Abstract
Background
Protease-Activated Receptor 2 (PAR2), a G-protein-coupled receptor, has been proved to be enhanced in human coronary atherosclerosis lesions. We aimed to investigate whether PAR2 actively participates in the atherosclerosis process.
Material/Methods
PAR2 expression was assessed in blood samples by RT-qPCR from healthy controls and patients with atherosclerosis. Human vascular smooth muscle cells (VSMCs) were treated with oxidative low-density lipoprotein (ox-LDL). After PAR2 overexpression by transfection, cell proliferation was determined by CCK-8, and cell migration was evaluated by Transwell assay. The protein expressions associated with cell growth and migration were measured by Western blot. The distribution of α-SMA in VSMCs was evaluated by immunofluorescence.
Results
Expression of PAR2 was higher in patients with atherosclerosis compared with normal controls. PAR2 mRNA and protein expression was increased in ox-LDL-treated VSMCs compared with control cells. Induced overexpression of PAR2 in VSMCs led to a reduction in α-SMA expression compared to controls. In addition, PAR2 overexpression caused increased migration compared to normal controls, and upregulated MMP9 and MMP14 expression. PAR-2 overexpression promoted cell proliferation compared to control cells, and increased expression levels of CDK2, and CyclinE1, but reduced levels of p27. We preliminary explored the potential mechanism of PAR2, and results showed that overexpression of PAR2 increased expression levels of VEGFA and Angiopoietin 2 compared to controls. Moreover, overexpression of PAR2 enhanced production of tissue factor and IL-8 compared to normal controls.
Conclusions
PAR2 promotes cell proliferation and disrupts the quiescent condition of VSMCs, which may be a potential therapeutic target for atherosclerosis.
MeSH Keywords: Atherosclerosis; Cell Dedifferentiation; Cell Migration Assays; Cell Proliferation; Receptor, PAR-2
Background
Atherosclerotic lesion advancement mainly depends on neovascularization, lipid-rich macrophages, cell death, calcification, and vascular remodeling. Among various risk factors, the cholesterol level is a sufficient and critical factor driving atherosclerosis. As a chronic inflammatory process, it always shows endothelium dysfunction, uptake of oxidized low-density lipoprotein (LDL), and intimal hyperplasia. The initiation of intimal hyperplasia is mainly led by proliferation and migration of vascular smooth muscle cells (VSMCs) [1,2]. Research indicates that ox-LDL must be present in atherosclerotic lesions to activate various angiogenic signaling pathways such as the sphingomyelinase-2/sphingosine kinase-1 pathway, and reactive oxygen species [3]. Therefore, better understanding of the mechanisms of angiogenesis in VSMCs is an important strategy for the prevention of atherosclerosis.
Proteinase-activated receptor 2 (PAR2), a 7-transmembrane G-protein-coupled receptor, was first found in endothelial cells and later in smooth muscle cells [4,5]. Protease-activated receptors (PARs) include PAR1, PAR2, PAR3, and PAR4. All PARs can be activated by Thrombin, except for PAR2. Recent investigations found that PAR2 can be activated by Cathepsin S, Tissue factor-VIIa protease complex, and trypsin [6–8]. Atherosclerosis lesions are usually induced by various cardiovascular diseases [9]. PAR2 has been reported to be upregulated in human coronary atherosclerotic lesions [10]. A PAR2 antagonist, FSLLRY-NH2, is used in type 2 diabetic mice, and the results show that PAR2 is downregulated in coronary arterioles and regulates endothelial dysfunction mediated by promoting TNFα production and NADPH oxidase activation [11]. Transient receptor potential vanilloid receptors may contribute to PAR2-induced protection for cardiac ischemia/reperfusion (I/R) injury, expressed mainly on the epicardial surface and blood vessels of I/R model mice [12]. Tryptase-induced cardiac fibroblasts were shown to be mediated in vitro by the PAR2/cyclooxygenase-2 (COX-2) signaling pathway [13]. Also, PAR2−/−ApoE−/− mice showed fewer atherosclerotic lesions involving lipid deposition, collagen formation, and inflammatory molecule production [14]. PAR2 deficiency reduced atherosclerosis in the aortic sinus and aortic root and further reduced CCL2 and CXCL1 expression, resulting in less monocyte migration [15]. However, little is known about the mechanism by which PAR2 in human vascular smooth muscle cells are related to atherosclerosis.
PAR2 signaling activates macrophages and promotes vascular inflammation in atherogenesis [14]. A previous investigation revealed PAR2 has potential pro-atherogenic effects by activating VEGFR2 in human coronary smooth muscle cells (HCSMCs) overexpressing PAR2 [16]. An in vivo study found that PAR2 deficiency alleviates atherosclerosis [15]. However, there are still no research showing the effects of PAR2 on VSMCs proliferation and migration, which usually results in intima hyperplasia in blood vessels, promoting formation of atherosclerotic plaque. Here, we hypothesize that PAR2 participation in atherosclerosis is partly due to activating VSMC proliferation and migration.
