Significance
Despite decades of prenatal programming research showing that “the womb may be more important than the home” with respect to offspring health outcomes, no studies of which we are aware have considered multiple indicators of maternal stress to identify the types of maternal stress that most influence developing offspring. This study’s key contributions include the use of a data-driven procedure to specify types of maternal stress—psychological and subclinical physical health indicators—that predict offspring outcomes including sex at birth, risk of preterm birth, and fetal neurodevelopment. Social support is a key factor differentiating the stress groups and a malleable intervention target to improve offspring outcomes.
Keywords: prenatal stress, secondary sex ratio, social support, birth outcomes, neurodevelopment
Abstract
Maternal prenatal stress influences offspring neurodevelopment and birth outcomes including the ratio of males to females born; however, there is limited understanding of what types of stress matter, and for whom. Using a data-driven approach with 27 variables from questionnaires, ambulatory diaries, and physical assessments collected early in the singleton pregnancies of 187 women, 3 latent profiles of maternal prenatal stress emerged that were differentially associated with sex at birth, birth outcomes, and fetal neurodevelopment. Most women (66.8%) were in the healthy group (HG); 17.1% were in the psychologically stressed group (PSYG), evidencing clinically meaningful elevations in perceived stress, depression, and anxiety; and 16% were in the physically stressed group (PHSG) with relatively higher ambulatory blood pressure and increased caloric intake. The population normative male:female secondary sex ratio (105:100) was lower in the PSYG (2:3) and PHSG (4:9), and higher in the HG (23:18), consistent with research showing diminished male births in maternal stress contexts. PHSG versus HG infants were born 1.5 wk earlier (P < 0.05) with 22% compared to 5% born preterm. PHSG versus HG fetuses had decreased fetal heart rate–movement coupling (P < 0.05), which may indicate slower central nervous system development, and PSYG versus PHSG fetuses had more birth complications, consistent with previous findings among offspring of women with psychiatric illness. Social support most strongly differentiated the HG, PSYG, and PHSG groups, and higher social support was associated with increased odds of male versus female births. Stress phenotypes in pregnant women are associated with male vulnerability and poor fetal outcomes.
Decades of developmental origins of health and disease (DOHaD) research demonstrate that “the womb may be more important than the home” for offspring’s future health (1–3). The in utero environment influences fetal neurodevelopment (4), birth outcomes (5), and even the secondary sex ratio (SSR) or the ratio of males to females born (6). An estimated 30% of pregnant women report psychosocial stress in their daily lives including job strain and depressive or anxiety symptoms (7). Maternal psychosocial stress may increase the risk of preterm birth (PTB) (8–10), which is associated with greater infant mortality and physical as well as mental morbidity (11). Although many fetuses appear resilient to stress effects, prospective longitudinal research suggests that pregnant women in the top 15% for prenatal anxiety and depression have offspring with an estimated 2-fold increased risk for a mental disorder (e.g., attention deficit hyperactivity disorder [ADHD] or anxiety), and this effect extends from childhood through adolescence (12). Greater maternal prenatal psychosocial stress also is associated with small but statistically significant reductions in intelligence quotient scores and less advanced language development at 5.5 y old (13) as well as greater likelihood of autistic traits in school-age children (14). Even before birth, maternal psychosocial stress effects can be detected in indices of fetal neurodevelopment such as greater fetal heart rate (FHR) reactivity to stimuli, overall reduced heart rate variability, diminished habituation to stimuli, and differences in newborn amygdala volume and resting-state functional connectivity (15–22).
Allostatic load (AL) incorporates multiple subclinical physiological/physical parameters (e.g., diastolic and systolic blood pressure [BP], heart rate, body mass index [BMI], immune markers, and cortisol with consideration of relevant lifestyle factors [diet, exercise, and social support]) into a single index to measure the cumulative impact of “wear and tear” on the body of repeated activation, and associated dysregulation, of physiological systems stemming from chronically stressful situations (23, 24). Higher AL during pregnancy is associated with increased odds of both PTB (25, 26) and delivering a low-birth-weight infant (25, 27). Individual AL factors, including elevated maternal ambulatory BP and cortisol, have been associated with fetal neurodevelopment including lower resting FHR, increased FHR reactivity, and reduced FHR–movement coupling (28, 29).
Maternal stress also impacts sex at birth such that the typically male-biased SSR (105 male births:100 female births) (30) is diminished in the context of maternal prenatal stress (31–36), most likely as a consequence of male-sex-selective spontaneous fetal loss (33, 34, 37). Although the population-level stress-related decrease in the SSR is debated (38, 39), most agree that a dynamic expression of the SSR allows for evolutionary response to natural selection pressures (6) and that males are more vulnerable to in utero perturbations (40). The tendency for more PTBs among males (41, 42), and males’ increased risk for early neurodevelopmental disorders such as intellectual disability, autism, dyslexia, and ADHD (37, 40) and greater likelihood of telomere shortening (43) support this assertion.
This paper presents an approach to phenotyping maternal prenatal stress, simultaneously considering multiple indices of psychosocial and physical indices of stress to determine which kinds of stress matter most for fetal development and birth outcomes. AL approaches emphasize the physiological impact of chronic stress, but the single index overlooks specific intervention targets. Conversely, DOHaD studies relating individual stress indicators to child outcomes abound but may take an overly reductionist approach by failing to concomitantly consider multiple physical and psychosocial indicators of stress, and typically exclude important factors such as social support, which has been inversely related to increased odds of PTB (44). Bridging the AL and psychosocial stress literatures, we collected data on 27 indicators of maternal prenatal psychosocial, physical, and lifestyle stress and applied a data-driven approach to determine distinct types of maternal stress and how these profiles differentially influence birth outcomes (SSR, gestational age [GA] at birth, birth weight, birth complications, delivery type, neonatal intensive care unit [NICU] needs) and fetal neurodevelopment (FHR reactivity and fetal heart rate–movement coupling). To enhance the study’s clinical relevance, we also aimed to determine which variable(s) best differentiated the groups. Social determinants of health (e.g., socioeconomic status) were examined as profile covariates based on their associations with psychosocial and physical stress and the potential consequent implications for interventions.
Results
Identification of Maternal Prenatal Stress Groups.
Latent profile analysis (LPA), a procedure that employs maximum likelihood estimation to determine distinct subgroups based on a set of manifest continuous indicator variables (45–47), was used to determine mutually exclusive stress groups which were then assessed on their substantive, theoretical, and empirical merits. Multiple solutions specifying 2 to 4 profiles were examined, and the optimal number of profiles was determined using the size of the subgroups, the meaningfulness of the models, and model fit statistics. LPA indicators were selected from the first study assessment (mean weeks gestation = 19.73, SD = 5.73) and included psychosocial and AL information obtained primarily via routine screening and/or the medical record: demographic (age), physical health (prepregnancy BMI, ambulatory BP, mean arterial pressure, heart rate, and diurnal cortisol), stressors (perceived stress and positive and negative pregnancy experiences), mental health (negative daily mood, depression, anxiety, and posttraumatic stress disorder [PTSD]), lifestyle (daily overall calories, calories from fat, protein, carbohydrates, and added sugar, and exercise frequency), and perceived social support (tangible, appraisal, and belonging) factors. The 3-profile model yielded the lowest Bayesian information criterion (BIC), equivalent entropy values compared to the 2-profile model (and better than the 4-profile model), and a significant Lo–Mendell–Rubin (L-M-R) statistic; class probability statistics were 0.91 (profile 1), 0.88 (profile 2), and 0.96 (profile 3). Posterior probabilities were used to assign each participant 1 of the 3 mutually exclusive groups.
