Significance
There is limited understanding of what ramifications conflict events have on disease transmission and control in regions plagued by civil unrest and violence. Furthermore, the multifaceted nature of the conflict events during an epidemic is yet to be characterized. Using conflict data, ethnographic appraisal, and a mathematical model, we provide a descriptive timeline of the events during the ongoing Ebola outbreak in the Democratic Republic of the Congo. We quantified the unrest preceding a conflict event and its subsequent impact on control activities to demonstrate how conflict events are contributing to the persistence of the epidemic. Our model framework can be extended to other infectious diseases in areas that have experienced chronic conflict and violence.
Keywords: insecurity, healthcare workers, epidemiology, humanitarian crisis
Abstract
The interplay between civil unrest and disease transmission is not well understood. Violence targeting healthcare workers and Ebola treatment centers in the Democratic Republic of the Congo (DRC) has been thwarting the case isolation, treatment, and vaccination efforts. The extent to which conflict impedes public health response and contributes to incidence has not previously been evaluated. We construct a timeline of conflict events throughout the course of the epidemic and provide an ethnographic appraisal of the local conditions that preceded and followed conflict events. Informed by temporal incidence and conflict data as well as the ethnographic evidence, we developed a model of Ebola transmission and control to assess the impact of conflict on the epidemic in the eastern DRC from April 30, 2018, to June 23, 2019. We found that both the rapidity of case isolation and the population-level effectiveness of vaccination varied notably as a result of preceding unrest and subsequent impact of conflict events. Furthermore, conflict events were found to reverse an otherwise declining phase of the epidemic trajectory. Our model framework can be extended to other infectious diseases in the same and other regions of the world experiencing conflict and violence.
The Democratic Republic of the Congo (DRC) is in the midst of its most devastating and prolonged outbreak of Ebola. Despite the efforts of government and foreign aid organizations, there have already been 2,084 cases and 1,405 deaths since April 30, 2018 (1). In the absence of sustained downturn, risk of Ebola dissemination to bordering countries is rising. Concurrent with the Ebola outbreak, the eastern part of the DRC has been engulfed in civil unrest and conflict. The violence is centralized around politics, ethnicity, land ownership, and economics (2, 3). In particular, mining regions in the provinces of North Kivu and Ituri have been destabilized by disagreements regarding entitlement, which have led to conflict from more than 70 armed groups (2–6). The most violent armed groups include the Ugandan Allied Democratic Forces (ADF) and the Maï-Maï Kilalo (7). In mid-April 2019, the Islamic State militant group carried out its first attack in the DRC (8).
The chronic conflict in North Kivu and Ituri has stymied surveillance, contact tracing, and vaccination (4, 9–12). Case identification and containment of Ebola becomes even more difficult in areas that are too dangerous for health workers to enter or work (5, 11). For example, violent episodes characterized by kidnappings and killings prohibited healthcare professionals from operating around the major road connecting the North Kivu and Ituri provinces (7). Moreover, health care workers and Ebola treatment centers (ETCs) were targets of attacks throughout the outbreak that have resulted in at least 10 deaths and many more gravely injured among healthcare workers thus far (4, 6, 9).
The Ebola outbreak in North Kivu was declared just 8 d after the end of the 2018 epidemic in the Equateur province. The Equateur epidemic was successfully controlled within 2 1/2 mo due to adequate contact tracing and the deployment of the recombinant vesicular stomatitis virus–Zaire Ebola virus vaccine (13). The vaccination campaign not only played an important role in curtailing the epidemic expeditiously (14), it also facilitated public awareness of the disease and improved practice of Ebola safety precautions (15). By contrast, the sociopolitical crisis in eastern DRC has hampered the contact tracing that is a prerequisite to ring vaccination that aims to vaccinate contacts of Ebola patients who are at risk for infection.
While the likelihood of the ongoing conflict amplifying the outbreak has been discussed (10, 11, 16–18), interplay between the conflict and the disease transmission is yet to be assessed. During the course of the epidemic, several conflict events including attacks on ETCs or healthcare workers and healthcare workers protests had direct impact on the public health response. We define such incidents as disruptive events. Understanding how the multifaceted nature of conflict impacts transmission and control is imperative for effective control strategies. However, there is minimal information available about the local conditions before a disruptive event occurs as well as how quickly the response returns to status quo. To assess the ways in which and the extent to which these disruptive events obstruct disease control during the current outbreak, we provide a timeline of conflict events in the context of outbreak and an ethnographic appraisal of the local conditions surrounding ETC attacks. Further, we use a transmission model of Ebola informed by ethnographic evidence and conflict data to evaluate the impact of disruptive events on effectiveness of case isolation and vaccination as well as on the temporal trajectory of the outbreak.
Results
Impact of Conflict on Progression of the Epidemic.
