Abstract
The sense of touch and proprioception are critical to movement control. After spinal cord injury, these senses may be restored with direct, electrical microstimulation of the brain as part of a complete sensorimotor neuroprosthesis. The present study was designed to test, in part, the hypothesis that the cuneate nucleus (CN) of the brainstem is a suitable site to encode somatosensory information. Two rhesus macaques were implanted with microelectrode arrays providing chronic access to the CN. The monkeys were trained on an active touch oddity task to detect vibrotactile stimuli. When the vibrotactile stimuli were replaced with electrical stimuli delivered to the CN, initial detection probabilities were near chance. Detection performance improved over time, reaching a plateau after about 10 daily sessions. At plateau performance, the monkeys exhibited detection probabilities that were 68–80% higher than the chance probability. Finally, detection probability was quantified as a function of stimulus amplitude. The resulting psychometric curve showed a detection threshold of 45 μA for 100-Hz stimulus trains. These behavioral data are the first to show that artificial CN activation is sufficient for perception. The results are consistent with our hypothesis and motivate future tests of the CN as a somatosensory encoding site.
I. Introduction
Somatosensation is critically lacking in demonstrations of neurally-controlled prosthetic arms in paralyzed individuals [1, 2]. The result is motor performance well below what is needed for widespread clinical adoption of this technology [3]. Prior work on providing artificial sensation through electrical stimulation of the brain has almost exclusively targeted primary somatosensory cortex (S1) [4–12]. However, this may not be the optimal target due to an inability of electrical stimulation to appropriately activate its distributed representations [13]. Alternative, upstream sensory targets remain largely unexplored.
The cuneate nucleus (CN) in the dorsal brainstem is upstream of S1 in the somatosensory pathway, receiving primary afferent input from the upper body and projecting predominantly to the thalamus. Its supraspinal location makes it a suitable sensory encoding site for individuals with spinal cord injury. Furthermore, its compact representations may be more reliably activated artificially. Due to technical challenges, the primate CN has previously been accessed only acutely in anesthetized animals. Recently, our lab demonstrated the first successful chronic interface to the CN of macaques [14, 15]. This technique allows us to now characterize, with behavioral experiments, the percepts elicited by CN microstimulation.
In particular, the present study quantified the detectability of CN microstimuli in two rhesus macaques. We show that both animals could detect these artificial stimuli. We further document the learning curve to switch from detecting natural to artificial stimuli and the threshold current amplitude.
II. Methods
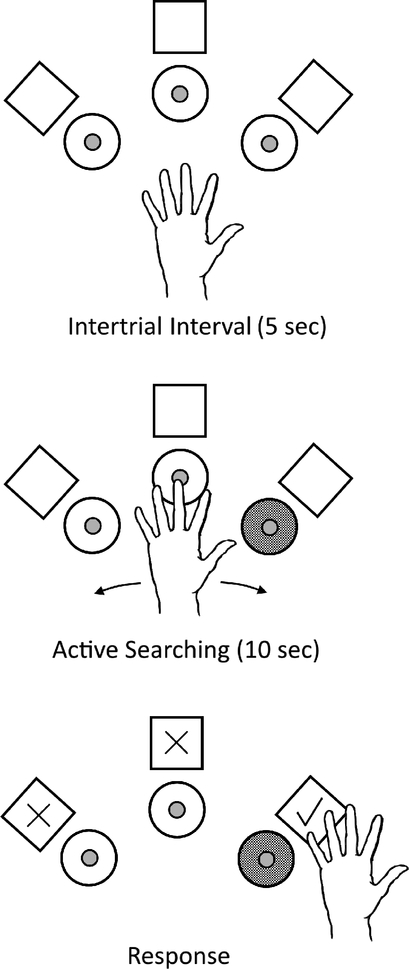
All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Two male rhesus macaques (Macaca mulatta), monkeys A and E, were trained on an active touch oddity task (ATOT) to quantify detection of vibrotactile stimuli. On each trial, the monkeys moved their left hand to determine which of three actuators (Haptuator Mark II, Tactile Labs Inc.) was vibrating and responded by pressing the corresponding capacitive touch sensor behind the chosen actuator (Fig. 1). Only one actuator (i.e. the oddity), chosen pseudorandomly, was active on each trial and the monkeys had up to 10 s to make their choice. Additional capacitive touch sensors were integrated with the actuators both to record the sequence in which the motors were explored and to gate the vibration. The oddity actuator was only on when the sensor indicated the monkey was touching it. Correct responses were rewarded with food and all trials were followed by a 5-s intertrial interval.
