Significance
RNA processing including covalent modifications (e.g., the addition of the methyl-7-guanosine [m7G] “cap” on the 5′ end of transcripts) centrally influences the proteome. For example, eIF4E recruits RNAs for translation by binding the m7G cap. eIF4E is engaged and controlled by the binding of factors to its dorsal surface while leaving its m7G cap-binding site free for RNA recruitment. Here, we unexpectedly found that a small viral protein, viral genome-linked protein (VPg), directly binds the cap-binding site of eIF4E, indicating that eIF4E can additionally be controlled through direct competition with its cap-binding site. Furthermore, VPg–RNA conjugates also bind eIF4E and are templates for translation, suggesting that VPg may substitute for the m7G cap during infection.
Keywords: VPg, m7 cap, potyvirus, translation, eIF4E
Abstract
Viruses have transformed our understanding of mammalian RNA processing, including facilitating the discovery of the methyl-7-guanosine (m7G) cap on the 5′ end of RNAs. The m7G cap is required for RNAs to bind the eukaryotic translation initiation factor eIF4E and associate with the translation machinery across plant and animal kingdoms. The potyvirus-derived viral genome-linked protein (VPg) is covalently bound to the 5′ end of viral genomic RNA (gRNA) and associates with host eIF4E for successful infection. Divergent models to explain these observations proposed either an unknown mode of eIF4E engagement or a competition of VPg for the m7G cap-binding site. To dissect these possibilities, we resolved the structure of VPg, revealing a previously unknown 3-dimensional (3D) fold, and characterized the VPg–eIF4E complex using NMR and biophysical techniques. VPg directly bound the cap-binding site of eIF4E and competed for m7G cap analog binding. In human cells, VPg inhibited eIF4E-dependent RNA export, translation, and oncogenic transformation. Moreover, VPg formed trimeric complexes with eIF4E–eIF4G, eIF4E bound VPg–luciferase RNA conjugates, and these VPg–RNA conjugates were templates for translation. Informatic analyses revealed structural similarities between VPg and the human kinesin EG5. Consistently, EG5 directly bound eIF4E in a similar manner to VPg, demonstrating that this form of engagement is relevant beyond potyviruses. In all, we revealed an unprecedented modality for control and engagement of eIF4E and show that VPg–RNA conjugates functionally engage eIF4E. As such, potyvirus VPg provides a unique model system to interrogate eIF4E.
The eukaryotic translation initiation factor eIF4E plays important roles in posttranscriptional control in plant and animals (1). Its association with the methyl-7-guanosine (m7G) “cap” on the 5′ end of RNAs allows eIF4E to recruit transcripts to the RNA processing machinery (2). To date, the m7G cap is generally accepted as the universal 5′ adaptor for RNAs in eukaryotes (3), with the exception of (i) the structurally related m3G cap, which is also used by nematodes (4), and (ii) with lower-frequency, nicotinamide adenine dinucleotide (NAD) and related analogs that destabilize transcripts and thus, are probably not involved in active translation (5). Through its m7G cap-binding activity, eIF4E recruits specific transcripts to the translation machinery in the cytoplasm and promotes the nuclear export of selected RNAs from the nucleus (6, 7). Both activities contribute to modulation of the proteome and in mammals, to its oncogenic activity (6, 7). For instance, eIF4E is dysregulated in many human cancers (6). In humans, targeting eIF4E with a cap competitor, the guanosine analog ribavirin, impairs its biochemical activities correlating with clinical responses in early-phase trials in leukemia, prostate, head, and neck cancers among others (8–13). Thus, the cap-binding activity of eIF4E can be targeted in patients to provide clinical benefit, highlighting its critical importance.
Viruses have paved the way for our understanding of many aspects of host-cell RNA processing, including m7G capping. Indeed, studies into cytoplasmic polyhedrosis virus (CPV) infection in silkworm and vaccinia virus (VV) in mammalian cells were critical for the elucidation of the m7G cap structure over 40 y ago (3, 14, 15). Here, we exploited unusual features of potyvirus biochemistry to unearth unknown strategies that can be implemented to engage eIF4E. Potyviruses are members of the picorna-like plant viruses. Their infection of mainstay crops has devastating economic consequences (16). Genetic studies revealed that potyviruses require host-cell translation machinery to replicate, and specifically, these reports have associated the potyviral protein genome linked (viral genome-linked protein [VPg]) with host plant eIF4E (17–21). Indeed, mutations in plant eIF4E are associated with potyviral resistance (18–20). VPgs exist in other virus families, such as poliovirus (22). The VPg designation is based on the covalent linkage of viral RNA to VPg. For the case of potyviruses, the 5′ end of the genomic RNA (gRNA) is covalently attached to the hydroxyl of tyrosine 64 (potato virus Y [PVY] numbering) (23–25). The genetic interaction between VPg and eIF4E is only reported for potyviruses (20), while other virus families typically use these RNA conjugates for replication (22). Consistent with this, potyviral VPgs only show significant sequence homology with each other and not with VPgs from other families (SI Appendix, Fig. S1).
While genetic studies linked VPg and eIF4E, conclusions from biochemical studies were highly divergent, leaving the mechanism as to how VPg coopts eIF4E activity unsettled. Some groups reported that PVY VPg binds m7G cap–eIF4E–eIF4G, forming a quaternary complex, which suggests that VPg utilizes a novel surface on eIF4E for binding and thereby, engaging its activity (17, 26). Supporting this model, mutation of the cap-binding site in wheat eIF4E (W123A, W102 in human eIF4E) did not reduce the ability of eIF4E to bind VPg but did reduce binding to the 7-methylguanosine diphosphate (m7GDP) cap analog. This suggested that VPg bound to a part of eIF4E not previously known to be involved in its control or engagement (26). By contrast, another study provided evidence that PVY VPg directly competes for the cap-binding site on plant eIF4E (21). Other reports suggested that PVY VPg interacts with the eIF4F (eIF4E–eIF4G–eIF4A/B) complex to stimulate cap-independent internal ribosomal entry site (IRES)-mediated translation, but the exact eIF4F component required was not ascertained, and whether IRES-mediated translation is relevant to potyviruses is not clear (27). Just as the mode of binding to eIF4E is controversial, there are divergent models regarding the molecular basis for the VPg–eIF4E interaction. One study proposed that residues 41 to 93 of PVY VPg were used for binding to eIF4E (21), while others reported that residues on a predicted long amphipathic helix spanning residues 90 to 125 were required (28). Efforts to solve the VPg structure had been to date unsuccessful. Modeling efforts yielded disparate solutions, including a helical bundle (29), a long amphipathic helix for the VPg-binding site for eIF4E (28), and a model based on the FOK1a kinase structure, which included a β-sheet core with flexible helices (30). Adding to the confusion, multiple biophysical studies proposed that VPg is an intrinsically disordered protein (21, 30–33).
While the mechanistic underpinnings of the interplay between VPg and eIF4E remains controversial, it is clear that eIF4E is required for infection and that there is a genetic interaction between eIF4E and VPg. In this way, VPg provides a unique model system to interrogate the modalities required for the engagement and control of eIF4E. No structure of any potyvirus protein exist. Here, we report the structure of the potyvirus protein, VPg, and characterized the VPg–eIF4E complex using high-resolution NMR and biophysical methods. We demonstrated that VPg binds the cap-binding site of eIF4E and forms trimeric complexes with eIF4E and eIF4G; furthermore, we demonstrated that VPg–RNA conjugates directly bind eIF4E and were templates for translation consistent with the requirement of potyviruses for VPg–eIF4E interactions for their lifecycle. Furthermore, the structural similarities of VPg to human proteins suggest that this modality for engagement of eIF4E could be conserved across kingdoms.
Results and Discussion
VPg Adopts a Previously Unknown Protein Fold.
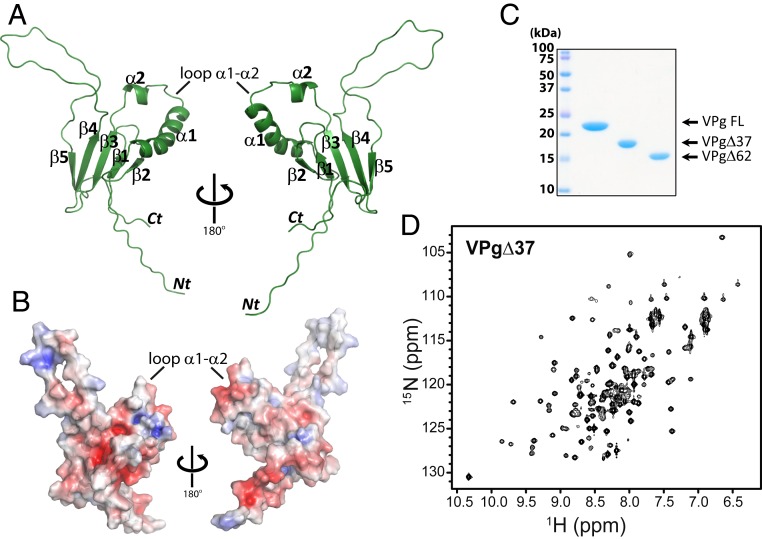
We determined the structure of PVY VPg protein in order to understand its molecular relationship with eIF4E and thus, its role in RNA recruitment to the host-cell translation machinery. We studied PVY VPg, since it is the archetypal potyvirus and thus, representative of the family (SI Appendix, Fig. S1B). We generated full-length and truncated forms of PVY VPg protein using bacterial expression constructs and purified these from the soluble fraction with the quality of the proteins confirmed by SDS/PAGE (Sodium Dodecyl Sulphate-PolyAcrylamide Gel Electrophoresis) (Fig. 1C) (34), NMR (Fig. 1D and SI Appendix, Fig. S2), and mass spectrometry (MS) (SI Appendix, Fig. S3A). We recently reported the NMR assignments for a VPg construct in which the first 37 residues were removed to improve stability (VPgΔ37) (34). Here, the 3-dimensional (3D) solution structure of this construct was determined by using an automated procedure for iterative nuclear Overhauser effect (NOE) assignment using CYANA (35). The structure of VPg is shown in Fig. 1 A and B and SI Appendix, Fig. S3B, and the structural statistics are in SI Appendix, Table S1. The rmsd for the ordered regions was 0.68 Å for the backbone atoms of the top 20 structures.