Material and Methods
Blood samples
Our study was approved by the Ethics Review Committees of the First Hospital of Hebei Medical University according to the Helsinki of Declaration (No. 2015033). All patients provided signed informed consent. Blood samples were collected from 30 patients with atherosclerosis and 30 normal healthy volunteers (Supplementary Table 1). The diagnosis of atherosclerosis in all patients was verified by 3 independent pathologists according to coronary angiography, with at least 1 lesion in a coronary artery or branches. Healthy individuals were randomly recruited among people attending regular health check-ups, with an age range similar to that of the selected atherosclerosis patients. We excluded patients who had received coronary artery bypass graft surgery, or who had congestive heart failure, renal failure, liver disease, malignant cancer, or inflammatory disease.
Cell lines
Human vascular smooth muscle cells (VSMCs) were purchased from ATCC (USA) and cultured in F12K culture medium supplemented with 10% fetal bovine serum (Gibco, USA). Cells were incubated at 37°C in a 5% CO2 atmosphere. VSMCs were treated with 100 μg/ml ox-LDL for 24 h. Overexpression of PAR2 was performed by transfecting pcDNA3.1 (Invitrogen, USA) encoding PAR2. The transfection of vectors of pcDNA3.1 was performed as negative control (NC). Lipofectamine 2000 (Invitrogen) was used to construct the plasmid according to standard protocol.
Quantitative real-time polymerase chain reaction (qRT-PCR)
VSMCs were seeded at 7×105 cells/well in 6-well plates. Cells were treated with ox-LDL or transfected with PAR2-pcDNA3.1. TRIzol reagent (ThermoFisher Scientific, USA) was used to extract the total RNA of cells and blood samples 24 h later. The RNA concentration was detected and calculated using NanoDrop ND-2000 (Thermo Scientific, USA). The RNA was reversed to cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen, USA), and the cDNA was later used for the qRT-PCR. The primers of PAR2 and 18s were:
PAR2 forward primer, 5′-TGGATGAGTTTTCTGCATCTGTCC-3′;
and reverse primer, 5′-CGTGATGTTCAGGGCAGGAATG-3′;
18s forward primer, 5′-CGGCTACCACATCCAAGGAA-3′,
and reverse primer, 5′-CCTGTATTGTTATTTTTCGTCACTACCT-3′.
The expression of PAR2 was normalized by 18s. mRNA levels were evaluated using the StepOne Plus real-time PCR system (Applied Biosystems, USA).
Western blot analysis
We seeded 1×106 cells into each 6-cm culture plate. Treated cells were later isolated using a lysis buffer (Sigma). Subsequently, proteins (30 μg) were separated by SDS-PAGE gel (10% and 12%). After proteins were well separated, they were transferred to polyvinylidene fluoride membranes (Millipore, USA) for 2 h and blocked with 5% (v/v TBST) skimmed milk for 1 h. The membranes were incubated with primary antibody at 4°C overnight: rabbit anti-PAR2 (#6976), MMP9 (#13667), MMP14 (#13130), and GAPDH (#5174) were purchased from Cell Signaling Technology (USA), and VEGFA (ab52917) and Angiopoietin 2 (ab8452) were purchased from Abcam (USA). HRP-linked anti-rabbit (#7074; Cell Signaling Technology) secondary antibodies were incubated at room temperature for 2 h. All results were validated through enhanced chemiluminescence detection method.
Cell proliferation
Cells were transfected with PAR2-pcDNA3.1, and then cultured in 96-well plates at a concentration of 5×103 cells/well incubated at 37 °C and 5% CO2 for 24 h after transfection. Cell viability was evaluated using the CCK-8 Kit (Real Times Technology, Beijing, China). Cells were incubated with 10 μl CCK-8 solution for 1 h at 37°C. Optical density was assessed at a wavelength of 490 nm.
Transwell assay to assess cell migration
Cells were overexpressed with PAR2 48 h before cell seeding. Subsequently, cells were planted into the upper chambers (8 μm; Millipore, USA) of 24-well plates at a concentration of 5×104 cells with serum-free medium, and complete medium (F12K supplemented with 10% FBS) was added to the bottom chambers. The cells were fixed and stained 24 h later using methanol and 0.1% crystal violet solution, respectively. Transferred cells were counted by microscope at 200× magnification.