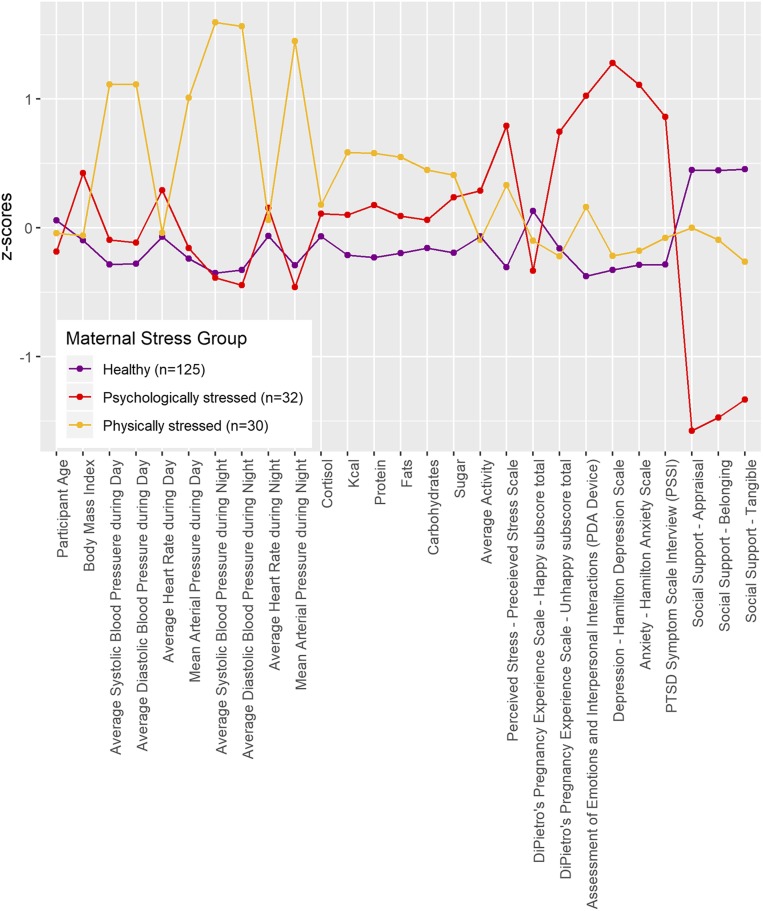
Fig. 1 depicts differences in the 3 stress groups using Z scores across the indicator variables. ANOVA was used to examine how the 3 profiles differed on indicator variables (SI Appendix, Table S1). The largest profile (66.8% of the sample; n = 125) could be considered both physically and psychologically “healthy” (healthy group, HG). A smaller group of “psychologically stressed” (psychologically stressed group, PSYG) pregnant women (17.1%; n = 32) had significantly higher scores on measures of perceived stress, unhappy pregnancy experiences, daily negative affect, depression, anxiety, and PTSD symptoms than the other 2 groups; PSYG also had higher prepregnancy BMI compared to HG. Relative to HG and PSYG, a smaller group of “physically stressed” (physically stressed group, PHSG) pregnant women (16%; n = 30), had higher average ambulatory systolic and diastolic BP and mean arterial pressure during the day and evening. PHSG also consumed significantly more overall calories, protein, fat, and sugar as recorded via diary compared to those in HG. All profiles differed significantly from each other on each of the 3 indices of social support. Groups did not differ on maternal age, diurnal cortisol, or physical activity. We may have had limited variability to detect age effects, as only 20% of the current sample was 35 to 44 y of age and none were 45 y of age or older. Additionally, assessing cortisol on a single day early in pregnancy may have contributed to the lack of findings (48). Finally, PDA (personal digital assistant)-rated physical activity relied on self-report and did not differentiate type or vigor of activity.
Fig. 1.
Maternal stress indicator variables are depicted according to latent profile membership. Each indicator was standardized using its sample mean and SD and the means of these indicators are displayed by maternal stress group. Significant differences between groups are noted in SI Appendix, Table S1.
Differences in Stress Indicators.
Consistent with work showing that elevations in BMI are associated with psychological stress such as PTSD (49), PSYG had higher BMI relative to HG (P < 0.01), with a trend for higher BMI relative to PHSG (P = 0.057). PHSG’s systolic and diastolic daytime BP values of 121 mmHG and 87 mmHG, respectively, and nighttime BP values of 120 mmHG and 86 mmHG, respectively, were significantly higher than those in the HG and PSYG (P < 0.001) and are at the low end of newly defined pregnancy-specific stage 1 hypertension (50), although caution is warranted as ambulatory measurement used here produces higher BP values than office-based mercury sphygmomanometer readings (51). PHSG women consumed significantly more calories including from fat, protein, and sugar compared to the HG and at 2,665 calories per day were slightly above the 2,500 calories per day recommended as an upper limit (52).
Levels of perceived stress in both stress groups were similar to those found in a previous study of pregnant women diagnosed with depression or anxiety (PTSD Symptom Scale [PSS] total score >25) while scores in the HG (<20) were consistent with scores for those without psychiatric conditions (53) (SI Appendix, Table S1). Similarly, the PSYG’s scores of 15 and 13 on the Hamilton Depression Rating Scale (HRSD) and Hamilton Anxiety Rating Scale (HRSA), respectively, are consistent with mild-to-moderate depression and anxiety in pregnant women and well above the clinical cut scores of 8 (54, 55), while the HG scored below the cut scores at 5 on both scales.
Birth Outcomes by Stress Groups.
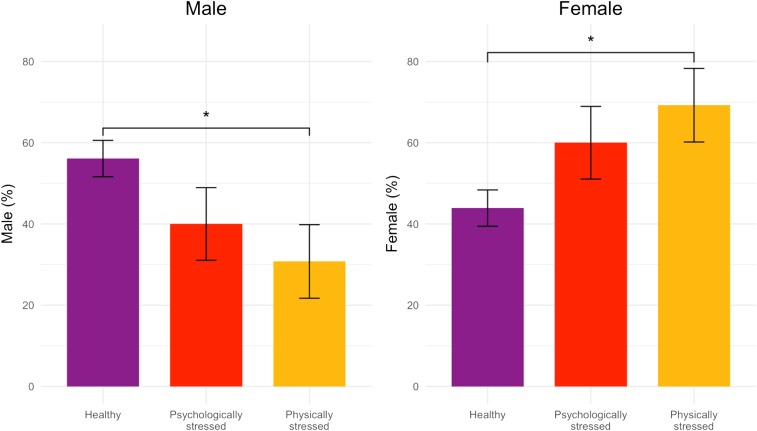
χ2 and ANOVA were used to compare the profiles on select categorical and continuous birth outcomes and third-trimester fetal neurodevelopment. For variables that differed significantly between profiles, we conducted post hoc pairwise group comparisons using least significant differences for continuous variables or Fisher’s exact test for categorical variables. In the overall sample, the sex ratio was 1:1 [49.7% male and 50.3% female, χ2(1) = 0.00, P = 0.1]; however, within groups the SSR differed (HG 23:18, PSYG 2:3, PHSG 4:9). As shown in Fig. 2 and SI Appendix, Table S2, compared to the HG, both stressed groups had a lower percent of male births [56%, 40%, and 31% for HG, PSYG, and PHSG, respectively; χ2(2, n = 179) = 6.87 (P < 0.05)].
Fig. 2.
χ2 differences in fetal sex (percent) by maternal stress group (*P < 0.05) with SE bars; 8 of 187 participants were missing fetal sex. For both sexes, significant differences were only observed between the healthy and physically stressed groups.
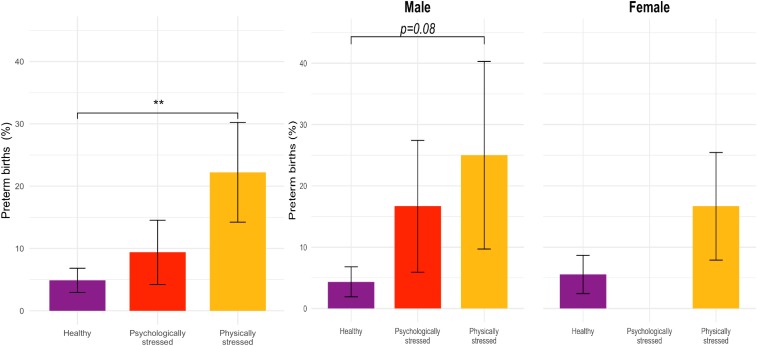
The overall rate of PTB was 8%; however, there were significant differences in PTB across the stress groups [χ2(2, n = 182) = 8.87, P = 0.01], with 22% of PHSG fetuses born before 37 wk compared to only 5% of HG fetuses (Fig. 3). With respect to GA at birth, PHSG fetuses were born 1.5 wk earlier than HG fetuses (d = 0.57, t = −2.31, P = 0.02). For the whole sample, there was no sex difference in PTB [odds ratio, OR = 0.84, χ2(1, n = 179) = 0.00, P = 0.98], yet there was a trend for male fetuses in both stress groups to be born earlier than those in the HG [F(2, 86) = 2.47, P = 0.09]. Although the occurrence was low (n = 4), fetal demise was strongly associated with maternal stress group [χ2(2, n =182) = 8.62, P = 0.01] such that 2 fetal deaths occurred in the PHSG group, 2 in the PSYG, and none in the HG.
Fig. 3.