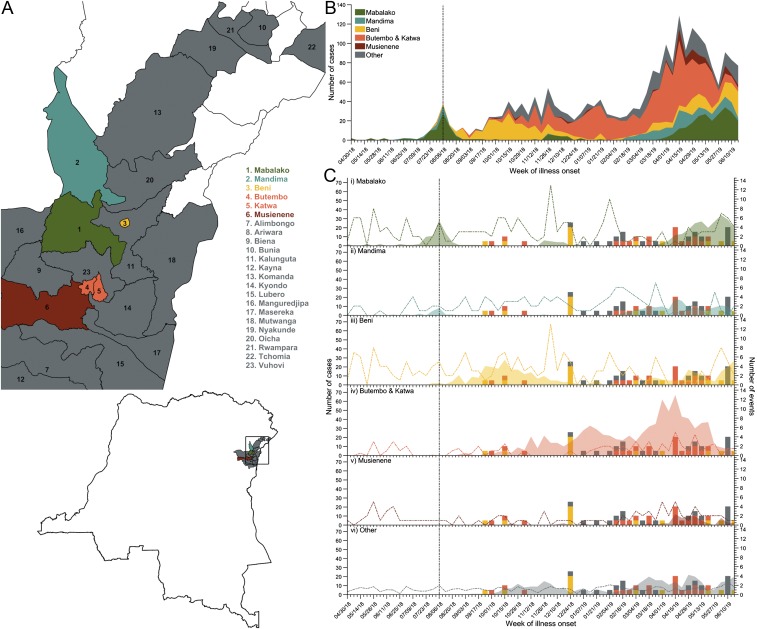
The North Kivu province has been experiencing an elevated frequency of conflict events (Fig. 1C) since December 2017 (5, 6, 19, 20). This period of civil unrest inhibited case detection and delayed reporting of the outbreak by 3 mo (11). The Mabalako health zone in the North Kivu province was the initial epicenter of the Ebola outbreak, with cases identified in the Mandima health zone of Ituri and Beni, Butembo, and Katwa health zones of North Kivu over the next 3 to 4 mo (Fig. 1 A and B). The frequency of conflict in these health zones spiked before their first peak in incidence (Fig. 1C).
Fig. 1.
The health zones affected by the Ebola outbreak in North Kivu and Ituri provinces. (A) The location of the health zones affected by the ongoing Ebola outbreak in North Kivu and Ituri provinces. Five health zones most heavily affected by outbreak are Mabalako (green), Butembo and Katwa (orange), Beni (yellow), Mandima (teal), and Musienene (dark red). Isolated cases occurred in the health zones (gray) of Alimbongo, Biena, Bunia, Kalunguta, Kayna, Komanda, Kyondo, Lubero, Mangurujipa, Masereka, Mutwanga, Nyankunde, Oicha, Rwampara, Tchomia, and Vuhovi. (B) The weekly Ebola incidence in North Kivu and Ituri stratified by health zone. (C) The weekly Ebola incidence in the Mabalako (i), Mandima (ii), Beni (iii), Butembo and Katwa (iv), Musienene (v), and other affected health zones (vi) compared to the timeline of disruptive events (colored bars) and all local conflict events (colored dashed line) based on the incidence data reported by the WHO and the conflict events reported during the outbreak. Vaccination was initiated on August 12, 2018 (vertical black dashed line).
While Ebola was almost controlled in the Mabalako and Mandima health zones by the end of August 2018 (Fig. 1), incidence rose precipitately in the Beni health zone between August and October. The surge was coincident with the intensified conflict in the area (Fig. 1C and SI Appendix, SI Methods and Table S2). Specifically, Beni experienced a deadly attack by the ADF on September 22, 2018, that resulted in a 5-d ville morte starting September 24, characterized by suspension of conflict and movement restrictions (SI Appendix, SI Methods and Table S2) (21). During this period, case isolation and ring vaccination were severely obstructed, such that only 20% of contacts could be traced (21, 22). There was another ADF attack in Beni on November 6, and healthcare workers were attacked in Butembo on October 2 and October 20 (SI Appendix, SI Methods and Table S2). By November 12, the epicenter shifted to Butembo and Katwa health zones (Fig. 1B).
Toward the end of 2018, the animosity over the postponement of an election in the area was directed toward the public health response teams due to their connection with the government. For example, residents associated the Médecins Sans Frontières (MSF) teams with the DRC Ministry of Health, eroding local cooperation and sparking violence (SI Appendix, Narrative). ETCs were destroyed in Beni and Oicha, and roads were barricaded in Butembo (6). These events likely promoted the spike in incidence during the week of January 14, 2019, in Butembo and Katwa (Fig. 1 B and C). Nine more attacks on ETCs occurred between February 1 and March 31, 2019 (Fig. 1C and SI Appendix, SI Methods and Table S2). As a result, Butembo and Katwa experienced another peak in incidence during the heightened violence. In addition, there was a resurgence of Ebola cases in the Beni, Mabalako, and Mandima health zones over this period.
Attacks on ETCs.