Immunofluorescence
Cells were overexpressed with PAR2 48 h before cell seeding. VSMCs cells were cultured on 24-well chamber slides with 1×104 cells in 1 ml cultured medium in 24-well plates, and 24 h later, cells were fixed with paraformaldehyde (4%) for 10 min. Subsequently, cells were permeabilized with 0.1% Triton X-100 for 20 min at 25°C. After blocking cells for 30 min with 5% bovine serum albumin, cells were treated with anti-α-SMA antibody (Cell Signaling Technology, USA) at 4°C overnight, followed by treatment with Anti-rabbit IgG (H+L), F(ab’)2 Fragment (Alexa Fluor® 488 Conjugate; Cell Signaling Technology) at 25°C for 1 h. The nuclei were then stained by 4′,6-diamidino-2-phenylindole (DAPI; sigma-Aldrich) for 7 min in the dark. Confocal laser scanning microscopy (Olympus) was performed to valid the immunofluorescence results.
Tissue factor detection kit
Human Coagulation Factor III/Tissue Factor Quantikine ELISA kits were purchased from R&D systems (USA). PAR2 were overexpressed in VSMCs cells, and the cultured medium was collected after overexpression for 48 h. Tissue factor (TF) from cell supernatant was detected according to the manufacturer’s instructions.
IL-8 ELISA kit
Human IL-8/CXCL8 Quantikine ELISA kits were purchased from R&D Systems (USA). The supernatant from the cultured medium of PAR2-overexpressed VSMCs cells was collected after 48 h. IL-8 from cell supernatant was detected according to the manufacturer’s instructions.
Statistical analysis
All experiments were replicated 3 times (total n=9 from 3 independent experiments). Data are presented as mean±SD. Statistical analysis between groups was performed using the t test, and multiple comparisons were analyzed using one-way ANOVA. All analyses were performed in GraphPad Prism. Image J (version 1.3.7, USA) software was used for densitometry of Western blotting. P<0.05 was considered as statistically significant.
Results
PAR2 is associated with atherosclerosis
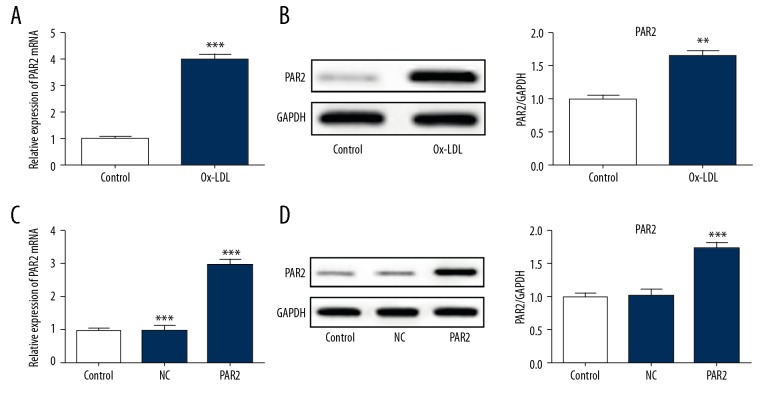
As shown in Figure 1, PAR2 was highly upregulated in atherosclerosis compared with healthy control. In addition, PAR2 expression was dramatically increased in ox-LDL-induced VSMCs, as shown by immunoblotting and qRT-PCR analysis (Figure 2A, 2B). To investigate whether PAR2 is associated with atherosclerosis progression, PAR2-pcDNA3.1 was transfected into VSMCs cells. The gene and protein expression levels of PAR2 were i significantly higher than in untreated cells and pcDNA control (Figure 2C, 2D).
Figure 1.
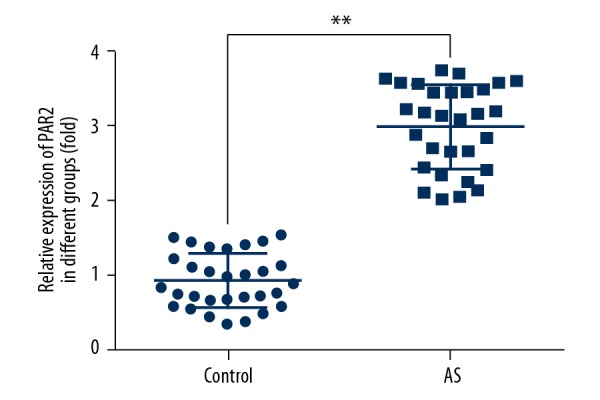
Increased expression of PAR2 in atherosclerosis patients (AS) compared to controls. qRT-PCR was performed to assess the expression levels of PAR2. ** p<0.01 versus controls.
Figure 2.
ox-LDL induction in the PAR2 expression in VSMCs. (A, B) Cells were treated with ox-LDL (100 μg/ml) for 24 h. qRT-PCR and Western blotting were performed to estimate the expression of PAR2. ** p<0.01 versus control. (C, D) Cells were transfected with PAR2-pcDNA3.1 or NC for 48 h. Transfection efficacy was detected by qRT-PCR and Western blotting. NC, pcDNA3.1 vector as the negative control. ** p<0.01, *** p<0.001 versus control or NC.
