Abstract
Background
Microglia, the resident macrophages of central nervous system, have been initially categorized into two opposite phenotypes: classical activation related to pro-inflammatory responses and alternative activation corresponding with anti-inflammatory reactions and tissue remodeling. The correlation between metabolic pattern and microglial activation has been identified. However, little is known about the mechanism of metabolism-mediated microglia polarization and pro-inflammatory effect.
Methods
Metabolic alteration was analyzed in different phenotypes of microglia in vitro. LPS-induced neuroinflammation and sickness behavior mouse model was used to investigate the effect of lactate on classical microglial activation in vivo.
Results
Glycolysis-related regulators, monocarboxylate transporter 1 (MCT1), MCT4, and pro-glycolytic enzyme 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3), were specifically increased in LPS-stimulated primary microglia and microglia cell line BV2. Knockdown of MCT1 suppressed glycolysis rate and decreased LPS-induced expression of iNOS, interleukin-1β (IL-1β), IL-6, and phosphorylation of STAT1 in BV2 cells. Importantly, MCT1 promoted PFKFB3 expression via hypoxia-inducible factor-1α (Hif-1α), and overexpression of PFKFB3 restored the classical activation of BV2 cells suppressed by MCT1 silence. All above strongly suggested that MCT1/PFKFB3 might accelerate LPS-induced classical polarization of microglia probably by promoting glycolysis. Interestingly, additional administration of moderate lactate, which may block the transport function of MCT1, decreased LPS-induced classical activation and expression of PFKFB3 in BV2 cells. Intracerebroventricular injection of lactate ameliorated LPS-induced sickness behavior and classical polarization of microglia in mice.
Conclusions
Our results demonstrate the key role of MCT1 in microglial classical activation and neuroinflammation in pathological conditions. In addition, lactate administration may be a potential therapy to suppress neuroinflammation by altering microglial polarization.
Keywords: Classical microglial polarization, MCT1, PFKFB3, Glycolysis, Lactate, Neuroinflammation
Introduction
Microglia, the tissue-resident macrophages in the central nervous system (CNS), play important roles in phagocytosis, cell migration, antigen presentation, and cytokine production [1]. Accumulated evidences have demonstrated the critical role of microglia in neuroinflammation in multiple neurologic diseases, such as Alzheimer’s disease and ischemic stroke [1–5]. Similar to macrophages in peripheral tissues, microglial activation in the CNS is heterogeneous, which can be categorized into two opposite types: M1-like phenotype (classical activation) and M2-like phenotype (alternative activation). M1 like phenotype represents pro-inflammatory responses, including the release of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and reactive oxygen species (ROS). M2-like phenotype plays critical roles in tissue repair and neuroprotection, and exhibits anti-inflammatory reactions by expressing IL-10 and transforming growth factor-β (TGF-β) [6–9]. iNOS, TNF-α, IL-1β, IL-6, and STAT1 are important markers of M1 phenotype, while M2 markers mainly include Arg1 and CD206. Although the M1/M2 polarization is oversimplified to describe the heterogenecity of activated microglia, M1/M2 balance is helpful to understand the dual characters of microglia in neuroinflammation.
Increasing evidences support the critical role of multiple metabolic pathways in macrophage reprogramming [10, 11]. Rather than depending on mitochondrial ATP production, M1-like macrophages display high glycolysis rates, which can be induced by the expression of glucose transporter 1 (GLUT1) and pro-glycolytic 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) [10, 12]. Monocarboxylate transporters (MCTs) are also important regulators in glycolysis by transporting redundant lactate outside of the cellular. Conversely, M2-like phenotype displays low glycolysis rates without expression of PFKFB3 but appears high rates of mitochondrial fatty acid oxidation and oxidative phosphorylation [10, 13, 14]. Actually, artificial reprogramming of macrophage polarization is somewhat successful in clinic [15]. Therefore, glycolysis-related regulators influencing macrophage/microglia polarization may benefit therapy for different inflammatory diseases including neuroinflammation such as ischemic stroke. Recent data have confirmed the link between metabolism and microglia polarization similar to traditional macrophages. Indeed, a strengthening of glycolysis is observed in M1-like microglia, which is dependent on the increase of hexokinase and lactate dehydrogenase activity, and high expression of GLUT1 [16, 17]. However, it is still unknown whether glycolysis alteration could directly regulate microglial polarization. Considering that glucose is the main energy source in CNS, it is necessary to fully understand the role of glucose metabolism in microglial polarization. MCTs, the main transporters of lactate, are regarded as the special step of anaerobic glycolysis but not TCA cycle and oxidative phosphorylation. Evidences reveal that MCT1 protein is increased in N11 microglial cells after stimulation with TNF-α [18]. However, the key role of MCTs and glycolytic metabolite lactate in microglial activation remains unclear.
In order to address the role of MCTs in microglial polarization and evaluate the potential role of microglia metabolic reprogramming in limiting its pro-inflammatory effect, lentivirus-mediated MCT silencing and overexpression of PFKFB3 were applied in LPS or IL-4-stimulated microglia. Besides, the open field test was used to investigate the effect of lactate on LPS-induced neuroinflammation and sickness behaviors in mice. Our data demonstrated the critical role of MCT1/PFKFB3-promoted glycolysis in classical microglia activation and pro-inflammatory effect.
Materials and methods
Animals
Male C57BL/6 mice (8 weeks old), weighting 25–30 g, were used in this study. Mice were housed at 22 °C on a 12-h light/dark cycle with abundant water and food. All procedures referred to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Shandong University.
