliferative capacity of cells | Population doublings |
| Romanek et al. [98] | Pig (BM-MSC treated with a high hydrostatic pressure (HHP) before freezing) | Cells treated with HHP showed better proliferation rate | Cell count |
| Mitchell et al. [32] | Horse | No effect on proliferation | Cell staining with CellTrace label |
| Colony-forming unit ability | |||
| Human | |||
| Verdanova et al. [25] | Human | Best number of colonies obtained when cells were frozen with 5% DMSO with 5% sericin in culture medium | Cells seeded 60 cm Petri dishes for 2 weeks, Crystal Violet stained and colonies counted (light microscope) |
| Other species | |||
| Ock and Rho, [51] | Pig | All cryopreserved cells showed significantly lower numbers of colonies compared to fresh; Lower DMSO produced higher number of colonies | Cells seeded in 6-well plates for 2 weeks, 4% Giemsa stained and colonies counted (light microscope) |
| Mitchell et al. [32] | Horse | No effect on colony-forming unit ability | Cells seeded in 10 cm plates for 1 week, Crystal Violet stained and colonies counted (light microscope) |
The key results on bone-marrow derived mesenchymal stem cell proliferation are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Metabolic activity
Table 6 lists the six studies which examined post-thaw BM-MSCS metabolic activity. Three methods were equally used to determine cell metabolic activity; AlamarBlue, Presoblue and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) reduction-based assays. From the data collated it could be seen that two-thirds of experiments performed reported impaired metabolic activity post-thaw.
Table 6.
Bone-marrow derived mesenchymal stem cell studies evaluating post-thaw metabolic activity
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Human | |||
| Liu et al. [28] | Human | Reduced-DMSO freezing solution gives comparable metabolic activity to 10% DMSO | AlamarBlue assay |
| Chinnadurai et al. [20] | Human | No reduction in metabolic fitness | Calcium uptake; PrestoBlue reduction |
| Chinnadurai et al. [68] | Human | The addition of various concentrations of hPL (human platelet lysate) did not significantly enhance MSC metabolic activity | PrestoBlue reduction |
| Other species | |||
| Liu et al. [29] | Rat, mouse and calf | In general, non-cryopreserved cells showed higher overall metabolic activities than the cryopreserved; Reduced DMSO (5%) with 2% PEG, 3% trehalose and 2% albumin give superior results to 10% DMSO | AlamarBlue assay |
| Nitsch et al. [97] | Monkey | Lower metabolic activity for cryopreserved cells compared with fresh; Enhnaced levels of metabolic activity obtained for 5% and 10% DSMO levels | MTT assay (24 h, 48, 72 and 96 h post-thaw) |
| Lauterboeck et al. [49] | Monkey | Cells’ metabolic activity was impaired up until 48 h post-thaw; Partial recovery at 72 h and full recovery observed at 96 h | MTT assay (24 h, 48, 72 and 96 h post-thaw) |
The key results on bone-marrow derived mesenchymal stem cell metabolic activity after cryopreservation are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Apoptosis and senescence levels
Typically assessed using flow cytometry, the induction of apoptosis is evident when considering the six studies entered in Table 7. However, cryopreservation does not seem to induce senescence (refer Table 7) although more studies are needed to draw a firm conclusion.
Table 7.
The induction of apoptosis in post-thaw BM-MSCs
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Apoptosis | |||
| Human | |||
| Liu et al. [28] | Human | Serum-free reduced-DMSO freezing solution gives comparable apoptotic percentage to 10% DMSO | Flow cytometry |
| Ginis et al. [50] | Human | Lower percentage of apoptotic cells obtained with Annexin V and Hoechst staining compared to caspase 3 assay: using caspase 3, the percentage of apoptotic cells was between 13 and 17% for CryStor media compared to 3% for conventional freezing media | Flow cytometry—Annexin V, Hoechst, Caspase 3 activity |
| Chinnadurai et al. [20] | Human | Higher percentage of apoptotic cells in cryopreserved MSC than live MSC | Flow cytometry |
| Moll et al. [38] | Human | Apoptosis increased by cryopreservation when exposed to human serum | Flow cytometry—Annexin V, PI staining |
| Other species | |||
| Ock and Rho [51] | Pig | Bak and Bcl2 gene expression in cryopreserved cells was higher than fresh at 3 h post-thaw: Bak and Bcl2 gene expression in cryopreserved cells was comparable to fresh after culturing thawed cells up to 90% confluence: Bcl2 antigen expression level was comparable to fresh after culturing thawed cells up to 90% confluence | RT-qPCR for Bak and Bcl2: Flow cytometry; Bcl2 antigen |
| Romanek et al. [98] | Pig (BM-MSC treated with HHP before freezing) | No significant difference between control (without HHP) and cells subjected to HHP pre-freeze | Flow cytometry—Annexin V: Fluorescence microscopy |
| Senescence | |||
| Human | |||
| Mamidi et al. [33] | Human | No difference in the level of senescent cells | Β-galactosidase assay |
| Al-Saqi et al. [66] | Human | There were signs of senescence (but could be due to culture medium rather than cryopreservation medium) | Β-galactosidase assay (analysed 2 passages after cryopreservation) |
| Pollock et al. [67] | Human | Immediate pre-freeze senescence levels show similar trends but higher levels compared to pre-freeze At 48 h post-thaw, level of senescent cells dropped significantly comparing to immediately post-thaw | Beta-glo assay |
The key results on bone-marrow derived mesenchymal stem cell apoptotic activity post-thaw are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Attachment and migration
Only four studies assessed BM-MSCs attachment ability post-thaw, and these are recorded in Table 8. This table indicates that frozen cells have lower adherence capability post-thaw. Only one study assessed post-thaw cell migration [52]. It concluded that cryopreservation has no effect on post-thaw cell migration ability.
Table 8.
