Significance
Bloodborne parasites develop very sophisticated mechanisms to survive in the bloodstream, causing serious diseases. The mechanisms involved in intravascular clearance of bloodborne parasites are largely unknown. Understanding the underlying mechanisms is fundamental for developing strategies to treat the diseases. In the current study, we reveal that CRIg, a macrophage receptor previously shown to bind C3b/iC3b in vitro, plays an essential role in intravascular clearance of the bloodborne parasites African trypanosomes. More importantly, we demonstrate that CRIg, through interactions with complement deposited on the parasites in vivo, rather than parasites themselves, captures parasites under flow conditions. Thus, CRIg functions as a complement receptor in vivo to capture bloodborne pathogens and may represent a target for treatment.
Keywords: CRIg, complement, intravital imaging, bloodborne parasites, African trypanosomes
Abstract
Although CRIg was originally identified as a macrophage receptor for binding complement C3b/iC3b in vitro, recent studies reveal that CRIg functions as a pattern recognition receptor in vivo for Kupffer cells (KCs) to directly bind bacterial pathogens in a complement-independent manner. This raises the critical question of whether CRIg captures circulating pathogens through interactions with complement in vivo under flow conditions. Furthermore, the role of CRIg during parasitic infection is unknown. Taking advantage of intravital microscopy and using African trypanosomes as a model, we studied the role of CRIg in intravascular clearance of bloodborne parasites. Complement C3 is required for intravascular clearance of African trypanosomes by KCs, preventing the early mortality of infected mice. Moreover, antibodies are essential for complement-mediated capture of circulating parasites by KCs. Interestingly, reduced antibody production was observed in the absence of complement C3 during infection. We further demonstrate that CRIg but not CR3 is critically involved in KC-mediated capture of circulating parasites, accounting for parasitemia control and host survival. Of note, CRIg cannot directly catch circulating parasites and antibody-induced complement activation is indispensable for CRIg-mediated parasite capture. Thus, we provide evidence that CRIg, by interacting with complement in vivo, plays an essential role in intravascular clearance of bloodborne parasites. Targeting CRIg may be considered as a therapeutic strategy.
The liver is the largest solid organ in the body and receives 30% of the body’s total volume of blood per minute (1). Kupffer cells (KCs) are liver resident macrophages and constitute 80–90% of the total tissue macrophages in the body (1, 2). Residing within the lumen of the liver sinusoids, KCs are exposed directly to the blood and have the capability of catching pathogens under flow conditions (1, 2). As such, KCs play a prominent role in the elimination of circulating pathogens, particularly pathogens derived from the digestive tract.
As a major component of host innate immunity, complement plays a central role in macrophage-mediated phagocytosis of pathogens. Complement can be activated by 3 pathways: the alternative, classical, and mannose binding lectin pathways (3, 4). Following activation, C3b/iC3b are deposited on the surface of pathogens, serving as ligands for complement receptors expressed on phagocytes including macrophages (5). Initially, 4 complement receptors were described: CR1, CR2, CR3, and CR4 (6). Later, another complement receptor, CRIg, was identified to be expressed on a restricted population of macrophages including KCs in mice and humans, but not on splenic or alveolar macrophages (7–9). In addition to CRIg, classical CR3, but not CR1, CR2, or CR4 is expressed on KCs (8). In contrast to CR3, which binds to iC3b, CRIg binds to both C3b and iC3b in vitro (7, 8). It has been recently reported that CRIg−/− mice exhibited significantly reduced capture of bacterial pathogens including Staphylococcus aureus and Listeria monocytogenes by KCs, demonstrating that CRIg plays an important role in KC-mediated bacterial capture (8, 10, 11). Paradoxically, absence of complement did not affect liver capture of S. aureus or L. monocytogenes (8, 10–12), suggesting that CRIg captures circulating bacterial pathogens in a manner independent of complement. Indeed, recent studies reveal that CRIg functions as a macrophage pattern recognition receptor to directly bind and capture circulating gram-positive bacteria in vivo (11). Thus, although CRIg was originally identified as a macrophage receptor for recognition of C3b/iC3b in vitro, it remains unknown as to whether CRIg captures bloodborne pathogens through interactions with complement in vivo under flow conditions.