Cell cultures and treatment
Primary microglial cells were obtained from the brain of C57BL/6 mouse pups postnatal days 1–3 [9, 19]. In short, the cerebral cortex without the hippocampus and striatum was digested with 0.25% trypsin and DNase for 15 min at 37 °C. Cells were filtered with 70-μm nylon mesh and seeded into T75 flask (Corning). Resultant cells were maintained with DMEM and 10% fetal bovine serum supplemented with 1% of a penicillin/streptomycin solution. After 2–3 weeks, microglia were removed from flask by shaking. BV2 microglial cells were cultured with DMEM and supplemented with 10% heat-inactivated fetal bovine serum, 1% of a penicillin/streptomycin solution, and 2 mM L-glutamine. All cells were kept at 37 °C under 5% CO2 atmosphere. Hif-1α inhibitor KC7F2 (40 μM, dissolved in dimethyl sulfoxide, MCE, HY-18777) and MCT1 substrate L-lactate (5 mM, dissolved in PBS, Sigma, L7022) or pyruvate (5 mM, dissolved in PBS, Sigma, P5280) were used in this study.
RNA isolation and real-time quantitative PCR
After treatment, cells were used to extract RNA with TRIzol-A+ RNA isolation reagent (TIANGEN). The total RNA was applied to synthesized cDNA by the Revert Aid First Strand cDNA Synthesis Kit (Fermentas). All protocols were performed according to the manufacturer’s instructions. Quantitative RT-PCR was performed in a Cycler (Bio-Rad) using SYBR Green reagent (TIANGEN) to quantification. The β-actin mRNA levels were used to normalization according to 2-ΔΔCT method. The primer sequences used were listed in Table 1.
Table 1.
Sequence of primers used for quantitative real-time PCR
| Symbol | GenBank | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|---|
| iNOS | NM_001313921 | GAGCGAGTTGTGGATTGTC | CTGCCTATCCGTCTCGTC |
| Arg1 | NM_007482 | ATCTGCCAAAGACATCGT | CATCACCTTGCCAATCCC |
| MCT1 | NM_009196 | GAGGTCCTATCAGCAGTATCTT | CCAGTGGTCGCTTCTTGT |
| MCT2 | NM_011391 | CACTGGCTCCTTTCAATC | CTGGCTTTCTTCAGAGTTG |
| MCT4 | NM_001038653 | ACTTCAACAAGCGTCGCCCTAT | TCAGTCCCTCCGCCTACCTG |
| PFKFB3 | NM_001177752 | AGCCTCTTGACCCTGATA | TTCTTGCCTCTGCTGGAC |
| IL-1β | NM_008361 | ATTGTGGCTGTGGAGAAG | ATCTCGGAGCCTGTAGTG |
| IL-6 | NM_001314054 | TGCCTTCTTGGGACTGAT | TTGCCATTGCACAACTCTTT |
| TNF-α | NM_001278601 | GGCGGTGCCTATGTCTCA | CCTCCACTTGGTGGTTTGT |
| Hif-1α | NM_001313919 | CATCATCTCTCTGGATTTTG | GAAGAGGGAAACATTACATC |
| β-actin | NM_007393 | CGTTGACATCCGTAAAGACCTC | CCACCGATCCACACAGAGTAC |
Western blotting
In vitro, BV2 cells were harvested at the indicated days and lysed in ice-old TNE buffer (150 mM NaCl; 10 mM Tris, pH 8.0; 1 mM EDTA; 10% glycerol; 1% NP-40 with phosphatase protease inhibitors). Lysates were centrifuged at 14,000×g at 4 °C for 10 min. Protein lysates were boiled in sample buffer and resolved on 10% SDS-PAGE gels. The following primary antibodies were used in this study: rabbit anti-iNOS (Abcam, ab15323, 1:100), rabbit anti-MCT1 (Millipore, AB3540P, 1:1000), mouse anti-MCT4 (Santa Cruz, sc-376140, 1:200), rabbit anti-PFKFB3 (Proteintech, 13763-1-AP, 1:2000), rabbit anti-STAT1 (Bioworld, BS1333, 1:500), rabbit anti-phospho-STAT1 (CST, 9167, 1:1000), and mouse anti-β-actin (Proteintech, 60008-1-Ig, 1:2000). The appropriate HRP-conjugated second antibodies (1:5000) were from Calbiochem. The blots were reacted with an ECL chemiluminescence system (Millipore) and the gray intensity analysis was performed using the Quantity One software.
Immunofluorescence staining
Mice were anesthetized with 5% chloral-hydrate and perfused with 4% paraformaldehyde in PBS. Brains were removed and infiltrated with 30% sucrose for dehydration. Then brains were coated with OCT compound and sliced (40 μm). Slices were blocked in 5% normal goat serum in PBS for 1 h and incubated with primary antibodies against rabbit anti-Iba1 (Wako, 019-19741, 1:1000) or rabbit anti-CD86 (Abcam, ab53004, 1:200) overnight at 4 °C. After washing in PBS for 4 times, slices were incubated with anti-rabbit Alexa Fluor 488 or anti-rabbit Alexa Fluor 594 (Invitrogen) for 1 h at room temperature. Images were photographed using fluorescent microscopy (Nikon 80i) and Imaging computer program (NIS-Elements BR, Nikon). Finally, photographs were analyzed using the NIH Image J software.
Lentivirus-mediated overexpression and RNA interference
BV2 cells were infected with lentivirus for 24 h before treatment with lipopolysaccharide (LPS, 1 μg/ml, Sigma) or recombinant mouse IL-4 (20 ng/ml, Proteintech). The siRNA sequences used for lentivirus were as followed: MCT1 siRNA: 5′-GCA ACG ACC AGT GAA GTA T-3 ′; MCT2 siRNA: 5′-GGA AGA GAG AGA AGG CAA A-3′; MCT4 siRNA: 5′-GGT CTT TGT GGT GAG CTA T-3′. The RNA interference plasmids used to package lentivirus were purchased from Thermo Scientific Open Biosysterms. The pUltra vector was used to package PFKFB3-overexpression lentivirus.