χ2 differences in PTB (percent <37 wk) by maternal stress groups (**P < 0.01) with SE bars are presented for all participants (Left, n = 182) and separately by fetal sex (Right, n = 179). In the overall sample, only the healthy and physically stressed groups differed significantly.
There were nearly significant overall group differences in pre-, peri-, or postnatal complications [χ2(2, n =181) = 5.67, P = 0.06] (SI Appendix, Fig. S1); follow-up pairwise comparisons indicated that the PSYG was significantly more likely to report complications compared to the PHSG [χ2(1, n = 58) = 3.85, P < 0.05]. There was a tendency for this pattern to hold for female fetuses [χ2(2, n =87) = 5.74, P = 0.06] but not for males (P = 0.29), in part because for males both stress groups tended to have higher rates of complications compared to HG [63% in PSYG, 50% in PHYG, χ2(1, n =19) = 0.02, P = 0.90]. There were no group differences in delivery type [χ2(4, n =162) = 6.90, P = 0.14], birth weight [F(2, 167) = 0.38, P = 0.68], NICU involvement [χ2(2, n =149) = 1.09, P = 0.58], or mothers’ or babies’ length of hospital stay [F(2, 120) = 0.14, P = 0.87 and F(2, 105) = 2.61, P = 0.08, respectively]. For babies’ length of hospital stay, after removing one outlier with greater than 10-d duration, there was no group difference [F(2, 104) = 0.60, P = 0.60]. There was no interaction with baby sex by length of hospital stay for babies [F(2, 98) = 0.76, P = 0.47] or for mothers [F(2, 109) = 0.38, P = 0.69].
Fetal Neurodevelopment by Stress Groups.
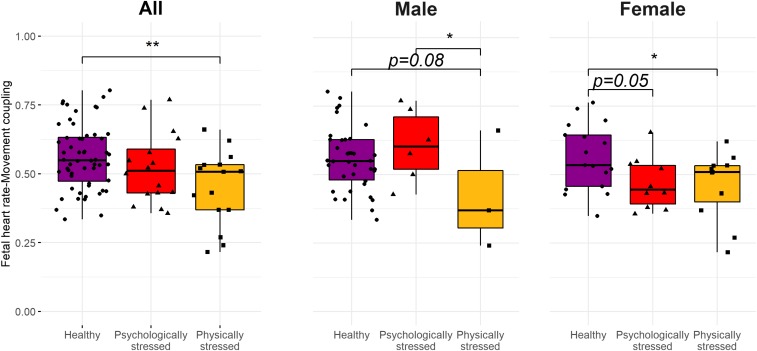
Based on a subset of offspring with usable fetal data (n = 87), there was a significant group difference in fetal heart rate–movement coupling [F(2, 85) = 4.92, P < 0.05]: PHSG versus HG fetuses had significantly less coupling, an index reflecting central nervous system integration of the autonomic and somatic systems (d = −0.90, t = −3.09, P < 0.01; Fig. 4). When examined by fetal sex, both stress groups had lower coupling levels relative to the HG among females (d = −0.81, t = −2.02, P = 0.05 in PSYG; d = −0.87, t = −2.26, P = 0.03 in PHSG), but there were no group difference among males [F(2, 44) = 2.23, P = 0.12]; findings may reflect fewer males in the stress groups. Among the subset of fetuses with usable data (n = 118), FHR reactivity did not differ by stress group [F(2, 118) = 1.25, P = 0.29]. However, female fetuses in the PHSG had higher reactivity than the other groups [F(2, 59) = 4.28, P = 0.02; PHSG versus HG: d = 0.93, t(59) = 2.80, P = 0.007; PHSG versus PSYG: d = 0.95, t(59) = 2.43, P = 0.02], while no difference was found in males [F(2, 55) = 1.55, P = 0.22].
Fig. 4.
Third-trimester FHR–movement coupling by maternal stress groups are presented for all available participants (Left, n = 87) and by fetal sex (Right, n = 85) (*P < 0.05; **P < 0.01). Fetal FHR and fetal movement were simultaneously assessed via a transabdominal Doppler during a 5-min laboratory stressor task and cross-correlations were computed to estimate “coupling.”
To ensure that effects of group membership on birth and fetal outcomes were not entirely accounted for by poor maternal physical health, a sensitivity analysis was conducted removing the 12 women who reported prenatal diabetes mellitus, vascular problems, or preeclampsia (SI Appendix, Table S3). The effects of group membership remained significant for SSR and PTB but shifted from significant to trend level for GA at birth and fetal heart rate–movement coupling; pairwise comparisons remained significant even when overall P values shifted to trend level.
Variables That Most Strongly Discriminated Stress Groups.
To enhance the study’s clinical relevance with respect to the identification of potential targets for intervention, we assessed which of the 27 indicator variables best differentiated the stress groups. We performed a partial least square discriminant analysis. Based on 5-fold cross-validation, the model with 2 components was selected. A variable importance in projection (VIP) score reflects the relative importance or explanatory power of the 27 stress indicators in differentiating the stress groups. We extracted the VIP scores on the first and most discriminant component of the final model. The variables with VIP scores greater than 1 are considered most relevant for differentiating the 3 stress groups (56). SI Appendix, Fig. S2 shows the VIP scores of the 27 variables sorted by VIP scores. Eight variables were considered important (>1) and the 3 social support variables—appraisal: people with whom to talk; belonging: people with whom to spend time; and tangible: people on whom to rely for material help—had the 3 highest VIP scores. When a composite social support score was used, the VIP score for the cumulative social support score was 3, well above those for other indicators (SI Appendix, Fig. S3). Other indicators with VIP scores greater than 1 included depression, PDA-rated negative affect, anxiety, and PTSD symptoms. Across all 3 social support subscales with potential scores ranging from 0 to 40, PSYG had the lowest (17, 18), PHSG next (22–26), and HG highest (26–29) (SI Appendix, Table S1 and Fig. S4), which significantly differed from each other [appraisal: F(2,158) = 108.84, P < 0.001; belonging F(2,158) = 86.27, P < 0.001; tangible F(2,158) = 69.63, P < 0.001].
Social Support and Outcomes.
As social support indices were the strongest differentiators of the stress groups, we investigated if maternal stress group effects on our key perinatal outcomes would remain when controlling for the composite social support score. As shown in SI Appendix, Table S4, when variation in social support was included in the statistical models, maternal stress group differences in SSR and GA at birth were no longer significant, became weaker for PTB, yet remained robust for fetal coupling.
To further probe the impact of social support, we examined social support in relation to the likelihood of having a male versus female baby. Quantiles were used to categorize each social support variable into 4 groups and logistic regression was used to compare odds of male birth across the 4 groups. The lowest social support group was used as the reference group. As shown in SI Appendix, Fig. S5, having more social support was associated with higher odds of male to female births (composite social support 1-unit increase, OR 1.02, P = 0.05); the odds of male birth were significantly higher in the high versus low group on the belonging subscale (OR = 2.56, P = 0.04) and trend level in the high versus low group on the tangible subscale (OR = 2.33, P = 0.08). Logistic regression conducted with continuous social support variables showed that the odds of male births increase by 8% per 1-point increase in the tangible support score (OR = 1.08, P = 0.02), 7% per 1-point increase in belonging score (OR = 1.07, P = 0.05), and 4% per 1-point increase in appraisal score (OR = 1.04, P = 0.24).
Social Determinants of Health among Stress Groups.
There were significant demographic correlates of profile membership, many of which are routinely identified as social determinants of health and contributors to future health trajectories (57, 58) (SI Appendix, Table S5). Women in the PSYG were more likely to be Hispanic and report fewer years of education, a lower household income, and increased likelihood of receiving needs-based public assistance compared to women in the other 2 profiles. Women in the PSYG also reported more prior pregnancies and abortions compared to HG and had more children than women in both of the other groups. Women in the PSYG reported higher levels of childhood emotional abuse and physical neglect compared to women in the other 2 profiles, and all 3 groups differed significantly on childhood emotional neglect such that women in the PSYG reported the highest levels, PHSG moderate levels, and HG lowest levels. Women in the PSYG reported less warm caregiving and more overprotective caregiving from their mothers compared to those in PHSG and HG, and more overprotective caregiving from their fathers compared to the HG.