Bloodborne parasites have developed very sophisticated mechanisms to survive in the bloodstream, causing serious diseases. The role of CRIg in the elimination of bloodborne parasites has not been elucidated. One such bloodborne parasite is African trypanosomes, which infect both humans and animals (13, 14). African trypanosomiasis is often fatal if left untreated and is mainly found in sub-Saharan Africa where ∼70 million people are at risk for contracting the disease (15, 16). Elimination of these parasites from the bloodstream is crucial to control the disease. It is well established that the liver is the major site for clearance of those parasites circulating in the bloodstream (17, 18). We have previously shown that IgM and IgG antibodies specific for trypanosomes mediate phagocytosis of the organisms by KCs (19). However, the mechanisms underlying the elimination of circulating trypanosomes by KCs have not been fully elucidated. In particular, the role of complement in intravascular clearance of the parasites in vivo under flow conditions is largely unknown.
In the current study, taking the advantage of intravital microscopy (IVM) and using African trypanosomes as a model, we studied the dynamic interactions of KCs with bloodborne parasites in real-time. We identified the essential role of CRIg in intravascular clearance of bloodborne parasites. More importantly, we proved that CRIg, by interacting with complement in vivo, catches circulating pathogens under flow conditions.
Results
Real-Time Imaging of the Capture of African Trypanosomes by Macrophages In Vitro and In Vivo.
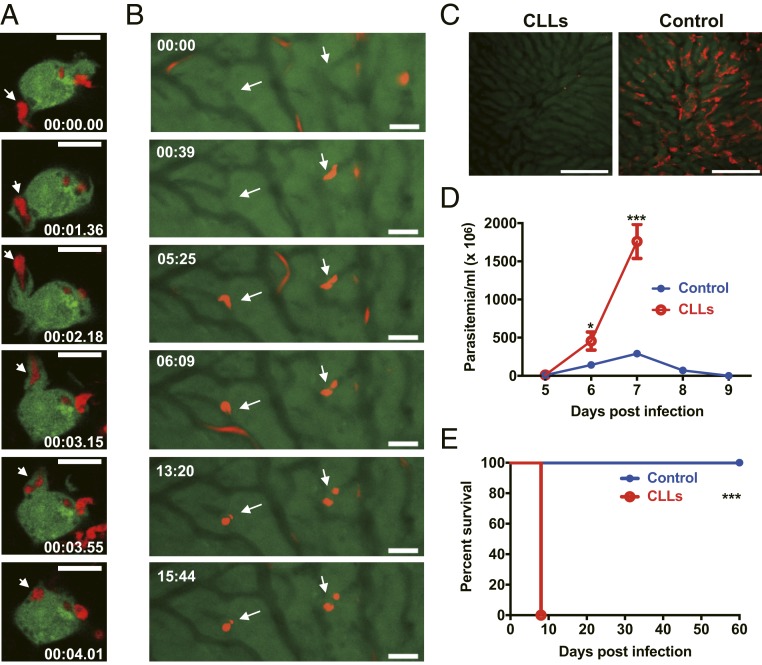
We first examined the kinetics of the capture of African trypanosomes by macrophages in vitro using monomorphic dTomato-expressing Trypanosoma brucei brucei (T. b. brucei). In a series of images, a parasite was initially attached to a macrophage; the macrophage used an extension of the cytoplasm (pseudopod) to catch and internalize the parasite where it was then visible as a round body, suggesting it was located within a phagosome (Fig. 1A and Movie S1). Internalization of the parasite was observed in all examined macrophages cultured in vitro. Using IVM, we next visualized the kinetics of the capture of circulating T. b. brucei in the liver on day 5 after infection. Like the in vitro observation, moving parasites were seen to be arrested in the liver sinusoids and then became round bodies, which were likely located within KCs (Fig. 1B and Movie S2). The capture of the moving parasites in the liver was detected in all 6 mice observed. We next treated mice with clodronate liposomes (CLLs) to deplete mononuclear phagocytes, particularly KCs, as described previously (11) and infected mice with polymorphic Trypanosoma congolense. IVM showed that KCs were almost completely depleted by CLLs (Fig. 1C) and partially repopulated on day 7 after depletion (SI Appendix, Fig. S1). Mice treated with CLLs could not control the first wave of parasitemia and displayed significantly higher parasitemia compared to control mice during infection with T. congolense (Fig. 1D). Mice treated with CLLs succumbed to infection on day 8 post infection (Fig. 1E), while control mice can survive more than 100 d after infection (20), indicative of the essential role of mononuclear phagocytes in disease control. CLLs deplete not only KCs, but also spleen macrophages and monocytes. We infected mice with or without a splenectomy and found that removal of the spleen did not significantly affect intravascular clearance of trypanosomes in our experimental setting (SI Appendix, Fig. S2). To rule out the role of monocytes, we infected CCR2−/− mice [lacking Ly6Chi monocytes (21)], Nur77−/− mice [lacking Ly6Clow monocytes (22)], and Nur77−/− mice depleted of Ly6Chi monocytes by anti-CCR2 mAb (23). We found that loss of Ly6Chi and/or Ly6Clow monocytes did not significantly affect parasitemia during T. congolense infection (SI Appendix, Fig. S3). Collectively, these results demonstrate that KCs play a prominent role in intravascular clearance of African trypanosomes.