Extracellular acidification rate (ECAR) assay
For real-time analysis of glycolysis, XFe 96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA) was used to monitor ECAR in BV2 microglial cells as described previously [20]. In brief, BV2 cells infected with lentivirus were plated onto a Seahorse XFe 96-well cell culture microplate (3 × 104 cells per well). After sedimentation for 1 h, BV2 cells were stimulated with LPS or vehicle and incubated at 37 °C in 5% CO2 for 24 h. Then culture medium was removed, and each well was washed two times with bicarbonate-free glycolysis stress media containing glutamine (pH 7.4). The cells were cultured at 37 °C without CO2 for 1 h. For ECAR measurement, the BV2 cells were monitored under basal conditions and in response to glucose (10 mM), oligomycin (2 μM), and 2-deoxyglucose (2-DG) (50 mM). The data obtained were normalized with cell number counts. Glycolysis values meant (maximum rate measurement before oligomycin injection) − (last rate measurement before glucose injection) while glycolytic capacity values represented (maximum rate measurement after oligomycin injection) − (last rate measurement before glucose injection).
Cell viability test
The cell viability of microglia was determined by the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The cells were seeded in the 96-well plates and treated as indicated. MTT solution was added to each well, and incubated for 3 h at 37 °C under 5% CO2 atmosphere. The supernatant media were removed and dimethyl sulfoxide (DMSO) was used to dissolve the formazan crystals. Absorbance of the soluble formazan dye was measured at 490 nm with a microplate reader.
Surgery, microinjection and open-field behavior test
After anesthetized with 5% chloral-hydrate, the mice were implanted a guide cannulas to the lateral ventricle. The coordinates were as follows: anteroposterior (AP) − 0.6 mm; lateral (L), +1.5 mm; dorsoventral (V), − 1.9 mm [21]. A stylus was placed into the guide cannula to avoid clogging. After recovery for 1 week, the mice were injected intraperitoneally with LPS (1 mg/kg). The microinjection of lactate (dissolved in 0.1 M PBS, 100 mM, 2 μL) or vehicle (0.1 M PBS) was performed by an infusion cannula, which is connected to a microsyringe and a microinjection pump (KD Scientific). The concentration of lactate was used as described previously [22].
After treatment with LPS for 8 h, open field test were used to evaluate the behavioral activity of mice [23]. The apparatus consists of a 40-cm × 40-cm black box and a camera at the top. Behavioral activity was recorded for 10 min after mice were placed in center of the field. A video tracking system (Smart) was used to score the total distance and resting time of each animal [24, 25]. The rearing activity was monitored by the camera. The mice were sacrificed after open field assay, and the brains were removed and used for immunofluorescence staining and RNA isolation.
Statistical analysis
The SPSS 19.0 statistical program was used for data analysis. Statistical comparisons were performed by Student’s t test or ANOVA, followed by the LSD or Dunnett’s T3 post hoc test. p < 0.05 was considered significant. Values were presented as the mean ± SEM.
Results
MCT1, MCT4, and PFKFB3 were increased after LPS stimulation but not IL-4 in both BV2 microglia cell line and primary microglia
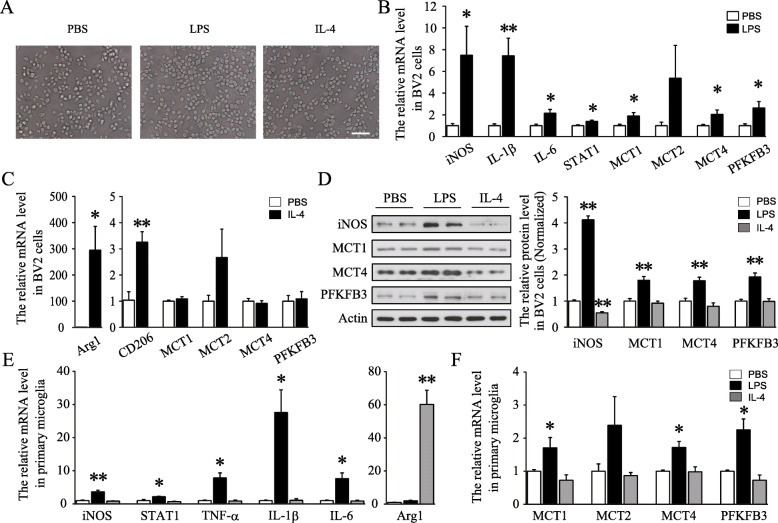
Accumulated data showed the increase of glycolysis in IFNγ/LPS-induced classical M1 microglia [10, 16]. To validate the effect of glycolysis-related regulators such as MCTs and PFKFB3 on microglia polarization, BV2 microglia cell line was stimulated with LPS or IL-4, respectively. As previously reported, the morphology of LPS-treated BV2 cells showed large soma and fewer branches than control cells (Fig. 1a). Quantitative RT-PCR further verified the classical M1 and alternative M2 phenotype of BV2 cells after LPS or IL-4 stimulation respectively. LPS specially increased the mRNA levels of classical microglia markers, including iNOS, IL-1β, IL-6, and STAT1, while IL-4 largely increased the expression of Arg1 and CD206, which were alternative phenotype markers (Fig. 1b, c). Importantly, LPS, but not IL-4, specially increased the levels of MCT1, MCT4, and PFKFB3 in BV2 cells (Fig. 1b–d). However, the expression of MCT2 was increased in both M1 and M2 microglia, although no significant difference was found.
Fig. 1.