Fig. 1.
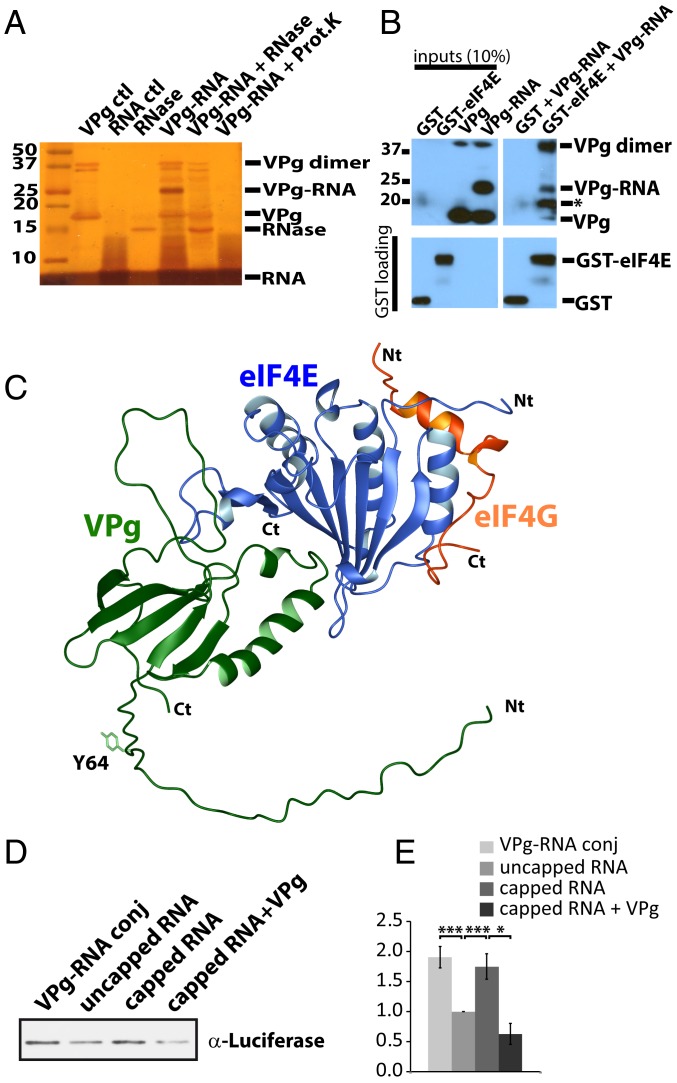
Structure of the PVY VPg protein. (A) Cartoon representation of the closest to the average structure in the ensemble for VPgΔ37. The family of best 20 structures is shown in SI Appendix, Fig. S3B. Residues starting at F60 shown as residues 37 to 70 are disordered. (B) Surface rendering of VPg structure. Red indicates negative charged area, blue indicates positive, and white are hydrophobic. (C) SDS/PAGE gel of full-length (FL) and VPg truncation constructs used for NMR analysis; molecular mass markers are shown. (D) 1H-15N HSQC spectrum of VPgΔ37.
The VPg structure is unlike the previously proposed models. VPgΔ37 adopts a well-folded core as well as 2 substantial unstructured regions at the N and C termini (residues 38 to 70) and a flexible loop between β4- and β5-strands (residues 145 to 165) (Fig. 1A and SI Appendix, Fig. S3B). We note that VPg is not an intrinsically disordered protein. Specifically, there were many long-range NOEs, indicating a tertiary structure (SI Appendix, Table S1), and furthermore, values for the 15N-1H heteronuclear NOE and chemical shift index also indicated the presence of structured elements (34). The VPg structure is composed of a 5-stranded β-sheet with 2 consecutive α-helices between β-strands 2 and 3 (Fig. 1A). Our structure of PVY VPg does not resemble VPg structures from other virus families, consistent with the lack of sequence conservation observed (SI Appendix, Fig. S1A). Residue Y64, which is covalently attached to gRNA during infection (23–25), is located within the flexible N terminus but close to the folded domain, which starts at residue 72 (Fig. 1A and SI Appendix, Fig. S1B). Inspection of the structure revealed that there were no elements within VPg that possessed any structural similarity to reported eIF4E-binding motifs (e.g., the helical turn describing the eIF4E consensus motif [YXXXXLΦ, where X is any residue and Φ is any hydrophobic] or RING (really interesting new gene) domains [6]).
Elucidation of the eIF4E Binding Site on VPg.
We used NMR and pulldown studies to garner information regarding the eIF4E–VPg complex structure (see below). We selected human eIF4E for these studies because of the extensive knowledge accumulated regarding its structure, allosteric effects of ligands, and dynamics (36–38). Importantly, human cap-bound eIF4E is highly homologous in sequence (SI Appendix, Fig. S4A) and structure (SI Appendix, Fig. S4B) to the 3 cap-bound eIF4E plant structures solved (rmsd ∼ 0.7 Å for 175 atom pairs). Both human and melon eIF4E structures were available in the apo- and cap-bound forms as well as ternary complexes with eIF4G (38, 39). These structures are highly homologous, indicating that these were conserved across kingdoms. The large positive surface used to bind the phosphates of the m7G cap in the unoccupied cap-binding site was conserved across kingdoms (SI Appendix, Fig. S4C). Given the overall similarity between structures coupled with the deeper structural understanding of human eIF4E, we studied the VPg–human eIF4E complex to ascertain how VPg engaged and/or controlled eIF4E.
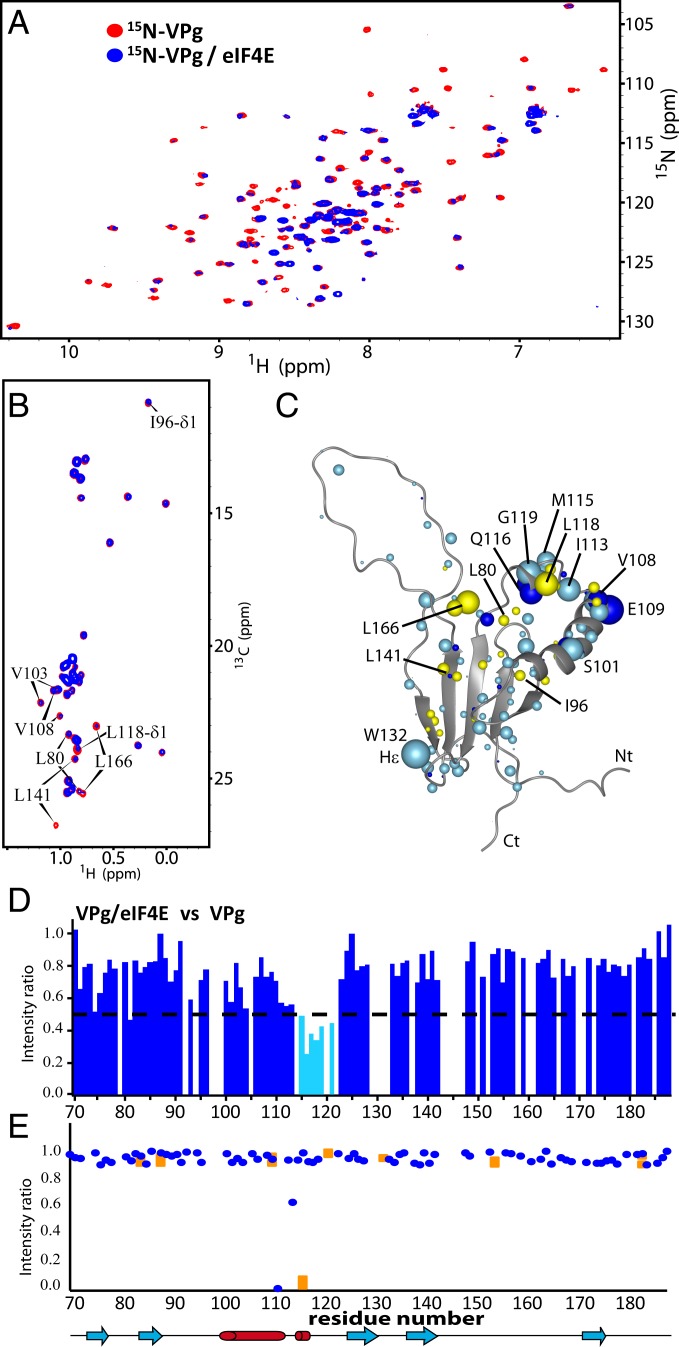
We identified the VPgΔ37 and eIF4E interface using a combination of NMR-based strategies and mutagenesis. We used cap-free eIF4E as a starting point for these studies. First, using 50 μM 15N-,13C-, or specifically labeled Ile, Leu and Val methyl groups (ILV) VPgΔ37, we monitored the effects of addition of unlabeled eIF4E (150 μM) on signal broadening and chemical shift perturbation (CSP) of the amide nitrogens, methyl carbons, and carbonyl groups using the 1H-15N HSQC (heteronuclear single quantum coherence), the methyl region of the 1H-13C spectra, or the H/C projection of the 3D-HNCO experiment, respectively (Fig. 2 A and B and SI Appendix, Fig. S5). We observed substantial broadening for residues located in the loop E108-G119 of VPgΔ37, and in particular, for carbonyls of E108, R109, and Q116 and amides of R109, D111, I113, and M115-G119, including the side-chain amide of Q116 (Fig. 2 C and E). Methyl carbons in the region V103-L118 also exhibited broadening (Fig. 2B) in addition to residues neighboring this loop (L80 and L166). The flexible regions of VPgΔ37 (i.e., the large loop [N145-E165] and both N [S38-E70] and C termini [A182-E188]) were not altered by eIF4E, indicating that they are unlikely to be involved in the interaction. Consistent with this observation, deletion of the first 62 residues in VPg did not impair binding to eIF4E (SI Appendix, Fig. S3 C and E).
Fig. 2.