Bone-marrow derived Mesenchymal Stem Cell studies evaluating cellular attachment post-thaw
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Attachment | |||
| Human | |||
| Heng [30] | Human | Level of adherent cells was 39.8 ± 0.9%; increased by approx. 10% with Y-27632 | MTT assay performed 24 h post-thawing |
| Chinnadurai et al. [20] | Human | 40% reduction in adhesion to fibronectin; 80% reduction in adhesion to endothelial cells; No reduction in the surface expression of adhesion molecules | After 2 h in static and 1 h in vascular flow conditions using microscopy (light); Flow cytometry for adhesion molecules |
| Other species | |||
| Li et al. [96] | Dog | Decreased adhesion capacity post-thaw; recovery of adhesion capacity after culturing for several passages | Adherent cell count (hemo-cytometer) at 4, 8, 12 and 24 h post-thaw |
| Tokumoto et al. [48] | Monkey | Limited influence of cryopreservation on cell adhesion capabilities | Adherent cell count (hemo-cytometer) |
| Migration | |||
| Human | |||
| Yuan et al. [52] | Human (BM-MSC engineered to express TRAIL) | No effect on migration potential | Trans-well plates |
The effects of cryopreservation on bone-marrow derived mesenchymal stem cell attachment are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Paracrine function
Paracrine function is related to two main MSCs activities namely immunomodulation and angiogenesis. In total, 10 studies (Refer Table 9) assessed BM-MSCs paracrine function with immunomodulation being the most frequently assessed with eight studies. The results of these studies are equally balanced with four of them reporting no effect of cryopreservation on BM-MSCs post-thaw immunomodulatory potential and four reporting an impaired potential. Angiogenesis potential and secretion of growth factors were only assessed by one study each with no effect of cryopreservation reported.
Table 9.
Published experimental studies detailing BM-MSCs post-thaw paracrine function
| Study | Species | Results post-thaw | Method of assessment |
|---|---|---|---|
| Immunomodulatory potential | |||
| Human | |||
| Zhao et al. [94] | Human (with chronic myeloid leukaemia) | No effect on immunomodulatory potential | Mixed leukocyte reaction inc. T-cell proliferation |
| François et al. [45] | Human | Impaired inhibition of proliferation of activated T cells; low IDO protein expression in response to INF-γ stimulation; up-regulation of heat shock proteins | T-cell proliferation assay (CD3/CD28); Western blot IDO; RT-qPCR IDO, CCL2, IL-6 |
| Holubova et al. [69] | Human | No effect on immunomodulatory potential | T-cell proliferation (PHA) |
| Moll et al. [38] | Human | Impaired immunomodulatory properties | RT-qPCR IDO, IL-6; Western blot IDO; Instant blood mediated inflammatory reaction (IBMIR) |
| Luetzkendorf et al. [40] | Human | No effect on immunomodulatory potential | Co-culture with PBMC; T-cell proliferation (PHA) |
| Chinnadurai et al. [68] | Human | Freeze-thawing attenuates immunosuppressive properties of human MSC independent of freezing methods or freezing media; Thawed MSC can suppress T-cell proliferation in the absence of cell contact; IFNɣ pre-licensing prior to cryopreservation enhances thawed MSC’s immunosuppressive properties | Co-culture with PBMC; T-cell proliferation (CD3/CD28 & SEB); RT-qPCR IDO, Hsp |
| Gramlich et al. [18] | Human | No effect on immunomodulatory potential | Co-culture with PBMC; T-cell proliferation (CD3/CD28); IDO activity assay (kynurenine) |
| Lechanteur et al. [34] | Human | Impaired immunomodulatory properties | Co-culture with PBMC; T-cell proliferation (CD3/CD28); IDO activity assay (kynurenine) |
| Angiogenesis potential | |||
| Human | |||
| Haack-Sorensen et al. [19] | Human | No effect on the capacity of MSC to differentiate into endothelial cells; Retained VEGF responsiveness | In vitro angiogenesis; RT-qPCR, KDR, vWF, INSIG |
| Growth factor secretion | |||
| Human | |||
| Gramlich et al. [18] | Human | Small changes in growth factor secretion between fresh and cryopreserved cells | Human antibody-mediated growth factor array |
Summary of the effects of cryopreservation on bone-marrow derived mesenchymal stem cell paracrine function are presented in this table. For further details on the cryopreservation experimental details refer to either Table 1 or Additional file 2 which provide the individual freezing protocols outlined in the extracted papers alongside the concentration and passage of cells at the point of cryopreservation and the process of thawing
Discussion
A recent analysis of MSC-based clinical trials showed that although no safety concerns surround MSC infusion, the translation from bench to bedside is still confronted by what the authors called the ‘Achilles heel’; donor heterogeneity, ex vivo expansion, immunogenicity and cryopreservation [53]. There is no doubt that cryopreservation is essential for MSC therapy translation, both autologous and allogeneic, and is still one the limitations to be addressed.
Cryopreservation by slow freezing can cause two types of cell damage; physical and molecular. Physical injuries were the first to be identified and include ice nucleation, solution effects, osmotic shock, cold shock as well as cryoprotectant toxicity [14]. Molecular injuries encompass the effect of cryopreservation on gene expression, protein levels, cell functionality, the induction of stress response as well as post-thaw epigenetic changes [54, 55].
In the case of MSCs, studying the effect of molecular injuries and how to mitigate them is a twofold problem. Firstly, investigating molecular injuries is still a developing branch of cryobiology. In fact, immediately post-thaw cell viability has always been the most assessed cell attribute in cryopreservation studies. However, it has been shown that signs of cellular damage may take some time to manifest (cryopreservation-induced delayed-onset cell death [54]). Leading to viability and functional losses which are compounded by a lack of detection and reporting in immediate post-thaw analysis. Approaches to tackle molecular injuries, intracellular-like freezing solutions and anti-apoptotic compounds, can be deployed yet research in this area is still at an early stage [54].