Figure 1.
Active touch oddity task (ATOT). The task consisted of three vibrotactile actuators (circles) and three response sensors (squares). One actuator was chosen as the oddity on each trial (gray circle). The objective was to move the hand to find the active actuator and indicate this choice by pressing the corresponding response sensor (checked square).
Following initial training on this task, the monkeys underwent a sterile surgical procedure to implant a headpost to stabilize the head during the experimental sessions. The animals were then re-trained to perform the ATOT under head-fixed conditions. Next, a second sterile surgical procedure was performed to implant a 32-channel floating microelectrode array (FMA, Microprobes for Life Science) into the left CN, using a technique developed previously [15]. The array had a 250-μm inter-electrode spacing and had platinum-iridium electrodes of four different lengths interspersed throughout the array (1.5, 2.0, 2.5, and 3.0 mm).
After implantation, electrophysiological sessions were conducted to verify the placement of the array in the CN. Punctate mechanical stimuli were delivered to the skin with a hand-held, force-sensing probe (REB7, Loadstar Sensors) while recording neuronal responses on the FMA (ZC32, PZ2, RZ2, Tucker-Davis Technologies). The site of maximal response for each recorded unit (i.e. the unit’s receptive field) was identified. Finally, behavioral sessions were conducted over several weeks. In these daily sessions, the monkeys performed the ATOT but with the vibrotactile stimulation replaced by CN microstimulation (0.2-ms/phase biphasic pulses, 100-Hz pulse frequency, 80-μA pulse amplitude unless noted otherwise; IZ2 stimulator, Tucker-Davis Technologies). Bipolar stimulation was delivered between pairs of electrodes with confirmed placement in hand or arm representations of the CN. As in training, the CN microstimuli were only delivered when the touch sensor signaled that the monkey was touching the pseudorandomly-chosen oddity location. The stimulus train lasted for as long as the touch was maintained, resulting in variable stimulus durations. Catch trials, in which no microstimulation was delivered, were included at random times in each session to evaluate chance performance on the task.
The location of the FMA in monkey A was confirmed to be in portions of the CN and the more medial gracile nucleus in a gross anatomical examination following euthanasia. No analysis of array location has been performed to date in monkey E as experiments are ongoing.
III. Results
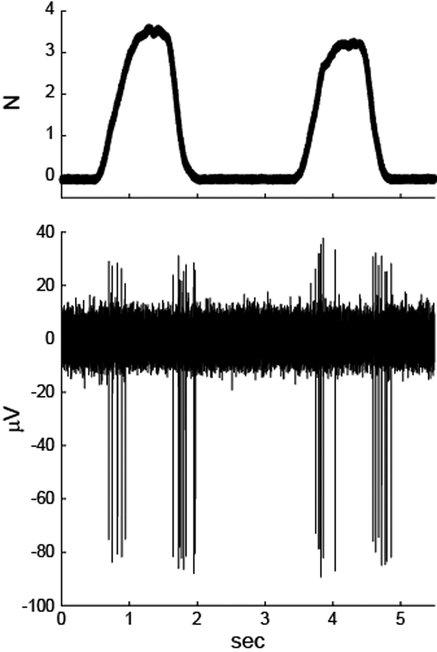
Monkeys A and E were implanted chronically with a FMA in the left CN. To assess the location of the electrodes, receptive fields (RFs) of recorded units were identified. An example is shown in Figure 2. This unit responded maximally to force pulses delivered on the extensor surface of the left forearm. The response, with spike bursts at the onset and offset of the stimulus, was similar to that of rapidly adapting primary afferents. RFs were compiled across several sessions to identify a subset of the 32-electrodes in each monkey that were in hand and arm representations of the CN. Microstimulation was restricted to these electrodes in the subsequent behavioral experiments.
Figure 2.
Stimulus-response data of one example CN neuron from monkey A. Force pulses were applied to the extensor surface of the forearm, near the elbow (top). The neuron responded with bursts of action potentials at the beginning and end of each pulse (bottom).
