Short abstract
Background
Hyalinizing clear cell carcinomas (HCCCs) are rare, low-grade, malignant tumors which most often arise from the minor salivary glands primarily in palate and tongue but can arise in any location with minor salivary glands including the nasopharynx.
Methods
A case report of primary nasopharyngeal HCCC is presented. Because of the rarity of this tumor and location, a literature search was conducted to determine the most common presenting symptoms, treatment strategies, and outcomes.
Results
A 48-year-old man underwent biopsy of a 4.5 cm mass of the right nasopharynx with pathology suggesting an intermediate grade mucoepidermoid carcinoma. After discussing management with the patient, an endoscopic resection was performed. Final pathology revealed an HCCC which was confirmed after negative Mastermind-like 2 (MAML2) and positive Ewing sarcoma breakpoint region 1 (ESWR1) gene rearrangements on fluorescence in situ hybridization (FISH) studies. Literature review of other nasopharyngeal HCCC cases shows diverse presentation and overall excellent prognosis through surgical and radiation therapy.
Conclusion
HCCCs are rare, low-grade malignant tumors of the minor salivary glands and can present as a nasopharyngeal mass. Presenting symptoms are diverse but frequently involve otologic and sinonasal disturbances. HCCC is an indolent tumor with an excellent prognostic outcome when treated appropriately with surgical resection and adjuvant radiotherapy.
Keywords: hyalinizing clear cell carcinomas, skull base, nasopharynx, endoscopic
Introduction
Salivary gland tumors make up less than 0.5% of all malignancies, and hyalinizing clear cell carcinomas (HCCCs) account for 1% of all salivary gland tumors, making a nasopharyngeal HCCC exceptionally rare.1–3 HCCCs are low-grade malignant tumors originating from minor salivary glands.1–7 They most frequently arise from the palate and tongue, but primary tumors have been reported in the nasal cavity, oral mucosa, parotid gland, and rarely, the nasopharynx.1–3,7,8 A case series by Kauzman et al. showed a 1% prevalence of nasopharyngeal HCCC out of 98 cases of HCCC in English literature.1
HCCC is more common in females and typically presents as an indolent small mass.1–3 Local or distant metastases at presentation are uncommon, with no cases (0 of 10) of nasopharyngeal HCCC presenting with distant metastases.1–3,5 When tumors arise in the nasopharynx, presenting symptoms are otorrhea, nasal congestion, epistaxis, and tinnitus.1–11 Treatment typically includes surgical resection and neck dissection if lymphadenopathy is noted on initial evaluation.1,3,4 Overall, the prognosis of HCCC is favorable, especially if negative margins are achieved.1,3,7 Positive surgical margins and particularly aggressive tumors are frequently treated with radiotherapy to decrease risk of recurrence.1,7
Grossly, HCCCs are firm, whitish tumors with varying degrees of erythema.1,7 Histologically, they are characterized as an infiltrative neoplasm typically composed of monomorphic clear cells and peripheral polygonal cells with eosinophilic cytoplasm.1,3,5,7 These clear cells display little or mild nuclear pleomorphism with low mitotic activity.1,3,5,9 The tumor can demonstrate glandular formation with mucinous differentiation and typically grows in trabeculae, cords, and nests formed by clear, oncocytic, polygonal cells, surrounded by hyalinized fibrous bands and myxoid stroma.1–3,6,7,9 HCCC stains positive on immunohistochemistry for periodic acid-Schiff stain, low- and high-molecular weight keratins, epithelial membrane antigen, and p63.1–3,5,7,9,12
This immunohistochemistry and histopathology is present in many tumor variants including but not limited to mucoepidermoid carcinoma (MEC), myoepithelial carcinoma, and acinic cell carcinoma, making HCCC a difficult diagnosis.1–3 Investigation of chromosomal rearrangements using fluorescence in situ hybridization (FISH) aides in the final diagnosis as Mastermind-like 2 (MAML2) and Ewing sarcoma breakpoint region 1 (ESWR1) gene fusions are associated with MECs and HCCCs, respectively.1–3,5,6,9
Case Report
A 48-year-old man presented with a chief complaint of nasal congestion and for evaluation of a right-sided sinonasal mass. He has a history of right-sided conductive hearing loss, hypertension, and a 5-year history of eustachian tube dysfunction requiring multiple sets of pressure equalization tubes.