Secondly, establishing and standardising potency markers and assays to characterise MSCs is still a challenge [24]. In fact, MSCs possess variability in their gene expression profiles, differentiation and expansion potential and phenotype depending on tissue origin, cell isolation and expansion procedures [56] as well as donor characteristics [57]. In 2006, the ISCT published a guideline on minimal criteria to define MSC; plastic adherence, expression of certain surface markers and lack of others and tri-lineage differentiation [47]. In 2013, these criteria were expanded to include a fourth parameter, quantification of MSC immune functional potency [58]. In 2016, the society suggested “a matrix assay approach: quantitative RNA analysis of selected gene products; flow cytometry analysis of functionally relevant surface markers and protein-based assay of the secretome” in order to fulfil the fourth criterion [59].
Currently, there are no standard markers or potency assays to typify MSCs or evaluate their post-thaw potency despite much discussion within the scientific community [24, 60]. Therefore, research laboratories follow differing protocols which makes data evaluation complex. As both research areas (freezing molecular injuries and MSC characterisation) develop so will the methodology of evaluating MSC cryopreservation.
There are profound variabilities in the whole cryopreservation process from freezing media formulation, method of freezing and thawing and duration of storage to passage number and cell concentration at freezing. However, despite all these variabilities and all the species included, there was evidence showing that four BM-MSCs attributes are stable and unaffected by the stresses imposed by freezing and thawing and these are: cell morphology, marker expression, proliferation potential and tri-lineage differentiation capability (although chondrogenesis was only assessed by five independent studies). The four other attributes viability, attachment and migration, genomic stability and paracrine function were governed by either conflicting results or by low assessment frequency.
All studies have employed a strategy to evade freezing and thawing physical damage. Freezing BM-MSCs at a slow cooling rate (at least at the start of the cooling process) and thawing at a high rate was followed by almost all the studies in this review. In terms of media formulation, DMSO remains the most commonly used cryoprotectant to protect BM-MSCs (used by 90% of studies analysed). However, DMSO is associated with adverse effects such as cardiac side effects [61, 62] and severe neurotoxicity [63, 64] when infused in patients. Consequently, reducing or eliminating DMSO, depending on clinical outcome, may become a requisite.
Mixing other potential cryoprotectants with DMSO at percentages < 10% indicates that this traditionally held protectant and percentage can both be altered [26–28]. This is an interesting result which indicates that eliminating DMSO from freezing solution is a viable option and is worthy of further evaluation based on a wider post-thaw MSC functional characteristics. Another important aspect of MSC cryopreservation is the fact that FBS is commonly added to freezing solutions for its benefits to stabilise the cell membrane and adjust cell osmotic pressure [65]. The main issue in using FBS is that it is animal-derived and may cause a xenogenic reaction if infused in patients [65]. Cryopreserving hBM-MSCs in xeno-free media was assessed across 13 studies [18, 26, 34, 38, 40, 48–50, 52, 66–69]. Verdanova et al. reported using Sericin (a protein derived from silkworm cocoon) as an FBS substitute for preserving human BM-MSCs [25]. The possibility of overlapping xeno-free and low or no DMSO exists in one study where monkey BM-MSCs were successfully frozen in xeno-free solution composed of methylcellulose, poloxamer, α-tocopherol and only 2.5% DMSO [49]. Such a freezing solution would be ideal because it is not only xeno-free but also contains a very low DMSO concentration.
Cell concentration at freezing and the duration of storage are not well studied, and it is not well documented if it plays a role in the performance of the end-product. The only study which tested cell viability after freezing at three different cell concentrations found no significant variation [52]. In regard to duration of storage, of note is the study where BM-MSCs were stored for more than 10 years without losing multipotency [37]. This could well be a suggestion that once cells enter a quiescent state, its duration is not of great significance.
The evaluation of the cryopreservation process is certainly an existing challenge. Our understanding of the biology of MSCs is evolving and so are the possibilities of the application of these cells in the medical field. Yet, as stated recently “Significant challenge remains the development of a relevant potency assay” [34]. These assays must be quick, easy and should not require trained personnel if they are to be used to release each cell batch in a clinical setting and if they are to fit with operation theatre logistics (thawing, testing and infusing within a couple of hours). In theory, potency assays could be therapy-specific and must indicate cell functionality; in other words, “mechanism(s) of action” [70]. How much these assays correlate with the in vivo niche is also of great importance. In addition, “the assay should be able to differentiate between sufficiently potent and sub-potent batches, a (semi-)quantitative assay is required” [56]. This thawing-infusing scenario would be realistic only if cryopreservation methods have improved to give an optimal product immediately after thawing.
Cell morphology (shape and size) can give indication on cell’s health as well as whether they have committed to differentiate or not. The absence of change in BM-MSCs morphology after cryopreservation can indicate that the freeze-thaw process does not cause differentiation or change in cell phenotype. This conclusion is further evidenced by the absence of effect of cryopreservation on BM-MSCs marker expression post-thaw.
No such firm conclusion can be drawn when it comes to viability. This is of real importance given that viability is one of the release criteria for cell therapies. In fact, viability has and will always be the primary indicator on cryopreservation success. It is an easy, cheap and fast measurement but is has some limitations. The various methods used across labs to measure viability and the lack of a unified reporting structure makes it hard to compare results. Although ≥ 90% viability for fresh MSC product and ≥ 70% viability for cryopreserved MSC product are generally considered the benchmark for clinical application [57], different labs report viability maintenance or loss of viability based on comparison with pre-freeze viability or on comparison with freezing with 10% DMSO (refer Table 1). In addition, the common measurement time-point, only immediately post-thaw could be misleading due to the late manifestation of the effect of current cryopreservation protocols on cells.
In fact, the initiation of apoptotic events is evident according to the data in Table 7. Despite the importance of this cryopreservation-related cell death, it is surprising how limited the investigation of this molecular pathway in thawed BM-MSCs is and the strategies to reduce it are. Only two studies utilized strategies to prevent post-thaw apoptosis (molecular injury). The addition of Rho-associated kinase inhibitor Y-27632 in the freezing medium and in the post-thaw culture medium did not improve hBM-MSCs viability immediately but recorded enhanced recoveries at 24-h post-thaw [30]. However, the addition of Caspase inhibitor z-VAD-fmk in the freezing media did not prove to be beneficial for equine, ovine and rodent BM-MSCs as assessed by viability immediately, at 24 and 48 h post-thaw [31].