Discussion
This data-driven approach to phenotyping prenatal maternal stress among women who, for the vast majority, were otherwise designated as having medically healthy pregnancies went beyond studies relating AL or individual stress indicators to birth outcomes by identifying maternal stress phenotypes that have unique effects on birth outcomes. Three profiles emerged: an HG, a PSYG, defined by clinically relevant mood and mental health symptoms, and a PHSG, distinguished by physical indicators of stress largely independent of any medical condition. Of the 27 psychosocial, physical, and lifestyle indicators considered, social support—or lack thereof—contributed most to differentiating the 3 groups. In both stress groups, the SSR—typically more males born than females—was reduced compared to both population norms and the HG, and greater maternal social support increased the odds of having a male. Compared to the HG, the PHSG had elevated PTB risk and lower fetal heat rate–movement coupling; however, the PSYG had more perinatal complications than the PHSG.
The population-normative male:female SSR, typically 105:100 (30), was lower in the PSYG (2:3) and PHSG (4:9) and higher in the HG (23:18), suggesting that male births are less common in the presence of maternal stress. These results are consistent with studies suggesting that male fetuses versus females are less likely to survive in suboptimal conditions (34, 59–63). Decreased male survival in utero in the context of poor maternal conditions has been observed in other mammals (64), and population-level data suggest that the relative number of male births tends to decrease in the context of maternal exposure to earthquakes and social upheaval, including President Kennedy’s assassination and the 9/11 terrorist attacks in New York (31–36). Diminished male fetal survival is poorly understood (59) and not without controversy (38, 39). SSR stress-related effects are frequently interpreted via evolutionary models as a process of “culling the weak” so that the fittest procreate (37) as well as supported by DOHaD research on prenatal programming which shows early male vulnerability proximal to the in utero exposure (65). Potential biological explanations for the decreased SSR in the presence of maternal stress abound. For instance, male fetuses are slower to mature than female fetuses, resulting in a prolonged period of vulnerability (40), and X-linked genes that may confer protection by fostering adaptability to survive (66, 67) are expressed at higher levels in the female placenta (40). Additionally, recent work showing a male bias in maternal-stress-linked newborn telomere shortening in cord blood suggests a possible pathway via accelerated aging and cell senescence (43, 68). Finally, masculinization of the brain typically cooccurs with the sensitive period during which maternal immune activation can negatively influence brain development (69). As with all biomedical research findings, these SSR results would not necessarily apply uniformly to all individuals.
Findings that the 3 social support indicators most strongly discriminated the groups and that total social support was associated with increased likelihood of having a male were unexpected. PSYG had the lowest levels of all 3 forms of social support (others with whom to talk, spend time, and on whom to rely for material help), which is consistent with studies suggesting that mental health problems and low social support highly correlate (70–72). However, PHSG also had lower social support compared to HG, which is consistent with data suggesting that high AL is associated with unsatisfactory social relationships and support (73). Lack of social support during pregnancy is associated with poor birth outcomes as well as perinatal depression (44, 74–77). Preclinical (78) and translational studies (79, 80) indicate that social isolation hampers effective hypothalamic–pituitary–adrenal-axis regulation, reduces brain-derived neurotrophic factor expression, and amplifies proinflammatory processes identified at the molecular level—each potentially involved in maternal mental and physical health and contributing to offspring outcomes (81). From the perspective of solution-oriented research (82), these findings suggest that social support is a modifiable target for clinical engagement that could benefit women and their future children (83).
The PHSG had elevated rates of PTB compared to the HG (22% versus 5%), a striking difference as the PTB rate in the PHSG was more than twice the national average of 9.9% (84) and PHSG infants were born 1.5 wk earlier on average than HG infants. PTB is a significant cause of infant mortality (85) and increased physical and mental morbidity (11). Even among those born at term (37 wk or later), subsequent childhood academic achievement is more advanced among those born closer to 40 wk (86). Population-level data indicate that disease burden among 3- to 5-y-old children is significantly higher among those born preterm or early term (37 to 38 wk) compared to those born full-term (87); thus, a difference of 1.5 wk can impact important health and educational outcomes. In this sample of healthy women, routinely measured indicators of physical stress revealed a phenotype of women whose fetuses were at higher risk of PTB. Prior null results of significant associations between maternal prenatal stress and birth outcomes (44, 87) may reflect minimal inclusion of physiological or physical indicators of stress, potentially forming a unique maternal prenatal stress group (9). The present findings indicate that an AL approach to characterizing maternal prenatal stress should be included in studies to fully capture the varied manifestations of stress, a matter of urgency in the context of the racial and ethnic disparities on the rise in the US maternal morbidity and mortality rates and poor birth outcomes (89–91).
Results that third-trimester fetuses in the PHSG relative to the HG evidenced reductions in fetal heart rate and movement coupling may suggest slower central nervous system development, as greater third-trimester coupling has been associated with faster auditory-evoked responses among newborns measured within 2 wk of birth (92). Results suggesting that third-trimester PSYG fetuses had a heart rate increase during maternal exposure to an acute laboratory stressor while HG fetuses had a (nonsignificant) heart rate decrease are consistent with our prior studies showing greater FHR reactivity among fetuses of women with elevated trait anxiety and diagnosed anxiety and depression (15, 16, 20, 93).
For both fetal neurodevelopmental outcomes examined, trends for sex differences emerged consistent with heightened female adaptation to altered prenatal environmental conditions, which frequently is hypothesized as functioning to ensure survival (37, 65–67, 81). Specifically, female fetuses in both stress groups, though not males, showed reduced coupling relative to HG females, and for FHR reactivity the overall pattern held for females but not for males. Alternatively, males born in the 2 stress groups may be especially robust, able to accommodate and develop despite altered prenatal environmental conditions.
Social determinants of health that were most prevalent in the PSYG, including minority status, low income, and financial need, are consistent with numerous studies suggesting that socioeconomic disparities predict poor perinatal mental health (94–96). Additionally, relative to the other groups, PSYG women reported more early life adversity, which has been implicated in adult psychopathology (97), including for pregnant women (98, 99). Maternal exposure to childhood adversity may alter gestational biology to negatively affect women as well as the health of future children (57, 58, 100–103), while greater childhood emotional support has been associated with positive offspring outcomes (e.g., longer newborn telomere length) (43). Findings underscore the need for prevention and early intervention for adversity to improve women’s future maternal health and the heath of the next generation.
Limitations of the current study are as follows. First, several racial/ethnic groups were underrepresented in the current sample and will be important to include in future studies to ensure generalizability of these observations. Second, although each pregnancy was well-characterized using multiple methods of assessment across a wide range of psychosocial and AL factors, the sample sizes for the 2 stress groups were relatively small and the sample sizes for subgroup analyses (e.g., sex differences) and fetal neurodevelopment analyses were even smaller; thus, results should be interpreted with caution. Third, a large number of analyses were run and most P values would not hold up to correction for multiple tests so results must be replicated to ensure confidence in findings. Fourth, participants were women with singleton, largely healthy pregnancies; therefore, findings may not generalize to women with higher-risk pregnancies. Fourth, although this data-driven procedure yielded an important means of assessing maternal prenatal stress, we did not include all possible indicators of maternal stress (e.g., inflammation, oxidative stress, and telomere length) and results could differ based on the inclusion of other variables. Fifth, preconception stress variables were not measured and may be important to include in future studies. Sixth, although we included a single item on whether fathers were involved, future work should attempt to more comprehensively characterize the role of fathers in maternal prenatal stress and perinatal outcomes.
Results of the current study highlight a number of avenues for future research. First, although the current study determined latent profiles based on markers assessed early in pregnancy because we anticipated that early identification of those at risk for poorer outcomes would allow for time to implement meaningful interventions to change outcomes, future work could examine how stress markers and stress group membership changes over the course of pregnancy to provide a more nuanced understanding of these malleable stress markers and the best point at which to intervene. Second, consistent with the recent emphasis to leverage randomized control trials to experimentally test findings from observational studies (e.g., ClinicalTrials.gov identifier NCT03011801), these findings provide stress, anxiety, depression, and subclinical health parameters to guide clinical intervention studies aimed at mitigating maternal prenatal stress effects on reproductive outcomes. Although replication is necessary, this study lays the groundwork for the development of a standardized data collection tool to index maternal prenatal stress using routinely collected information such as the markers assessed here that could be an important next step in advancing maternal prenatal health screening. Third, although outside the scope of the current study, to understand whether a count of risk factors or certain combinations of risk factors better predict perinatal outcomes, it could be useful to compare an AL approach to operationalizing maternal stress to our data-driven, person-centered method.