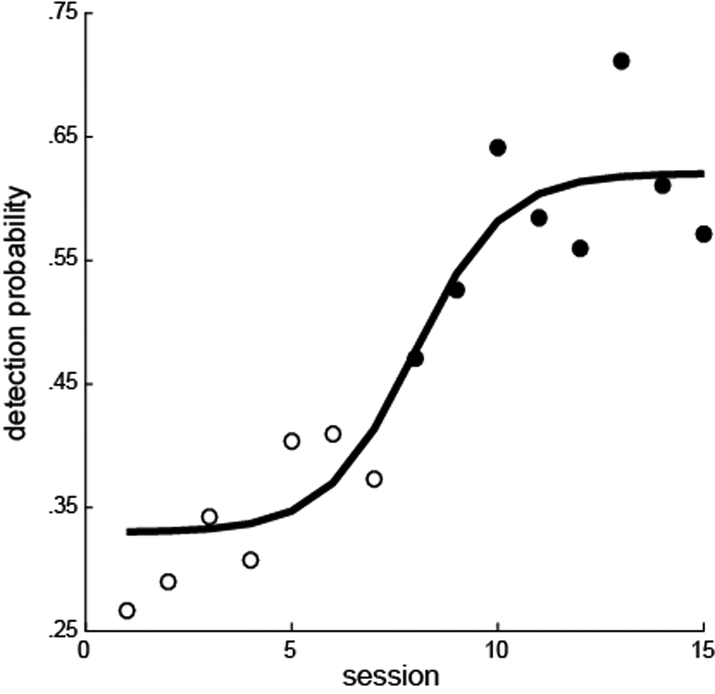
The monkeys were trained on the vibrotactile version of the detection task: the ATOT. Following implantation and RF mapping, vibrotactile stimuli were replaced with microstimulation delivered to the CN. In the first few sessions following this switch in stimulus modality, the monkeys’ detection performance in the ATOT was at chance levels. This suggests that CN microstimulation did not match the sensation of the vibrotactile input on which the monkeys were trained. In subsequent sessions, the animals’ performance improved as they learned, through operant conditioning, to detect the artificial sensations produced by CN microstimulation. Monkey A had interruptions in the testing sessions making it difficult to assess the transition in performance from natural to artificial stimuli. However, monkey E showed a clear learning curve after the first introduction of CN microstimuli (Fig. 3). The first significant improvement in detection probability over chance occurred on the eighth daily session (X2(1) = .0156). Plateau performance was seen by the tenth session.
Figure 3.
Performance of monkey E as he learned to detect CN microstimuli (80 μA, 100 Hz). Filled circles indicate the sessions in which the performance significfantly differed from performance on catch trials (chi-squared tests, p < .05). A logistic function fit to the data is shown.
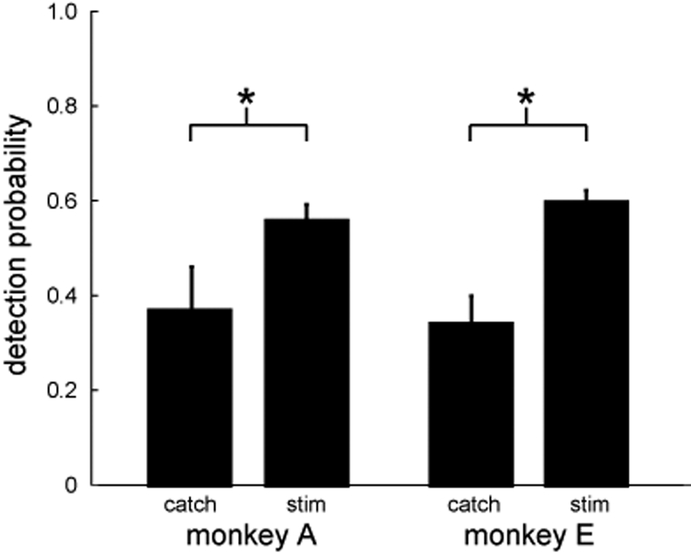
After the initial learning phase, both monkeys performed the CN stimulus detection task consistently above chance (Fig. 4). Monkey A had an average post-learning detection probability of 0.56, a 68% increase over chance performance. Monkey E had an average post-learning detection probability of 0.59, an 80% increase. These probabilities were significantly higher than on catch trials (chi-squared tests, p<0.05), in which no stimulation was delivered and the monkey had to simply choose a random response.
Figure 4.
Summary of detection probability of CN microstimuli (80 μA, 100 Hz) across all post-learning sessions. Error bars indicate the 95% confidence interval on the mean. Significant differences were observed between stimulus and catch trials for both monkeys (chi-squared tests, p<0.05).