An expert workshop on preservation and stability of cell therapy products was held in May 2015 [71]. Assessing post-thaw viability was one of the topics discussed and the limitations/conflicts mentioned above were identified. The group advised that assessing cell viability post-thaw should “go beyond simple enumeration of cell numbers to facilitate a greater understanding of the cell system in question”. In addition, the group realised the need for more advanced and accurate methods to assess cell viability that can be linked to cell function [71].
Interestingly, impaired cell metabolism can be closely associated with apoptotic pathways through the Bcl-2 family proteins which initiates apoptosis in metabolically stressed cells through utilization of autophagy as a nutrient source before ultimately undergoing necrosis [72, 73]. From the data presented in Table 6 some impairment of BM-MSCs metabolic activity post-thaw is evident. Therefore, a link between defective metabolic activity in thawed BM-MSCs and a higher level of apoptosis post-thaw could be postulated. This may indicate new pathways to mitigate against these post-thaw phenomena.
More research is needed if cells are to be thawed and immediately infused in patients. Infusing apoptotic cells will hinder MSCs therapeutic benefits. Moreover, it is vital that therapeutic cells emerge potent from freezing to be capable to survive in a damaged tissue where they will encounter a hostile environment with mechanical, hypoxia and nutritional stresses, the host immune response and inflammation. These factors are known to cause a huge loss in MSC viability after transplantation as well as poor engraftment [74]. According to Table 8, frozen cells have lower adherence capability. One study has tried to examine this post-thaw phenomena in more detail lending evidence to a disruption of F-actin polymerization rather than shedding of surface adhesion receptors [20]. Advancing our knowledge in this area will help manufacture clinical MSCs with improved regenerative engraftment, although the concept of MSCs proliferating, differentiating and engrafting in host tissue as their primary therapeutic modality has been recently challenged [75].
Generally, it can be said that cryopreservation does not affect BM-MSCs proliferative capacity (Table 5). However, intriguing evidence is presented by Ginis et al. [50] on the potential cell selection that the cryopreservation process may enforce. In this study, BM-MSCs post-thaw proliferation potential was higher than that at pre-freeze. The authors justified their results as “selection of stronger cells after cryopreservation” and suggested that their results should “alarm a scientific community”. This same theory has also been mentioned by Baust et al. [76] who stated, “unstudied but of concern is the potential for the preservation process to select for increased resistance to preservation stresses”.
From Table 5, it can be concluded that there is a strong agreement that cryopreservation does not affect BM-MSCs differentiation potential. This conclusion is of value to fulfil one of the 2006 ISCT criteria for MSCs. However, differentiation potential may become less important for cell therapy. For example, in heart disease, MSCs’ initial mechanism of regeneration was outlined as differentiation into cardiomyocytes and incorporation into the host tissue. Recently, this outline has been updated to shed a light on a more effective regeneration mechanism and that is their associated paracrine signalling [75]. In fact, paracrine signalling is not confined to cardiac regeneration but is now generally considered as the MSCs main mode of action. Caplan [77] suggested that it is “time to change the name” of MSCs to “Medicinal Signalling Cells” in order to better describe the secretion of trophic factors. According to Gonzalez et al. [78] “80% of the therapeutic effect of stem cells is attributed to paracrine actions”.
The MSCs secretome is composed of growth factors and cytokines either soluble or engulfed in exosomes and/or vesicles [75, 79, 80]. MSC paracrine signalling is described as exerting plethora of effects including induction of angiogenesis, regulation of immune response and inflammation, modulation of cell differentiation and proliferation, extracellular matrix formation, neuroprotective and neurotrophic effects, anti-apoptotic, anti-tumour and anti-microbial activities [81, 82]. Yet despite such a compelling list of activities no comprehensive evaluation of the MSC secretome, and or its cell-free utility, has been conducted [83]. Data on the BM-MSCs secretome upon thawing is very limited and so is the data on angiogenic potential. There are only two studies which concluded that no effect of cryopreservation was observed (Table 9).
At an injury site, MSC contribute to the creation of an anti-inflammatory environment by suppressing the activation and proliferation of pro-inflammatory cells and promoting anti-inflammatory cells [84]. This modulation of both the innate and adaptive immune system can be accomplished in two ways: via cell–cell interaction and cell–cell communication through an array of soluble factors including indoleamine 2,3-dioxygenase (IDO) and/or extracellular vesicles [85]. These characteristics were the main contributors in raising the MSC profile as therapeutically valuable. As discussed above, MSC immune function has now become an essential indicator on MSC function. In view of this, it is expected that more studies on MSC immunomodulatory properties, and assays to evaluate them will emerge. Recently, Chinnadurai et al. [86] tested BM-MSCs potency using a matrix approach and concluded that cryopreservation negatively affects cells’ secretome with T cell proliferation. According to Table 9, eight studies assessed BM-MSCs immune function post-thaw with a balance of four concluding damaging effect and four concluding no effect.
Conclusion
This systematic review has highlighted areas of agreement and deviation that currently exist around BM-MSCs properties and functions after cryopreservation (Fig. 4).
Fig. 4.