The binding surface on VPg used to interact with eIF4E. (A) 1H-15N HSQC of 50 μM 15N-labeled VPg in the absence (red) or presence (blue) of a 3-molar excess of unlabeled eIF4E. (B) Constant time 1H-13C HSQC spectrum of ILV-labeled VPg (50 μM) in the absence (red) or presence of 2-molar excess eIF4E (blue). (C) VPg residues are perturbed by eIF4E binding. Light blue indicates 1H CSP or broadening, dark blue indicates 1H methyl CSP, and yellow indicates broadening from the 1H-13C projection of the 3D HNCO spectra (SI Appendix, Fig. S5). (D) Per residue plot of backbone amide line broadening of 15N-labeled VPgΔ37 in response to binding of eIF4E (extracted from A). Residues that undergo line broadening below the dashed line are in cyan. Note that empty spaces correspond to residues that overlap, residues that are not assigned, or Proline residues. (E) Plot of the intensity ratios of the cross-peaks in the TCS experiment for the backbone (blue) and asparagine/glutamine side-chain (orange) resonances for VPg.
Second, we used transferred cross-saturation (TCS) experiments (40) to directly detect through space interactions between eIF4E and VPg. In this experiment, the labeled VPg was in 4-fold excess relative to unlabeled eIF4E (90 μM eIF4E, 360 μM VPg). Consistent with the above NMR data, we observed substantial reductions in signal intensity of the amide side chain of residue Q116 and to a lesser extent, of the backbone amides of D111 and E114 (Fig. 2D), indicating that these residues were close in space to eIF4E. We then used mutagenesis to validate the NMR data. VPgΔ37 mutants M115A/Q116A and D111K/E114K/Q116K had weaker affinity for eIF4E compared with wild-type VPgΔ37 as observed by increased intensities of the NMR signals (SI Appendix, Fig. S6). These spectra were acquired under identical concentrations and ratios; thus, differences in intensities in comparing spectra directly reflect affinity. To support the delineation of the binding surface, we also mutated residues outside of the predicted binding site and examined their impact. Consistently, these mutations (E86K/E87K, D92K, or E98K/E102K) did not significantly impact the VPg–eIF4E interaction (SI Appendix, Fig. S6D). None of the mutants described above disrupted the VPg structure as observed by NMR or circular dichroism (CD) (SI Appendix, Fig. S3D). Altogether, TCS, CSP, signal broadening, and mutational studies indicate that the α1–α2 loop forms the surface on VPg that binds to eIF4E.
Mapping of the VPg Binding Site on eIF4E.
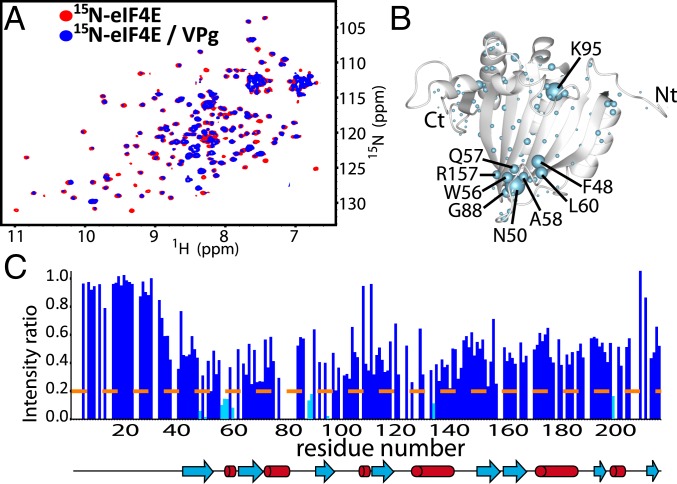
We used the same strategy to determine the binding site of VPgΔ37 on eIF4E. We monitored spectral perturbations of 50 μM 15N- or 13C-eIF4E samples as a function of unlabeled VPgΔ37 addition to a maximum of 150 μM eIF4E (Fig. 3 and SI Appendix, Fig. S7). The most substantial broadening was detected around the cap-binding site, particularly for backbone residues in the F48-L60 loop (Fig. 3C) which also contains W56, one of the tryptophan residues that binds the m7G cap. Other proton amides around the cap-binding pocket of eIF4E were also broadened (i.e., the K95 and the H200). As shown for VPgΔ37 (Fig. 2D), the empty spaces indicate residues that were not quantified due to spectral overlap (Fig. 3C). Consistently, we also observed carbon chemical shifts for methyl groups located directly behind the phosphate-binding region of the cap-binding site of eIF4E (I63 and L85) (SI Appendix, Fig. S7). Given that these changes were concentrated in the cap-binding site, we individually mutated 2 parts of this site: the m7G moiety-binding region, which includes W56, and the phosphate-binding region, which includes R157, K159, and K162 that form a positively charged patch. The R157E/K159E/K162E triple mutation (4ETrMut) substantially reduced the interaction of eIF4E with VPgΔ37 as did the W56A mutation (SI Appendix, Figs. S8 A–C and S9). These findings were confirmed by glutathione S-transferase (GST) pulldown experiments using murine eIF4E, which is only 4 residues different from human eIF4E (SI Appendix, Fig. S8E). Importantly, none of these mutants altered the overall fold of eIF4E as assessed by HSQC and CD (SI Appendix, Fig. S8 A–D). Importantly, W56 and R157/K159/K162 comprise one end of the cap-binding site and are relatively close in space in both the apo- and cap-bound eIF4E forms (<11 Å). We note that the W102A mutation, allied to W123A in wheat, barely affects the ability of eIF4E to bind VPg (SI Appendix, Fig. S9). In apo-eIF4E, W102 is at the other end of the cap-binding site, while upon cap binding, this residue moves to be in close proximity with W56, R157, K159, and K162 (38). In all, our NMR data strongly indicate that the VPg-binding site on eIF4E is clustered in the region of the cap-binding site, including W56, R157, K159, and K162.
Fig. 3.
Interaction surface used by eIF4E to bind VPg. (A) 1H-15N HSQC of 15N-labeled eIF4E (50 μM) in the absence (red) or presence (blue) of 3-molar excess VPg. (B) Broadening and CSPs mapped onto the apo-eIF4E structure (PDB ID code 2GPQ) depicted as light blue balls. (C) Per residue plot of backbone amide line broadening of 50 μM eIF4E in response to binding of VPgΔ37 (extracted from A). Residues that undergo line broadening below the dashed line are in cyan.
The VPg–eIF4E Complex.
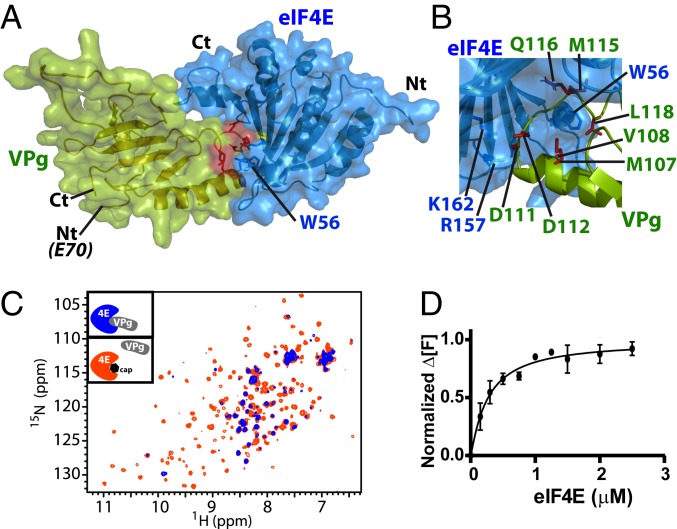
We used the above NMR and mutagenesis data to generate a model of the eIF4E–VPgΔ37 complex using the restraint-driven docking program HADDOCK (41). We note that the intermediate NMR exchange regimen observed for the complex meant that it was impossible to observe NOEs due to broadening of signals for residues at the interface. The resulting HADDOCK calculation led to a solution where 150 of 200 structures converged to the same eIF4E–VPgΔ37 complex. Of these 150 structures, the backbone rmsd for the complex was ∼0.8 Å, and the buried surface area for the complex was ∼1,960 Å2 (Fig. 4A). In this complex, W56 is buried in a hydrophobic pocket formed by M107, V108, M115, and L118 in the helix–loop–helix structure of VPg. Also, the positively charged residues (R157 and K162) in the phosphate-binding pocket of eIF4E are facing D111 and D112 of VPg (Fig. 4B).
Fig. 4.
(A) Restraint-driven model of the VPg (green) and eIF4E (blue) complex. Red highlights W56 from eIF4E involved in the association with VPg (SI Appendix, Fig. S8C). (B) Close-up view of the complex highlighting the positive patch on eIF4E and its interaction with negative residues on VPg (rotation of 120° compared with A). This region was confirmed to bind eIF4E by mutation (SI Appendix, Fig. S8 B and E). (C) Overlay of the HSQC spectra of 15N-labeled eIF4E (50 μM) in the presence of 3-fold excess of unlabeled VPg (blue) and after addition of 20-fold molar excess of m7GDP relative to eIF4E (orange), demonstrating that VPg and the cap compete for overlapping binding surfaces on eIF4E. (D) eIF4E specifically binds to VPg with submicromolar affinity (∼0.3 μM). Normalized change in fluorescence at emission of 484 nm (Δ[F]) as a function of eIF4E concentration for 0.5 μM VPgΔ37. Measurements were carried out 3 independent times.
To independently validate our model, we performed cross-linking mass spectrometry (XL-MS) on cross-linked heterodimers that were isolated by size exclusion chromatography. According to our model, only 3 lysines of VPg are located near the interface of the heterodimer (K105, K106, and K138), and hence, a low number of cross-links between both proteins was expected. Furthermore, most of the cross-linker was absorbed by intramolecular cross-link between VPg K105-K106 (extracted ion chromatogram [XIC] values in SI Appendix, Table S2), thus contributing greatly to a reduction of interprotein cross-link occurrences between VPg and eIF4E. Nevertheless, consistent with our HADDOCK model, the most frequently observed cross-link (15 occurrences), which also had the highest score (5.33), bridged K106 of VPgΔ37 with K159 of eIF4E, for which the Euclidean distance measured by the Xwalk software (42) was found to be 16.0 Å (SI Appendix, Table S2). This most frequent cross-link was consistent with the magnitude of line broadening in the different HSQCs, TCS, and mutagenesis data defining the binding sites for both eIF4E and VPg. The second most frequent interprotein cross-link only occurred 4 times and was found between eIF4E–K192 and VPg–K47, the latter of which is in a highly flexible region of VPg. The presence of this cross-link is consistent with previous studies that showed that the N terminus of VPg was involved in binding to eIF4E (21). Finally, a cross-link between eIF4E–K192 and VPgΔ37–K138 was observed but only with 2 occurrences. As observed in the apo form of eIF4E, the K192-containing loop and a fortiori, its side chain are flexible (Protein Data Bank [PDB] ID code 2GPQ), positioning these 2 lysines between 25 and 37 Å apart (calculated by the Xwalk software). Thus, in some conformations, these lysines would be accessible. Taken together, the XL-MS data support our HADDOCK model based on NMR and mutagenesis restraints.