The current study leveraged a data-driven approach to maternal stress during pregnancy that bridges the AL and psychosocial literatures relevant to less optimal pregnancy outcomes and children’s future well-being. The key contributions of this study are the identification of 2 maternal prenatal stress phenotypes, one characterized by clinically significant psychological stress and the other by subclinical physical stress, which were associated with fetal and birth outcomes, and to which males seem more vulnerable— including which sex is born, risk of PTB, and variations in fetal neurodevelopment relevant to future functioning. Social support—a potential clinical target—is a key factor defining stress phenotypes and should be included in future DOHaD studies. Maternal mental health matters, and the womb is an influential first home.
Materials and Methods
Participants.
Pregnant women between the ages 18 and 45 y participated in the study between September 2011 and September 2016. They were recruited in the first or second trimester through the Departments of Obstetrics and Gynecology at Columbia University Medical Center (CUMC) and flyers posted in the CUMC vicinity. All had a healthy pregnancy at the time of recruitment. Participants were excluded if they acknowledged smoking or use of recreational drugs, lacked fluency in English, were pregnant with multiples, or reported use of the following: nitrates, steroids, beta blockers, triptans, or psychiatric medications. Inclusion criteria included self-reported good health of self and fetus and current enrollment in standard prenatal care.
We enrolled a total of 187 participants as part of a study designed to observe mood and stress during pregnancy. All participants provided written informed consent, and all procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute/CUMC. The final dataset included all 187 participants who had a mean age of 29.6 (SD = 6.2; range = 19.5 to 44.9 y). More than two-thirds of the sample (69%; n = 129) identified as Hispanic/Latina. The mean number of years of education was 14.9 (SD = 3.1), and one-third reported a family income under $25,000. Nearly half reported being on Medicaid (n = 93), 43% (n = 81) reported Women Infant and Child (WIC), and 25% (n = 46) reported other public assistance. Three-quarters of the sample (n = 141) had been pregnant previously.
Procedures.
Participants had 3 assessment sessions at 12 to 22 wk, 23 to 28 wk, and 34 to 36 wk of pregnancy. Study sessions consisted of maternal mood assessment (self-report and interview based), lifestyle habits, as well as initiation of the collection of 24-h ambulatory BP, 48-h salivary cortisol, and mood using a PDA. Only data from the first session (12 to 22 wk) were included as indicators to generate stress groups. Fetal assessments occurred at the third session. For fetal testing, participants remained in a semirecumbent position and completed a 5-min resting period (baseline, on which the coupling variable was based) and a 5-min validated laboratory stressor task [the Stroop color–word matching task (104) or 5-min Respbar task (a controlled breathing exercise following a computer prompt)]. The order of presentation of Stroop and Respbar tasks was counterbalanced across participants and controlled for in analyses of FHR reactivity defined as FHR during baseline – FHR during the stressor task. Birth outcomes were obtained from the medical records.
LPA Indicator Variables.
Demographic characteristics.
Maternal age was self-reported at enrollment.
Stressors and stress perceptions.
Happy and unhappy pregnancy experiences were assessed via the Pregnancy Experiences Scale (105), a 41-item measure of maternal exposures to daily, ongoing uplifts and hassles specific to pregnancy (e.g., thinking about nursery arrangements, how much baby is moving). Participants rate how happy, uplifted, or positive or how unhappy, negative, or upset they feel for each on a scale from 0 (not at all) to 3 (a great deal). Perceived stress was assessed via the Perceived Stress Scale (106), a 14-item measure of the degree to which life situations during the previous month are perceived as stressful. Participants respond to items on a 5-point Likert scale ranging from 0 (never) to 4 (very often), and higher scores reflect greater perceived stress. The scale has adequate reliability (Cronbach’s alphas of 0.84 to 0.86) (106).
Mental health indicators.
Negative mood states were collected via a PDA every 30 min for a 24-h period timed to begin with the laboratory assessment. Using a 5-point Likert scale ranging from 1 (low) to 5 (high), participants rated themselves according to 4 negative mood states: angry, frustrated, irritated, and stressed. A weight was created by dividing the number of diary entries at each session by the total number of diary entries across all study sessions. The average for the 4 items was calculated for each participant and multiplied by the weight at that session. Participants were incentivized to provide ratings, earning $0.10 per rating. Depression and anxiety were measured via the HRSD (107) and the HRSA (108), respectively, administered during laboratory visits. The validity and reliability of the HRSD and HRSA are well established (109–111). Current symptoms of PTSD were measured via the PSS Interview (PSS-I) during laboratory visits. The PSS-I (112, 113) is a semistructured self-report interview consisting of 17 items that correspond to the DSM-IV symptoms of PTSD. The PSS-I has been found to be reliable and valid in civilian trauma survivors (113).
Physical indicators.
Prepregnancy BMI was calculated from participants’ self-reported weight in pounds and measured height.
Ambulatory BP, heart rate, and mean arterial pressure.
Participants were outfitted with a Spacelabs Healthcare 90207 ABP Monitor (Spacelabs Healthcare), an instrument with documented reliability, validity (51), and acceptability (114) in pregnant populations, with which measures of ambulatory systolic and diastolic BP, mean arterial pressure, and heart rate were collected every 30 min over the subsequent 24-h period. During instrumentation, cuff size was adjusted for upper arm dimensions, and 2 readings were compared to an initial measurement via sphygmomanometer with the requirement that readings fall within 10 mmHg of one another.
Maternal diurnal cortisol.
Forty-eight hour salivary cortisol collection began during the first day of the study session. Subsequent samples on the second day were collected at waking; at 45 min, 2.5 h, 3.5 h, and 8 h after waking; and at 10:00 PM or before going to bed. The Medication Event Monitoring System track cap (Aardex) was used to monitor collection times. After collection, cotton was placed in a Salivette tube (Sarstedt), returned to the laboratory, and frozen at 280 °C. Cortisol was measured by ultraperformance liquid chromatography–tandem mass spectrometry assay developed by the Irving Institute for Clinical and Translational Research, CUMC. The lower limit of quantitation was 100 pg/mL. Intraassay and interassay coefficients of variation are less than 3.4% and 3.6% over the analytical measurement range (100 to 50,000 pg/mL). The diurnal slope was calculated using linear curve fitting based on the least squared error method for the participants with more than 2 valid measurements after 45 min from wakening up.
Lifestyle indicators.
Nutrition data regarding macronutrients (overall calories as well as calories from protein, fat, and carbohydrates) and added sugar were acquired via the Automated Self-Administered 24 h Dietary Recall (ASA24). The ASA24 is an internet-based questionnaire provided by the National Cancer Institute (115) that asks participants to recall food intake over the preceding 24 h using detailed probes and portion-size food images. The ASA24 generates estimates using 3 databases: the USDA’s Food and Nutrient Database for Dietary Surveys, MyPyramid Equivalents Database (MPED), and the Center for Nutrition Policy and Promotion’s MPED Addendum. Physical activity was collected via the PDA every 30 min for a 24-h period, timed to begin with the laboratory assessment. Participants were asked how active they were in the past 30 min on a scale from 0 (not active) to 3 (very active). The total physical activity score used in analyses was an average of scores from the first visit.
Social support.
Perceived availability of social support was measured by 3 of the 4 Interpersonal Support Evaluation List (ISEL) subscales (116): tangible support, appraisal support, and belonging support. Tangible support refers to material aid, appraisal support refers to emotional support, and belonging support is the perception of being a member of a social group (117). Each subscale contains 10 items with 4 response options: “definitely true,” “probably true,” “probably false,” or “definitely false.” Higher scores reflect greater perceived levels of support. The ISEL has shown good reliability and validity (116–118).
Outcome Measures.
Fetal behavioral assessment.