Based on our firsthand experience treating patients in one of the ETCs in North Kivu, we provide a narrative of local conditions preceding attacks on ETC centers in Katwa and Butembo and their subsequent impact on public health response (see Methods and SI Appendix for detailed narrative).
Events Leading up to the ETC Attacks in Butembo and Katwa.
Butembo and Katwa health zones have been roiled by civil war for many years, with about 20 different armed groups of various origins operating in this area. In the months leading up to the attacks in Butembo and Katwa, there was mounting hostility, reflected by attacks on health care centers, just 2 h away in Beni. As the situation in Beni improved, the violence migrated to the Butembo and Katwa area.
Two major signs indicated the pending attack on the ETC in Katwa on February 24, 2019. The first sign was a change in public behavior toward the MSF team. Until the week preceding the attack, the MSF team would positively interact with the local residents during their 30 min walk to the ETC. On February 17, residents began shouting “Ebola, Ebola, Ebola” at the MSF team. Simultaneously, there was a marked drop in suspected cases referred to the ETC. The ETC had been receiving 35 to 40 suspected Ebola cases a week. However, on the day before the attack, only 1 suspected Ebola case was referred, and on the day of the attack, only 2. Rumors about foreigners experimenting on locals, taking organs, and filling the bodies with concrete and Ebola being a fabrication were also circulating.
Attack on the ETC in Katwa.
The ETC that was attacked on February 24 was located near a village close to a forested area in Katwa, DRC, and began operating in January 2019. This ETC was ∼100 m by 100 m, had capacity for case isolation of 70 patients, and was managed by MSF France and MSF Belgium. The costly equipment for the polymerase chain reaction was not present as the Ministry of Public Health did not trust the security of the ETCs. On the day of the attack, 30 healthcare workers were treating 4 confirmed Ebola cases and 6 suspected cases. At 10:40 PM, dozens of people surrounded the ETC and began throwing stones. At 11:00 PM, they started a fire around the ETC and attempted to ignite the chlorine stock.
Events Following the ETC Attack in Katwa.
After the attack, posters of threats were disseminated throughout the city of Katwa demanding that the ETC healthcare workers leave and warning locals against visiting ETCs (for an example of such poster, please refer to SI Appendix, Narrative on conflict and violence). The 4 confirmed Ebola cases were transferred to the Butembo ETC, ∼20 min away. After closure of the ETC in Katwa, the nearby Ebola transit center that had ∼20 beds was quickly converted into an ETC. Under the management of Ministry of Public Health and funded by the World Health Organization (WHO)/UNICEF and international communities, the ETC in Katwa was opened again 5 wk after the attack. The security at the ETC was fortified and became resemblant of a military base.
Attack on the ETC in Butembo.
The ETC in Butembo with a capacity of 85 beds was opened by MSF Swiss in September of 2018. On February 27, 2019, at 6:00 PM the ETC was attacked, and the attack was more elaborately orchestrated than the one in Katwa. The assailants attempted to force entrance with 2 cars and shot at people. Once in the ETC, the attackers did not enter the “red zone” where confirmed cases reside but went straight for and shot at the power supply. The loss of power resulted in 2 patients dying from a lack of oxygen. The assailants repeatedly demanded an intensive care unit specialist by name.
Events Following the Attack in Butembo.
On Saturday, March 9, 2019, 10 d after the first attack, the Butembo ETC was attacked again despite being heavily guarded by the police and army. During this attack, a policeman was killed, and several healthcare workers were wounded. After the second attack, security at ETC was further fortified with an expanded military presence.
Quantitative Impact of Conflict on Disease Control in North Kivu.
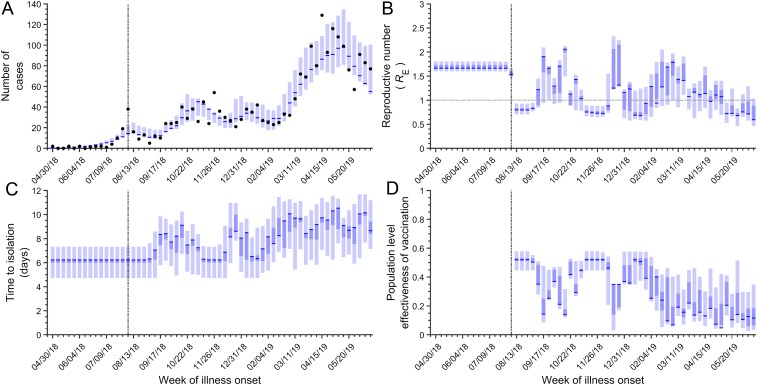
We fit our model to weekly Ebola incidence data from North Kivu and Ituri (Fig. 2A, Methods, and SI Appendix, SI Methods). We used the fitted model to estimate the effective reproductive number RE, defined as the average number of secondary cases that an infection generates. A value of RE greater than 1 indicates a growing epidemic, whereas the epidemic is in decline when it is less than 1. We further calculated the time between symptom onset and case isolation, as well as the population-level effectiveness of vaccination over the course of the outbreak. We report the maximum likelihood estimates and their corresponding confidence intervals. Weekly changes in these epidemiological parameters were used to assess the impact of conflict on disease control measures during the epidemic (Fig. 2 B–D).