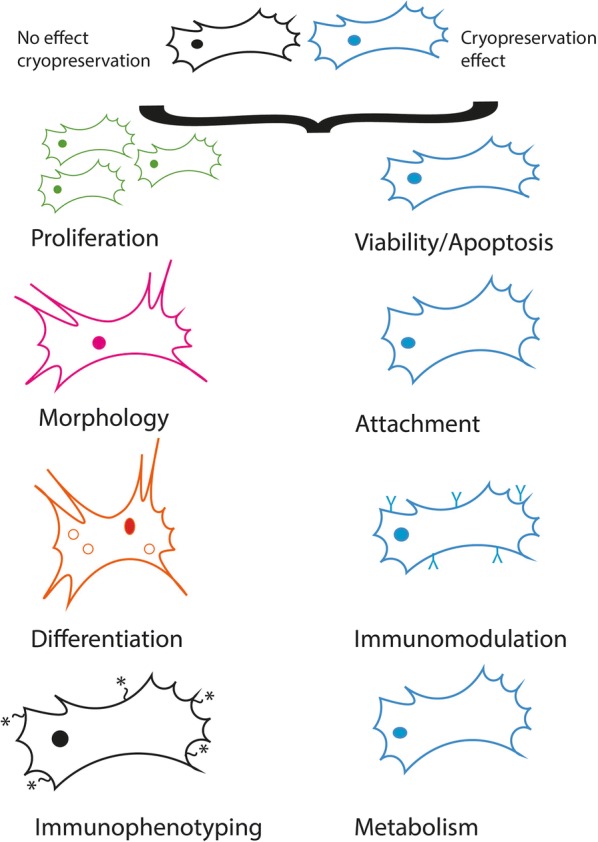
Effects of cryopreservation on BM-MSC in terms of cellular attributes and function. From the systematic analysis performed, cryopreservation appears to influence viability and apoptosis, cellular attachment, immunomodulation and metabolism (cell schematics shown in blue on right-hand side). Whereas, no common significant effects mediated by cryopreservation have been documented in proliferation, morphology, differentiation or immunophenotyping (cell schematics given in green, red, orange and black on the left-hand side)
With MSC-based therapies expected to offer treatment choices for several conditions and diseases that are currently incurable and presently no MSC drug yet approved by the FDA the biological community must work towards reproducible and reliable data sets to achieve regulatory accepted drug status. With cryopreservation effects being fully identified and assessed in terms of therapeutic evaluation.
Continuing variance in our scientific approaches facilitates unapproved therapies and stem cell tourism [87, 88]; leading both the FDA and ISCT to urge both caution for individuals and request enhanced rigor, reproducibility and visibility from the scientific community [59, 89, 90]. “Successful new therapies come at a considerable cost that cannot easily be sustained without evaluation and guidance” [89].
This review has been limited to one tissue source and that is bone marrow, yet we know that MSCs can be isolated from almost all tissues especially adipose and umbilical cord [17]. Including more tissue sources was beyond the scope of this review yet it is important to document the impact of cryopreservation on those MSC sources also.
Supplementary information
Additional file 1. Table of cellular attribute data from studies extracted for the systematic review. It is a grid identifying which cell attributes each of the forty-one studies assessed. Of note each of the 41 studies may appear more than once depending on the attributes they assessed. Where a study undertook an assessment of a cellular attribute a cross is placed in the grid. Studies are arranged by species: human [chronologically and then alphabetically] and animals from most to least frequent species [chronologically and then alphabetically]). Column headers: Morphology; Morph. Viability; Via. Immunophenotyping; IP. Differentiation; Diff. Colony forming unit frequency; CFUF. Growth; Total growth. Metabolism; Met. Apoptosis; Apo. Attachment; Attach. Immunomodulation; Immuno. Paracrine; Para. Angiogenesis; Angio. Migration; Migr.
Additional file 2. Tabulated information relating to the freezing details extracted from the relevant studies. It shows the details of the individual freezing protocols outlined in the 41 retained studies. The method of freezing is given in detail alongside the species information, the concentration and passage of cells at the point of cryopreservation and the process of thawing. These details are common to the results tables (Tables 1, 2, 3, 4, 5, 6, 7, 8, 9).
Acknowledgements
None.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- α-MEM
alpha modified eagle medium
- ADMEM
advanced Dulbecco’s medium eagle media
- COOH-PLLs
carboxylated poly-l-lysine
- DMEM
Dulbecco’s medium eagle media
- DMEM/F12
1:1 mixture of Dulbecco’s Medium and Ham’s F-12 medium
- DMSO
dimethylsulfoxide
- EMEA
DMEM, low glucose with HEPES 1×
- FBS
fetal bovine serum
- FCS
fetal calf serum
- H
hour(s)
- hPL
human platelet lysate
- IMDM
Iscove’s modified Dulbecco’s medium
- INF-γ
interferon-gamma
- MEM
modified eagle medium
- microM
micromolar
- Min
minute(s)
- NA
not available
- P
passage
- PEG
polyethylene glycol
- PHA
phytohemagglutinin
- pHPL
pooled human platelet lysate
- PI
propidium iodide
- SEB
Staphylococcal enterotoxin B
Authors’ contributions
ECA and SB jointly contributed to the conception of the manuscript. Searches and generation of the manuscript were carried out by SB. ECA contributed throughout the preparation and edited the manuscript. All authors read and approved the final manuscript.
Funding
Financial support from the Engineering and Physical Research Council (EP/L015072/1), UK and SSEHS, Loughborough University is acknowledged.
Availability of data and materials
All data generated by this systematic search are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12967-019-02136-7.