For the fetal assessment, participants were in a semirecumbent position as fetal movement (FM) and FHR were acquired on the first day of the second and third sessions. Data were obtained using a Toitu MT 325 fetal actocardiograph (Toitu Co.). The Toitu detects FHR and FM via a single transabdominal Doppler transducer and processes this signal through a series of filters. The detection of FM uses these filters to remove frequency components of the Doppler signal that are associated with FHR and maternal somatic activity and has been shown to be reliable (119–122). FHR and FM data were collected from the output port of the Toitu MT 325 and digitized at 50 Hz using a 16-bit A/D card (National Instruments 16XE50). Data were analyzed offline using custom MATLAB programs (http://www.math-works.com/) developed for this project. Two fetal variables were of interest: mean FHR during maternal tasks compared to baseline (FHR reactivity) and FM/FHR cross-correlation (“coupling”). As a first step in preprocessing, FHR below 80 beats per minute (bpm) or above 200 bpm was linearly interpolated and then low-pass-filtered at 3 Hz using a 16-point finite impulse response filter. The mean of the resulting FHR was taken over noninterpolated values. Filtered FHR was further examined for artifact in the following way: Times at which the absolute sample-to-sample (20 ms) change in FHR exceeded 5 bpm were found and FHR was marked as artifact until it returned to within 5 bpm of the previous value. The resultant gaps were linearly interpolated. Because the FHR/FM coupling of interest occurs on time scales fewer than 4 min, we estimated cross-correlation of FHR and FM for the 5-min period by averaging cross-correlations taken for 4-min overlapping (50%) segments. This is akin to averaging over fast Fourier transformation in the Welch method of power spectrum estimation (123). FHR–FM cross-correlations were computed as follows: 1) FHR and FM over the entire record were first band-pass-filtered between 0.002 and 0.05 Hz using a 400-point FIR filter; 2) FHR was further smoothed by subtracting a local regression of 10% span; 3) decelerations of FHR were set to zero; and 4) FM was z-scored (124). Before taking the cross-correlation for each 4-min segment, a Hanning window was applied. Based upon hundreds of similar studies (28), our group has developed criteria used to screen data for artifact. Specifically, individual segments were excluded from the average if any of 3 conditions applied: 1) Total interpolated FHR exceeded 50% of segment length; 2) an interpolated gap of greater than 30 s occurred; or 3) cumulative time of interpolated gaps between 2 s and 30 s exceeded 1 min. As an aid to postprocessing, the mean percentage of interpolated data within each segment and the number of nonexcluded segments were recorded. Finally, as a further control for artifact, any segment with maximum cross-correlation at a lag of less than −15 s or greater than 0 s was not included in the average spectral power or cross-correlation calculation because true physiological FHR–FM coupling is within this range, with FM leading FHR (125). For fetal neurodevelopmental measures, any data points more than 1.5 interquartile ranges below the first quartile or above the third quartile were considered as outliers and removed from statistical analyses. Twenty-eight women did not attend the study session at 34 to 36 wk, resulting in missing fetal data, while 23 to 60 fetuses had missing data due to quality issues depending on the specific fetal neurodevelopmental assessment.
Birth outcomes.
Medical records were coded for delivery type (vaginal, assisted vaginal, caesarian); GA (weeks); birth weight (grams); pre-, peri-, or postnatal complications (e.g., infection, preeclampsia, vascular problems, or diabetes mellitus); NICU involvement (yes/no); and baby’s and mother’s length of hospital stay (days). GA and birth weight were correlated (r = 0.66, P < 0.001); therefore, sensitivity analyses were run to correct birth weight for GA. Specifically, standardized residuals from a linear regression model predicting birth weight from GA were used as a dependent variable in an ANOVA with latent profile groups as the independent variable; significant differences were not observed.
Social determinants of health related to profile membership.
Participants self-reported Hispanic ethnicity, years of education, household income, and receipt of Medicaid, WIC, and other public assistance. They also reported whether the baby’s father was involved, whether this was their first pregnancy, and the number of children, miscarriages, and abortions they had. Participants reported on their own history of 5 types of childhood maltreatment (emotional, sexual, and physical abuse as well as emotional and physical neglect) using the Childhood Trauma Questionnaire—Short Form (CTQ-SF) (126). Respondents are asked to rate on a 5-point Liker-type scale (1 = never and 5 = very often) how frequently they experienced certain events. Past research demonstrates that the CTQ-SF has good sensitivity and excellent convergent and discriminant validity (127–129). Participants also reported on their relationships with their own mothers and fathers using the Care and Overprotection subscales of the Parental Bonding Instrument (PBI) (130), a 25-item scale describing parents’ behaviors during their childhood from “very like,” “moderately like,” “moderately unlike,” and “very unlike.” The PBI has shown good reliability and validity (131, 132).
Statistical Considerations.
Missing data.
Although this study was designed to assess women once during each trimester, we allowed women to enroll later than the first trimester and thus have some missing data on first assessment LPA indicators. Those who were missing at least one first trimester data point (n = 79) did not differ from those with complete first assessment data on age, race/ethnicity, years of education, or income. Missing data were handled via full information maximum likelihood estimation, which is asymptotically equivalent to multiple imputation (133). There were no demographic differences in mean week of entry into the study by latent profile, F(2,183) = 1.54, P = 0.22, further supporting that missing data did not influence the LPA.
Model fit statistics.
The Akaike information criterion and BIC are relative fit statistics wherein lower values indicate a better-fitting model (134). Entropy values indicate how well the model identifies separate latent profiles; values above 0.80 provide good separation between profiles (45). The L-M-R test is a comparative fit statistic; significant values indicate how many profiles should be extracted by testing the parsimony of the current model against the model with one less profile (135). Class probability statistics summarize how well participants can be classified into one group or another based on a given model; values closer to 1 indicate better classification (136).
Supplementary Material
Acknowledgments
We thank all of the women for their participation in the study as well as Rachel Yehuda, PhD, for comments on earlier versions of the manuscript. We also acknowledge NIH Grant R01MH092580 awarded to Principal Investigators C.M., F.A.C., and B.T. for funding the project.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See Commentary on page 23877.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905890116/-/DCSupplemental.
References
- 1.Kim D. R., Bale T. L., Epperson C. N., Prenatal programming of mental illness: Current understanding of relationship and mechanisms. Curr. Psychiatry Rep. 17, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale T. L., et al. , Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker D. J., The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc. Biol. Sci. 262, 37–43 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Bronson S. L., Bale T. L., The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41, 207–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan T. K., Role of the placenta in preterm birth: A review. Am. J. Perinatol. 33, 258–266 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Orzack S. H., et al. , The human sex ratio from conception to birth. Proc. Natl. Acad. Sci. U.S.A. 112, E2102–E2111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomans E. M., et al. , Psychosocial stress during pregnancy is related to adverse birth outcomes: Results from a large multi-ethnic community-based birth cohort. Eur. J. Public Health 23, 485–491 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Gemmill A., et al. , Association of preterm births among US latina women with the 2016 presidential election. JAMA Netw. Open 2, e197084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman M. C., Mazzoni S. E., Wagner B. D., Laudenslager M. L., Ross R. G., Measures of maternal stress and mood in relation to preterm birth. Obstet. Gynecol. 127, 545–552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadhwa P. D., Sandman C. A., Garite T. J., The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Prog. Brain Res. 133, 131–142 (2001). [DOI] [PubMed] [Google Scholar]
- 11.D’Onofrio B. M., et al. , Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry 70, 1231–1240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell K. J., Glover V., Barker E. D., O’Connor T. G., The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev. Psychopathol. 26, 393–403 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Laplante D. P., Brunet A., Schmitz N., Ciampi A., King S., Project Ice Storm: Prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J. Am. Acad. Child Adolesc. Psychiatry 47, 1063–1072 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Walder D. J., et al. , Prenatal maternal stress predicts autism traits in 6(1/2) year-old children: Project Ice Storm. Psychiatry Res. 219, 353–360 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Monk C., et al. , Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Dev. Psychobiol. 53, 221–233 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monk C., et al. , Fetal heart rate reactivity differs by women’s psychiatric status: An early marker for developmental risk? J. Am. Acad. Child Adolesc. Psychiatry 43, 283–290 (2004). [DOI] [PubMed] [Google Scholar]
- 17.DiPietro J. A., Hodgson D. M., Costigan K. A., Hilton S. C., Johnson T. R., Fetal neurobehavioral development. Child Dev. 67, 2553–2567 (1996). [PubMed] [Google Scholar]