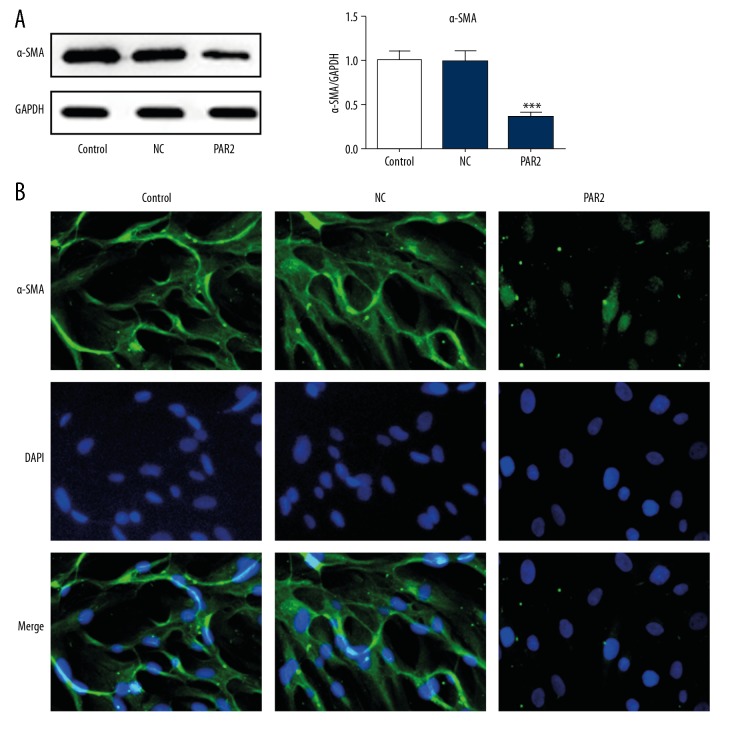
PAR2 is associated with VSMCs dedifferentiation
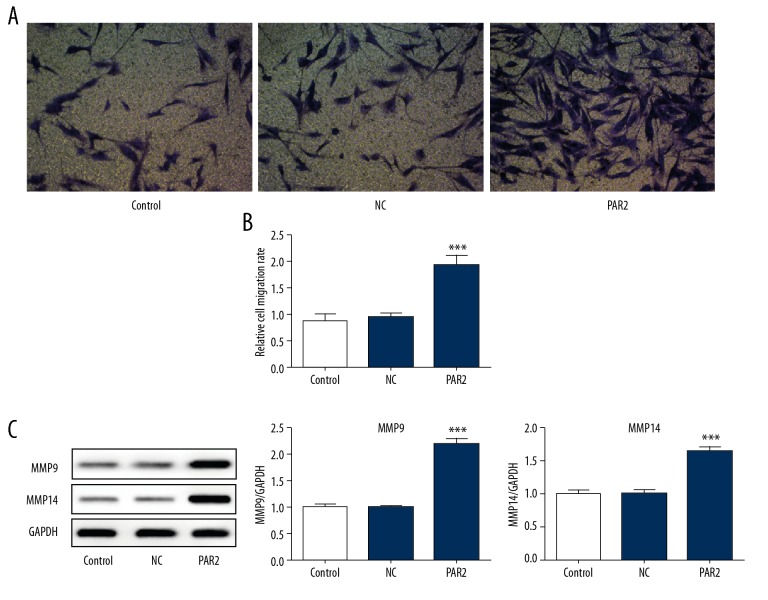
Modulation of phenotype in VSMCs, including decreased contractile marker and increased migration, proliferation, and extracellular matrix synthesis, is associated with atherosclerosis progression [17]. The expression of α-SMA was significantly decreased in PAR2-overexpressed VSMCs (Figure 3A). Consistently, immunofluorescence staining showed that PAR2 overexpression in VSMCs significantly reduced the level of α-SMA, which is used as a contractile phenotype marker (Figure 3B). There were significantly more migrating cells in PAR2-overexpressed VSMCs compared with control or NC (Figure 4A, 4B), and the expression levels of MMP9 and MMP14 were dramatically increased compared with control or NC (Figure 4C). In short, PAR2 dedifferentiates the quiescent contractile phenotype of VSMCs and promotes cell migration.
Figure 3.
Effect of PAR2 on α-SMA expression in VSMCs. Cells were transfected with PAR2-pcDNA3.1 or NC. (A) The expression levels of α-SMA were validated by Western blotting. ** p<0.01 versus control or NC. (B) Immunofluorescence assay was also performed to detect the alteration of α-SMA.
Figure 4.
VSMCs overexpression of PAR2 results in increased migration and MMP9/14 activity. (A) Transwell assay of cell migration. ** p<0.01 versus control or NC. Representative western blot analysis (B) and quantification (C) of MMP9 and MMP14 in VSMCs transfected with PAR2-pcDNA3.1 or NC. *** p<0.001 versus control.