The VPg–eIF4E complex presented here provides a molecular basis for understanding the genetic studies of eIF4E and VPg. The positions of eIF4E mutants that impart resistance to potyvirus infection in plants are mapped onto our eIF4E–VPg complex structure (SI Appendix, Fig. S8F). These mutants are generally located in the loop containing W56 (using human eIF4E amino acid numbering) or in the nearby phosphate-binding site (43, 44), which overlays with the proposed VPg-binding site. Interestingly, the engineered wheat W123A mutant (or human W102A), which did not disrupt the eIF4E–VPg interaction (27) (SI Appendix, Fig. S9), was found in the upper part of the cap-binding site and was not included in the experimentally defined eIF4E-binding surface used to interact with VPg, but it is clearly important for m7G cap binding. Additionally, the VPg residues, which interacted with eIF4E, were consistent with substitutions in this region being critical for viral pathogenicity (S101-N121 of PVY–VPg) (SI Appendix, Fig. S8G) (43–45).
We next determined whether VPgΔ37 bound plant eIF4E in a similar manner to human eIF4E. Previous biophysical studies showed that wheat eIF(iso)4E bound to VPg, and hence, we focused on this homolog for our studies (27, 46). Given that there were no NMR assignments available for eIF(iso)4E, we examined the interaction via 1H-15N HSQCs using 35 μM 15N-labeled VPgΔ37 with unlabeled eIF(iso)4E (105 μM). We found that VPg used the same binding region (I113-N121) to bind eIF(iso)4E (SI Appendix, Fig. S10 B and C) as it used to associate with human eIF4E (Fig. 2), indicating that this was conserved across kingdoms. Superimposing our homology model of wheat eIF(iso)4E onto the wheat eIF4E structure revealed that the orientation of the set of positive residues (corresponding to the R157, K159, and K162) and W56 (human numbering) was the same in plant and human eIF4Es (SI Appendix, Figs. S4 and S10A). Thus, the basic principles for the VPg–eIF4E interaction seem evolutionarily conserved.
VPg Binds eIF4E in the Presence of an eIF4G Peptide.
eIF4G is a major platform protein in the translation initiation complex, recruiting not only eIF4E–RNA complexes but other cofactors to the small ribosomal subunit in order to initiate translation (47, 48). To determine whether eIF4E–VPg complexes bind eIF4G, we carried out NMR experiments where VPgΔ37 was added to 15N-labeled eIF4E in complex with an eIF4G peptide containing the consensus binding site (4Gp). Both VPgΔ37 and 4Gp were used in a 3-fold molar excess (150 μM) compared with eIF4E (50 μM); 4Gp binds on the dorsal surface of eIF4E (SI Appendix, Fig. S11D), a region distal to the cap-binding and VPg-binding pocket in eIF4E. Interaction of both partners affects eIF4E’s signals differently in the HSQC, serving as reporters for binding. Indeed, on VPgΔ37 addition, most signals were broadened (SI Appendix, Fig. S11 B and F), while 4Gp addition induces peak shifting only for residues in the proximity to its binding site on eIF4E. In particular, addition of 4Gp perturbed residues W73, Y34, I35, K36, L75, and L137 on the dorsal surface of eIF4E as expected (SI Appendix, Fig. S11 A and E and Fig. 5C), which were not altered by VPgΔ37 binding. Overall, on addition of 4Gp to the eIF4E–VPgΔ37 complex, we observed 2 phenomena (SI Appendix, Fig. S11 C and G): 1) signals remain broad, indicating that VPgΔ37 was still bound to eIF4E, and 2) residues close to the 4Gp-binding site (see above) were shifted similarly as in the eIF4E/4Gp complex. Thus, our NMR data analysis revealed that eIF4E associates with a 4Gp and VPgΔ37 simultaneously.
Fig. 5.
(A) Silver-stained SDS/PAGE gel showing the conjugation between VPgΔ37 (Y64C, C150A) and the 19-mer RNA with a 5′ maleimide group. No β-mercaptoethanol or DTT (dithiothreitol) was present in order to preserve the conjugate. VPg, the RNA fragment, and the conjugated form are shown (lanes 1, 2, and 4, respectively). Validation of the conjugate is shown by treatment with either RNase A (lane 5) or proteinase K (Prot.K; lane 6). The 19-mer RNA is diffuse because of the percentage of acrylamide used. Position of the RNase A protein is shown in lane 3 for comparison. We note the presence of dimeric forms through C64 in the absence of reductant. (B) Association of the VPg–RNA conjugate with GST–eIF4E but not GST alone by western blot using an anti-His tag antibody (Upper). As expected, the VPg dimer also binds GST–eIF4E. An asterisk is shown to highlight a VPg–RNA degradation product that occurred during the GST pulldown. GST loading is shown below by western blot using an anti-GST antibody. The samples were run on the same gel, with unrelated samples removed for clarity. (C) Model representing eIF4G (orange; PDB ID code 5T46) derived from the crystal structure of the eIF4E–eIF4G complex with the VPgΔ37 (green) and eIF4E (blue) as displayed in Fig. 4A. The Y64, which is covalently attached to gRNA during infection, is shown. Similarly, the VPg–RNA conjugates made in vitro were also at the same position, C64 (in the text). (D) Western blot for Luciferase protein produced in in vitro translation reactions using wheat germ lysates and different luciferase RNAs: conjugated to VPg (VPg–RNA conj), uncapped, m7G-capped, and m7G-capped luciferase RNA in the presence of 10 μM VPg protein (capped RNA + VPg protein). Loading of different luciferase RNAs was confirmed by qRT-PCR (SI Appendix, Fig. S14D). (E) Quantification of western blots for in vitro translation assays described in D. Data were derived from 3 independent experiments. Mean intensities ± SDs are shown. P values are from Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001).
m7G Cap Analogs Compete for VPg Binding to eIF4E.
Given that VPg and the m7G cap analogs bound overlapping surfaces on eIF4E, we explored whether the cap analog m7GDP and VPgΔ37 competed for binding of 15N-eIF4E by HSQC experiments. Addition of 20-fold excess m7GDP to preformed VPgΔ37–eIF4E complexes (50 μM eIF4E, 150 μM VPgΔ37, 1 mM m7GDP) led to the reemergence of eIF4E resonances but now in their m7GDP-bound positions (Fig. 4C and SI Appendix, Fig. S12 A–C). This indicated that the m7GDP cap analog and VPgΔ37 competed for eIF4E on the same site. Importantly, VPgΔ37 did not bind to m7GDP cap itself as demonstrated by the observation that HSQC spectra of 15N-labeled VPg protein were unchanged in the presence or absence of the m7GDP (SI Appendix, Fig. S13D). Thus, the m7GDP cap analog competed for VPg binding in vitro. This was confirmed using the reverse titration in which we monitored changes to the 15N transverse relaxation-optimized spectroscopy (TROSY) HSQC of 2H-15N VPg (50 μM) on addition of unlabeled eIF4E (up to 175 μM) in the absence and presence of 1 mM m7GDP. Residues at the proposed VPg-binding site (D111, I113, M115, Q116, L118, G119, and N121) were substantially broadened upon eIF4E addition to VPg (SI Appendix, Fig. S13A). However, in the presence of 1 mM m7GDP (SI Appendix, Fig. S13 B and C), these peaks reappeared, indicating that m7GDP competes for the same binding site on eIF4E as VPg. The same experiment was performed with wheat eIF(iso)4E and yielded equivalent results (SI Appendix, Fig. S10D). Thus, VPg utilized the cap-binding site on both human and plant eIF4E for recognition and competed for cap analogs.
To gain a quantitative understanding of this interaction, we determined the dissociation constants for eIF4E–VPg and eIF4E cap analogs. Unfortunately, NMR titrations of the eIF4E–VPg complex via 1H-15N HSQC techniques resulted in disappearance of peaks, making determination of Kd values challenging for a variety of technical reasons, including the inability to obtain information of the completely broadened state where binding would be saturated. Using isothermal calorimetry (ITC), we determined the Kd for the human eIF4E–m7GDP cap analog. Consistent with previous results in phosphate buffer used in the above NMR experiments, we obtained a Kd of 0.37 ± 0.02 μM, which is consistent with previous literature reports for this interaction in phosphate buffer (49). When evaluating the Kd for the eIF4E–VPgΔ37 interaction by ITC using the same buffer conditions, we observed no heat release, suggesting that the interaction is mainly entropic. Thus, we used a fluorescence assay, whereby VPg was labeled with N-(iodoacetyl)-N′-(5-sulfo-1-naphtyl)ethylenediamine (IAEDANS) (33), an organic fluorophore that fluoresces at 490 nm, a region where the VPg and eIF4E proteins produced no signal. We observed a Kd of 0.26 ± 0.07 μM (Fig. 4D) and a Hill coefficient of ∼1.1, indicating a 1:1 ratio of VPg–eIF4E complexes, consistent with our NMR studies. This affinity is very similar to literature values for wheat eIF4E and VPg (∼0.3 μM) (27). Thus, the affinities of eIF4E for VPg and the m7G cap analog were very similar, consistent with the competition that we observed in our above NMR studies.
VPg–RNA Conjugates Directly Bind eIF4E and Were Templates for Translation.