LPS-induced special increase of MCT1, MCT4, and PFKFB3 in BV2 cell line and primary microglia. a Representative morphology of BV2 cells after treatment with PBS, LPS, or IL-4. Scale bar = 200 μm. b Quantification of the mRNA levels of classical microglial markers and glycolysis-related regulatory factors after treatment with LPS for 24 h (n = 6–8 per group and errors represent S.E.M; t test). c Quantification of the mRNA levels of alternative M2 markers and glycolysis-related regulatory factors after treatment of IL-4 for 24 h (n = 5 per group; t test). d Representative immunoblots and relative quantification of iNOS, MCT1, MCT4, and PFKFB3 after treatment with PBS, LPS, or IL-4 (n = 6 per group; one-way ANOVA). e, f Quantification of the mRNA levels of these genes in primary cultured microglia in each group (n = 6 per group one-way ANOVA). *p < 0.05, **p < 0.01 versus PBS-treated group
As shown in Fig. 1e, the phenotypes of LPS- and IL-4-induced classical or alternative microglial activation were further confirmed in cultured primary microglia. Besides, compared with control group, LPS significantly enhanced the expression of MCT1, MCT4, and PFKFB3, while no significant change was found in IL-4-stimulated primary microglia (Fig. 1f). Furthermore, in vivo experiments also displayed that intraperitoneal injection of LPS induced expression of MCT1 and PFKFB3 in the hippocampus (Additional file 1: Figure S1). Collectively, the specific increase of MCT1, MCT4, and PFKFB3 in LPS-stimulated microglia suggested a close relationship between increase of glycolysis and activation of classical microglia.
Knockdown of MCT1 suppressed LPS-stimulated microglial classical polarization and glycolysis rate
To further investigate the role of MCTs in classical polarization of microglia, lentiviruses expressing siRNA targeting MCT1, MCT2, or MCT4 (Lenti-siMCT1, Lenti-siMCT2, and Lenti-siMCT4) and a control lentivirus with a scrambled sequence (Lenti-siCtr) were applied. Although each lentivirus effectively suppressed expression of corresponding MCT (Fig. 2a), only treatment with Lenti-siMCT1, but not Lenti-siMCT2 or Lenti-siMCT4, significantly downregulated LPS-induced expression of iNOS mRNA (Fig. 2b). Furthermore, compared with LPS-treated group, Lenti-siMCT1 reduced the mRNA levels of IL-1β, IL-6, and STAT1, suggesting the involvement of MCT1 in LPS-induced classical polarization of microglia (Fig. 2c).
Fig. 2.
Knockdown of MCT1 inhibited LPS-stimulated classical microglial polarization and glycolysis rate in BV2 cells. a The interference efficiency of Lenti-siMCT1, Lenti-siMCT2, and Lenti-siMCT4, respectively, in BV2 cells (n = 6 per group and errors represent S.E.M; t test). b Quantification of the mRNA level of iNOS after treated with indicated lentivirus with or without LPS stimulation (n = 6–7 per group). c Quantification of the mRNA levels of IL-1β, IL-6, and STAT1, respectively, in each group (n = 5 per group). d Quantification of lactate levels in the cultured media in each group (n = 4 per group). e Quantification of the mRNA level of PFKFB3 in each group (n = 5–6 per group). f, g Representative immunoblots and relative quantification of iNOS, PFKFB3, and MCT1 in each group (n = 6 per group). h, i The real-time extracellular acidification rate was measured by the sequential addition of glucose, oligomycin, and 2-DG in each group (n = 5 per group). *p and #p < 0.05, **p and ##p < 0.01, two-way ANOVA followed by post hoc. Lenti-siCtr, Lenti-siMCT1, Lenti-siMCT2, and Lenti-siMCT4 are abbreviated as siCtr, siMCT1, siMCT2 and siMCT4, respectively
To explore whether MCT1-mediated classical polarization of microglia is dependent on glycolysis, lactate in the cultured media was detected using the L-lactate assay kit. As shown in Fig. 2d, LPS treatment increased the production of lactate while knockdown of MCT1 reversed LPS-increased release of lactate. In accordance, Lenti-siMCT1 also significantly blocked LPS-induced expression of pro-glycolytic PFKFB3 enzyme (Fig. 2e–g). Further, ECAR assay showed that the glycolysis was enhanced in M1-polarized BV2 cells, displaying as increased ECAR value, while increased glycolysis was largely diminished after knockdown of MCT1 in LPS-stimulated BV2 cells (Fig. 2h, i). All above data suggested that MCT1 may promote classical microglial polarization via enhancement of glycolysis.
MCT1 regulated LPS-stimulated classical microglial polarization via PFKFB3
Hypoxia-inducible factor-1 (Hif-1) is a crucial transcription factor during classical microglial polarization [26, 27]. In this study, we found that the protein level of Hif-1α was increased after LPS stimulation and significantly decreased after knockdown of MCT1 in BV2 cells (Fig 3a). Hif-1α is also regarded as an important regulator of glycolysis and its target genes include glucose transporter-1 (GLUT1) and PFKFB3 [27, 28]. KC7F2, a specific inhibitor of Hif-1α, was used to investigate the effect of Hif-1α in MCT1-regulated PFKFB3 expression. We found that treatment with KC7F2 reversed MCT1-increased expression of PFKFB3 in LPS-treated BV2 cells (Fig 3b), suggesting that MCT1 may regulate expression of PFKFB3 in a Hif-1α dependent manner. In addition, previous study has proved that knockdown of PFKFB3, the key enzyme in glycolysis, attenuated LPS-induced classical M1 polarization of macrophages [29]. Lentirivus-mediated MCT1 shRNA and lentivirus-mediated overexpression of PFKFB3 (Lenti-PFKFB3) were applied to address the role of PFKFB3 in MCT1-mediated classical microglial polarization. Results showed that, under LPS stimulation, overexpression of PFKFB3 significantly rescued the decreases of iNOS, IL-1β, and IL-6 mediated by Lenti-siMCT1 (Fig. 3c, d). However, either overexpression of PFKFB3 or knockdown of MCT1 showed no effect on the expression of Arg1 and CD206 (Additional file 1: Figure S2A, B). Besides, knockdown of MCT1 or overexpression of PFKFB3 had no effect on BV2 cell viability (Additional file 1: Figure S2C). Collectively, our data demonstrated the crucial role of PFKFB3 in MCT1-promoted classical polarization of microglia.