- 18.Sandman C. A., et al. , Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Dev. Neurosci. 25, 41–49 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Dieter J. N. I., et al. , Maternal depression and increased fetal activity. J. Obstet. Gynaecol. 21, 468–473 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Posner J., et al. , Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry 6, e935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham A. M., et al. , Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 Years of age. Biol. Psychiatry 83, 109–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham A. M., et al. , Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry 85, 172–181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen B. S., Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 840, 33–44 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Juster R. P., McEwen B. S., Lupien S. J., Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 35, 2–16 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Barrett E. S., et al. , Allostatic load, a measure of chronic physiological stress, is associated with pregnancy outcomes, but not fertility, among women with unexplained infertility. Hum. Reprod. 33, 1757–1766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson D. M., et al. , Allostatic load and preterm birth. Int. J. Mol. Sci. 16, 29856–29874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hux V. J., Catov J. M., Roberts J. M., Allostatic load in women with a history of low birth weight infants: The national health and nutrition examination survey. J. Women’s Health 23, 1039–1045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle C., et al. , Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Dev. Psychobiol. 57, 607–625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duthie L., Reynolds R. M., Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 98, 106–115 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Nations United,“Live births by age of mother, sex of the child and urban/rural residence: latest available year, 1995–2004. Table 10” in Demographic Yearbook (United Nations, New York, 2004). [Google Scholar]
- 31.Grech V., Zammit D., The President Kennedy assassination and the male to female birth ratio. Early Hum. Dev. 103, 119–121 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Catalano R., Bruckner T., Gould J., Eskenazi B., Anderson E., Sex ratios in California following the terrorist attacks of September 11, 2001. Hum. Reprod. 20, 1221–1227 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Catalano R., Bruckner T., Marks A. R., Eskenazi B., Exogenous shocks to the human sex ratio: The case of September 11, 2001 in New York City. Hum. Reprod. 21, 3127–3131 (2006). [DOI] [PubMed] [Google Scholar]
- 34.James W. H., Proximate causes of the variation of the human sex ratio at birth. Early Hum. Dev. 91, 795–799 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Chason R. J., et al. , Preconception stress and the secondary sex ratio: A prospective cohort study. Fertil. Steril. 98, 937–941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catalano R., Bruckner T., Anderson E., Gould J. B., Fetal death sex ratios: A test of the economic stress hypothesis. Int. J. Epidemiol. 34, 944–948 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Sandman C. A., Glynn L. M., Davis E. P., Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 75, 327–335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcox A. J., Baird D. D., Invited commentary: Natural versus unnatural sex ratios–A quandary of modern times. Am. J. Epidemiol. 174, 1332–1334, discussion 1335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnettler S., Klüsener S., Economic stress or random variation? Revisiting German reunification as a natural experiment to investigate the effect of economic contraction on sex ratios at birth. Environ. Health 13, 117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bale T. L., The placenta and neurodevelopment: Sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 18, 459–464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeitlin J., Ancel P. Y., Larroque B., Kaminski M.; EPIPAGE Study , Fetal sex and indicated very preterm birth: Results of the EPIPAGE study. Am. J. Obstet. Gynecol. 190, 1322–1325 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Zeitlin J., et al. , Fetal sex and preterm birth: Are males at greater risk? Hum. Reprod. 17, 2762–2768 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Bosquet Enlow M., et al. , Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology 95, 74–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grobman W. A., et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b) Network , Racial disparities in adverse pregnancy outcomes and psychosocial stress. Obstet. Gynecol. 131, 328–335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berlin K. S., Williams N. A., Parra G. R., An introduction to latent variable mixture modeling (part 1): Overview and cross-sectional latent class and latent profile analyses. J. Pediatr. Psychol. 39, 174–187 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Lanza S. T., Cooper B. R., Latent class analysis for developmental research. Child Dev. Perspect. 10, 59–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanza S. T., Rhoades B. L., Latent class analysis: An alternative perspective on subgroup analysis in prevention and treatment. Prev. Sci. 14, 157–168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giesbrecht G. F., Campbell T., Letourneau N., Kooistra L., Kaplan B.; APrON Study Team , Psychological distress and salivary cortisol covary within persons during pregnancy. Psychoneuroendocrinology 37, 270–279 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Glover D. A., Stuber M., Poland R. E., Allostatic load in women with and without PTSD symptoms. Psychiatry 69, 191–203 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton E. F., Hauspurg A., Caritis S. N., Powers R. W., Catov J. M., Maternal outcomes associated with lower range stage 1 hypertension. Obstet. Gynecol. 132, 843–849 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown M. A., et al. , Ambulatory blood pressure monitoring in pregnancy: What is normal? Am. J. Obstet. Gynecol. 178, 836–842 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Elliott-Sale K. J., Graham A., Hanley S. J., Blumenthal S., Sale C., Modern dietary guidelines for healthy pregnancy; maximising maternal and foetal outcomes and limiting excessive gestational weight gain. Eur. J. Sport Sci. 19, 62–70 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Evans L. M., Myers M. M., Monk C., Pregnant women’s cortisol is elevated with anxiety and depression–But only when comorbid. Arch. Women Ment. Health 11, 239–248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji S., et al. , Validity of depression rating scales during pregnancy and the postpartum period: Impact of trimester and parity. J. Psychiatr. Res. 45, 213–219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misri S., et al. , Antenatal depression and anxiety affect postpartum parenting stress: A longitudinal, prospective study. Can. J. Psychiatry 55, 222–228 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Li H. D., Xu Q. S., Liang Y. Z., libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemometr. Intell. Lab 176, 34–43 (2018). [Google Scholar]
- 57.Gluckman P. D., Hanson M. A., Cooper C., Thornburg K. L., Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messer L. C., Boone-Heinonen J., Mponwane L., Wallack L., Thornburg K. L., Developmental programming: Priming disease susceptibility for subsequent generations. Curr. Epidemiol. Rep. 2, 37–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kraemer S., The fragile male. BMJ 321, 1609–1612 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein A. D., Barnett P. G., Sellen D. W., Maternal undernutrition and the sex ratio at birth in Ethiopia: Evidence from a national sample. Proc. Biol. Sci. 271 (suppl. 3), S37–S39 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cameron E. Z., Facultative adjustment of mammalian sex ratios in support of the Trivers-Willard hypothesis: Evidence for a mechanism. Proc. Biol. Sci. 271, 1723–1728 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheldon B. C., Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Grant V. J., Could maternal testosterone levels govern mammalian sex ratio deviations? J. Theor. Biol. 246, 708–719 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Trivers R. L., Willard D. E., Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92 (1973). [DOI] [PubMed] [Google Scholar]
- 65.Hodes G. E., Epperson C. N., Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatry 86, 421–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clifton V. L., Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31 (suppl), S33–S39 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Eriksson J. G., Kajantie E., Osmond C., Thornburg K., Barker D. J., Boys live dangerously in the womb. Am. J. Hum. Biol. 22, 330–335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin J., et al. , In vitro proinflammatory gene expression predicts in vivo telomere shortening: A preliminary study. Psychoneuroendocrinology 96, 179–187 (2018). [DOI] [PubMed] [Google Scholar]
- 69.McCarthy M. M., Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology 44, 38–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lancaster C. A., et al. , Risk factors for depressive symptoms during pregnancy: A systematic review. Am. J. Obstet. Gynecol. 202, 5–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morikawa M., et al. , Relationship between social support during pregnancy and postpartum depressive state: A prospective cohort study. Sci. Rep. 5, 10520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nylen K. J., O’Hara M. W., Engeldinger J., Perceived social support interacts with prenatal depression to predict birth outcomes. J. Behav. Med. 36, 427–440 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Brooks K. P., et al. , Social relationships and allostatic load in the MIDUS study. Health Psychol. 33, 1373–1381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Séguin L., Potvin L., St-Denis M., Loiselle J., Chronic stressors, social support, and depression during pregnancy. Obstet. Gynecol. 85, 583–589 (1995). [DOI] [PubMed] [Google Scholar]
- 75.Biaggi A., Conroy S., Pawlby S., Pariante C. M., Identifying the women at risk of antenatal anxiety and depression: A systematic review. J. Affect. Disord. 191, 62–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoffman S., Hatch M. C., Stress, social support and pregnancy outcome: A reassessment based on recent research. Paediatr. Perinat. Epidemiol. 10, 380–405 (1996). [DOI] [PubMed] [Google Scholar]