References
- 1.Friedenstein AJ, Chailakhjan RK, Lalykin KS. The development of fibroblast colonies in marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 3.Prockop D. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 5.Afanasyev BV, Elstner EE, Zander ARAJ. friedenstein, founder of the mesenchymal stem cell concept. Cell Ther Transplant. 2009;1(3):35–38. [Google Scholar]
- 6.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen M. Marrow stromal stem cells. J Cell Sci. 1988;10:63–76. doi: 10.1242/jcs.1988.Supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 9.Mizukami A, Swiech K. Mesenchymal stromal cells: from discovery to manufacturing and commercialization. Stem Cells Int. 2018 doi: 10.1155/2018/4083921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–564. [PubMed] [Google Scholar]
- 11.Lotfinejad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B. Immunomodulatory nature and site specific mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull. 2014;4(1):5–13. doi: 10.5681/apb.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation best practices: a cornerstone in the supply chain of cell-based therapies—MSC model case study. Cell Gene Ther Insights. 2017;3(10):853–871. doi: 10.18609/cgti.2017.082. [DOI] [Google Scholar]
- 14.Woods EJ, Thirumala S, Badhe-Buchanan SS, Clarke D, Mathew AJ. Off the shelf cellular therapeutics: factors to consider during cryopreservation and storage of human cells for clinical use. Cytotherapy. 2016;18(6):697–711. doi: 10.1016/j.jcyt.2016.03.295. [DOI] [PubMed] [Google Scholar]
- 15.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Coopman K. Large-scale compatible methods for the preservation of human embryonic stem cells: current perspectives. Biotechnol Prog. 2011;27(6):1511–1521. doi: 10.1002/btpr.680. [DOI] [PubMed] [Google Scholar]
- 17.Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Gramlich OW, Burand AJ, Brown AJ, Deutsch RJ, Kuehn MH, Ankrum JA. Cryopreserved mesenchymal stromal cells maintain potency in a retinal ischemia/reperfusion injury model: toward an off-the-shelf therapy. Sci Rep. 2016;6:26463. doi: 10.1038/srep26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haack-Sorensen M, Bindslev L, Mortensen S, Friis T, Kastrup J. The influence of freezing and storage on the characteristics and functions of human mesenchymal stromal cells isolated for clinical use. Cytotherapy. 2007;9(4):328–337. doi: 10.1080/14653240701322235. [DOI] [PubMed] [Google Scholar]
- 20.Chinnadurai R, Garcia MA, Sakurai Y, Lam WA, Kirk AD, Galipeau J, et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Rep. 2014;3(1):60–72. doi: 10.1016/j.stemcr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moll G, Geißler S, Catar R, Ignatowicz L, Hoogduijn MJ, Strunk D, et al. Advances in experimental medicine and biology. Cham: Springer; 2016. Cryopreserved or fresh mesenchymal stromal cells: only a matter of taste or key to unleash the full clinical potential of MSC therapy? pp. 77–98. [DOI] [PubMed] [Google Scholar]
- 22.Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17(2):125–127. doi: 10.1016/j.jcyt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Trento C, Bernardo ME, Nagler A, et al. Manufacturing mesenchymal stromal cells for the treatment of graft-versus-host disease: a survey among centers affiliated with the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24(11):2365–2370. doi: 10.1016/j.bbmt.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahsoun S, Coopman K, Forsyth NR, Akam EC. The role of dissolved oxygen levels on human mesenchymal stem cell culture success, regulatory compliance, and therapeutic potential. Stem Cells Dev. 2018;27(19):1303–1321. doi: 10.1089/scd.2017.0291. [DOI] [PubMed] [Google Scholar]
- 25.Verdanova M, Pytlik R, Kalbacova MH. Evaluation of sericin as a fetal bovine serum-replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast-like cells. Biopreserv Biobank. 2014;12(2):99–105. doi: 10.1089/bio.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumura K, Hayashi F, Nagashima T, Hyon SH. Long-term cryopreservation of human mesenchymal stem cells using carboxylated poly-l-lysine without the addition of proteins or dimethyl sulfoxide. J Biomater Sci Polym Ed. 2013;24(12):1484–1497. doi: 10.1080/09205063.2013.771318. [DOI] [PubMed] [Google Scholar]
- 27.Naaldijk Y, Staude M, Fedorova V, Stolzing A. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol. 2012;12(1):49. doi: 10.1186/1472-6750-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Xu X, Ma X, Martin-Rendon E, Watt S, Cui Z. Cryopreservation of human bone marrow-derived mesenchymal stem cells with reduced dimethylsulfoxide and well-defined freezing solutions. Biotechnol Prog. 2010;26(6):1635–1643. doi: 10.1002/btpr.464. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Xu X, Xuehu M, Liu J, Cui Z. Effect of various freezing solutions on cryopreservation of mesenchymal stem cells from different animal species. Cryo-Letters. 2011;32(5):425–435. [PubMed] [Google Scholar]
- 30.Heng BC. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2009;41(5):376–380. doi: 10.1016/j.tice.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Renzi S, Lombardo T, Dotti S, Dessi SS, De Blasio P, Ferrari M. Mesenchymal stromal cell cryopreservation. Biopreserv Biobank. 2012;10(3):276–281. doi: 10.1089/bio.2012.0005. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell A, Rivas KA, Smith R, Watts AE. Cryopreservation of equine mesenchymal stem cells in 95% autologous serum and 5% DMSO does not alter post-thaw growth or morphology in vitro compared to fetal bovine serum or allogeneic serum at 20 or 95% and DMSO at 10 or 5% Stem Cell Res Ther. 2015;6(1):231. doi: 10.1186/s13287-015-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamidi MK, Nathan KG, Singh G, Thrichelvam ST, Mohd Yusof NAN, Fakharuzi NA, et al. Comparative cellular and molecular analyses of pooled bone marrow multipotent mesenchymal stromal cells during continuous passaging and after successive cryopreservation. J Cell Biochem. 2012;113(3):3153–3164. doi: 10.1002/jcb.24193. [DOI] [PubMed] [Google Scholar]
- 34.Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med. 2016;14(1):145. doi: 10.1186/s12967-016-0892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotobuki N, Hirose M, Takakura Y, Ohgushi H. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004;28(1):33–39. doi: 10.1111/j.1525-1594.2004.07320.x. [DOI] [PubMed] [Google Scholar]