Our above studies demonstrated that VPgΔ37 competed for m7G cap binding to eIF4E and in this way, could interfere with host-cell translation. However, they also suggested that VPgΔ37–RNA conjugates engaged eIF4E–eIF4G complexes possibly to be recruited to the translation machinery. As a first step to test this possibility, we investigated whether RNA conjugation disrupted the interaction with eIF4E or alternatively, if VPg–RNA conjugates could directly bind to eIF4E. During infection, VPgΔ37 is conjugated through the side-chain hydroxyl group of Y64 to the 5′ end of the viral gRNA (23–25). Y64 is in a flexible region distal to the eIF4E-binding site, consistent with the possibility that VPgΔ37 interacts with eIF4E and RNA simultaneously (Fig. 4A). Unfortunately, potyvirus VPg–gRNA conjugates are not available in purified forms to directly investigate this with the viral gRNA. Using maleimide chemistry (50), we thus conjugated a 19-mer fragment of luciferase RNA onto VPgΔ37 (SI Appendix, Fig. S14A). In this case, we generated VPgΔ37 Y64C for conjugation to the 5′ end of the RNA. In this same construct, we mutated the only naturally occurring cysteine C150, which is found in the flexible loop, to alanine in order to ensure a single conjugation site for the protein. This protein was folded as the wild type and binds eIF4E similarly to wild-type VPgΔ37 (SI Appendix, Fig. S14B). The 19-mer RNA segment with a maleimide group on its 5′ end was then cross-linked to the sulfur side chain of C64 (SI Appendix, Fig. S14A). We verified that the 19-mer RNA was conjugated using SDS/PAGE and silver staining, where the VPgΔ37(C150A/Y64C)–RNA conjugate was ∼6 kDa larger as expected. We further confirmed the presence of RNA by treating the conjugate with RNase (ribonuclease), which resulted in a reduction in size corresponding to the unmodified form of VPgΔ37(C150A/Y64C) (Fig. 5A). Similarly, treatment with proteinase K also disrupted the conjugate, leaving only the free 19-mer RNA band. Using eIF4E–GST pulldown, we observed that the VPgΔ37(C150A/Y64C)–RNA conjugate bound eIF4E but not the GST control (Fig. 5B). Thus, VPg recruited RNA to eIF4E.
Given the above findings, we examined the possibility that VPg–RNA conjugates were templates in in vitro translation assays. To produce conjugates with full-length messenger RNAs (mRNAs), we had to alter our strategy in order to conjugate full-length luciferase RNAs (∼1,800 nucleotides) to VPgΔ37(C150A/Y64C), which yielded a species of ∼500 kDa. Using in vitro transcription, guanosine-5′-monophosphorothioate (GMPS) was incorporated into the 5′ end of luciferase transcripts and subsequently coupled to 2,2′-pyridine disulfide using standard methods (51, 52). A disulfide exchange reaction of the resulting pyridyl-disulfide linkage on the 5′ end of the RNA was used for conjugation to VPgΔ37(C150A/Y64C) (53). To monitor the efficiency of conjugation, VPg–RNA conjugates were subjected to agarose gel electrophoresis due to their large size followed by immunoblotting (54) for the His tag of VPg (SI Appendix, Fig. S14C). Unconjugated VPgΔ37(C150A/Y64C) is shown for comparison. As observed, all of VPgΔ37(C150A/Y64C) was conjugated to the luciferase RNA, and no unconjugated RNA was detected after the reaction. For comparison, we generated luciferase transcripts using in vitro transcription without any modifications (referred to as uncapped) and also, generated capped luciferase transcripts using the VV-capping enzyme. Equal amounts of each luciferase RNA, confirmed by qRT-PCR (SI Appendix, Fig. S14D), were used as templates for in vitro translation assays, and the production of luciferase protein was monitored by western blot using an antiluciferase antibody with the experiments carried out 3 independent times (Fig. 5 D and E). We observed ∼2-fold more luciferase protein produced from m7G-capped luciferase RNA templates than uncapped templates as expected. The uncapped templates provided a lower bound for background translation, where it is well established that translation of uncapped RNAs occurs in in vitro systems but less efficiently than when RNAs are m7G capped (55). The levels of translation for VPg-capped luciferase transcripts were nearly identical to m7G-capped RNAs and ∼2-fold higher than observed for uncapped RNA (Fig. 5 D and E). This demonstrated that the VPg conjugation to the luciferase RNA did not interfere with its translation, indicating that VPg–luciferase RNA conjugates were templates for translation. Moreover, VPg–luciferase conjugates were translated with the same efficiency as capped RNAs, suggesting that VPg could functionally substitute for the m7G cap. These observations are consistent with our identification of VPg–eIF4E–eIF4G complexes (Fig. 6) and VPg–RNA–eIF4E complexes (Fig. 5). We note that the dynamic range of our assay was limited (2-fold between capped/VPg relative to uncapped RNA). Finally, the addition of free VPg (i.e., not conjugated to the RNA) reduced translation, consistent with our model of cap competition and previous reports (49).
Fig. 6.
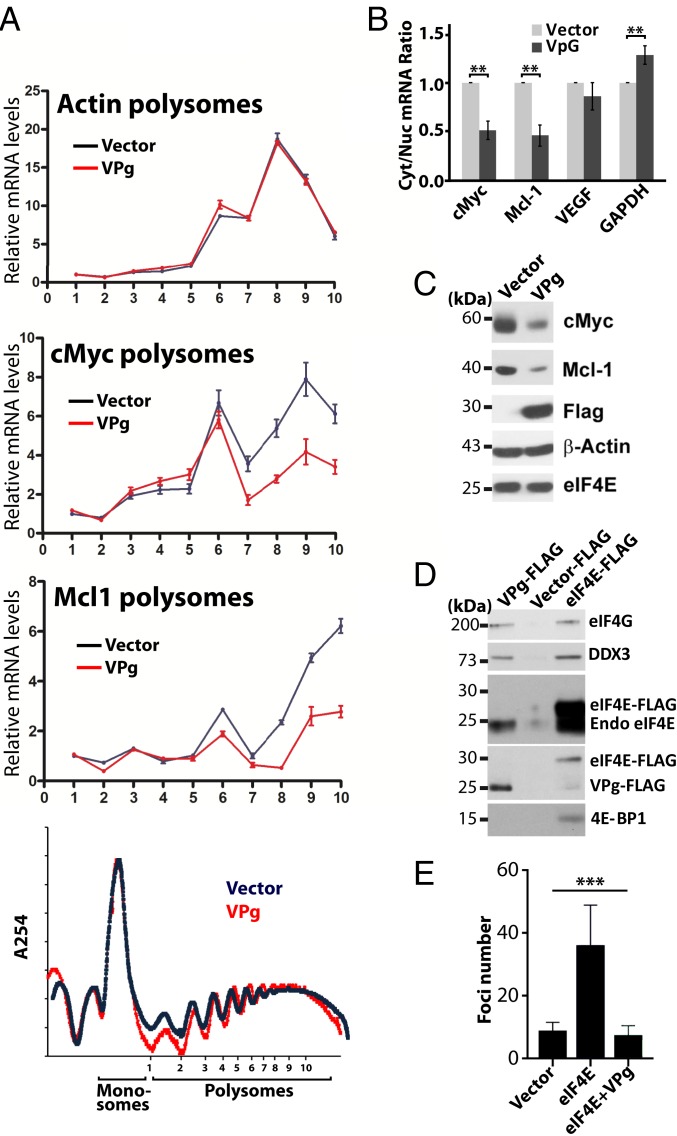
VPg represses eIF4E function in human cancer cells. (A) Polysome analyses of cells expressing VPg or vector controls indicates that VPg reduces translation efficiency of c-Myc and Mcl1 RNAs but not Actin, the negative control, without altering the global polysome profile (Lower). (B) VPg inhibited eIF4E-dependent mRNA export for targets RNAs. RNA levels were measured in nuclear and cytoplasmic fractions by qRT-PCR. While the increase in GAPDH was significant, it was so modest that it seems unlikely to be physiologically relevant. P values are shown. (C) Western blot analysis of the effects of VPg overexpression on eIF4E targets Mcl1 and cMyc. VPg–FLAG levels are given, and actin is provided as a loading control. Note that VPg does not lower endogenous eIF4E protein levels. (D) FLAG immunoprecipitations from cells overexpressing VPg–FLAG, eIF4E–FLAG, or controls. Blots were probed as indicated. (E) VPg overexpression suppresses formation of foci in eIF4E–Myc overexpressing cells. ANOVA (P < 0.0009) was conducted. Experiments were carried out 3 independent times; means ± SDs are shown in A, B, and E (***P < 0.001).
VPg Suppressed Cap-Dependent eIF4E Activities in Cells.
Next, we explored the impact of VPg on eIF4E activity in human cells to determine if the effects observed in vitro were recapitulated in cellulo. We postulated that VPg without RNA would potently inhibit cap-dependent activities of endogenous eIF4E in human cells by competing for host-cell transcripts. To test this hypothesis, we generated stable human osteosarcoma U2Os cell lines expressing either full-length VPg–FLAG or vector controls. U2Os cells were selected based on their well-characterized eIF4E activities (6, 56, 57). We investigated the effects of VPg on the nuclear functions in mRNA export and cytoplasmic functions in translation, because both functions require the ability of eIF4E to bind the m7G cap (6, 7).
We first examined translational efficiency using polysomal analysis in VPg–FLAG cells vs. vector controls. We monitored translation and RNA export of 2 well-characterized target transcripts of eIF4E: c-Myc and MCL1 (56, 58). VPg overexpression did not alter the overall polysomal profile, indicating that it did not interfere with the formation of ribosomes (Fig. 6A). However, VPg reduced translational efficiency for both MCL1 and c-Myc transcripts; in contrast, it did not alter the total RNA levels for either of these transcripts, and it did not affect the translation efficiency of Actin, an RNA insensitive to eIF4E (Fig. 6A and SI Appendix, Fig. S15A). We next explored whether the RNA export activity of eIF4E, which is also cap dependent, was impaired in VPg–FLAG cells relative to vector controls (Fig. 6B). Cells were fractionated into nuclear and cytoplasmic compartments, and RNAs were quantified by qRT-PCR (quality of fractionation was verified by semi-qPCR) (SI Appendix, Fig. S15C). VPg–FLAG overexpression impaired nuclear export of eIF4E target transcripts (e.g., MCL1 and c-Myc) by ∼2-fold but not eIF4E-independent transcripts, such as GAPDH and VEGF RNAs. Again, total levels of RNA were not altered for any of the examined transcripts as expected (SI Appendix, Fig. S15A). Consistent with VPg inhibiting both export and translation of these RNAs, protein levels for MCL1 and c-Myc were substantially decreased in VPg–FLAG cells relative to vector controls, while β-Actin protein levels were unchanged (Fig. 6C). This is also consistent with previous studies in plants that showed that potyvirus infection inhibited translation of specific RNAs (59), likely because only a subset of these is sensitive to eIF4E inhibition with other compensatory mechanisms coming into play, such as eIF3d-mediated cap-dependent translation for example (60). Thus, VPg inhibited activity of endogenous eIF4E.