Fig. 1.
Dynamics of the capture of African trypanosomes by macrophages in vitro and in vivo. (A) A series of confocal live-cell images showing the dynamics of the uptake of dTomato-expressing T. b. brucei (red, arrows) by murine J774 macrophages (green, stained by CSFE) in vitro. In the first image, the parasite was adhered to the macrophage. In the next images, the macrophage used a pseudopod to chase and catch the parasite. In the last image, the parasite was visualized as a round body inside the macrophage. The images are representative observations of at least 40 macrophages from 2 independent experiments. (Scale bars, 10 µm.) (B) A series of IVM images showing the kinetics of the capture of dTomato-expressing T. b. brucei (red, arrows) in the liver 5 d post i.p. infection of 1 × 103 parasites. Parasites were initially stopped in the sinusoids and then became round bodies. The images are representative observations from 6 mice. (Scale bars, 20 µm.) (C) Representative IVM images showing the depletion of KCs (red, labeled by anti-F4/80 mAb) 24 h after treatment with clodronate liposomes (CLLs). (Left) Treatment with CLLs. (Right) Treatment with PBS liposomes as control. (Scale bars, 200 µm.) (D and E) Administration of CLLs resulted in uncontrolled parasitemia (D) and early mortality (E) of mice (n = 5–6 per group) i.p. infected with 1 × 103 T. congolense. Data are expressed as mean ± SEM of 2 independent experiments. *P < 0.05, ***P < 0.001 by Student’s t test or log-rank test.
Complement C3 Mediates the Capture of Parasites by KCs, Preventing the Early Mortality of Infected Mice.
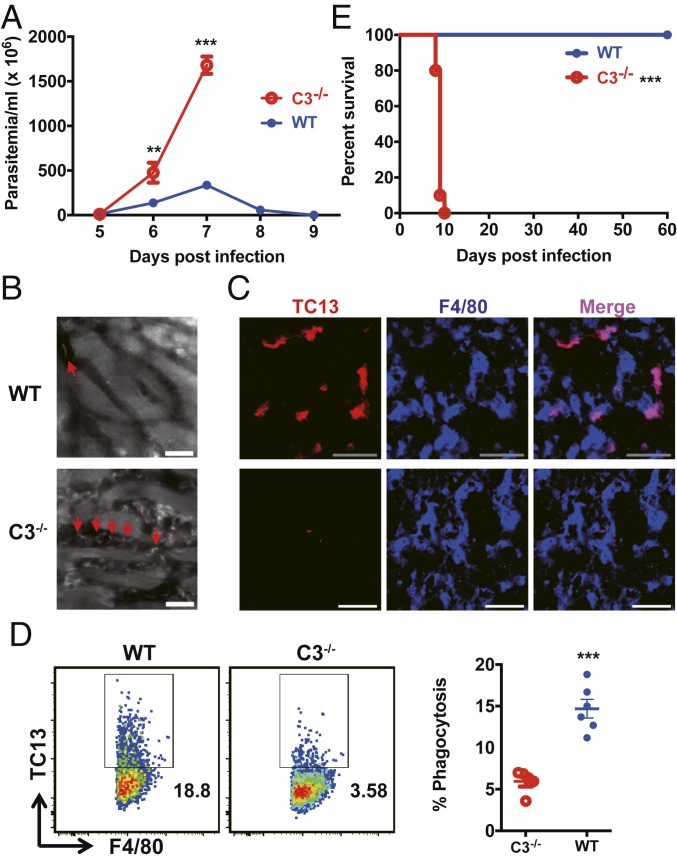
We next evaluated the role of complement in intravascular clearance of the parasites. Wild-type mice infected with T. congolense could control the first wave of parasitemia, while infected complement C3−/− mice could not and exhibited significantly higher parasitemia than wild-type mice (Fig. 2A). IVM on the livers of infected mice at the peak of parasitemia showed that there were more parasites in the liver sinusoids of infected C3−/− mice compared to infected wild-type mice (Fig. 2B and Movie S3). Using immunohistochemistry, we detected a large amount of parasite antigens in KCs of all infected wild-type mice, while parasite antigens were hardly observed in KCs of all infected C3−/− mice (Fig. 2C). Flow cytometry confirmed that the frequency of KCs containing parasite antigens in C3−/− mice were significantly lower compared to wild-type mice (Fig. 2D). All C3−/− mice infected with T. congolense died within 10 d and had a significantly shorter survival time than infected wild-type mice (Fig. 2E). In addition, C3−/− mice infected with dTomato-expressing T. b. brucei exhibited more parasites in liver sinusoids and survived significantly shorter than infected wild-type mice (SI Appendix, Fig. S4 and Movie S4). Collectively, these data demonstrate that complement is critically involved in the capture of parasites by KCs under flow conditions, preventing the early mortality of infected mice.