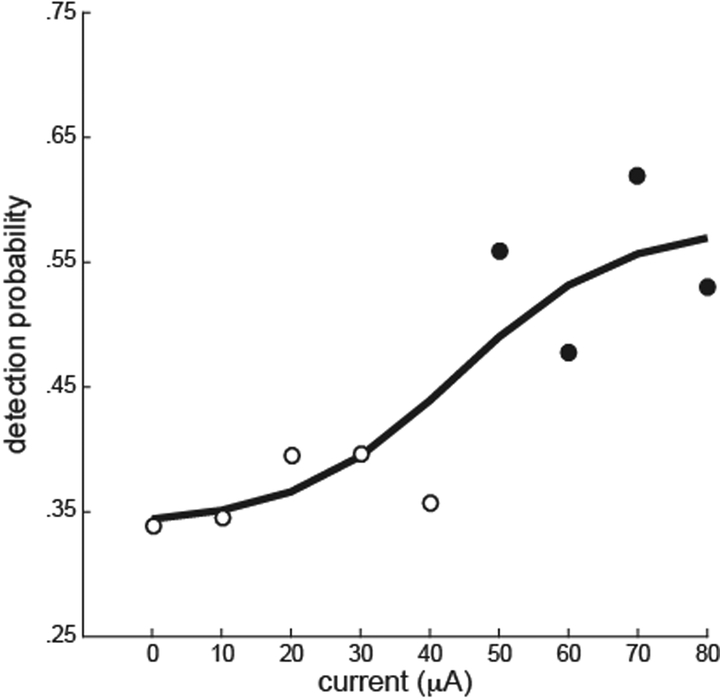
Finally, in monkey E, an experiment was performed to identify the threshold amplitude for detection. On each trial, the amplitude of the oddity stimulus was chosen at random from eight values ranging from 10 μA to 80 μA. In addition, 10% of the trials were catch trials in which stimulus amplitude was 0 μA. The results indicate a clear threshold at about 45 μA (Fig. 5). Detection probabilities at current amplitudes of 50 μA and above were significantly different from the detection probabilities on catch trials.
Figure 5.
Detection probability as a function of current amplitude. Pulse frequency was 100 Hz for all amplitudes. Data for 0 μA were from catch trials. Filled circles indicate the amplitudes in which the performance significantly differed from performance on catch trials (chi-squared tests, p < .05). A logistic function fit to the data is shown.
IV. Discussion
As we found in three previous macaques [14, 15], the chronic brainstem implants in the two monkeys of this study provided long-term electrophysiological access to the CN without causing adverse neurological effects. The novelty of this study was in showing that both monkeys learned to detect microstimulation delivered to the CN.
Learning is not always required to detect artificial stimuli. Perceptions arising from natural vibrotactile stimulation of the fingers and artificial electrical stimulation of S1 can be indistinguishable when properly calibrated [11, 16]. Here, we made no attempt to calibrate the CN stimuli. The resulting detection learning curve matched those of similar, non-biomimetic approaches targeting S1. In particular, macaques have required 5–15 sessions for perceptual performance with S1 stimuli to significantly exceed chance [5, 9, 10]. With CN stimuli in monkey E, it took 8 sessions.
Detectability of S1 stimuli is known to be dependent on stimulus parameters, including the amplitude, width, and frequency of the stimulus pulses [7]. Here we explored the effect of stimulus amplitude on detection and found a threshold of 45 μA for 100 Hz stimulus trains. At this same pulse frequency and same 0.2-ms pulse width, a recent study found an average detection threshold of ~ 38 μA for S1 microstimulation [7]. The difference may be accounted for by differences in pulse train duration, which also impacts detection thresholds.
The present study provides the first assessment of percepts evoked by microstimulation of the CN. However, additional work will be needed to determine whether the CN is a viable encoding site for sensorimotor prostheses. First, it will be critical to assess the discriminability of CN microstimuli, not just the detectability. Second, it will be important to directly compare, in the same subjects, the perceptual performance of encoding in the CN versus downstream targets such as the ventral posterolateral nucleus of the thalamus [17, 18] or S1. Does activation of the compact, simpler representations of the CN lead to more intuitive percepts than S1? Does electrical current spread make it difficult to independently activate the closely spaced CN representations? Ultimately, are the increased risks associated with a brainstem implant offset by superior perceptual performance relative to a cortical target? The results presented here suggest it is both possible and imperative to pursue these questions.
Acknowledgments
This research was supported by National Science Foundation grant CBET-1404041.
Contributor Information
Srihari Y. Sritharan, Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, 19104, USA.
Andrew G. Richardson, Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, 19104, USA.
Pauline K. Weigand, Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, 19104, USA.
Ivette Planell-Mendez, Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, 19104, USA..
Xilin Liu, Department of Electrical and Systems Engineering, University of Pennsylvania, Philadelphia, PA, 19104, USA..
Hongjie Zhu, Department of Electrical and Systems Engineering, University of Pennsylvania, Philadelphia, PA, 19104, USA..