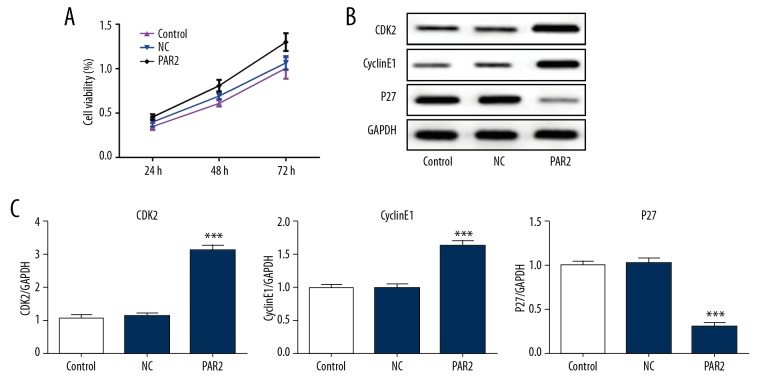
PAR2 promotes cell proliferation in VSMCs
In contrast with control or NC, PAR2 overexpression significantly increased the cell viability of VSMCs (Figure 5A). Activation of CDK2/CyclinE complex is essential for the cell cycle by promoting progression of cells from G1 phase to S phase. Overexpression of PAR2 significantly promoted the upregulation of CDK2 and CyclinE1 in VSMCs (Figure 5B, 5C). Moreover, the CDK2/CyclinE1 complex inhibitor, p27, was significantly inhibited by overexpression of PAR2 (Figure 5B, 5C). These data show that PAR2 promotes proliferation of VSMCs.
Figure 5.
PAR2 increased VSMCs proliferation. (A) CCK-8 demonstrated the effect of PAR2 on cell viability. ** p<0.01, *** p<0.001 versus control or NC. (B, C) Western blot analysis of PAR2 regulated CDK2, cyclin E1, and p27 expression. The results of western blotting were exhibited as band image (B) or quantitative analysis (C). *** p<0.001 versus control or NC.
Exploration of further mechanisms for PAR2 in VSMCs
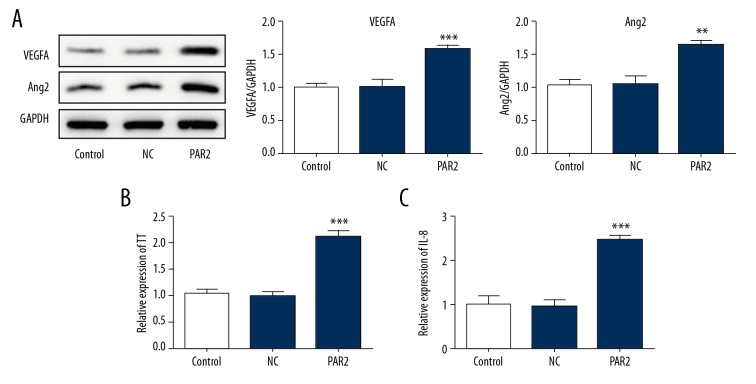
Figure 6A shows that overexpression of PAR2 upregulated the expression levels of VEGFA and Angiopoietin 2. Moreover, overexpression of PAR2 promoted the production of tissue factors (Figure 6B). A previous study found that expression of TF and PAR2 is accompanied by the expression of VEGF and IL-8. In the present study, IL-8 levels were significantly increased after VSMCs overexpressed PAR2 (Figure 6C).
Figure 6.
The potential mechanisms for the effects of PAR2. (A) Expression of VEGFA and Angiopoietin 2 (Ang2) were estimated by western blotting. Production of tissue factor (TF) (B) and IL-8 (C) were analyzed by ELISA. *** P<0.001 versus control or NC. ** p<0.01, *** p<0.001.
Discussion
During atherosclerosis, the phenotype of VSMCs was switched from contractile to proliferative and migrative types [18]. In the present study, we show that upregulation of PAR2 promotes the proliferation and migration of the cells in VSMCs, and ox-LDL induces upregulation of PAR2 in VSMCs. These novel findings show that ox-LDL-induced PAR2 overexpression promotes VSMCs proliferation and migration and elucidate the intercellular mechanism underlying the role of VSMCs in progression of atherosclerosis.
Ox-LDL has been proved to be a critical endogenous ligand that has various pathogenic roles. Ox-LDL is associated with formation of atherosclerotic plaque via induction of macrophage foam cell formation, as well as smooth muscle cell migration, proliferation, and inflammation [19,20]. A previous study found that VSMCs induced by ox-LDL can form foam-like cells, which contributes to the fatty streaks in atherosclerotic plaque [21]. In the present study, ox-LDL was used to stimulate VSMCs, and the expression of PAR2 was significantly upregulated. Furthermore, our results indicate that overexpression of PAR2 promotes proliferation, migration, and upregulation of the pro-inflammatory factor IL-8 in VSMCs.