Fig. 2.
Complement C3 mediates parasite capture by KCs, preventing the early mortality of mice during T. congolense infection. (A) Parasitemia of WT and C3−/− mice (n = 5 per group) i.p. infected with 1 × 103 T. congolense. (B) Representative IVM images showing the parasites (arrows) in the liver sinusoids of WT and C3−/− mice 7 d after i.p. infection with 1 × 103 T. congolense. The parasites in the sinusoids were labeled by i.v. administration of rhodamine 6G 10 min prior to imaging. (Scale bars, 20 µm.) (C) Representative images of immunohistological staining showing parasite TC13 antigens (red) in KCs (blue, labeled by anti-F4/80 mAb) of WT and C3−/− mice 7 d post i.p. infection with 1 × 103 T. congolense. (Scale bars, 50 µm.) (D) WT and C3−/− mice (n = 5–6 per group) were i.p. infected with 1 × 103 T. congolense; flow cytometry was performed to detect the capture of the parasites by KCs (labeled by anti-F4/80 mAb) in the liver. (Left) Representative dot plots. (Right) Quantification. (E) The survival of WT and C3−/− mice (n = 10 per group) after i.p. infection with 1 × 103 T. congolense. Data are expressed as mean ± SEM of 2 independent experiments. **P < 0.01, ***P < 0.001 by Student’s t test or log-rank test.
Antibodies Are Essential for Complement-Mediated Capture of Parasites by KCs.
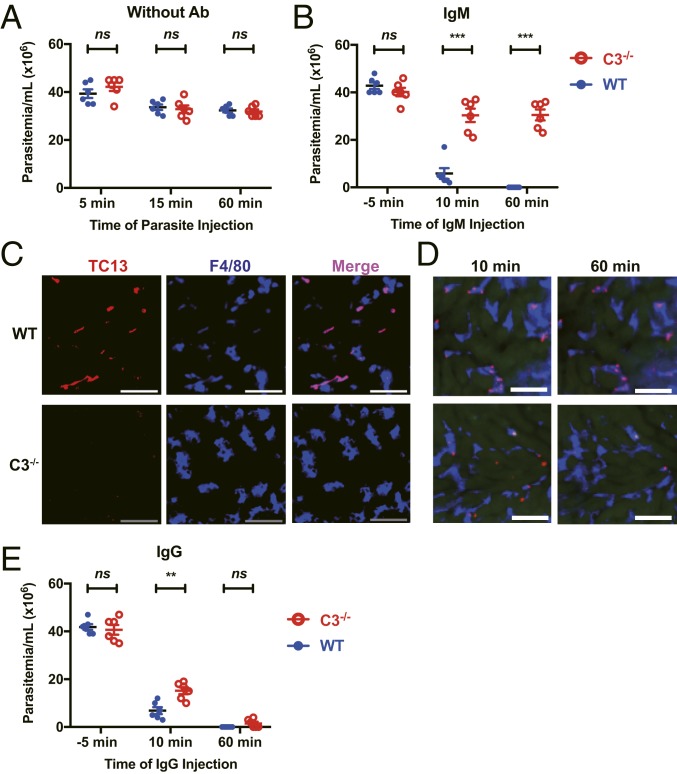
We next examined antibody production during the infection. The results showed that IgM and IgG antibodies were detected starting on day 3 following infection with African trypanosomes (SI Appendix, Fig. S5). This result prompted us to examine the role of antibodies in complement-mediated capture of the parasites by KCs. First, we compared intravascular clearance of T. congolense between wild-type and C3−/− mice in the absence of antibodies specific for the parasites. We injected mice with T. congolense via the tail vein. There was no significant difference in the parasitemia between infected wild-type and C3−/− mice (Fig. 3A). Next, wild-type and C3−/− mice were i.v. injected with T. congolense, followed by i.v. injection with IgM monoclonal antibody (mAb) specific for T. congolense. After IgM mAb injection, the parasitemia in wild-type mice was significantly lower compared to C3−/− mice at 15 min post injection and undetectable at 60 min (Fig. 3B), indicative of antibody-mediated activation of complement, which led to intravascular clearance of the parasites. In consistency with parasitemia, abundant parasite antigens were detected in KCs of all infected wild-type mice using immunohistochemistry, whereas parasite antigens were hardly seen in KCs of all infected C3−/− mice following IgM mAb treatment (Fig. 3C). This observation was further confirmed by IVM (Fig. 3D and Movie S5). In addition, infected C3−/− mice treated with IgM mAb failed to control the parasitemia and survived significantly shorter than wild-type mice treated with IgM mAb (SI Appendix, Fig. S6). To confirm the fact that IgM-mediated parasite clearance is dependent on KCs, splenectomized mice with or without KC depletion were i.v. infected with T. congolense, followed by i.v. injection with IgM mAb. The results showed that KC depletion significantly affected IgM-mediated parasite clearance in the blood (SI Appendix, Fig. S7).