Fig. 3.
Overexpression of PFKFB3 rescued MCT1 silence-induced suppression of classical microglial polarization in BV2 cells. a Representative immunoblots and relative quantification of Hif-1α in each group (n = 4 per group and errors represent S.E.M). b The overexpression efficiency of Lenti-MCT1 and quantification of the mRNA level of PFKFB3 in each group (n = 4 per group). KC7F2, an inhibitor of Hif-1α, 30 mM. c, Quantification of the mRNA level of iNOS, IL-1β, and IL-6 after treatment with Lenti-siMCT1 and Lenti-PFKFB3 with or without LPS (n = 6–9 per group). d, e Representative immunoblots and relative quantification of iNOS and STAT1 protein, respectively, 24 h after LPS stimulation in each group (n = 6 per group). f Representative immunoblots and relative quantification of STAT1 and p-STAT1 after stimulation with LPS for 3 h in each group (n = 5–6 per group). *p, #p, and &p < 0.05, **p, ##p, and &&p < 0.01, NS represents no significant difference; two-way ANOVA followed by post hoc
STAT1 has been reported to drive classical macrophage polarization and inflammatory gene expression [30–32]. Therefore, we addressed the effects of MCT1 on regulating STAT1 signaling. Consistently, compared with control group, MCT1 interference decreased LPS-induced expression of STAT1 protein, which was rescued by overexpression of PFKFB3 (Fig. 3e). However, as previous report [33], short stimulation of LPS (within 3 h) led to significant increase of p-STAT1 in cultured microglia although there was no significant change of total STAT1 protein. Moreover, compared with vehicle group, MCT1 knockdown inhibited LPS-induced phosphorylation of STAT1 which was blocked by PFKFB3 overexpression (Fig. 3f). All these results validated that MCT1 might promote classical microglial polarization via a pro-glycolysis manner.
Lactate suppressed peripheral LPS-induced classical microglial polarization and neuroinflammation in mice
MCT1 is a transmembrane transporter of monocarboxylates and regarded as the key factor of anaerobic glycolysis to transport lactate into or outside of cell by facilitated diffusion [34]. Therefore, exogenous lactate stimulation may prevent MCT1-mediated excretion of lactate. Recently, lactate has been regarded as a substitute of glucose as a neuronal energy source to regulate neuronal activity and learning and memory [35]. Then the role of lactate on microglial function was assayed in this study. We found that exogenous lactate stimulation greatly suppressed the LPS-induced expression of iNOS and PFKFB3 in BV2 cells (Fig. 4a, b). Compared with stimulation of pyruvate, another substrate of MCT1, exogenous lactate seems to show larger effect on the decrease of IL-1β, IL-6, and Hif-1α (Fig. 4c–e). The above data suggested that lactate may mimic MCT1 interference effect on classical microglial polarization and neuroinflammation in vitro.
Fig. 4.
Lactate suppressed LPS-induced classical microglial polarization in BV2 cells. a, b Quantification of the mRNA levels of iNOS and PFKFB3 in the treatment of lactate and LPS in BV2 cells (n = 9 per group and errors represent S.E.M). *p < 0.05, **p, and ##p < 0.01, two-way ANOVA followed by post hoc. c–e Quantification of the mRNA levels of IL-1β, IL-6, and Hif-1α in the treatment of lactate, pyruvate, and LPS in BV2 cells (n = 4–7 per group one-way ANOVA)
To verify its role in vivo, lactate was injected intracerebroventricularly after peripheral administration with LPS. The substantia nigra consists of dopaminergic neuron and is an important brain region in regulation of locomotion. It has reported that the substantia nigra is susceptible to microglia-mediated inflammatory insult [25, 36]. The hippocampus plays critical roles in learning and memory, and neuroinlammatory processes in the hippocamous could lead to serious cognitive impairment [37]. In this study, we found that injection of LPS increased mRNA levels of pro-inflammatory markers in both the hippocampus and substantia nigra (Fig. 5a, b). Intracerebroventricular injection of lactate significantly reduced LPS-induced expression of M1-like phenotype markers, and also moderately enhanced the expression of Arg1 and CD206. In accordance with the quantitative RT-PCR data, the immunofluorescence results also showed that lactate significantly inhibited LPS increased the number of Iba1+ and CD86+ cells in the hippocampus (Fig. 5c, d) and substantia nigra (Additional file 1: Figure S3A,B). These data revealed that increased levels of lactate in the brain could reduce LPS-induced classical microglial polarization and neuroinflammation.
Fig. 5.