Fig. 2.
The model results for the disease dynamics and effectiveness of control measures. (A) The incidence predicted by the model (blue) compared to the observed weekly incidence (black dots). (B) RE (blue) compared to an RE threshold of 1 (horizontal gray dashed line). (C) The time from symptom onset to isolation (blue). (D) The effectiveness of vaccination (blue). In A–D, the maximum likelihood estimates are depicted by blue lines, 95% confidence intervals are depicted by lighter blue, and the interquartile range is depicted by the darker blue area. Vaccination was initiated on August 12, 2018 (vertical black dashed line). Results are based on 1,000 realizations.
The average time from symptom onset to isolation between August 12, 2018, and June 23, 2019, is estimated to be 8.13 d [95% confidence interval (CI) 5.92 to 9.33]. Disruptive events extended the time to isolation by 1.91 d (95% CI 1.21 to 2.08) on average. During the first week of vaccine deployment, prior to any disruptive events, the effectiveness of vaccination was 52.0% (95% CI 44.6 to 57.8%). We found that the disruptive conflict events dampened the effectiveness of vaccination to a minimum of 4.8% (95% CI 2.7 to 14.0%) over the course of the epidemic. On average, the effectiveness of vaccination was 29.5% (95% CI 28.7 to 37.6%).
After the initiation of the vaccination campaign on August 12, 2018, RE decreased from 1.66 (95% CI 1.60 to 1.80) to 0.80 (95% CI 0.71 to 0.93). There was an ADF attack in Beni on September 22, followed by a 5-d ville morte on September 24. During the week of September 17, RE rebounded to 1.90 (95% CI 0.94 to 2.10) (Figs. 2B and 3A). After a transient decline, an attack on a healthcare worker in Butembo and a second ville morte in Beni in October occurred (Fig. 3B), and RE rose again and remained elevated until November 5 (Fig. 2B). After the effects of these disruptive events dissipated, RE dropped to 0.72 (95% CI 0.66 to 0.87).
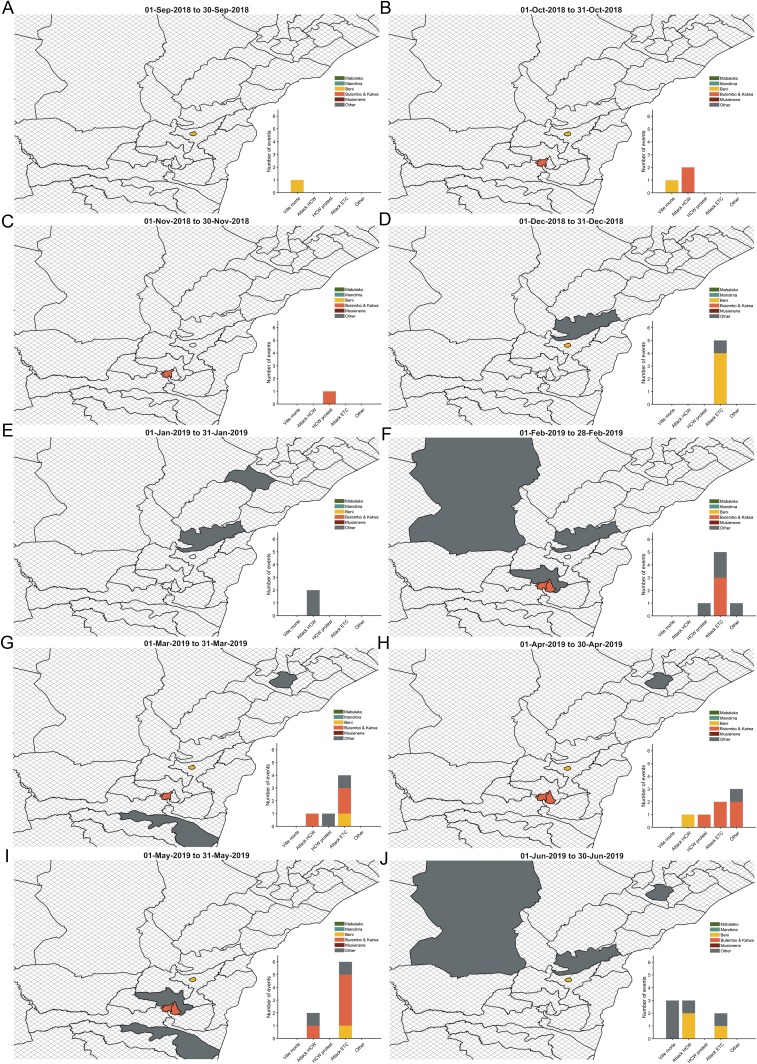
Fig. 3.