Fig. 3.
Antibodies are required for complement-mediated capture of parasites by KCs. (A) WT and C3−/− mice (n = 6 per group) were i.v. infected with 5 × 107 T. congolense. The parasitemia was determined 5, 15, and 60 min post infection. (B and C) WT and C3−/− mice (n = 6 per group) were i.v. infected with 5 × 107 T. congolense. Ten min later, the mice were i.v. injected with anti-TC13 IgM mAb. The parasitemia was determined −5, 10, and 60 min post mAb administration (B). Immunohistological staining was performed on liver sections 60 min after anti-TC13 IgM mAb administration to label KCs (blue, labeled by anti-F4/80 mAb) and TC13 antigens (red) (C). (D) Representative IVM images showing the capture of T. congolense (red) by KCs (blue, labeled by anti-F4/80 mAb) in the liver 10 and 60 min after i.v. administration of anti-TC13 IgM mAb. Ten min prior to mAb administration, the mice were i.v. infected with 5 × 107 T. congolense labeled in vitro by TRITC. (E) WT and C3−/− mice (n = 6 per group) were i.v. infected with 5 × 107 T. congolense. Ten min later, the mice were i.v. injected with anti-TC13 IgG mAb. The parasitemia was determined −5, 10, and 60 min post mAb administration. (Scale bars, 50 µm.) Data are expressed as mean ± SEM of 2 independent experiments. ns, not significant; **P < 0.01, ***P < 0.001 by Student’s t test or log-rank test.
As with IgM, following IgG mAb injection, wild-type mice exhibited significantly lower parasitemia at 15 min post injection; however, both wild-type and C3−/− mice displayed undetectable levels of parasitemia at 60 min (Fig. 3E), probably reflecting the occurrence of Fc-mediated capture of the parasites, especially, in the absence of complement. Accordingly, abundant parasite antigens were detected in KCs of both infected wild-type and C3−/− mice (SI Appendix, Fig. S8A). Interestingly, although the parasitemia of both C3−/− and wild-type mice were undetectable following IgG mAb treatment (Fig. 3E), infected C3−/− mice but not wild-type mice redeveloped parasitemia in the next few days and died significantly earlier than infected wild-type mice (SI Appendix, Fig. S8 B and C), indicating impaired clearance of parasites in C3−/− mice even in the presence of IgG antibodies. These results suggest that antibody-mediated complement activation plays a dominant role in intravascular clearance of parasites, although the Fc-receptor of IgG antibodies is also likely involved in the clearance.
Collectively, these data demonstrate that antibodies are required for complement-mediated capture of trypanosomes, resulting in intravascular clearance of the parasites by KCs.
Reduced Antibody Production in the Absence of Complement C3 during Infection.