To further dissect the role of VPg in eIF4E-dependent translation in cells, we explored whether VPg, via eIF4E, also interacted with endogenous eIF4G (Fig. 6D). We immunoprecipitated VPg–FLAG using an FLAG antibody and compared this with vector or eIF4E–FLAG-expressing cells. We observed that VPg–FLAG immunoprecipitated with endogenous eIF4E, endogenous eIF4G, and the RNA helicase DDX3, which is part of the active translation complex, but not with 4E-BP1, the inhibitor of eIF4E. Thus, VPg engaged active translation initiation complexes. As expected, eIF4E–FLAG bound to eIF4G, DDX3, and 4E-BP1, while no proteins were found in the negative control FLAG immunoprecipitations from vector controls. This suggests that eIF4G–eIF4E–VPg–RNA complexes readily formed in human cells, which is consistent with in vitro translation assays, where addition of VPgΔ37, when not conjugated to RNA, impeded translation of luciferase RNAs (Fig. 5 D and E). Taken together, VPg is able to impair host-cell RNA export and translation by competing with eIF4E for host-cell m7G-capped RNAs as well as during infection when, conjugated to gRNA, VPg is positioned to recruit gRNA to the translation machinery.
Given that VPg inhibited the mRNA export and translation activities of eIF4E, both of which contribute to its oncogenic potential and require cap binding (6, 7), we explored the possibility that VPg inhibited foci formation in eIF4E-overexpressing cells. In this case, stable U2Os cell lines were generated expressing eIF4E–Myc, VPg–FLAG + eIF4E–Myc, or vector controls. As expected, eIF4E–Myc increased foci formation ∼3-fold over vector. VPg–FLAG + eIF4E–Myc cells produced ∼3-fold fewer foci relative to eIF4E–Myc, comparable with vector controls (Fig. 6E and SI Appendix, Fig. S15B). While the initial focus of our VPg studies was to discover biochemical principles with regard to engagement of eIF4E, it is clear that the unusual properties of VPg could be exploited in the future to inhibit the oncogenic activity of eIF4E in human cancer cells.
Human Homologs Based on Structural Similarity with VPg.
Given that potyvirus VPg did not adopt a fold like VPgs from other virus families (consistent with the lack of sequence homology) (SI Appendix, Fig. S1A) or like known eIF4E-binding partners, we used the DALI server to ascertain if the VPg fold was similar to any others in the protein databank. The top 10 DALI hits included human kinesin 5 family member KIF11/EG5 (top hit) and prokaryotic or yeast-derived ribosomal large subunit protein 1 complexed to 23S ribosomal RNA or a viral IRES (SI Appendix, Table S3). With regard to the top hit, VPg is about half the size of EG5, where the homologous region includes the motor domain (SI Appendix, Fig. S16A). Given the structural homology, we investigated whether EG5 bound eIF4E. Using NMR, we found that EG5 directly bound eIF4E, leading to a similar pattern of spectral broadening observed for VPgΔ37 (SI Appendix, Fig. S16 C and E). Like VPgΔ37, excess m7G cap competed for eIF4E in preformed EG5–eIF4E complexes, leading to reemergence of cross-peaks at cap-bound positions (SI Appendix, Fig. S16D). Furthermore, peak broadening of the 15N-labeled eIF4E TrMut was significantly reduced compared with wild-type eIF4E on addition of EG5 (SI Appendix, Fig. S16E), again supporting that both EG5 and VPg interact with the cap-binding surface on eIF4E. Interestingly, this interaction could have important implications for newly identified functions of EG5 (i.e., it interacted with the RNA-binding ZBP protein to traffic actin transcripts to modulate cell motility and additionally, was found to associate with and traffic ribosomes) (61–63). Thus, our studies into potyviruses revealed a previously unknown eIF4E partner protein EG5 and a means for human proteins to engage eIF4E.
Conclusions
These studies leveraged previous plant genetic investigations to reveal the existence of a fundamentally different modality to engage and control eIF4E. The traditional view is that eIF4E is only controlled or engaged by interactions with its dorsal surface as observed for eIF4G. The notion that eIF4E could be inhibited by proteins competing for the cap-binding site was not previously considered. Consistent with this, VPg impaired host-cell eIF4E-dependent RNA export and translation through binding the cap-binding site to prevent host-cell RNA association with eIF4E. Our studies with the human EG5 protein suggest that this modality is not restricted to potyvirus but rather, is conserved across kingdoms. Based on these findings, VPg represents a previously unknown class of inhibitor of human eIF4E, which could be leveraged in future for therapeutic purposes.
Importantly, previous genetic studies demonstrated the requirement for VPg–eIF4E interactions and thus, presumably, the host-cell host translation machinery for successful viral infection. Given our data, one possibility is that free VPg (not conjugated to RNA) sequesters eIF4E–eIF4G complexes to allow for either IRES-mediated or some other form of translation to be engaged for VPg–gRNA conjugates. Another nonmutually exclusive possibility based on our findings is that VPg–gRNA conjugates are recruited by eIF4E–eIF4G complexes to the translation machinery. In this model, VPg would substitute for the m7G RNA cap on the gRNA, mediating a form of m7G cap-independent, eIF4E-dependent translation. In all, our studies demonstrated the existence of a fundamentally different form of cap competitor (VPg and EG5) than m7G cap analogs and identified an unanticipated binding surface for proteins to engage eIF4E. Furthermore, our findings suggest that VPg could substitute as the cap for potyvirus gRNA, which in physical nature, is a significant departure from the m7G cap first identified using CPV and VV over 40 y ago (3, 14, 15) or recently discovered adenosine nucleotides (5). Investigating these possibilities will be interesting areas of future study.
Materials and Methods
Protein Purification.
Unlabeled and labeled constructs of PVY VPg encoding residues 38 to 188 were expressed and purified as described previously (34). VPg mutants and deletion constructs (38 to 188 and 63 to 188) were generated using Quick Change mutagenesis (Bio Basic Inc.). eIF4E was expressed both in pET-28a for NMR and biophysics and in pGEX-6p1 for GST pulldown experiments (64). Wheat eIF(iso)4E was expressed in pET28a and purified similarly as human eIF4E. Human EG5 was overexpressed in a pGEX-6p1 construct. All constructs were verified by sequencing. After overnight induction at 20 °C, pelleted cells were resuspended in Tris Buffer (TB) buffer (10 mM Tris, pH 7.5, 250 mM KCl, 0.1% Triton X-100, 1 mM MgCl2, 1 mM DTT) and lysed using sonication (8 rounds of 10 s at high power using the Sonic Dismembrator Model 500 from Fisher); the supernatant of the lysate was added to preequilibrated glutathione Sepharose 4B beads for affinity purification. After extensive washing with TB buffer containing 500 mM KCl, the protein was cleaved overnight with the Prescission Protease in TB buffer, eluted, and further purified by gel filtration (Superdex 75). High levels of purity (>95%) were obtained.
NMR Spectroscopy.
NMR experiments were performed on Bruker Avance III spectrometers running at 600 or 800 MHz equipped with 5-mm QCIP (quadruple resonance probe with phosphorus) or 5-mm TCI (triple resonance inverse probe) cryoprobes, respectively. For structure determination, typical sample conditions were 0.4 to 0.5 mM VPg in 50 mM phosphate buffer, 150 mM NaCl, 1 mM DTT, and 0.02% NaN3 with either 7 or 100% D2O (pH 7.5), and all spectra were ran at 20 °C. 3D NOESY (NOE spectroscopy) spectra were acquired using nonuniform sampling (NUS) with 30% Poisson Gap sampling (65). For NMR titrations, concentrations for labeled and unlabeled proteins were 50 and 150 μM, respectively, and when the m7GDP cap analog was added, a 20-molar excess to eIF4E was used. Spectra were processed with NMRPipe (66) and SMILE (67) and analyzed with SPARKY (68).
HADDOCK-Derived Complex Structure of VpG–eIF4E.
The HADDOCK2.2 webserver (41) was used to generate restraint-driven docking for interaction between eIF4E and VPg using the standard protocols with the VPg structure reported here and eIF4E (PDB ID code 2GPQ). Default HADDOCK settings were used for the docking, generating a final 200 structures. The final models were clustered based on the fraction of common contacts using a 0.60 cutoff.
XL-MS.
Cross-linked sample was prepared by mixing equimolar amounts of eIF4E and His-tagged VPgΔ37 (0.15 μmol) with 1 mM DSS (4,4-Dimethyl-4-silapentane-1-sulfonic acid). After a 15-min incubation at room temperature, the reaction was stopped using NH4HCO3 and further purified by gel filtration chromatography (Superdex-75 column). Proteins were digested overnight at 37 °C using a 1:20 trypsin-to-protein ratio, and trypsin was inactivated with 0.5% trifluoroacetic acid. A series of samples (4 μg per well) was loaded into a 2-mg sorbent 96-well plate Oasis MCX µElution plate (Waters), washed, and eluted according to SI Appendix, Table S4 to enrich for multiply charged peptides. All samples were loaded at 600 nL min−1 on a 17-cm × 75-µm inner diameter PicoFrit fused silica capillary column (New Objective) and packed in house with Jupiter 5 µm C18 300 Å (Phenomenex). The column was mounted in an Easy-nLC II system (Proxeon Biosystems) and coupled to an Orbitrap Fusion mass spectrometer (ThermoFisher Scientific) equipped with a Nanospray Flex Ion source (Proxeon Biosystems). MS raw files were analyzed using SIM-XL (69, 70). MS2 spectra were visually inspected, and theoretical surface-accessible solvent distances for each compiled cross-link were measured using Xwalk (42).
CD.
CD spectra were collected on a Jasco-810 spectropolarimeter using a 0.1-cm quartz cuvette (Hellma) on pure proteins at 20 μM at room temperature. Relative ellipticity was converted to mean residue molar ellipticity according to Fasman (71).
Fluorescence Spectroscopy.