Milin Zhang, Department of Electrical and Systems Engineering, University of Pennsylvania, Philadelphia, PA, 19104, USA..
Jan Van der Spiegel, Department of Electrical and Systems Engineering, University of Pennsylvania, Philadelphia, PA, 19104, USA..
Timothy H. Lucas, Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, 19104, USA.
References
- [1].Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. , “High-performance neuroprosthetic control by an individual with tetraplegia,” Lancet, December 13 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. , “Reach and grasp by people with tetraplegia using a neurally controlled robotic arm,” Nature, vol. 485, pp. 372–5, May 17 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bensmaia SJ and Miller LE, “Restoring sensorimotor function through intracortical interfaces: progress and looming challenges,” Nat Rev Neurosci, vol. 15, pp. 313–25, May 2014. [DOI] [PubMed] [Google Scholar]
- [4].Dadarlat MC, O’Doherty JE, and Sabes PN, “A learning-based approach to artificial sensory feedback leads to optimal integration,” Nat Neurosci, vol. 18, pp. 138–44, January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fitzsimmons NA, Drake W, Hanson TL, Lebedev MA, and Nicolelis MA, “Primate reaching cued by multichannel spatiotemporal cortical microstimulation,” J Neurosci, vol. 27, pp. 5593–602, May 23 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnson LA, Wander JD, Sarma D, Su DK, Fetz EE, and Ojemann JG, “Direct electrical stimulation of the somatosensory cortex in humans using electrocorticography electrodes: a qualitative and quantitative report,” J Neural Eng, vol. 10, p. 036021, June 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim S, Callier T, Tabot GA, Gaunt RA, Tenore FV, and Bensmaia SJ, “Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex,” Proc Natl Acad Sci U S A, vol. 112, pp. 15202–15207, December 8 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Klaes C, Shi Y, Kellis S, Minxha J, Revechkis B, and Andersen RA, “A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback,” J Neural Eng, vol. 11, p. 056024, October 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].O’Doherty JE, Lebedev MA, Hanson TL, Fitzsimmons NA, and Nicolelis MA, “A brain-machine interface instructed by direct intracortical microstimulation,” Front Integr Neurosci, vol. 3, p. 20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Doherty JE, Lebedev MA, Li Z, and Nicolelis MA, “Virtual active touch using randomly patterned intracortical microstimulation,” IEEE Trans Neural Syst Rehabil Eng, vol. 20, pp. 85–93, January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tabot GA, Dammann JF, Berg JA, Tenore FV, Boback JL, Vogelstein RJ, et al. , “Restoring the sense of touch with a prosthetic hand through a brain interface,” Proc Natl Acad Sci U S A, vol. 110, pp. 18279–84, November 5 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zaaimi B, Ruiz-Torres R, Solla SA, and Miller LE, “Multi-electrode stimulation in somatosensory cortex increases probability of detection,” J Neural Eng, vol. 10, p. 056013, October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Weber DJ, Friesen R, and Miller LE, “Interfacing the somatosensory system to restore touch and proprioception: essential considerations,” J Mot Behav, vol. 44, pp. 403–18, November 2012. [DOI] [PubMed] [Google Scholar]
- [14].Richardson AG, Weigand PK, Sritharan SY, and Lucas TH, “Somatosensory encoding with cuneate nucleus microstimulation: effects on downstream cortical activity,” in 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 2015, pp. 695–698. [Google Scholar]
- [15].Richardson AG, Weigand PK, Sritharan SY, and Lucas TH, “A chronic neural interface to the macaque dorsal column nuclei,” J Neurophysiol, vol. 115, pp. 2255–64, May 1 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Romo R, Hernandez A, Zainos A, and Salinas E, “Somatosensory discrimination based on cortical microstimulation,” Nature, vol. 392, pp. 387–90, March 26 1998. [DOI] [PubMed] [Google Scholar]
- [17].Heming EA, Choo R, Davies JN, and Kiss ZH, “Designing a thalamic somatosensory neural prosthesis: consistency and persistence of percepts evoked by electrical stimulation,” IEEE Trans Neural Syst Rehabil Eng, vol. 19, pp. 477–82, October 2011. [DOI] [PubMed] [Google Scholar]
- [18].Song W and Semework M, “Tactile representation in somatosensory thalamus (VPL) and cortex (S1) of awake primate and the plasticity induced by VPL neuroprosthetic stimulation,” Brain Res, vol. 1625, pp. 301–13, November 2 2015. [DOI] [PubMed] [Google Scholar]