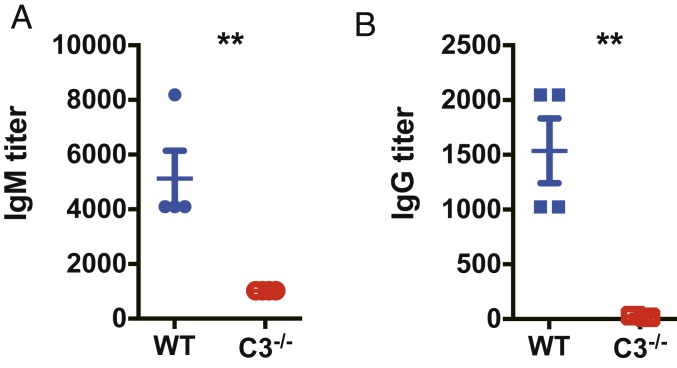
We next examined antibody production in the absence of complement C3 during infection. Wild-type and C3−/− mice were infected with T. congolense and serum levels of IgM and total IgG antibodies specific for the parasites were detected on day 7 after infection. The infected C3−/− mice exhibited significantly lower plasma levels of IgM and IgG antibodies compared to infected wild-type mice (Fig. 4). Thus, antibody production was significantly reduced in the absence of complement C3 during infection with T. congolense.
Fig. 4.
Reduced antibody production in C3−/− mice during T. congolense infection. WT and C3−/− mice (n = 4 per group) were i.p. infected with 1 × 103 T. congolense. Sera were collected 7 d post infection. Serum titers of IgM (A) and IgG (B) specific for TC13 were measured by ELISA; cutoff points were determined based on O.D. values of samples from naïve mice. Data are expressed as mean ± SEM of 2 independent experiments. **P < 0.01 by Student’s t test.
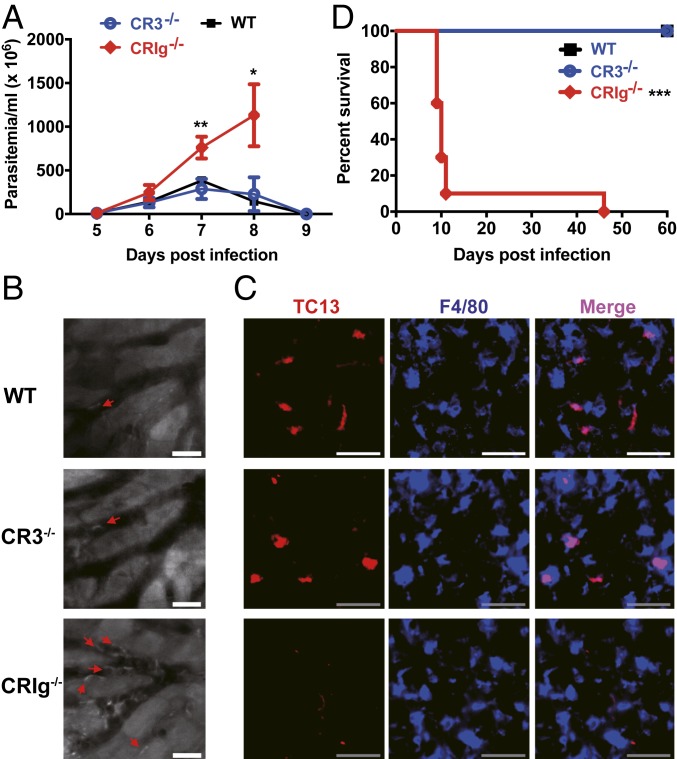
CRIg but Not CR3 Mediates Parasite Capture by KCs, Which Is Essential for Parasitemia Control and Host Survival.
Having demonstrated the essential role of complement in intravascular clearance of parasites by KCs, we next identified the receptor involved in this process. In addition to expressing complement receptor CR3, KCs have recently been shown to express another complement receptor, CRIg (8). We first i.v. infected CR3−/− and wild-type mice with T. congolense and then treated the mice with IgM mAb and found that KCs of all CR3−/− mice contained comparable parasite antigens (SI Appendix, Fig. S9), indicative of fully functioning parasite phagocytosis by CR3 deficient KCs. Following i.p. infection, CRIg−/− mice, but not CR3−/− mice, could not control the first wave of the parasitemia and displayed significantly higher parasitemia than wild-type mice (Fig. 5A). More parasites were seen in the liver sinusoids of all infected CRIg−/− mice compared to wild-type and CR3−/− mice as revealed by IVM (Fig. 5B and Movie S6). Immunohistochemistry showed abundant parasite antigens in KCs of all infected wild-type and CR3−/− mice, whereas parasite antigens were hardly seen in KCs of all infected CRIg−/− mice (Fig. 5C). More importantly, infected CRIg−/− but not CR3−/− mice had significantly shorter survival times than infected wild-type mice (Fig. 5D). Collectively, these results demonstrate that CRIg, but not CR3, is involved in the capture of circulating parasites by KCs.
Fig. 5.