Intracerebroventricular injection of lactate reduced classical microglial polarization and neuroinflammation in the hippocampus and substantia nigra. a, b Quantification of the mRNA levels of microglial M1 and M2 markers in the hippocampus and substantia nigra, respectively, after intraperitoneal injection of LPS and intraventricular injection of L-lactate (n = 6–9 per group). c, d Immunostaining of classical microglia markers, CD86, and Iba1, and quantification of Iba1+ cells and CD86+ cells in the hippocampus in each group (n = 5–6 per group). *p and #p < 0.05, **p and ##p < 0.01, two-way ANOVA followed by post hoc. Scale bar = 50 μm
Lactate ameliorated sickness behavior during a peripheral inflammatory challenge
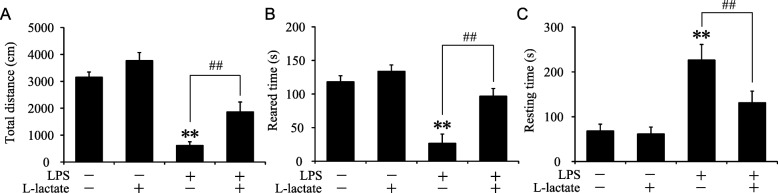
As reported, stimulation of the peripheral innate immune system can induce activation of microglia and secretion of cytokines. Moderate cytokines act on neuron substrates, which is beneficial for the host. However, excessive production of cytokines by microglia induced neuroinflammation and sickness behavior [38]. It has been reported that systemic injection of LPS-induced inflammatory response can lead to sickness behavior [25]. According to the previous studies, sickness behavior may include anorexia, weight loss, and social withdrawal and so on [39]. Here, open field assay was used to investigate the effect of lactate on LPS-induced sickness behavior. As shown in Fig. 6, LPS decreased the total distance and reared time greatly. Interestingly, mice with intracerebroventricular injection of lactate showed significant improvement on total distance and reared times (Fig. 6a, b). In contrast, resting times were largely decreased after treatment with lactate compared with mice in LPS-treated group (Fig. 6c). In conclusion, these data demonstrated that intracerebroventricular administration of lactate could ameliorate LPS-induced neuroinflammation and sickness behavior.
Fig. 6.
Intracerebroventricular injection of lactate ameliorated LPS-induced sickness behavior in mice. a–c Quantification of mice total distance, reared times, and resting times by open field tests after intraperitoneal injection of LPS and intraventricular injection of L-lactate (n = 8 per group and errors represent S.E.M, **p and ##p < 0.01; two-way ANOVA followed by post hoc)
Discussion
Diversity and plasticity are hallmarks of cells of the monocyte-macrophage lineage. Microglia has been considered as the resident macrophages in the CNS. Due to the critical effect of activated microglia on neuroinflammation, reprogramming microglial polarization has become a potential therapy strategy in neurologic diseases [1, 3, 40, 41]. The current study provided several new insights on the glycolysis-mediated microglial polarization. First, we reported that MCT1, MCT4, and PFKFB3, the key glycolysis-related regulators, were significantly increased in classical microglia induced by LPS stimulation. Second, MCT1/PFKFB3-promoted glycolysis directed classical microglial polarization, which may rely on phosphorylation of STAT1. Third, exogenous lactate may mimic MCT1 interference effect on classical microglial polarization and neuroinflammation in vitro. Intracerebroventricular administration of lactate suppressed LPS-induced classical microglial polarization, accompanied by amelioration of neuroinflammation and sickness behavior in mice. Significantly, our research identified MCT1 as a new therapeutic target to attenuate neuroinflammation by reprogramming microglial polarization through metabolism.
Glucose has been regarded as the main energy source in the brain. Although emerging evidences show that glucose metabolism exists in neuron and astrocyte [35, 42], little is known about the effect of glucose metabolism on microglial function during pathological condition. In a rat middle cerebral artery occlusion model, enhanced expression of MCT1 and MCT2 has been detected in activated microglia [18]. However, there is no certain evidence to display the explicit effect of MCTs-mediated glycolysis on microglial polarization. Here, we showed that MCT1, MCT4, and PFKFB3 were increased in microglia after stimulation with LPS, while no changes were found in IL-4-treated cells. Moreover, knockdown of MCT1 significantly suppressed the expression of classical M1 markers, iNOS, IL-1β, IL-6, and STAT1, suggesting that MCT1 directly determine classical microglial polarization. Furthermore, knockdown of MCT1 reduced LPS-increased release of lactate and expression of pro-glycolytic PFKFB3 enzyme, and stimulation of additional lactate could mimic MCT1 interference effect on microglial activation. It is reported that MCT1 has a much greater affinity for lactate than MCT4. Besides, the expression of MCT1 and MCT4 in microglia may be different. The above data suggested that microglia may rely more on MCT1 than MCT4 to transport lactate [34]. The ECAR assay also revealed that knockdown of MCT1 directly decreased glycolytic rate in LPS-stimulated BV2 cells. These results confirm that MCT1-directed classical microglial polarization may be dependent on pro-glycolytic regulation.
STATs are well-established potent transcriptional factors in the regulation of cell growth and differentiation, apoptosis, and inflammation in immune cells, which can be activated after phosphorylation [33, 43]. STAT1 has been identified as an important regulator in macrophage and microglia M1 polarization and inflammatory gene expression. An impaired expression of IFN regulatory factor-1 and iNOS is detected in the macrophages derived from STAT1-/- mice after LPS stimulation [31]. In microglia, a rapid increase of p-STAT1 was displayed within 3 h after LPS stimulation. Our results showed that knockdown of MCT1 significantly decreased the level of p-STAT1 after LPS stimulation. Therefore, we conclude that MCT1-mediated glycolysis may drive microglia to classical polarization through promoting phosphorylation of STAT1. However, the mechanism of MCT1 regulating STAT1 requires further investigation in the future.
Some metabolic enzymes and metabolites in the glycolysis and pentose phosphate pathway (PPP) have been reported to direct distinct polarization of macrophages [10]. PFKFB3 is an active ubiquitous PFK2 isoenzyme and a crucial regulator to promote glycolysis. Inhibition of glycolysis flux via knockdown of PFKFB3 largely hinders M1-polarization induced by LPS/IFN-γ [29]. Our results showed that both MCT1 deficiency and treatment with additional lactate decreased LPS-induced expression of PFKFB3 in microglia. By using a specific Hif-1α inhibitor, we found that MCT1 may regulate the expression of PFKFB3 via a Hif-1α dependent manner in microglia. In LPS-stimulated microglia, overexpression of PFKFB3 prevented knockdown of MCT1-impaired classical polarization. Here, we speculated that PFKFB3 might regulate the protein synthesis of markers of microglial polarization and phosphorylation of STAT1 through regulation of glycolysis and related metabolites. Above all, we conclude that MCT1 mediated classical microglial polarization through upregulation of PFKFB3 and further enhanced glycolysis flux. As MCTs are transporters of lactate, knockdown of MCT1 would directly prevent the transport of lactate from intracellular to outside, leading to lactate accumulation. The high accumulation of intracellular lactate may be a negative feedback regulation factor of glycolysis, which can inhibit the expression of Hif-1α. Alternatively, lactate, regarded as a signaling regulator, may also modulate expression of Hif-1α [44, 45]. As reported, PFKFB3 is one of Hif-1α target genes [28]. According to our results, we believed that MCT1 may enhance PFKFB3-mediated glycolysis via Hif-1α and finally promote microglial classical polarization (Fig. 7).