- 36.Edamura K, Nakano R, Fujimoto K, Teshima K, Asano K, Tanaka S. Effects of cryopreservation on the cell viability, proliferative capacity and neuronal differentiation potential of canine bone marrow stromal cells. J Vet Med Sci. 2014;76(4):573–577. doi: 10.1292/jvms.13-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumazawa K, Sugimoto T, Yamazaki Y, Takeda A, Uchinuma E. Osteogenic potential, multipotency, and cytogenetic safety of human bone tissue-derived mesenchymal stromal cells (hBT-MSCs) after long-term cryopreservation. Kitasato Med J. 2014;44:95–103. [Google Scholar]
- 38.Moll G, Alm JJ, Davies LC, Von Bahr L, Heldring N, Stenbeck-Funke L, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heino TJ, Alm JJ, Moritz N, Aro HT. Comparison of the osteogenic capacity of minipig and human bone marrow-derived mesenchymal stem cells. J Orthop Res. 2012;30(7):1019–1025. doi: 10.1002/jor.22049. [DOI] [PubMed] [Google Scholar]
- 40.Luetzkendorf J, Nerger K, Hering J, Moegel A, Hoffmann K, Hoefers C, et al. Cryopreservation does not alter main characteristics of Good Manufacturing Process-grade human multipotent mesenchymal stromal cells including immunomodulating potential and lack of malignant transformation. Cytotherapy. 2015;17:186–198. doi: 10.1016/j.jcyt.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Hirose M, Kotobuki N, Machida H, Kitamura S, Ohgushi H, Tateishi T. Osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells after thawing in culture. Mater Sci Eng C. 2004;24(3):355–359. doi: 10.1016/j.msec.2003.12.011. [DOI] [Google Scholar]
- 42.Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology. 2014;69(2):342–347. doi: 10.1016/j.cryobiol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Bissoyi A, Nayak B, Pramanik K, Sarangi SK. Targeting cryopreservation-induced cell death: a review. Biopreserv Biobank. 2014;12(1):23–34. doi: 10.1089/bio.2013.0032. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho KAT, Cury CC, Oliveira L, Cattaned RII, Malvezzi M, Francisco JC, et al. Evaluation of bone marrow mesenchymal stem cell standard cryopreservation procedure efficiency. Transplant Proc. 2008;40:839–841. doi: 10.1016/j.transproceed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 45.François M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy. 2012;14(2):147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Yuan F, Li L, Zheng Y, Xiao Y, Yan F. Evaluation of canine bone marrow-derived mesenchymal stem cells after long-term cryopreservation. Zool Sci. 2013;30(12):1032–1037. doi: 10.2108/zsj.30.1032. [DOI] [PubMed] [Google Scholar]
- 47.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 48.Tokumoto S, Sotome S, Torigoe I, Omura K, Shinomiya K. Effects of cryopreservation on bone marrow derived mesenchymal cells of a nonhuman primate. J Med Dent Sci. 2008;55:137–143. [PubMed] [Google Scholar]
- 49.Lauterboeck L, Saha D, Chatterjee A, Hofmann N, Glasmacher B. Xeno-Free Cryopreservation of bone marrow-derived multipotent stromal cells from Callithrix jacchus. Biopreserv Biobank. 2016;14:530–538. doi: 10.1089/bio.2016.0038. [DOI] [PubMed] [Google Scholar]
- 50.Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18(6):453–463. doi: 10.1089/ten.tec.2011.0395. [DOI] [PubMed] [Google Scholar]
- 51.Ock SA, Rho GJ. Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCS) Cell Transplant. 2011;20(8):1231–1239. doi: 10.3727/096368910X552835. [DOI] [PubMed] [Google Scholar]
- 52.Yuan Z, Lourenco SDS, Sage EK, Kolluri KK, Lowdell MW, Janes SM. Cryopreservation of human mesenchymal stromal cells expressing TRAIL for human anti-cancer therapy. Cytotherapy. 2016;18(7):860–869. doi: 10.1016/j.jcyt.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Squillaro T, Peluso G, Galderisi U. clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 54.Baust JM, Corwin W, Snyder KK, Van Buskirk R, Baust JG. Biobanking and cryopreservation of stem cells. Cham: Springer; 2016. Cryopreservation: evolution of molecular based strategies; pp. 13–29. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee A, Saha D, Niemann H, Gryshkov O, Glasmacher B, Hofmann N. Effects of cryopreservation on the epigenetic profile of cells. Cryobiology. 2017;74:1–7. doi: 10.1016/j.cryobiol.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 56.De Wolf C, Van De Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19:784–797. doi: 10.1016/j.jcyt.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 57.Robb KP, Fitzgerald JC, Barry F, Viswanathan S. Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy. 2019;21(3):289–306. doi: 10.1016/j.jcyt.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells-The international society for cellular therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martino M, Morabito F, Messina G, Irrera G, Pucci G, Iacopino P. Fractionated infusions of cryopreserved stem cells may prevent DMSO- induced major cardiac complications in graft recipients. Haematologica. 1996;81(1):59–61. [PubMed] [Google Scholar]
- 62.Zenhäusern R, Tobler A, Leoncini L, Hess OM, Ferrari P. Fatal cardiac arrhythmia after infusion of dimethyl sulfoxide-cryopreserved hematopoietic stem cells in a patient with severe primary cardiac amyloidosis and end-stage renal failure. Ann Hematol. 2000;79:523–526. doi: 10.1007/s002770000186. [DOI] [PubMed] [Google Scholar]
- 63.Stamatovic D, Balint B, Tukic LJ, Elez M, Tarabar O, Ostojic G, et al. Severe neurotoxicity following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2011;46:1110. [Google Scholar]
- 64.Windrum P, Morris TCM. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31:315. doi: 10.1038/sj.bmt.1703848. [DOI] [PubMed] [Google Scholar]
- 65.Yong KW, Wan Safwani WKZ, Xu F, Wan Abas WAB, Choi JR, Pingguan-Murphy B. Cryopreservation of human mesenchymal stem cells for clinical applications: current methods and challenges. Biopreserv Biobank. 2015;13(4):231–239. doi: 10.1089/bio.2014.0104. [DOI] [PubMed] [Google Scholar]
- 66.Al-Saqi SH, Saliem M, Quezada HC, Ekblad Å, Jonasson AF, Hovatta O, et al. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2015;16(2):181–193. doi: 10.1007/s10561-014-9463-8. [DOI] [PubMed] [Google Scholar]