- 77.Webster J., Linnane J. W., Dibley L. M., Pritchard M., Improving antenatal recognition of women at risk for postnatal depression. Aust. N. Z. J. Obstet. Gynaecol. 40, 409–412 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Faraji J., et al. , Lack of social support raises stress vulnerability in rats with a history of ancestral stress. Sci. Rep. 7, 5277 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cacioppo J. T., Cacioppo S., Capitanio J. P., Cole S. W., The neuroendocrinology of social isolation. Annu. Rev. Psychol. 66, 733–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cole S. W., et al. , Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology 62, 11–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monk C., Lugo-Candelas C., Trumpff C., Prenatal developmental origins of future psychopathology: Mechanisms and pathways. Annu. Rev. Clin. Psychol. 15, 317–344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson T. N., Sirard J. R., Preventing childhood obesity: A solution-oriented research paradigm. Am. J. Prev. Med. 28 (suppl. 2), 194–201 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Ickovics J. R., et al. , Effects of group prenatal care on psychosocial risk in pregnancy: Results from a randomised controlled trial. Psychol. Health 26, 235–250 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin J. A., Hamilton B. E., Osterman M. J. K., Driscoll A. K., Drake P., Births: Final data for 2016. Natl. Vital Stat. Rep. 67, 1–55 (2018). [PubMed] [Google Scholar]
- 85.Callaghan W. M., MacDorman M. F., Rasmussen S. A., Qin C., Lackritz E. M., The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 118, 1566–1573 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Noble K. G., Fifer W. P., Rauh V. A., Nomura Y., Andrews H. F., Academic achievement varies with gestational age among children born at term. Pediatrics 130, e257–e264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyle E. M., et al. , Effects of gestational age at birth on health outcomes at 3 and 5 years of age: Population based cohort study. BMJ 344, e896 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lima S. A. M., et al. , Is the risk of low birth weight or preterm labor greater when maternal stress is experienced during pregnancy? A systematic review and meta-analysis of cohort studies. PLoS One 13, e0200594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller G. E., et al. , Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav. Immun. 64, 276–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dominguez T. P., Dunkel-Schetter C., Glynn L. M., Hobel C., Sandman C. A., Racial differences in birth outcomes: The role of general, pregnancy, and racism stress. Health Psychol. 27, 194–203 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Louis J. M., Menard M. K., Gee R. E., Racial and ethnic disparities in maternal morbidity and mortality. Obstet. Gynecol. 125, 690–694 (2015). [DOI] [PubMed] [Google Scholar]
- 92.DiPietro J. A., et al. , Prenatal antecedents of newborn neurological maturation. Child Dev. 81, 115–130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monk C., et al. , Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Dev. Psychobiol. 36, 67–77 (2000). [PubMed] [Google Scholar]
- 94.Fisher J., et al. , Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: A systematic review. Bull. World Health Organ. 90, 139G–149G (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muzik M., Rosenblum K., Motherhood in the Face of Trauma: Pathways Towards Healing and Growth (Springer, 2018). [Google Scholar]
- 96.Lu M. C., We can do better: Improving perinatal health in America. J. Women’s Health 19, 569–574 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Shonkoff J. P., Garner A. S.; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics , The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129, e232–e246 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Choi K. W., Sikkema K. J., Childhood maltreatment and perinatal mood and anxiety disorders: A systematic review. Trauma Violence Abuse 17, 427–453 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Meltzer-Brody S., Boschloo L., Jones I., Sullivan P. F., Penninx B. W., The EPDS-lifetime: Assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Arch. Women Ment. Health 16, 465–473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buss C., et al. , Intergenerational transmission of maternal childhood maltreatment exposure: Implications for fetal brain development. J. Am. Acad. Child Adolesc. Psychiatry 56, 373–382 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moog N. K., et al. , Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol. Psychiatry 83, 120–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yehuda R., Lehrner A., Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry 17, 243–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bowers M. E., Yehuda R., Intergenerational transmission of stress in humans. Neuropsychopharmacology 41, 232–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Renaud P., Blondin J.-P., The stress of Stroop performance: Physiological and emotional responses to color-word interference, task pacing, and pacing speed. Int. J. Psychophysiol. 27, 87–97 (1997). [DOI] [PubMed] [Google Scholar]
- 105.DiPietro J. A., Ghera M. M., Costigan K., Hawkins M., Measuring the ups and downs of pregnancy stress. J. Psychosom. Obstet. Gynaecol. 25, 189–201 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Cohen S., Kamarck T., Mermelstein R., A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396 (1983). [PubMed] [Google Scholar]
- 107.Hamilton M., A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamilton M., The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55 (1959). [DOI] [PubMed] [Google Scholar]
- 109.Maier W., Buller R., Philipp M., Heuser I., The Hamilton anxiety scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 14, 61–68 (1988). [DOI] [PubMed] [Google Scholar]
- 110.Ramos-Brieva J. A., Cordero-Villafafila A., A new validation of the Hamilton rating scale for depression. J. Psychiatr. Res. 22, 21–28 (1988). [DOI] [PubMed] [Google Scholar]
- 111.Trajković G., et al. , Reliability of the Hamilton rating scale for depression: A meta-analysis over a period of 49 years. Psychiatry Res. 189, 1–9 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Foa E. B., Riggs D. S., Dancu C. V., Rothbaum B. O., Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J. Trauma. Stress 6, 459–473 (1993). [Google Scholar]
- 113.Foa E. B., Tolin D. F., Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale. J. Trauma. Stress 13, 181–191 (2000). [DOI] [PubMed] [Google Scholar]
- 114.Walker S. P., Permezel M. J., Brennecke S. P., Tuttle L. K., Higgins J. R., Patient satisfaction with the SpaceLabs 90207 ambulatory blood pressure monitor in pregnancy. Hypertens. Pregnancy 23, 295–301 (2004). [DOI] [PubMed] [Google Scholar]
- 115.Subar A. F., et al. , The automated self-administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 112, 1134–1137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cohen S., Hoberman H. M., Positive events and social supports as buffers of life change stress. J. Appl. Soc. Psychol. 13, 99–125 (1983). [Google Scholar]
- 117.Brookings J. B., Bolton B., Confirmatory factor analysis of the interpersonal support evaluation list. Am. J. Community Psychol. 16, 137–147 (1988). [DOI] [PubMed] [Google Scholar]
- 118.Cohen S., Wills T. A., Stress, social support, and the buffering hypothesis. Psychol. Bull. 98, 310–357 (1985). [PubMed] [Google Scholar]
- 119.Besinger R. E., Johnson T. R., Doppler recording of fetal movement: Clinical correlation with real-time ultrasound. Obstet. Gynecol. 74, 277–280 (1989). [PubMed] [Google Scholar]
- 120.DiPietro J. A., et al. , Fetal neurobehavioral development: A tale of two cities. Dev. Psychol. 40, 445–456 (2004). [DOI] [PubMed] [Google Scholar]
- 121.Dipietro J. A., Irizarry R. A., Costigan K. A., Gurewitsch E. D., The psychophysiology of the maternal-fetal relationship. Psychophysiology 41, 510–520 (2004). [DOI] [PubMed] [Google Scholar]
- 122.DiPietro J. A., Costigan K. A., Pressman E. K., Fetal movement detection: Comparison of the Toitu actograph with ultrasound from 20 weeks gestation. J. Matern. Fetal Med. 8, 237–242 (1999). [DOI] [PubMed] [Google Scholar]
- 123.Bendat J. S., Piersol A. G., Random data analysis and measurement procedures. Meas. Sci. Technol. 11, 1825 (2000). [Google Scholar]
- 124.Dipietro J. A., Irizarry R. A., Hawkins M., Costigan K. A., Pressman E. K., Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. Am. J. Obstet. Gynecol. 185, 1421–1428 (2001). [DOI] [PubMed] [Google Scholar]
- 125.DiPietro J. A., Hodgson D. M., Costigan K. A., Hilton S. C., Johnson T. R., Development of fetal movement–Fetal heart rate coupling from 20 weeks through term. Early Hum. Dev. 44, 139–151 (1996). [DOI] [PubMed] [Google Scholar]
- 126.Bernstein D. P., Fink L., Childhood Trauma Questionnaire: A Retrospective Self-Report Manual (The Psychological Corporation, San Antonio, TX, 1998). [Google Scholar]
- 127.Bernstein D. P., Ahluvalia T., Pogge D., Handelsman L., Validity of the childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36, 340–348 (1997). [DOI] [PubMed] [Google Scholar]
- 128.Bernstein D. P., et al. , Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136 (1994). [DOI] [PubMed] [Google Scholar]
- 129.Scher C. D., Stein M. B., Asmundson G. J., McCreary D. R., Forde D. R., The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. J. Trauma. Stress 14, 843–857 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Parker G., Tupling H., Brown L. B., A parental bonding instrument. Br. J. Med. Psychol. 52, 1–10 (1979). [Google Scholar]
- 131.Wilhelm K., Parker G., Reliability of the parental bonding instrument and intimate bond measure scales. Aust. N. Z. J. Psychiatry 24, 199–202 (1990). [DOI] [PubMed] [Google Scholar]
- 132.Murphy E., Wickramaratne P., Weissman M., The stability of parental bonding reports: A 20-year follow-up. J. Affect. Disord. 125, 307–315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Graham J. W., Missing data analysis: Making it work in the real world. Annu. Rev. Psychol. 60, 549–576 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Burnham K. P., Anderson D. R., Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304 (2004). [Google Scholar]
- 135.Nylund K. L., Asparouhov T., Muthén B. O., Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo Simulation Study. Struct. Equ. Modeling 14, 535–569 (2007). [Google Scholar]
- 136.Nagin D. S., Odgers C. L., Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 6, 109–138 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.