The disruptive events during the months between September 2018 and June 2019. The map depicts the location of the disruptive events, and the bars represent the number of disruptive events. We examined the disruptive events occurring in (A) September 2018, (B) October 2018, (C) November 2018, (D) December 2018, (E) January 2019, (F) February 2019, (G) March 2019, (H) April 2019, (I) May 2019, and (J) June 2019. Five health zones most heavily affected by outbreak are Mabalako (green), Butembo and Katwa (orange), Beni (yellow), Mandima (teal), and Musienene (dark red). Isolated cases occurred in the health zones (gray) of Alimbongo, Biena, Bunia, Kalunguta, Kayna, Komanda, Kyondo, Lubero, Mangurujipa, Masereka, Mutwanga, Nyankunde, Oicha, Rwampara, Tchomia, and Vuhovi. The cross-hatch health zones denote areas where no disruptive events occurred during the month.
In December, when public resentment toward the government was at its highest over delays in the election, there were multiple attacks on ETCs in Beni and Oicha (Fig. 3C). Between the weeks of December 3 and December 17, the effectiveness of vaccination decreased from 52.0% (95% CI 44.6 to 57.1%) to 34.9% (95% CI 2.9 to 35.0%), and RE increased from 0.72 (95% CI 0.66 to 0.87) to 1.25 (95% CI 1.22 to 2.33). From that point, RE declined gradually through mid-January before increasing again (Fig. 2B). Between February and late March, there were multiple attacks on ETCs and healthcare workers, as well as healthcare worker protests. Throughout this series of attacks, RE remained above 1 (Figs. 2B and 3 D and E). Another eruption of violence including an attack on healthcare workers in Beni, as well as attacks on ETCs, was accompanied by a rise in RE again in early April (Figs. 2B and 3F). The subsequent months of May and June were characterized by several disruptive events, a low effectiveness of vaccination, and fluctuations of RE around 1 (Fig. 2 B and D).
Discussion
Understanding the behavioral response of individuals during the course of an epidemic can inform how an outbreak unfolds (23). Based on our firsthand experience treating patients in North Kivu, we provided an ethnographic narrative and a descriptive portrait of the violent events that unfolded. We integrated this ethnographic appraisal along with incidence and conflict data to calibrate a mathematical model to further characterize the extent to which conflict impeded disease control in North Kivu and Ituri.
The ethnographic evidence suggested that just prior to the ETC attack in Katwa, there was a stark change in the behavior of the local community. After the attack on the Katwa ETC, threats were made demanding the Ebola response teams leave the entire region or risk further violence. Two days later the threat was followed through with the destruction of the Butembo ETC. In each instance, rapid efforts to resume treatment and control were made, and ETCs were often operational again within a couple of weeks.
We found that the time from symptom onset to case isolation varied widely as it evolved over the course of the epidemic, tracking the conflict events. Arising from delayed and incomplete contact tracing, a substantial proportion of cases identified were not in known chains of transmission (12, 24). The isolation of cases during the early stages of infection is critical to curtailing Ebola transmission (25). However, the heavy security around disease control teams has raised suspicions that the response to the outbreak is politically motivated (10). Mistrust of government and the public health response among civilians compounded hostility (5) and fueled reluctance of sick people to seek treatment (26). Integrating humanitarian work with the response as well as community engagement is needed to improve trust among the residents (10).
Although vaccination has contributed to the decline in incidence, our modeling results indicated that disruptive events reduced the population-level effectiveness of vaccination by 43% overall. Consistent with this result, spatial analysis of the 2018 Equateur outbreak found that the effectiveness of the vaccination campaign in preventing disease introduction would have decreased by 36% if it was delayed by 1 wk (14). From a survey conducted in Beni and Butembo during the first 2 wk of September 2018, 63% of the respondents said that they would receive the vaccine (27). However, almost half of the cases in Butembo and Katwa during February were not in known chains of transmission (12).
Our results suggest that declining incidence was repeatedly reversed by conflict events. For example, public health responders were not able to follow up with the vast majority of the contacts of Ebola cases (21) during the ville morte after the ADF attack on September 22, 2018, in Beni (21, 22). Hostility mounted with the postponement of elections on December 27, 2018, and led to a prolonged period of frequent conflict events including destruction of 5 ETCs. The presidential election was ostensibly postponed to limit transmission (28). However, the postponement not only instigated a cascade of violence but also gave credence to the rumors that the outbreak was fabricated by politicians.
A relatively simple epidemiological model was utilized to evaluate the effects of conflict on Ebola control measures. The model framework can be seamlessly extended to examine the impact of conflict on the progression of an epidemic for a variety of diseases and regions. For example, violence in DRC has contributed not only to the spread of Ebola but also to the spread of pneumonic plague, sleeping sickness, and river blindness (29, 30). Similarly, the conflict in Yemen is contributing to an explosive cholera outbreak (31, 32). In Nigeria and Pakistan, violence contributes to the inability to eradicate polio (33–35). More generally, many regions around the globe are plagued by chronic violence and conflict, inflaming the spread of disease (36). Due to the volatile nature of conflict as well as the complexity of sociopolitical dynamics in the region, we did not forecast the epidemic. We utilized existing conflict and incidence data to understand the interplay between disease transmission and violence retrospectively. The availability of more granular spatially explicit data on conflict and incidence could facilitate future studies to explore the interrelated evolution of epidemiological trajectories and fluctuations in violence.