Fig. 7.
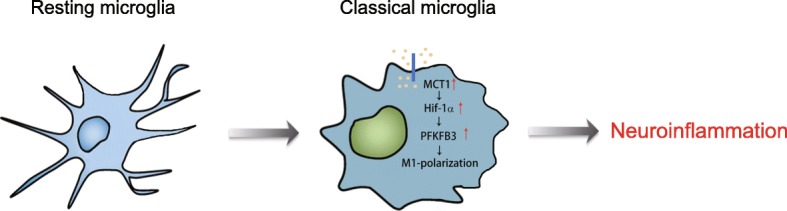
Schematic representation of MCT1/PFKFB3-mediated classical microglial activation and pro-inflammatory effect
More importantly, our in vitro and in vivo experiments showed that treatment with lactate prevented microglia from classical polarization and attenuated neuroinflammation, which mimicked the effect of MCT1 interference. Evidences revealed that systemic administration of LPS was able to induce microglial M1 polarization and neuroinflammation, and finally led to sickness behavior [25]. Astonishingly, the increased levels of lactate in the brain attenuated LPS-induced sickness behavior. This result indicated that lactate may improve mice behavioral disorder through suppressing LPS-induced classical microglial polarization and neuroinflammation.
Conclusions
In short, the present findings demonstrate that MCT1 enhances the expression of PFKFB3 via Hif-1α and thereby promotes classical microglial activation and pro-inflammatory effect. Exogenous lactate can mimic MCT1 interference effect on classical microglial polarization and neuroinflammation in vitro. Importantly, intracerebroventricular administration of lactate suppressed LPS-induced classical microglial polarization, accompanied by amelioration of neuroinflammation and sickness behavior in mice. Our data strongly suggested that attenuation of glycolysis flux targeting MCT1 in classical microglia maybe a potential therapy for brain diseases in the future.
Supplementary information
Additional file 1: Figure S1. Intraperitoneal injection of LPS increased the expression of MCT1 and PFKFB3 in the hippocampus. A, Immunostaining of Iba1 (green) and MCT1 (Red) in the hippocampus in PBS- and LPS-treated groups (n = 4 per group). The white arrow represents Iba1 and MCT1-positive cells. B, Immunostaining of PFKFB3 in the hippocampus in each group (n = 4 per group). Scale bar = 50 μm. Figure S2. MCT1 and PFKFB3 have no effect on alternative microglial polarization and cell viability. A, The overexpression efficiency of Lenti-PFKFB3 in BV2 cells (n = 8 per group and errors represent S.E.M, **p < 0.01; t test). B, Quantification of the mRNA level of Arg1 and CD206 after treatment with Lenti-siMCT1 and Lenti-PFKFB3 under PBS and LPS-stimulated conditions (n = 8 per group and errors represent S.E.M). C, Knockdown of MCT1 or overexpression of PFKFB3 has no effect on BV2 cell viability (n = 5 per group and errors represent S.E.M). Figure S3. Intracerebroventricular injection of lactate reduced classical microglial polarization in the substantia nigra. A, B, Immunostaining of classical microglia markers, CD86 and Iba1, and quantification of Iba1+ cells and CD86+ cells in the hippocampus in each group (n = 5-6 per group). **p and ##p < 0.01, two-way ANOVA followed by post hoc. Scale bar = 50 μm.
Acknowledgements
We thank School of Basic Medical Sciences of Shandong University for supplying experimental places and materials.
Abbreviations
- MCT1
Monocarboxylate transporter 1
- PFKFB3
6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3
- IL-1β
Interleukin-1β
- Hif-1α
Hypoxia-inducible factor-1α
- CNS
Central nervous system
- TNF-α
Tumor necrosis factor-α
- ROS
Reactive oxygen species
- TGF-β
Transforming growth factor-β
- GLUT1
Glucose transporter 1
- ECAR
Extracellular acidification rate
- DMSO
Dimethyl sulfoxide
- PPP
Pentose phosphate pathway
- MTT
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
Authors’ contributions
CHM and LFG designed the experiments, analyzed the results, and revised the paper. LK finished the majority of this work and wrote the paper. ZHW, XHL, and YW provided lots of technical supports and participated in the discussion of the results. All authors approved the final manuscript.
Funding
This work was supported in part by grants from the NSFC of China (81370527), the National Natural Science Fund for Outstanding Youth Fund (81425012), the Programme for ZR2015HZ005 and 2016ZDJS07A17, and Collaborative Innovation Center for Marine Biomass Fibers, Materials and Textiles of Shandong Province.
Availability of data and materials
All data are available upon request.
Ethics approval and consent to participate
All procedures referred to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Shandong University.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lifen Gao, Phone: 86-531-88382038, Email: glfflg@sdu.edu.cn.
Chunhong Ma, Phone: 86-531-88382038, Email: machunhong@sdu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12974-019-1648-4.