CRIg is essential for intravascular clearance of parasites by KCs, enhancing the survival of infected mice. (A) The parasitemia of WT, CR3−/−, and CRIg−/− mice (n = 4–5 per group) i.p. infected with 1 × 103 T. congolense. (B) Representative IVM images showing the parasites in the liver sinusoids of WT, CR3−/−, and CRIg−/− mice 7 d post i.p. infection with 1 × 103 T. congolense. The parasites (arrows) in the sinusoids were labeled by i.v. administration of rhodamine 6G 10 min prior to imaging. (Scale bars, 20 µm.) (C) Representative images of immunohistological staining showing parasite TC13 antigens (red) in liver KCs (blue, labeled by anti-F4/80 mAb) of WT, CR3−/−, and CRIg−/− mice 7 d post i.p. infection with 1 × 103 T. congolense. (Scale bars, 50 µm.) (D) The survival of WT, CR3−/−, and CRIg−/− mice (n = 10 per group) i.p. infected with 1 × 103 T. congolense. Data are expressed as mean ± SEM of 2 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test or log-rank test.
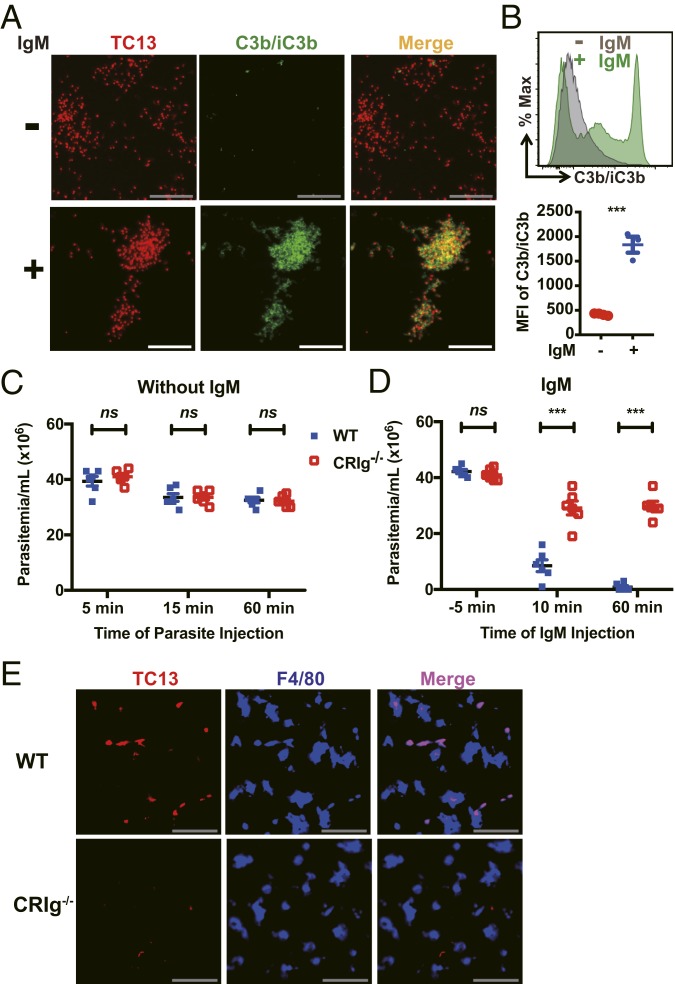
CRIg, by Interacting with Complement Deposited on the Parasites Rather than the Parasites Themselves, Captures Parasites In Vivo under Flow Conditions.
Although both complement and CRIg were involved in the elimination of parasites from vasculature, the above results cannot rule out the possibility that complement and CRIg function independently, as it has been recently shown that CRIg functions as a pattern recognition receptor to directly bind to lipoteichoic acid of bacteria in a complement independent manner (11). To address this issue, mice depleted of KCs were infected with T. congolense, followed by treatment with or without IgM mAb. As expected, abundant C3b/iC3b was detected on the parasites purified from mice treated with IgM mAb (Fig. 6 A and B and Movie S7), confirming the activation of complement by antibodies. In contrast, we did not detect C3b/iC3b on the parasites purified from mice without antibody treatment; however, it is possible that the deposition occurred but was not detected due to the rapid internalization (24, 25). We next i.v. infected wild-type and CRIg−/− mice with T. congolense and then treated the mice with or without IgM mAb. Without IgM mAb administration, there was no significant difference in parasitemia between wild-type and CRIg−/− mice (Fig. 6C), suggesting that CRIg expressed on KCs does not function as a pattern recognition receptor to directly bind the parasites. In contrast, injection of IgM mAb, which activated complement as shown in Figs. 3 and 6 A and B, led to clearance of the parasites in the blood of wild-type but not CRIg−/− mice (Fig. 6D). In addition, injection of IgM mAb resulted in efficient capture of parasites by KCs in all wild-type but not CRIg−/− mice (Fig. 6E). Thus, antibody-induced C3b/iC3b deposition on parasites is indispensable for CRIg-mediated parasite capture by KCs. Collectively, our results demonstrate that CRIg plays an essential role in KC-mediated intravascular clearance of bloodborne parasites by interacting with complement deposited on parasites rather than the parasites themselves.