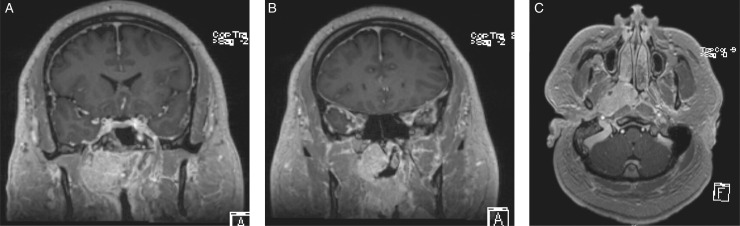
Nasal endoscopy demonstrated a large polypoid mass with prominent vessels obstructing the right nasal cavity. The tumor encompassed the posterior septum extending into the contralateral nasal cavity. A magnetic resonance imaging (MRI) showed a well-circumscribed 4.5 × 2.4 × 4 cm mass centered in the right fossa of Rosenmuller with anterior extension to the basal lamalle as well as the contralateral side (Figure 1). The torus tubarius was obliterated from mass effect, and he was noted to have a right-sided mastoid effusion, visualized on computed tomography and MRI. On physical examination, he had a pressure equalization tube in place with clear thick fluid. A biopsy of the mass was taken and was suggestive of MEC of intermediate grade.
Figure 1.
Cross-sectional imaging of the tumor on T1-weighted images with contrast. Coronal cuts (A and B) show the tumor extending along the nasopharynx, infratemporal fossa, and abutting the skull base. The tumor did not invade the skull base. Axial cut (C) demonstrates the lateral extension of the tumor into the distal portion of the eustachian tube along the pterygoids.
After discussing management with the patient including open, endoscopic, and primary radiation therapy, the patient underwent right-sided neck dissection at which time the carotid artery was controlled and then a subsequent endoscopic endonasal resection of the mass with a posterior septectomy and a nasal septal flap for coverage of the carotid artery. Despite a well-encapsulated tumor on MRI, the tumor was adherent to surrounding structure and residual tumor was left on the carotid artery.
Final pathology of the biopsy was consistent with a high-grade HCCC with ductal and mucinous differentiation and necrosis (Figure 2). FISH results were negative for MAML2 gene rearrangement and positive for EWSR1 rearrangement, confirming the diagnosis.
Figure 2.
Tumor histology. A, H&E ×40 low-power view demonstrating an infiltrative carcinoma growing in solid nests and cords with areas of glandular formation in a background of hyalinized stroma. B, H&E ×100 multiple areas of glandular formation (ductal differentiation) present in a background of hyalinized stroma. Areas of cytoplasmic clearing more typical of HCC can be appreciated in the more solid growth pattern. C, H&E ×100 focal areas of the tumor demonstrated more classic morphology, with clear cells juxtaposed to peritumoral hyaline stroma. D, H&E ×200 minor component consisting of clear cells juxtaposed to peritumoral hyaline stroma. E, H&E ×200 evidence of mucinous differentiation with mucin production.
Given the diagnosis and positive margins, the patient underwent intensity-modulated radiation therapy of 66 Gy targeting the nasopharynx and regional lymphatics over 6 weeks of duration. There were no signs of recurrent or residual disease 9 weeks of posttreatment.
Methods
Data on the case report were collected from the electronic medical record. PubMed and MEDLINE databases were queried for “Hyalinizing Clear Cell Carcinoma” and “nasopharynx.” The reference sections of these articles were used to identify additional cases of nasopharyngeal HCCC. A total of 268 abstracts were identified. Abstracts that did not include HCCC of nasopharyngeal origin were excluded, resulting in 6 case reports. Further investigation of bibliographic references resulted in 5 additional reports for a total of 13 nasopharyngeal HCCC cases found in the literature (Figure 3).
Figure 3.
Schematic of literature review. HCCC, hyalinizing clear cell carcinoma.