References
- 1.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, Lee SR, Choi SS, Yeo HG. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int. 2014;2014:297241. doi: 10.1155/2014/297241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemarchant S, Dunghana H, Pomeshchik Y, Leinonen H, Kolosowska N, Korhonen P, Kanninen KM, Garcia-Berrocoso T, Montaner J, Malm T, Koistinaho J. Anti-inflammatory effects of ADAMTS-4 in a mouse model of ischemic stroke. Glia. 2016;64:1492–1507. doi: 10.1002/glia.23017. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016;213:667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, Ffrench-Constant C M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 8.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Murakami K, Bando Y, Yoshida S. Interferon regulatory factor 7 participates in the M1-like microglial polarization switch. Glia. 2015;63:595–610. doi: 10.1002/glia.22770. [DOI] [PubMed] [Google Scholar]
- 10.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 11.Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 14.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, Mahy N. Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res. 2014;92:723–731. doi: 10.1002/jnr.23356. [DOI] [PubMed] [Google Scholar]
- 17.Holland R, McIntosh AL, Finucane OM, Mela V, Rubio-Araiz A, Timmons G, McCarthy SA, Gun'ko YK, Lynch MA. Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun. 2018;68:183–196. doi: 10.1016/j.bbi.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Moreira TJ, Pierre K, Maekawa F, Repond C, Cebere A, Liljequist S, Pellerin L. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J Cereb Blood Flow Metab. 2009;29:1273–1283. doi: 10.1038/jcbfm.2009.50. [DOI] [PubMed] [Google Scholar]
- 19.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Giri S, Kumar A. 5-Aminoimidazole-4-carboxamide ribonucleoside-mediated adenosine monophosphate-activated protein kinase activation induces protective innate responses in bacterial endophthalmitis. Cell Microbiol. 2016;18:1815–1830. doi: 10.1111/cmi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–1789. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- 23.Kang SS, Ren Y, Liu CC, Kurti A, Baker KE, Bu G, Asmann Y, Fryer JD. Lipocalin-2 protects the brain during inflammatory conditions. Mol Psychiatry. 2017. [DOI] [PMC free article] [PubMed]
- 24.Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Xu S, Qian Y, Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav Immun. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Bok S, Kim Y-E, Woo Y, Kim S, Kang S-J, Lee Y, Park SK, Weissman IL, Ahn G-O. Hypoxia-inducible factor-1α regulates microglial functions affecting neuronal survival in the acute phase of ischemic stroke in mice. Oncotarget. 2017;8:111508. doi: 10.18632/oncotarget.22851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merighi S, Borea PA, Stefanelli A, Bencivenni S, Castillo CA, Varani K, Gessi S: A2a and a2b adenosine receptors affect HIF-1Merighi S, Borea PA, Stefanelli A, Bencivenni S, C Glia 2015, 63:1933-1952. [DOI] [PubMed]
- 28.Obach M, Navarro-Sabaté À, Caro J, Kong X, Duran J, Gómez M, Perales JC, Ventura F, Rosa JL, Bartrons R. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem. 2004;279:53562–53570. doi: 10.1074/jbc.M406096200. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 30.Liu PW, Chen MF, Tsai AP, Lee TJ. STAT1 mediates oroxylin a inhibition of iNOS and pro-inflammatory cytokines expression in microglial BV-2 cells. PLoS One. 2012;7:e50363. doi: 10.1371/journal.pone.0050363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001;69:598–604. [PubMed] [Google Scholar]
- 32.Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood. 2005;106:3114–3122. doi: 10.1182/blood-2005-02-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Przanowski P, Dabrowski M, Ellert-Miklaszewska A, Kloss M, Mieczkowski J, Kaza B, Ronowicz A, Hu F, Piotrowski A, Kettenmann H, et al. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med (Berl) 2014;92:239–254. doi: 10.1007/s00109-013-1090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cerebral Blood Flow Metabol. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfau ML, Russo SJ. Neuroinflammation regulates cognitive impairment in socially defeated mice. Trends Neurosci. 2016;39:353–355. doi: 10.1016/j.tins.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, Henry C, Dantzer R, Johnson RW, Godbout J. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson MJ, Manzanero S, Borges K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia. 2015;56:895–905. doi: 10.1111/epi.12960. [DOI] [PubMed] [Google Scholar]
- 41.Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, Pu H, Li WW, Tang B, Wang Y, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benarroch EE. Brain glucose transporters: implications for neurologic disease. Neurology. 2014;82:1374–1379. doi: 10.1212/WNL.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 44.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I: Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. 2014, 111:12228-12233. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Intraperitoneal injection of LPS increased the expression of MCT1 and PFKFB3 in the hippocampus. A, Immunostaining of Iba1 (green) and MCT1 (Red) in the hippocampus in PBS- and LPS-treated groups (n = 4 per group). The white arrow represents Iba1 and MCT1-positive cells. B, Immunostaining of PFKFB3 in the hippocampus in each group (n = 4 per group). Scale bar = 50 μm. Figure S2. MCT1 and PFKFB3 have no effect on alternative microglial polarization and cell viability. A, The overexpression efficiency of Lenti-PFKFB3 in BV2 cells (n = 8 per group and errors represent S.E.M, **p < 0.01; t test). B, Quantification of the mRNA level of Arg1 and CD206 after treatment with Lenti-siMCT1 and Lenti-PFKFB3 under PBS and LPS-stimulated conditions (n = 8 per group and errors represent S.E.M). C, Knockdown of MCT1 or overexpression of PFKFB3 has no effect on BV2 cell viability (n = 5 per group and errors represent S.E.M). Figure S3. Intracerebroventricular injection of lactate reduced classical microglial polarization in the substantia nigra. A, B, Immunostaining of classical microglia markers, CD86 and Iba1, and quantification of Iba1+ cells and CD86+ cells in the hippocampus in each group (n = 5-6 per group). **p and ##p < 0.01, two-way ANOVA followed by post hoc. Scale bar = 50 μm.
Data Availability Statement
All data are available upon request.