Controlling disease outbreaks in conflict zones is already formidable, but when healthcare providers become the target of violence, it becomes even more daunting. As local violence is beyond the control of health professionals, it is paramount to determine how the health sector can maximize its chances of fulfilling programmatic objectives in the midst of conflict. Engendering trust among locals early in an outbreak through community engagement is fundamental to ensure that frontline workers providing treatment, conducting contact tracing, and distributing vaccines can work efficiently. As the volatility in the region has been unabating, Ebola continues to spread. There is an urgent need for comprehensive response strategies to bolster the trust of the local government and healthcare professionals.
Methods
Narrative.
B.-A.G., who communicated the ethnographic assessments, was deployed as a senior Ebola physician with MSF in Katwa, DRC. His description of the events were used to 1) inform model components that would be influenced by conflict, 2) guide the shape of the functional form representing the reduction in effectiveness of public health response surrounding a disruptive event, and 3) provide insight into the role that hostility had throughout this outbreak.
Disease Dynamics.
We developed a modified susceptible–latent–infectious–recovered transmission model for the Ebola outbreak in the DRC. The latently infected (E) and infectious (I) populations were subdivided into nE and nI classes, respectively, based on Erlang distribution (37) (SI Appendix, SI Methods). Susceptible individuals become latently infected at rate
where Ii(t) is the prevalence for stage i of infection, β is the rate of infection, and Ω(t) is a saturation function. The saturation function accounts for the potential increasing concern about becoming infected and increased awareness about the disease which would affect the rate of disease transmission (38) (SI Methods).
When infected with Ebola, the individual remains in the latent phase of infection for an average of 1/α days before becoming infectious. The average time from symptom onset to isolation for an infectious individual is 1/γ days. The time to isolation in the model accounts for the duration of infectiousness in the community for Ebola cases, which includes individuals who are not isolated and either recover or die. The impact of vaccination is incorporated in the model by reducing the rate of infection by the effectiveness of vaccination, denoted ε (SI Methods).
Conflict.
We assessed the impact of disruptive events on the transmission dynamics. The dates and description of these events were obtained from the Armed Conflict Location and Event Data Project dataset for DRC from April 30, 2018, to July 14, 2019 (6), as well as numerous crisis reports from MSF and the United Nations (39–43) (SI Methods). These events impaired the intervention efforts and the seeking of medical care, thereby delaying case isolation and eroding the population-level effectiveness of the vaccination campaign in the model (SI Methods). The magnitude of the impact of each event is dependent on its type and location. Events were classified as a ville morte, attacks on healthcare workers, healthcare workers protests, attacks on ETC, and other (SI Methods, Table S2). The locations of events were classified based on the reporting of health zone-level incidence data by the WHO as Mabalako, Mandima, Beni, Butembo and Katwa, Musienne, and the other affected health zones (44).
Events were usually preceded by a noticeable change in behavior of the local residents and marked decline in referral of suspected cases to ETCs. Following these events, there was a transient decline in the effectiveness of disease control measures. Thus, there are periods before and after the event where the contact tracing and vaccination efforts are impaired. To account for the influence of an event on the effectiveness of disease control, we modeled a linear increase beginning d days before the event and an exponential decay after the event, with the effects being compounded for each additional event (SI Methods).
Fitting.
The model was fit to weekly Ebola incidence data from WHO reports between April 30, 2018, and June 23, 2019, using a Bayesian melding approach (45). We used a negative binomial distribution for the likelihood function of incidence (46, 47). The duration of the latent period (1/α) was set to be 9.4 d (48), the average duration estimated for the West African outbreak. Using empirical estimates of the distributions of the latent period and time from symptom onset to hospitalization from the 2013 to 2016 West African outbreak (48), we calculated the shape of the Erlang distribution (SI Methods). The posterior distribution from the model fitting provided estimates of the RE, the time from symptom onset to case isolation and the population-level effectiveness of vaccination as these parameters evolved over the course of the epidemic (SI Methods).
Supplementary Material
Acknowledgments
We gratefully acknowledge funding from the National Institutes of Health (UO1-GM087719), the Burnett and Stender families’ endowment, the Notsew Orm Sands Foundation, and the Fogarty International Center.
Footnotes
The authors declare no competing interest.
See Commentary on page 23880.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913980116/-/DCSupplemental.
References
- 1.World Health Organization , Ebola virus disease–Democratic Republic of the Congo: Disease outbreak news: Update 13 June 2019. https://www.who.int/csr/don/13-june-2019-ebola-drc/en/. Accessed 17 June 2019.