Results
Eleven case reports of primary nasopharyngeal HCCC were reviewed (Table 1), resulting in a total of 14 patient cases including the current report.2–11 Four patients lacked specified data in multiple categories, resulting in 10 patients with relevant demographic and clinical information. Of these 10 patients, 7 (70%) were women with an average age of 47.8 (range: 22–77 years). However, this small sample size limits our ability to suggest female predilection. Presenting symptoms of these 10 patients included epistaxis, otorrhea, hearing dysfunction, nasal congestion, weight loss, and 1 asymptomatic individual. Duration of symptoms prior to presentation ranged from 2 months to 5 years.
Table 1.
Published Cases of Nasopharyngeal HCCC.
| Study | Age/Sex | Presentation | Primary Tumor | Metastasis | Surgical Approach | Radiation | Outcome |
|---|---|---|---|---|---|---|---|
| Current Study | 48/Male | Otorrhea, eustachian tube dysfunction 5 years | 4.5 × 2.4 × 4 cm, Right Rosenmuller Fossa with extension to right posterior choana | None | Extradural craniofacial resection and maxillectomy | 66 Gy to nasopharynx and regional lymphatics | NED after 9 weeks |
| Ceballos et al.2 | 38/Male | Nasal congestion, Nasal voice quality change, rhinorrhea 5 months | 3.2 × 4.5 × 4.4 cm, extension into left nasal cavity, masticator space, bilateral medial ptyergoid lamina, sphenoid sinus, skull base and foramen ovale | Local cervical lymph nodesa | Incomplete surgical excision | Performed, dosing unspecified | Unspecified |
| Nakashima et al.4 | 27/Female | Right tinnitus and hearing disability for 6 months, and otitis media with effusion | 30 × 25 mm, obstruction of right eustachian tube, no parapharyngeal extension | None | Trans-oral, trans-palatal wide surgical resection | None | NED after 2 years |
| Fukuda et al.3 | 63/Female | Asymptomatic | 15 × 15 × 10 mm, roof of nasopharynx | None | Trans-nasal biopsy with macroscopic resection | None | NED after 1 year |
| Cheng et al.8 | 63/Female | Repeated epistaxis, right sided tinnitus, and weight loss for 2 months | 3.99 × 3.42 × 4.85 cm, right lateral and posterior wall, extension to the oropharynx, nasopharyngeal and choana obstruction | None | Endoscopic Resection | Performed, dosing unspecified | NED after 1 year |
| Nakano et al.5 | 27/Female | Hearing loss and fullness in right ear | 25 mm, location unspecified | None | Surgical resection, Unspecified | None | NED after 18 months |
| Bilodeau et al.11 | Not reported individually | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified |
| Bilodeau et al.11 | Not reported individually | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified |
| Bilodeau et al.11 | Not reported individually | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified |
| Antonescu et al.6 | 77/Female | Unspecified | 3.8 cm, invasion of eustachian tube | None | Surgical resection, Unspecified | Performed, dosing unspecified | NED after 1 year |
| Tang et al.10 | 51/Female | Unspecified | Unspecified | None | Trans-palatal excision, YAG laser ablative therapy | 60 Gy in 24 fractions for 46 days after second recurrence | Multiple recurrences over 11 year period |
| Shah et al.9 | Not reported individually | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified | Unspecified |
| Dosemane et al.7 | 22/Female | Epistaxis and partial nasal obstruction for 2 months | 2 × 2 × 1.5 cm, nasopharyngeal roof with partial bilateral choanae occlusion | None | Endonasal endoscopic wide excision | 60 Gy 30 fractions over 6 weeks | NED after 3 years |
| Chapman et al.13 | 62/Male | Unspecified | 10 mm | None | Excisional biopsy with positive margins | Unspecified | NED after 5 months |
Abbreviations: Gy, gray; NED, no evidence of disease; YAG, yttrium, aluminum, and garnet.
Hypermetabolic lymphnodes without biopsy confirmation.