It has been reported that PAR2 deficiency is associated with attenuation of atherosclerosis [15], but the main cellular mechanism has been unknown. VSMCs have a quiescent contractile phenotype under normal conditions. When cells are stimulated, VSMCs switch to a dedifferentiated state by suppressing the expression of VSMC-specific markers, including alpha-smooth muscle actin (α-SMA), to increase the ability of cells to cell proliferate and migrate [22]. Except for the VSMCs dedifferentiation, the cell–cell and cell–matrix interactions were also induced by VSMCs proliferation and migration [23,24]. Our data show that α-SMA was decreased in PAR2-overexpression VSMCs. In addition, the expression levels of MMP9 and MMP14 were increased by overexpression of PAR2, suggesting that overexpression of PAR2 promotes ECM degradation, which is related to migration of VSMCs [25].
Thrombosis usually forms based on atherosclerosis. Tissue factor is sensitive to initiation of atherosclerotic plaque and induces thrombosis [26,27]. In astrocytoma samples, elevated expression of VEGF and IL-8 was positively correlated with TF and PAR2 expression [28]. Camerer et al. found that PAR2 can be activated by TF/FVIIa complex [29]. The present study shows that PAR2 overexpression promotes the production of TF. Whether the endogenous TF affects the biological effects of VSMCs in atherosclerosis requires further investigation. VSMCs migration is related to increased expression of chemoattractants, including VEGF. Furthermore, the neovascularization process is tightly associated with the acceleration of atherosclerotic plaque formation. Vascular endothelial growth factor-A (VEGFA) is essential in the formation of angiogenesis [30,31]. The expression of VEGFA was significantly increased in PAR2 overexpressed VSMCs compared with control and NC. VEGF receptor 2 is the primary signaling receptor of VEGFA. A study reported that PAR2-activated VEGFR exerts pro-atherogenic effects [16]. It appears that PAR2-activated VEGFA/VEGFR signaling exert pro-atherosclerosis effects, but this requires further study. Angiopoietin-2 levels are clinically relevant to atherosclerosis, and this has been confirmed in mouse models [32]. In the present study, Angiopoietin-2 expression was significantly upregulated by overexpression of PAR2. These data show the potential molecular mechanism of PAR2 in VSMCs. Whether PAR2 affects VSMCs in atherosclerosis progression via these signaling processes remains unknown.
Conclusions
We assessed the molecular and cellular mechanisms by which PAR2 affects cell proliferation and migration in development of atherosclerosis, showing its potential roles as a diagnostic and therapeutic target. Although PAR2 was significantly upregulated in ox-LDL-treated VSMCs, the relationship between PAR2 and ox-LDL requires further exploration, and the effects of PAR2 need to be confirmed by in vivo experiments.
Supplementary Data
Supplementary Table 1.
Baseline characteristics of cardiac atherosclerosis under angiography.
| Age (year) | Sex | Smoking history | Family history of atherosclerosis | History of myocardial infarctions | Number of blood vessel lesions | LM* | LAD* | LCX* | RCA* |
|---|---|---|---|---|---|---|---|---|---|
| 53 | Female | No | No | No | 3 | No | No | No | No |
| 51 | Female | No | No | No | 2 | No | Yes | Yes | No |
| 53 | Female | No | Yes | No | 3 | No | Yes | Yes | Yes |
| 54 | Female | No | Yes | No | 3 | No | Yes | Yes | No |
| 55 | Female | No | No | No | 3 | No | No | Yes | Yes |
| 46 | Female | No | No | No | 2 | No | Yes | No | No |
| 52 | Female | No | No | No | 3 | No | No | No | No |
| 55 | Female | No | No | No | 3 | No | No | No | No |
| 50 | Female | No | No | No | 3 | Yes | No | No | No |
| 51 | Female | No | No | No | 2 | No | Yes | No | No |
| 52 | Female | No | No | No | 2 | No | No | Yes | No |
| 54 | Female | No | No | No | 2 | No | Yes | No | Yes |
| 44 | Female | No | Yes | No | 2 | No | Yes | Yes | No |
| 50 | Female | No | No | No | 2 | No | No | No | Yes |
| 49 | Female | No | Yes | No | 3 | Yes | No | No | No |
| 42 | Male | Yes | No | No | 3 | No | Yes | Yes | No |
| 43 | Male | Yes | Yes | No | 2 | No | No | No | Yes |
| 43 | Male | No | Yes | No | 3 | No | No | Yes | Yes |
| 41 | Male | Yes | No | No | 3 | No | No | No | No |
| 40 | Male | Yes | No | No | 3 | No | No | No | Yes |
| 45 | Male | No | Yes | No | 2 | No | No | Yes | No |
| 45 | Male | Yes | No | No | 3 | No | No | Yes | No |
| 46 | Male | Yes | Yes | No | 2 | No | No | No | Yes |
| 45 | Male | No | No | No | 3 | Yes | No | No | No |
| 43 | Male | Yes | Yes | No | 3 | No | No | No | No |
| 45 | Male | Yes | Yes | Yes | 3 | No | No | Yes | No |
| 43 | Male | Yes | No | No | 2 | No | No | Yes | No |
Distribution of the location of lesions in LM, LAD, LCX, or RCA.