- 2.Mclean D., Impact of violence on medical and humanitarian services in North Kivu, DRC (Analysis Department, Medicins sans Frontiers, Brussels) (2017). https://msf-analysis.org/wp-content/uploads/2017/12/McLean-Kivu-Violence-Impact-Report-Jan17.pdf. Accessed 14 May 2019.
- 3.Büscher K., Urbanisation and the political geographies of violent struggle for power and control: Mining boomtowns in Eastern Congo. Rev. Int. Polit. Dev. 10, 302–324 (2018). [Google Scholar]
- 4.Moran B., Fighting Ebola in conflict in the DR Congo. Lancet 392, 1295–1296 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Matfess H., Layered insecurity in North Kivu: Violence and the Ebola response. Acled Data (2018). https://www.acleddata.com/2018/10/26/layered-insecurity-in-north-kivu-violence-and-the-ebola-response/. Accessed 28 March 2019.
- 6.Acled Data , Data export tool. Acled Datahttps://www.acleddata.com/data/. Accessed 12 April 2019.
- 7.Bedford J., Key considerations: The context of North Kivu province, DRC (Social Science in Humanitarian Action). https://reliefweb.int/sites/reliefweb.int/files/resources/SSHAP_North_Kivu_context.pdf. Accessed 29 March 2019. [Google Scholar]
- 8.Wembi S., Goldstein J., ISIS claims first attack in the Democratic Republic of Congo. NY Times, 19 April 2019. https://www.nytimes.com/2019/04/19/world/africa/isis-congo-attack.html. Accessed 22 April 2019.
- 9.The Lancet , DR Congo: Managing Ebola virus in war. Lancet 392, 1280 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Green A., DR Congo Ebola virus treatment centres attacked. Lancet 393, 1088 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Nakkazi E., DR Congo Ebola virus outbreak: Responding in a conflict zone. Lancet 392, 623 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Maxmen A., Violence propels Ebola outbreak towards 1,000 cases. Nature 567, 153–154 (2019). [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization , Ebola virus disease: Democratic Republic of the Congo: Declaration of the end of the outbreak. https://apps.who.int/iris/bitstream/handle/10665/273348/SITREP_EVD_DRC_20180725-eng.pdf?ua=1. Accessed 16 April 2019.
- 14.Wells C. R., et al. , Ebola vaccination in the Democratic Republic of the Congo. Proc. Natl. Acad. Sci. U.S.A. 116, 10178–10183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J., Congo’s Ebola outbreak is all but over. Did an experimental vaccine help? Science (2018). https://www.sciencemag.org/news/2018/07/congo-s-ebola-outbreak-all-over-did-experimental-vaccine-help. Accessed 27 March 2019. [Google Scholar]
- 16.Ilunga Kalenga O., et al. , The ongoing Ebola epidemic in the Democratic Republic of Congo, 2018-2019. N. Engl. J. Med. 381, 373–383 (2019). [DOI] [PubMed] [Google Scholar]
- 17.The Ebola Gbalo Research Group ,Responding to the Ebola virus disease outbreak in DR Congo: When will we learn from Sierra Leone? Lancet 393, 2647–2650 (2019). [DOI] [PubMed] [Google Scholar]
- 18.UN News, Ebola-hit DRC faces “perfect storm” as uptick in violence halts WHO operation (2018). https://news.un.org/en/story/2018/09/1020392. Accessed 4 June 2019.
- 19.Marcucci G., The war report 2018: Democratic Republic of the Congo: Conflict in the Eastern regions (Geneva Academy, 2019). https://www.geneva-academy.ch/joomlatools-files/docman-files/Democratic%20Republic%20of%20The%20Congo%20Conflict%20In%20The%20Eastern%20Regions.pdf. Accessed 8 August 2019.
- 20.Deutsche Welle , Rebels attack UN peacekeepers in DRC, with more than a dozen dead and scores wounded (2019). https://www.dw.com/en/rebels-attack-un-peacekeepers-in-drc-with-more-than-a-dozen-dead-and-scores-wounded/a-41718228. Accessed 8 August 2019.
- 21.World Health Organization , Dr. Peter Salama, WHO Deputy Director-General, Emergency Preparedness and Response (2018). https://www.who.int/ebola/WHO-RUSH_Ebola_DR_Congo_update_SALAMAp_UNOG_25SEP2018-final.pdf. Accessed May 16, 2019.
- 22.UN High Commissioner for Refugees, UNHCR alarm at recent attacks and rising displacement in eastern DRC–Democratic Republic of the Congo. ReliefWeb. https://reliefweb.int/report/democratic-republic-congo/unhcr-alarm-recent-attacks-and-rising-displacement-eastern-drc. Accessed 16 May 2019.