All primary tumors were located in the nasopharynx and demonstrated diverse paths of invasion and obstruction. One of 9 (10%) cases presented with local cervical lymph node involvement.2 The unanimous treatment approach was surgical resection with varying open and endoscopic methodologies utilized. Six of 9 (66.67%) patients were treated with adjuvant radiotherapy with only 1 patient experiencing multiple recurrences. Of note, however, this case reported incomplete initial surgical excision. The average follow-up was 1.42 years (with a range of 1-to-3 years) excluding this case and 1 reported by Tang et al. (which had multiple recurrences over an 11-year period).10 HCCC, when treated with complete surgical excision and adjuvant radiotherapy, exhibits an excellent prognosis with no evidence of mortality.
Discussion
This article highlights a case of nasopharyngeal HCCC. Diagnosis of HCCC is difficult given the histologic similarities to MEC; however, with genetic testing of ESWR1 and MAML2 gene fusions, the diagnosis is conclusive.1,3,5,6,9 Given the histological similarities between MEC and HCCC, it is likely that HCCCs have been underrepresented in clinical diagnosis before the advent and increased access to FISH assays. HCCC is a diagnosis of exclusion from the other possible tumors on a differential diagnosis (eg, MEC). Without routine utilization of FISH in diagnosis, this likely led to a lowered statistical prevalence of HCCC with its neoplastic mimics. Therefore, a positive ESWR1 translocation in conjunction with distinct morphology and immunohistochemistry are required to definitively diagnose HCCC. Although there is no standardized treatment regimen, positive surgical margins and particularly aggressive tumors are frequently treated with radiotherapy to decrease risk of recurrence. The distinction between an MEC and HCCC is subtle histologically and generally both would be treated with surgical resection with adjuvant radiotherapy reserved for particularly aggressive tumors or positive surgical margins; however, it is still important to have an accurate diagnosis to better counsel patients regarding their disease-related mortality, which is higher with an MEC.14 In addition, the distinction is important in any clinical studies, and therefore, we can assure we are truly looking at outcomes for a single disease. Currently, FISH analysis for both MAML2 and ESWR1 mutations are not routinely performed. The ESWR1 assay is typically reserved for indistinct morphology between HCCC and MEC in the setting of a known negative MAML2 fusion, mainly due to the current knowledge that MECs are overwhelmingly more common than HCCC.
HCCC is defined by a positive ESWR1 and negative MAML2 gene fusion.1,3,5,6,9 The underlying tumorigenicity of MEC is aberrant cAMP/CREP activation caused by the MECT1–MAML2 fusion gene.15 Despite the similar histologic appearance of clear cell and mucin production, HCCC is negative for this fusion gene and represents a distinct entity.6,12 The binding of the N-terminal region and promoter of ESWR1 and dimerization domain of activating transcription factor 1 (ATF1) form a constitutively active protein.12 This translocation results in overexpression of microthphalmia-associated transcription factor (MiTF), a transcription protein that promotes tumor cell growth and survival.12 Despite this fusion protein being diagnostic in defining HCCC, Hsieh et al. reported a series of HCCCs that were negative for MiTF on immunohistochemistry.16 This suggests a knowledge gap in the molecular tumorigenicity of HCCC beyond the understanding of the presence of the ESWR1–ATF1 fusion gene.