LM – left main; LAD – left anterior descending; LCX – left circumflex branch; RCA – right coronary artery.
Footnotes
Source of support: This study was supported by the Science and Technology Pillar Program of Hebei Province (No. 16277707D), the Natural Science Foundation of Hebei Province (No. H2017206358), and the Big Health Service Research Program and Biomedical Program of Hebei Province (17277754D)
Conflict of interests
None.
References
- 1.Zhang QZ, Guo YD, Li HM, et al. Protection against cerebral infarction by Withaferin A involves inhibition of neuronal apoptosis, activation of PI3K/Akt signaling pathway, and reduced intimal hyperplasia via inhibition of VSMC migration and matrix metalloproteinases. Adv Med Sci. 2017;62:186–92. doi: 10.1016/j.advms.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhao XS, Zheng B, Wen Y, et al. Salvianolic acid B inhibits Ang II-induced VSMC proliferation in vitro and intimal hyperplasia in vivo by downregulating miR-146a expression. Phytomedicine. 2018;58:152754. doi: 10.1016/j.phymed.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Camare C, Auge N, Pucelle M, et al. The neutral sphingomyelinase-2 is involved in angiogenic signaling triggered by oxidized LDL. Free Radic Biol Med. 2016;93:204–16. doi: 10.1016/j.freeradbiomed.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Mirza H, Yatsula V, Bahou WF. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest. 1996;97:1705–14. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molino M, Raghunath PN, Kuo A, et al. Differential expression of functional protease-activated receptor-2 (PAR-2) in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1998;18:825–32. doi: 10.1161/01.atv.18.5.825. [DOI] [PubMed] [Google Scholar]
- 6.Cattaruzza F, Lyo V, Jones E, et al. Cathepsin S is activated during colitis and causes visceral hyperalgesia by a PAR2-dependent mechanism in mice. Gastroenterology. 2011;141:1864–74e1–3. doi: 10.1053/j.gastro.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belting M, Dorrell MI, Sandgren S, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502–9. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 8.Carr MJ, Schechter NM, Undem BJ. Trypsin-induced, neurokinin-mediated contraction of guinea pig bronchus. Am J Respir Crit Care Med. 2000;162:1662–67. doi: 10.1164/ajrccm.162.5.9912099. [DOI] [PubMed] [Google Scholar]
- 9.Agarwala A, Virani S, Couper D, et al. Biomarkers and degree of atherosclerosis are independently associated with incident atherosclerotic cardiovascular disease in a primary prevention cohort: The ARIC study. Atherosclerosis. 2016;253:156–63. doi: 10.1016/j.atherosclerosis.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napoli C, de Nigris F, Wallace JL, et al. Evidence that protease activated receptor 2 expression is enhanced in human coronary atherosclerotic lesions. J Clin Pathol. 2004;57:513–16. doi: 10.1136/jcp.2003.015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y, Yang J, Zhang H, et al. Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–23. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong B, Wang DH. Protease-activated receptor 2-mediated protection of myocardial ischemia-reperfusion injury: Role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1681–90. doi: 10.1152/ajpregu.90746.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray DB, McLarty-Williams J, et al. Tryptase activates isolated adult cardiac fibroblasts via protease activated receptor-2 (PAR-2) J Cell Commun Signal. 2012;6:45–51. doi: 10.1007/s12079-011-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara T, Phuong PT, Fukuda D, et al. Protease-activated receptor-2 plays a critical role in vascular inflammation and atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2018;138:1706–19. doi: 10.1161/CIRCULATIONAHA.118.033544. [DOI] [PubMed] [Google Scholar]
- 15.Jones SM, Mann A, Conrad K, et al. PAR2 (protease-activated receptor 2) deficiency attenuates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:1271–82. doi: 10.1161/ATVBAHA.117.310082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indrakusuma I, Romacho T, Eckel J. Protease-activated receptor 2 promotes pro-atherogenic effects through transactivation of the VEGF receptor 2 in human vascular smooth muscle cells. Front Pharmacol. 2016;7:497. doi: 10.3389/fphar.2016.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–8. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–64. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Zhou S, Zhao T, et al. TRPM7 channel regulates ox-LDL-induced proliferation and migration of vascular smooth muscle cells via MEK-ERK pathways. FEBS Lett. 2016;590:520–32. doi: 10.1002/1873-3468.12088. [DOI] [PubMed] [Google Scholar]