- 67.Pollock K, Sumstad D, Kadidlo D, McKenna DH, Hubel A. Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy. 2015;17(1):38–45. doi: 10.1016/j.jcyt.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chinnadurai R, Copland IB, Garcia MA, Petersen CT, Lewis CN, Waller EK, et al. Cryopreserved MSCs are susceptible to T-cell mediated apoptosis which is partly rescued by IFNγ licensing Raghavan. Stem Cells. 2016;34(9):2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holubova M, Lysak D, Vlas T, Vannucci L, Jindra P. Expanded cryopreserved mesenchymal stromal cells as an optimal source for graft-versus-host disease treatment. Biologicals. 2014;42(3):139–144. doi: 10.1016/j.biologicals.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Bieback K, Kuçi S, Schäfer R. Production and quality testing of multipotent mesenchymal stromal cell therapeutics for clinical use. Transfusion. 2019;59:2164–2173. doi: 10.1111/trf.15483. [DOI] [PubMed] [Google Scholar]
- 71.Stacey GN, Connon CJ, Coopman K, Dickson AJ, Fuller B, Hunt CJ, et al. Preservation and stability of cell therapy products: recommendations from an expert workshop. Regen Med. 2017;12(5):553–564. doi: 10.2217/rme-2017-0073. [DOI] [PubMed] [Google Scholar]
- 72.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(2):237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 73.Mason EF, Rathmell JC. Cell metabolism: An essential link between cell growth and apoptosis. Biochim et Biophys Acta Mol Cell Res. 2011;1813:645–654. doi: 10.1016/j.bbamcr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G. Challenges and strategies for improving the regenerative effects of mesenchymal stromal cell-based therapies. Int J Mol Sci. 2017;18(10):2087. doi: 10.3390/ijms18102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward MR, Abadeh A, Connelly KA. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Transl Med. 2018;7(7):543–550. doi: 10.1002/sctm.17-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baust JG, Snyder KK, Van Buskirk R, Baust JM. Integrating molecular control to improve cryopreservation outcome. Biopreserv Biobank. 2017;15(2):134–141. doi: 10.1089/bio.2016.0119. [DOI] [PubMed] [Google Scholar]
- 77.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.González DA, Pando RH, Lim MÁG, Fraustro SA, Garcia AT. Stromal cells-structure, function, and therapeutic implications. London: IntechOpen; 2018. Therapeutic strategies of secretome of mesenchymal stem cell. [Google Scholar]
- 79.Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, et al. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol. 2016;7:24. doi: 10.3389/fphys.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallina C, Turinetto V, Giachino C. A new paradigm in cardiac regeneration: the mesenchymal stem cell secretome. Stem Cells Int. 2015;2015:765846. doi: 10.1155/2015/765846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62:216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 82.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konala VBR, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett. 2015;168:154–158. doi: 10.1016/j.imlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Chinnadurai R, Rajan D, Qayed M, Arafat D, Garcia M, Liu Y, et al. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22(9):2504–2517. doi: 10.1016/j.celrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fung M, Yuan Y, Atkins H, Shi Q, Bubela T. Responsible translation of stem cell research: an assessment of clinical trial registration and publications. Stem Cell Rep. 2017;8:1190–1201. doi: 10.1016/j.stemcr.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–749. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 90.Marks PW, Witten CM, Califf RM. Clarifying stem-cell therapy’s benefits and risks. N Engl J Med. 2017;376(11):1007–1009. doi: 10.1056/NEJMp1613723. [DOI] [PubMed] [Google Scholar]
- 91.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 92.Kotobuki N, Hirose M, Machida H, Katou Y, Muraki K, Takakura Y, et al. Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng. 2005;11:663–673. doi: 10.1089/ten.2005.11.663. [DOI] [PubMed] [Google Scholar]
- 93.Xiang Y, Zheng Q, Jia B, Huang G, Xie C, Pan J, et al. Ex vivo expansion, adipogenesis and neurogenesis of cryopreserved human bone marrow mesenchymal stem cells. Cell Biol Int. 2007;31:444–450. doi: 10.1016/j.cellbi.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 94.Zhao ZG, Li WM, Chen ZC, You Y, Zou P. hematopoeisis capacity immunomodulatory effect andex vivo expasion poetntial of mesenhcymal stem cells are not impaired by cryopreservation. Cancer Invest. 2008;26(4):391–400. doi: 10.1080/07357900701788049. [DOI] [PubMed] [Google Scholar]
- 95.Doan CC, Truong NH, Vu NB, Nguyen TT, Nguyen HM, Nguyen KG, et al. Isolation, culture and cryopreservation of human bone marrow-derived mesenchymal stem cells. Int J Plant Anim Environ Sci. 2012;2(2):83–90. [Google Scholar]
- 96.Li H, Yan F, Lei L, Li Y, Xiao Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs. 2009;190(2):94–101. doi: 10.1159/000166547. [DOI] [PubMed] [Google Scholar]
- 97.Nitsch S, Chatterjee A, Hofmann N, Glasmacher B. Impact of cryopreservation on histone modifications of mesenchymal stem cells. Biomedizinische Technik. 2014;59:S294–S297. [Google Scholar]
- 98.Romanek J, Opiela J, Lipiński D, Smorąg Z. Effect of high hydrostatic pressure applied before cryopreservation on the survival rate and quality of porcine mesenchymal stem cells after thawing. Anim Biotechnol. 2018;29(4):283–292. doi: 10.1080/10495398.2017.1381106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table of cellular attribute data from studies extracted for the systematic review. It is a grid identifying which cell attributes each of the forty-one studies assessed. Of note each of the 41 studies may appear more than once depending on the attributes they assessed. Where a study undertook an assessment of a cellular attribute a cross is placed in the grid. Studies are arranged by species: human [chronologically and then alphabetically] and animals from most to least frequent species [chronologically and then alphabetically]). Column headers: Morphology; Morph. Viability; Via. Immunophenotyping; IP. Differentiation; Diff. Colony forming unit frequency; CFUF. Growth; Total growth. Metabolism; Met. Apoptosis; Apo. Attachment; Attach. Immunomodulation; Immuno. Paracrine; Para. Angiogenesis; Angio. Migration; Migr.
Additional file 2. Tabulated information relating to the freezing details extracted from the relevant studies. It shows the details of the individual freezing protocols outlined in the 41 retained studies. The method of freezing is given in detail alongside the species information, the concentration and passage of cells at the point of cryopreservation and the process of thawing. These details are common to the results tables (Tables 1, 2, 3, 4, 5, 6, 7, 8, 9).
Data Availability Statement
All data generated by this systematic search are included in this published article.