In the 14 identified nasopharyngeal HCCCs, the most common presenting symptoms were otorrhea and tinnitus with occasional sinonasal disturbances. Surgical treatment was performed in 10 of 10 (100%) patients with specified information and postoperative radiation was utilized in 6 of 9 (66.67%).2–11 Although follow-up for our current case is limited, the available literature suggests an optimistic prognosis for the patient given the rare result of mortality across all head and neck HCCC. We identified 1 reported case of head and neck HCCC that results in death in the literature.17
Conclusion
HCCCs are rare tumors of the head and neck arising in the minor salivary glands. FISH assays for MAML2 and ESWR1 fusion genes can aid in a conclusive diagnosis and distinguish these tumors from MEC. Optimal management is uncertain; however, treatment strategies in the literature typically consisted of surgical resection and adjuvant radiation therapy. Overall outcomes are good with 1 (7.1%) reported recurrence and no reported disease-specific deaths.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was exempt by the institutional review board.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490 to A. J. K. and NIHT32-GM008719 and UL1TR002489 to W. H. S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ORCID iD
Madison J. Malfitano https://orcid.org/0000-0002-8820-1928
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Kauzman A, Tabet JC, Stiharu TI. Hyalinizing clear cell carcinoma: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:e26–e34. [DOI] [PubMed] [Google Scholar]
- 2.Ceballos Saenz C, Argyris PP, Manivel JC, Urias Barreras CM, Koutlas IG. Nasopharyngeal hyalinizing clear cell carcinoma: report of the histopathologic features of a case showing EWSR1 rearrangements by FISH and literature review. Int J Surg Pathol. 2014; 22:667–672. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda A, Tagami Y, Takasawa A, et al. Nasopharyngeal hyalinizing clear cell carcinoma with EWSR1 rearrangements diagnosed by fluorescence in situ hybridization. Auris Nasus Larynx. 2015; 42:412–415. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima T, Yasumatsu R, Yamauchi M, et al. Hyalinizing clear cell carcinoma of the nasopharynx operated by trans-oral and trans-palatal approach. J Laryngol Otol. 2015; 129 Suppl 2:S95–S97. [DOI] [PubMed] [Google Scholar]
- 5.Nakano T, Yamamoto H, Nishijima T, et al. Hyalinizing clear cell carcinoma with EWSR1-ATF1 fusion gene: report of three cases with molecular analyses. Virchows Arch. 2015; 466:37–43. [DOI] [PubMed] [Google Scholar]
- 6.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011; 50:559–570. [DOI] [PubMed] [Google Scholar]
- 7.Dosemane D, Lobo FD, Sreedharan SS. Primary clear cell carcinoma of the nasopharynx. J Cancer Res Ther. 2015; 11:928–930. [DOI] [PubMed] [Google Scholar]
- 8.Cheng LH, Lin YS, Lee JC. Primary clear cell carcinoma of the nasopharynx. Otolaryngol Head Neck Surg. 2008; 139:592–593. [DOI] [PubMed] [Google Scholar]
- 9.Shah AA, LeGallo RD, van Zante A, et al. EWSR1 genetic rearrangements in salivary gland tumors: a specific and very common feature of hyalinizing clear cell carcinoma. Am J Surg Pathol. 2013; 37:571–578. [DOI] [PubMed] [Google Scholar]
- 10.Tang SK, Wan SK, Chan JK. Hyalinizing clear cell carcinoma of salivary gland: report of a case with multiple recurrences over 12 years. Am J Surg Pathol. 1995; 19:240–241. [DOI] [PubMed] [Google Scholar]
- 11.Bilodeau EA, Weinreb I, Antonescu CR, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013; 37:1001–1005. [DOI] [PubMed] [Google Scholar]
- 12.Sidiropoulos M, Busam K, Guitart J, Laskin WB, Wagner AM, Gerami P. Superficial paramucosal clear cell sarcoma of the soft parts resembling melanoma in a 13-year-old boy. J Cutan Pathol. 2013; 40:265–268. [DOI] [PubMed] [Google Scholar]
- 13.Chapman E, Skalova A, Ptakova N, et al. Molecular profiling of hyalinizing clear cell carcinomas revealed a subset of tumors harboring a novel EWSR1-CREM fusion. Am J Surg Pathol. 2018; 42(9):1182–1189. [DOI] [PubMed] [Google Scholar]
- 14.Patel TD, Vázquez A, Patel DM, Baredes S, Eloy JA. A comparative analysis of sinonasal and salivary gland mucoepidermoid carcinoma using population‐based data. Int Forum Allergy Rhinol. 2015; 5(1):78–84. [DOI] [PubMed] [Google Scholar]
- 15.Coxon A, Rozenblum E, Park YS, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005; 65:7137–7144. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh MS, Wang H, Lee YH, Ko JY, Chang YL. Reevaluation of MAML2 fusion-negative mucoepidermoid carcinoma: a subgroup being actually hyalinizing clear cell carcinoma of the salivary gland with EWSR1 translocation. Hum Pathol. 2017; 61:9–18. [DOI] [PubMed] [Google Scholar]
- 17.Weinreb I. Hyalinizing clear cell carcinoma of salivary gland: a review and update. Head Neck Pathol. 2013; 7(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]