- 20.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low-density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–98. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang MJ, Zhou Y, Chen L, et al. Impaired SIRT1 promotes the migration of vascular smooth muscle cell-derived foam cells. Histochem Cell Biol. 2016;146:33–43. doi: 10.1007/s00418-016-1408-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang TM, Chen KC, Hsu PY, et al. microRNA let-7g suppresses PDGF-induced conversion of vascular smooth muscle cell into the synthetic phenotype. J Cell Mol Med. 2017;21:3592–601. doi: 10.1111/jcmm.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res. 2009;81:178–86. doi: 10.1093/cvr/cvn278. [DOI] [PubMed] [Google Scholar]
- 24.Barnes RH, 2nd, Akama T, Ohman MK, et al. Membrane-tethered metalloproteinase expressed by vascular smooth muscle cells limits the progression of proliferative atherosclerotic lesions. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.003693. pii: e003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murgai M, Ju W, Eason M, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. 2017;23:1176–90. doi: 10.1038/nm.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Miller C, Swarthout RF, et al. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–13. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Stevenson MJ, Brown JM, et al. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: Mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 28.Carneiro-Lobo TC, Lima MT, Mariano-Oliveira A, et al. Expression of tissue factor signaling pathway elements correlates with the production of vascular endothelial growth factor and interleukin-8 in human astrocytoma patients. Oncol Rep. 2014;31:679–86. doi: 10.3892/or.2013.2880. [DOI] [PubMed] [Google Scholar]
- 29.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–60. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–39. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 31.Waltenberger J. Modulation of growth factor action: Implications for the treatment of cardiovascular diseases. Circulation. 1997;96:4083–94. doi: 10.1161/01.cir.96.11.4083. [DOI] [PubMed] [Google Scholar]
- 32.Theelen TL, Lappalainen JP, Sluimer JC, et al. Angiopoietin-2 blocking antibodies reduce early atherosclerotic plaque development in mice. Atherosclerosis. 2015;241:297–304. doi: 10.1016/j.atherosclerosis.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Baseline characteristics of cardiac atherosclerosis under angiography.
| Age (year) | Sex | Smoking history | Family history of atherosclerosis | History of myocardial infarctions | Number of blood vessel lesions | LM* | LAD* | LCX* | RCA* |
|---|---|---|---|---|---|---|---|---|---|
| 53 | Female | No | No | No | 3 | No | No | No | No |
| 51 | Female | No | No | No | 2 | No | Yes | Yes | No |
| 53 | Female | No | Yes | No | 3 | No | Yes | Yes | Yes |
| 54 | Female | No | Yes | No | 3 | No | Yes | Yes | No |
| 55 | Female | No | No | No | 3 | No | No | Yes | Yes |
| 46 | Female | No | No | No | 2 | No | Yes | No | No |
| 52 | Female | No | No | No | 3 | No | No | No | No |
| 55 | Female | No | No | No | 3 | No | No | No | No |
| 50 | Female | No | No | No | 3 | Yes | No | No | No |
| 51 | Female | No | No | No | 2 | No | Yes | No | No |
| 52 | Female | No | No | No | 2 | No | No | Yes | No |
| 54 | Female | No | No | No | 2 | No | Yes | No | Yes |
| 44 | Female | No | Yes | No | 2 | No | Yes | Yes | No |
| 50 | Female | No | No | No | 2 | No | No | No | Yes |
| 49 | Female | No | Yes | No | 3 | Yes | No | No | No |
| 42 | Male | Yes | No | No | 3 | No | Yes | Yes | No |
| 43 | Male | Yes | Yes | No | 2 | No | No | No | Yes |
| 43 | Male | No | Yes | No | 3 | No | No | Yes | Yes |
| 41 | Male | Yes | No | No | 3 | No | No | No | No |
| 40 | Male | Yes | No | No | 3 | No | No | No | Yes |
| 45 | Male | No | Yes | No | 2 | No | No | Yes | No |
| 45 | Male | Yes | No | No | 3 | No | No | Yes | No |
| 46 | Male | Yes | Yes | No | 2 | No | No | No | Yes |
| 45 | Male | No | No | No | 3 | Yes | No | No | No |
| 43 | Male | Yes | Yes | No | 3 | No | No | No | No |
| 45 | Male | Yes | Yes | Yes | 3 | No | No | Yes | No |
| 43 | Male | Yes | No | No | 2 | No | No | Yes | No |
Distribution of the location of lesions in LM, LAD, LCX, or RCA.
LM – left main; LAD – left anterior descending; LCX – left circumflex branch; RCA – right coronary artery.