- 23.Epstein J. M., Parker J., Cummings D., Hammond R. A., Coupled contagion dynamics of fear and disease: Mathematical and computational explorations. PLoS One 3, e3955 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxmen A., Ebola detectives race to identify hidden sources of infection as outbreak spreads. Nature 564, 174–175 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Yamin D., et al. , Effect of Ebola progression on transmission and control in Liberia. Ann. Intern. Med. 162, 11–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Social Science in Humanitarian Action, Key considerations: changing behaviours & care-seeking practices in the Grand Nord, North Kivu, DRC–Democratic Republic of the Congo. ReliefWeb. https://reliefweb.int/report/democratic-republic-congo/key-considerations-changing-behaviours-care-seeking-practices-grand. Accessed 14 May 2019.
- 27.Vinck P., Pham P. N., Bindu K. K., Bedford J., Nilles E. J., Institutional trust and misinformation in the response to the 2018-19 Ebola outbreak in North Kivu, DR Congo: A population-based survey. Lancet Infect. Dis. 19, 529–536 (2019). [DOI] [PubMed] [Google Scholar]
- 28.The Lancet , Was DR Congo’s Ebola virus outbreak used as a political tool? Lancet 393, 104 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Gayer M., Legros D., Formenty P., Connolly M. A., Conflict and emerging infectious diseases. Emerg. Infect. Dis. 13, 1625–1631 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makenga Bof J. C., et al. , Onchocerciasis control in the Democratic Republic of Congo (DRC): Challenges in a post-war environment. Trop. Med. Int. Health 20, 48–62 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Rabaan A. A., Cholera: An overview with reference to the Yemen epidemic. Front. Med. 13, 213–228 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Federspiel F., Ali M., The cholera outbreak in Yemen: Lessons learned and way forward. BMC Public Health 18, 1338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Global Polio Eradication Initiative, Reaching the hard to reach: Ending polio in conflict zones. (2017). http://polioeradication.org/news-post/ending-polio-in-conflict-zones/. Accessed 27 May 2019.
- 34.SteelFisher G. K., et al. , Threats to polio eradication in high-conflict areas in Pakistan and Nigeria: A polling study of caregivers of children younger than 5 years. Lancet Infect. Dis. 15, 1183–1192 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Verma A. A., Jimenez M. P., Tangermann R. H., Subramanian S. V., Razak F., Insecurity, polio vaccination rates, and polio incidence in northwest Pakistan. Proc. Natl. Acad. Sci. U.S.A. 115, 1593–1598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotez P. J., Ten global “hotspots” for the neglected tropical diseases. PLoS Negl. Trop. Dis. 8, e2496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krylova O., Earn D. J. D., Effects of the infectious period distribution on predicted transitions in childhood disease dynamics. J. R. Soc. Interface 10, 20130098 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisman D. N., Hauck T. S., Tuite A. R., Greer A. L., An IDEA for short term outbreak projection: Nearcasting using the basic reproduction number. PLoS One 8, e83622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World ReliefWeb , Safeguarding Healthcare Monthly News Brief–Attacks on healthcare, February 2019. https://reliefweb.int/report/world/safeguarding-healthcare-monthly-news-brief-attacks-healthcare-february-2019. Accessed 12 April 2019.
- 40.Médecins Sans Frontières International , DRC Ebola outbreak crisis update. https://www.msf.org/drc-ebola-outbreak-crisis-update. Accessed 12 April 2019.
- 41.World ReliefWeb , Safeguarding Healthcare Monthly News Brief–Attacks on healthcare, January 2019. https://reliefweb.int/report/world/safeguarding-healthcare-monthly-news-brief-attacks-healthcare-january-2019. Accessed 12 April 2019.
- 42.World ReliefWeb , Safeguarding Healthcare Monthly News Brief–Attacks on healthcare, December 2018. https://reliefweb.int/report/world/safeguarding-healthcare-monthly-news-brief-attacks-healthcare-december-2018. Accessed 12 April 2019.
- 43.Médecins Sans Frontières , Northeast DRC Ebola outbreak, crisis info (2019). https://lakareutangranser.se/sites/default/files/north_kivu_ebola_crisis_info_6_0.pdf. Accessed 12 April 2019.
- 44.World Health Organization , Ebola virus disease—Democratic Republic of the Congo: Disease outbreak news: Update 4 July 2019 (2019). https://www.who.int/csr/don/04-july-2019-ebola-drc/en/. Accessed 6 August 2019.
- 45.World Health Organization , Ebola virus disease: Democratic Republic of the Congo: External situation report (2019). https://apps.who.int/iris/bitstream/handle/10665/311641/SITREP_EVD_DRC_20190331-eng.pdf?ua=1. Accessed 12 April 2019.
- 46.Kucharski A. J., et al. , Measuring the impact of Ebola control measures in Sierra Leone. Proc. Natl. Acad. Sci. U.S.A. 112, 14366–14371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ndeffo-Mbah M. L., Parpia A. S., Galvani A. P., Mitigating prenatal Zika virus infection in the Americas. Ann. Intern. Med. 165, 551–559 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Aylward B., et al. ; WHO Ebola Response Team , Ebola virus disease in West Africa—The first 9 months of the epidemic and forward projections. N. Engl. J. Med. 371, 1481–1495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.