Cysteine residues were reduced by buffer-exchanging VPg into phosphate-buffered saline (PBS) buffer at pH 7.4 (containing 50 μM tris(2-carboxyethyl)phosphine [TCEP] and purged with N2) using an NAP-5 column (Fisher). VPg was then labeled by adding 10-molar excess of the organic fluorophore IAEDANS dropwise while stirring. The reaction was left at 25 °C for 2 h in the dark and stopped by adding 10 mM DTT. Buffer exchange was performed over an NAP-5 column using a spin column (Amicon 10-kDa MWCO [molecular cutoff]; Fisher) to remove unbound dye. Fluorescence measurements were carried out as follows. Briefly, 0.5 μM labeled VPg was incubated with increasing concentrations of eIF4E (0 to 2.5 μM) in PBS buffer containing 1 mM DTT. Fluorescence measurements were performed in a 0.3 × 0.3-cm2 fluorescence cuvette (Hellma) using a Cary Eclipse Fluorescence Spectrophotometer (Agilent Tech.). The degree of IAEDANS labeling was determined by measuring the absorbance at 336 nm using the extinction coefficients 5,700 M−1 cm−1. The IAEDANS-labeled VPg displayed a maximum fluorescence intensity at 484 nm. The fluorescence intensity increased ∼45% on association of eIF4E. Binding isotherms were fit according to the Hill model. Measurements were carried out at least 3 independent times.
ITC.
ITC was performed with a Microcal ITC200 calorimeter operating at 20 °C. The data were analyzed with MicroCal Origin software. The protein concentration was 10 μM in the cell, while the m7GDP cap analog was 100 μM in the syringe. Experiments consisted of 16 injections of 2.5 μL at a rate of 0.5 μL s−1 at 180-s intervals. The first injection peak was discarded from the isotherm. The baseline was automatically generated by the MicroCal Origin package and corrected manually. The binding isotherm was fitted using the One Set of Sites model in the MicroCal Origin package.
RNA Conjugation.
VPgΔ37 was mutated in positions 64 (Y64C) and 150 (C150A) to present only 1 cysteine at the position where VPg is known to be covalently attached to gRNA during infection. The sulfhydryl group of C64 was conjugated with the maleimide functional group of a succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate oligonucleotide (GeneLink) as shown in SI Appendix, Fig. S14A. The reaction was performed at room temperature with a 20-fold excess of the modified oligonucleotide. More details are given in SI Appendix, Supplementary Methods.
To generate RNA templates suitable for translation, full-length luciferase RNA (∼1,800 nucleotides) was in vitro transcribed (using the T7 Megascript Kit; Ambion) and 5′ end primed with GMPS (Axxora; Biolog Life Science Institute) (51, 52). GMPS-primed RNAs were than coupled to 2,2′-pyridine disulfide (SIGMA) to produce pyridyl-disulfide linkage on the 5′ end of RNAs. Disulfide exchange chemistry between the thiol group from VpG and pyridyl-disulfide on the 5′ end on luciferase RNA was used to covalently attach RNA to purified His–VPg C150A/Y64C. A schematic diagram of these reactions is shown in SI Appendix, Fig. S14E (53), and a detailed protocol is given in SI Appendix, Supplementary Methods. Validation of the conjugates was carried out using formaldehyde-agarose gel electrophoresis followed by immunoblotting with anti-His antibodies.
In Vitro Translation Assays.
Wheat germ lysate system was used to assess the relative translation of VPg–luciferase RNA conjugates, m7G-capped luciferase RNA, or uncapped luciferase RNA. More details are in SI Appendix, Supplementary Methods.
Cell Culture and Transfection.
U2Os cells (ATCC) were maintained in 5% CO2 at 37 °C in DMEM (Dulbecco’s Modified Eagle Medium, Gibco BRL) supplemented with 10% FBS (Fetal Bovine Serum) and 1% penicillin-streptomycin (Invitrogen). Transfections for stable cell lines were performed using Trans IT-LT1 Transfection Reagent (Mirus) as specified by the manufacturer and selected in puromycin-containing medium (10 μg/mL) for pMSCVeIF4E–Myc and/or G418 (1 mg/mL) for 2Flag–VPg overexpressing cell lines. The identity of U2Os cell line has been authenticated using STR profiling (Montreal EpiTerapia Inc.).
Cellular Fractionation, mRNA Export Assay, and Polysomal Profiling.
Nucleocytoplasmic fractionations for mRNA export assays and polysomal profiling were done as previously described (56, 58, 72) and detailed in SI Appendix, Supplemental Methods. TRIzol reagent (ThermoFisher Scientific) was added to each fraction, and RNAs were extracted using a DirectZol RNA Miniprep kit (Zymo Research), including deoxyribonuclease (DNase) treatment. RNAs were reversed transcribed using M-MLV Reverse Transcriptase (ThermoFisher Scientific). SensiFastSybr Lo-Rox Mix (Bioline) was used for qRT-PCR analyses by relative standard curve method (Applied Biosystems User Bulletin #2).
Anchorage-Dependent Foci Assays.
Experiments were carried out as described previously. A total of 500 cells were seeded per 10-cm plate or 100 cells per well in 6-well plates for 14 d, and then, they were stained with Giemsa (Sigma-Aldrich).
Immunoprecipitations.
U2Os cells were fixed with 1% paraformaldehyde (PFA) for 10 min at room temperature (RT) and quenched with 0.15 M glycine for 5 min at RT. Cells were than washed 3 times with cold 1× PBS, lysed by sonication (4 rounds of 5 s at lowest power) in NucleoTrap (NT2) buffer (56), and centrifuged for 10 min at 12,000 × g. Lysates were precleared with Sephadex G beads (GE Healthcare) for 30 min at 4 °C, and 0.75 to 1 mg of precleared lysates were used for immunoprecipitation with 7 to 10 μg anti-Flag antibody (Sigma) overnight at 4 °C. After incubation, complexes were washed 6 times with NT2 buffer, eluted by boiling in Tris(hydroxymethyl)aminomethane (Tris) EDTA (ethylenediaminetetraacetic acid) containing 1% sodium dodecyl sulfate (SDS) and 12% β-mercaptoethanol, and analyzed by western blot.
Data Availability.
The processed spectra and atomic coordinates for VPg were deposited into the PDB and the Biological Magnetic Resonance Data Bank (PDB ID code 6NFW and Biological Magnetic Resonance Data Bank accession no. 27506).
Supplementary Material
Acknowledgments
We are grateful for helpful discussions and VPg and wheat eIF(iso)4E constructs from Dr. Jadwiga Chroboczek (Université Grenoble Alpes-Centre National de la Recherche Scientifique [UGA-CNRS]) and Dr. Karen Browning (University of Texas), respectively. We thank Jose Rafael Dimayacyac (Institute of Research in Immunology and Cancer) and Dr. Jack Kornblatt (Concordia University) for technical assistance. We also thank Dr. Tara Sprules at Quebec/Eastern Canada High-Field NMR facility for use of the 800-MHz NMR. K.L.B.B. acknowledges financial support from NIH Grants R01 CA80728 and R01 CA98571, Canadian Institutes of Health Research (CIHR) Grant PJT159785, the Canada Research Chair in Molecular Biology of the Cell Nucleus, and the Canadian Foundation for Innovation for upgrades to the 600-MHz instrument.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The NMR, atomic coordinates, chemical shifts, and restraints reported in the paper have been deposited in the Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu/; accession no. 27506) and the Protein Data Bank (https://www.rcsb.org; ID code 6NFW).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904752116/-/DCSupplemental.
References
- 1.Sesma A., Castresana C., Castellano M. M., Regulation of translation by TOR, eIF4E and eIF2α in plants: Current knowledge, challenges and future perspectives. Front. Plant Sci. 8, 644 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipowicz W., et al. , A protein binding the methylated 5′-terminal sequence, m7GpppN, of eukaryotic messenger RNA. Proc. Natl. Acad. Sci. U.S.A. 73, 1559–1563 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuichi Y., Discovery of m(7)G-cap in eukaryotic mRNAs. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 91, 394–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace A., et al. , The nematode eukaryotic translation initiation factor 4E/G complex works with a trans-spliced leader stem-loop to enable efficient translation of trimethylguanosine-capped RNAs. Mol. Cell. Biol. 30, 1958–1970 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grudzien-Nogalska E., et al. , Structural and mechanistic basis of mammalian Nudt12 RNA deNADding. Nat. Chem. Biol. 15, 575–582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll M., Borden K. L., The oncogene eIF4E: Using biochemical insights to target cancer. J. Interferon Cytokine Res. 33, 227–238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culjkovic-Kraljacic B., Borden K. L., Aiding and abetting cancer: mRNA export and the nuclear pore. Trends Cell Biol. 23, 328–335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka T., A clinical study to evaluate the efficacy and safety of docetaxel with ribavirin in patients with progressive castration resistant prostate cancer who have previously received docetaxel alone. J. Clin. Oncol. 35 (suppl. 15), e14010 (2017). [Google Scholar]
- 9.Dunn L. A., et al. , Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck 40, 233–241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assouline S., et al. , Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): A proof-of-principle clinical trial with ribavirin. Blood 114, 257–260 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Assouline S., et al. , A phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica 100, e7–e9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zahreddine H. A., et al. , The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 511, 90–93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kentsis A., Topisirovic I., Culjkovic B., Shao L., Borden K. L., Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc. Natl. Acad. Sci. U.S.A. 101, 18105–18110 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuichi Y., Shatkin A. J., A simple method for the preparation of [beta-32P]purine nucleoside triphosphase. Nucleic Acids Res. 4, 3341–3355 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei C. M., Moss B., Methylated nucleotides block 5′-terminus of vaccinia virus messenger RNA. Proc. Natl. Acad. Sci. U.S.A. 72, 318–322 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams M., et al. , “Family potyviridae” in Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses, King A. M. Q., Adams M. J., Carstens E. B., Lefkowitz E. J., Eds. (Elsevier Academic Press, San Diego, CA, 2011), pp. 1069–1089. [Google Scholar]
- 17.Léonard S., et al. , Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 74, 7730–7737 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastet A., Robaglia C., Gallois J. L., eIF4E resistance: Natural variation should guide gene editing. Trends Plant Sci. 22, 411–419 (2017). [DOI] [PubMed] [Google Scholar]
- 19.German-Retana S., et al. , Mutational analysis of plant cap-binding protein eIF4E reveals key amino acids involved in biochemical functions and potyvirus infection. J. Virol. 82, 7601–7612 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robaglia C., Caranta C., Translation initiation factors: A weak link in plant RNA virus infection. Trends Plant Sci. 11, 40–45 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Grzela R., et al. , Potyvirus terminal protein VPg, effector of host eukaryotic initiation factor eIF4E. Biochimie 88, 887–896 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Steil B. P., Barton D. J., Cis-active RNA elements (CREs) and picornavirus RNA replication. Virus Res. 139, 240–252 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy J. F., Klein P. G., Hunt A. G., Shaw J. G., Replacement of the tyrosine residue that links a potyviral VPg to the viral RNA is lethal. Virology 220, 535–538 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Murphy J. F., Rychlik W., Rhoads R. E., Hunt A. G., Shaw J. G., A tyrosine residue in the small nuclear inclusion protein of tobacco vein mottling virus links the VPg to the viral RNA. J. Virol. 65, 511–513 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oruetxebarria I., et al. , Identification of the genome-linked protein in virions of Potato virus A, with comparison to other members in genus potyvirus. Virus Res. 73, 103–112 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Michon T., Estevez Y., Walter J., German-Retana S., Le Gall O., The potyviral virus genome-linked protein VPg forms a ternary complex with the eukaryotic initiation factors eIF4E and eIF4G and reduces eIF4E affinity for a mRNA cap analogue. FEBS J. 273, 1312–1322 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Khan M. A., Miyoshi H., Gallie D. R., Goss D. J., Potyvirus genome-linked protein, VPg, directly affects wheat germ in vitro translation: Interactions with translation initiation factors eIF4F and eIFiso4F. J. Biol. Chem. 283, 1340–1349 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Roudet-Tavert G., et al. , Central domain of a potyvirus VPg is involved in the interaction with the host translation initiation factor eIF4E and the viral protein HcPro. J. Gen. Virol. 88, 1029–1033 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Płochocka D., Wełnicki M., Zielenkiewicz P., Ostoja-Zagórski W., Three-dimensional model of the potyviral genome-linked protein. Proc. Natl. Acad. Sci. U.S.A. 93, 12150–12154 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rantalainen K. I., Eskelin K., Tompa P., Mäkinen K., Structural flexibility allows the functional diversity of potyvirus genome-linked protein VPg. J. Virol. 85, 2449–2457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grzela R., et al. , Virulence factor of potato virus Y, genome-attached terminal protein VPg, is a highly disordered protein. J. Biol. Chem. 283, 213–221 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Hébrard E., et al. , Intrinsic disorder in viral proteins genome-linked: Experimental and predictive analyses. Virol. J. 6, 23 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter J., et al. , Hydrodynamic behavior of the intrinsically disordered potyvirus protein VPg, of the translation initiation factor eIF4E and of their binary complex. Int. J. Mol. Sci. 20, 1794–1805 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coutinho de Oliveira L., Volpon L., Osborne M. J., Borden K. L. B., Chemical shift assignment of the viral protein genome-linked (VPg) from potato virus Y. Biomol. NMR Assign. 13, 9–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Güntert P., Buchner L., Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 62, 453–471 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Salvi N., Papadopoulos E., Blackledge M., Wagner G., The role of dynamics and allostery in the inhibition of the eIF4E/eIF4G translation initiation factor complex. Angew. Chem. Int. Ed. Engl. 55, 7176–7179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpon L., Osborne M. J., Borden K. L. B., Biochemical and structural insights into the eukaryotic translation initiation factor eIF4E. Curr. Protein Pept. Sci. 20, 525–535 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Volpon L., Osborne M. J., Topisirovic I., Siddiqui N., Borden K. L., Cap-free structure of eIF4E suggests a basis for conformational regulation by its ligands. EMBO J. 25, 5138–5149 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miras M., et al. , Structure of eIF4E in complex with an eIF4G peptide supports a universal bipartite binding mode for protein translation. Plant Physiol. 174, 1476–1491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakanishi T., et al. , Determination of the interface of a large protein complex by transferred cross-saturation measurements. J. Mol. Biol. 318, 245–249 (2002). [DOI] [PubMed] [Google Scholar]
- 41.van Zundert G. C. P., et al. , The HADDOCK2.2 web server: User-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 428, 720–725 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Kahraman A., Malmström L., Aebersold R., Xwalk: Computing and visualizing distances in cross-linking experiments. Bioinformatics 27, 2163–2164 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayme V., et al. , Different mutations in the genome-linked protein VPg of potato virus Y confer virulence on the pvr2(3) resistance in pepper. Mol. Plant Microbe Interact. 19, 557–563 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Li G., et al. , Variability in eukaryotic initiation factor iso4E in Brassica rapa influences interactions with the viral protein linked to the genome of turnip mosaic virus. Sci. Rep. 8, 13588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moury B., et al. , Evolution of plant eukaryotic initiation factor 4E (eIF4E) and potyvirus genome-linked protein (VPg): A game of mirrors impacting resistance spectrum and durability. Infect. Genet. Evol. 27, 472–480 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Khan M. A., et al. , Interaction of genome-linked protein (VPg) of turnip mosaic virus with wheat germ translation initiation factors eIFiso4E and eIFiso4F. J. Biol. Chem. 281, 28002–28010 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Prévôt D., Darlix J. L., Ohlmann T., Conducting the initiation of protein synthesis: The role of eIF4G. Biol. Cell 95, 141–156 (2003). [DOI] [PubMed] [Google Scholar]
- 48.von Der Haar T., Ball P. D., McCarthy J. E., Stabilization of eukaryotic initiation factor 4E binding to the mRNA 5′-cap by domains of eIF4G. J. Biol. Chem. 275, 30551–30555 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Osborne M. J., et al. , eIF4E3 acts as a tumor suppressor by utilizing an atypical mode of methyl-7-guanosine cap recognition. Proc. Natl. Acad. Sci. U.S.A. 110, 3877–3882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh S. S., Kao P. M., McCue A. W., Chappelle H. L., Use of maleimide-thiol coupling chemistry for efficient syntheses of oligonucleotide-enzyme conjugate hybridization probes. Bioconjug. Chem. 1, 71–76 (1990). [DOI] [PubMed] [Google Scholar]
- 51.Sengle G., et al. , Synthesis, incorporation efficiency, and stability of disulfide bridged functional groups at RNA 5′-ends. Bioorg. Med. Chem. 8, 1317–1329 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Wu C. W., Eder P. S., Gopalan V., Behrman E. J., Kinetics of coupling reactions that generate monothiophosphate disulfides: Implications for modification of RNAs. Bioconjug. Chem. 12, 842–844 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Kerr J., Schlosser J. L., Griffin D. R., Wong D. Y., Kasko A. M., Steric effects in peptide and protein exchange with activated disulfides. Biomacromolecules 14, 2822–2829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margis R., Hans F., Pinck L., VPg Northern-immunoblots as a means for detection of viral RNAs in protoplasts or plants infected with grapevine fanleaf nepovirus. Arch. Virol. 131, 225–232 (1993). [DOI] [PubMed] [Google Scholar]
- 55.Pelham H. R., Translation of fragmented viral RNA in vitro: Initiation at multiple sites. FEBS Lett. 100, 195–199 (1979). [DOI] [PubMed] [Google Scholar]
- 56.Zahreddine H., The eukaryotic translation initiation factor eIF4E harnesses hyaluronan production to drive its malignant activity. eLife 6, e29830 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Culjkovic B., Topisirovic I., Skrabanek L., Ruiz-Gutierrez M., Borden K. L., eIF4E is a central node of an RNA regulon that governs cellular proliferation. J. Cell Biol. 175, 415–426 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Culjkovic-Kraljacic B., et al. , Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B-cell lymphomas. Blood 127, 858–868 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moeller J. R., et al. , Differential accumulation of host mRNAs on polyribosomes during obligate pathogen-plant interactions. Mol. Biosyst. 8, 2153–2165 (2012). [DOI] [PubMed] [Google Scholar]
- 60.de la Parra C., et al. , A widespread alternate form of cap-dependent mRNA translation initiation. Nat. Commun. 9, 3068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartoli K. M., Jakovljevic J., Woolford J. L. Jr, Saunders W. S., Kinesin molecular motor Eg5 functions during polypeptide synthesis. Mol. Biol. Cell 22, 3420–3430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chudinova E. M., Nadezhdina E. S., Interactions between the translation machinery and microtubules. Biochemistry (Mosc.) 83 (suppl. 1), S176–S189 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Song T., et al. , Specific interaction of KIF11 with ZBP1 regulates the transport of β-actin mRNA and cell motility. J. Cell Sci. 128, 1001–1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpon L., et al. , Importin 8 mediates m7G cap-sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. Proc. Natl. Acad. Sci. U.S.A. 113, 5263–5268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hyberts S. G., Takeuchi K., Wagner G., Poisson-gap sampling and forward maximum entropy reconstruction for enhancing the resolution and sensitivity of protein NMR data. J. Am. Chem. Soc. 132, 2145–2147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delaglio F., et al. , NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Ying J., Delaglio F., Torchia D. A., Bax A., Sparse multidimensional iterative lineshape-enhanced (SMILE) reconstruction of both non-uniformly sampled and conventional NMR data. J. Biomol. NMR 68, 101–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goddard T. D., Kneller D. G., Sparky 3 (University of California, San Francisco, CA, 2003). [Google Scholar]
- 69.Lima D. B., et al. , SIM-XL: A powerful and user-friendly tool for peptide cross-linking analysis. J. Proteomics 129, 51–55 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Lima D. B., et al. , Characterization of homodimer interfaces with cross-linking mass spectrometry and isotopically labeled proteins. Nat. Protoc. 13, 431–458 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Fasman G. D., Ed., Circular Dichroism and the Conformational Analysis of Biomolecules (Plenum Press, New York, NY, 1996). [Google Scholar]
- 72.Tcherkezian J., et al. , Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5'TOP mRNA translation. Genes Dev. 28, 357–371 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed spectra and atomic coordinates for VPg were deposited into the PDB and the Biological Magnetic Resonance Data Bank (PDB ID code 6NFW and Biological Magnetic Resonance Data Bank accession no. 27506).