Fig. 6.
CRIg functions as a complement receptor, and not a pattern recognition receptor, to capture circulating parasites in vivo. (A and B) WT mice were i.v. injected with CLLs to deplete KCs and 24 h later i.v. infected with 5 × 107 T. congolense (red) labeled by TRITC in vitro. Ten min post parasite injection, mice were i.v. administered with or without anti-TC13 IgM mAb (n = 3–4 mice per group). Twenty min after infection, blood was collected. Parasites were purified and then stained with anti-C3b/iC3b (green) mAb. The parasites were then subjected to microscopy (A) and flow cytometry (B) to determine the in vivo deposition of C3b/iC3b on the parasites. (C) Parasitemia of WT and CRIg−/− mice (n = 6 per group) 5, 15, and 60 min post i.v. infection with 5 × 107 T. congolense. (D and E) WT and CRIg−/− mice (n = 6 per group) were i.v. infected with 5 × 107 T. congolense. Ten min after infection, the mice were i.v. injected with anti-TC13 IgM mAb. Parasitemia was determined −5, 10, and 60 min post mAb administration (D). Immunohistological staining was performed on liver sections of infected mice 60 min after mAb administration to visualize parasite TC13 antigens (red) in KCs (blue, labeled by anti-F4/80 mAb) (E). (Scale bars, 50 µm.) Data are expressed as mean ± SEM of 2 independent experiments. ns, not significant; ***P < 0.001 by Student’s t test.
Discussion
The liver is believed to be important in the capture of circulating pathogens via KCs, preventing the systemic dissemination of pathogens from local infection sites such as the digestive tract (1, 2). In contrast to disseminating pathogens, bloodborne parasites such as African trypanosomes have developed very sophisticated mechanisms to survive in the bloodstream (26). Nevertheless, previous studies have shown that the liver can capture circulating African trypanosomes via KCs with the involvement of antibodies (17–19, 27). However, mechanisms involved in the capture of bloodborne parasites by KCs have not been fully elucidated. Notably, it remains incompletely understood whether complement is involved in the capture of parasites by KCs. In the current study, we demonstrate that antibody-mediated complement activation is essential for capture of circulating parasites by KCs. Interestingly, recent studies reveal that complement is dispensable for liver capture of bacterial pathogens such as S. aureus and L. monocytogenes in vivo under flow conditions (10–12), although it is well established that complement mediates the uptake of bacterial pathogens under static conditions (28, 29). Thus, the mechanisms involved in intravascular clearance of bloodborne parasites and disseminating bacteria are different.
CRIg was originally identified as a macrophage complement receptor for recognition of complement components C3b/iC3b deposited on pathogens in vitro (8). It is interesting to note that deficiency of CRIg leads to impaired liver capture of circulating bacterial pathogens such as S. aureus and L. monocytogenes (8, 10, 11), although deficiency or depletion of complement has no impact on the capturing of these bacteria by KCs in the liver (10–12). Indeed, recent studies reveal that CRIg functions as a pattern recognition receptor to directly bind and capture these bacterial pathogens via lipoteichoic acid recognition in a complement-independent manner (11). In addition, recent studies also reveal a “dual-track” mechanism for “fast” and “slow” clearance of bloodborne bacteria, involving scavenger receptors and CRIg, respectively (10). In the absence of complement, circulating bacteria are caught by KCs via scavenger receptors rather than CRIg (10). This explains why there was no difference in the liver capture of S. aureus between C3−/− mice and C3−/−CRIg−/− mice as reported earlier by Helmy et al. (8). Although CRIg was recently reported to mediate slow clearance of bacteria (10), it could not be ruled out that CRIg bound bacterial lipoteichoic acid and not C3b/iC3b in this experimental setting (10). Therefore, although CRIg was initially described as a macrophage receptor for binding C3b/iC3b in vitro, a critical gap in our understanding remains as to whether CRIg binds bloodborne pathogens by interacting with complement in vivo under flow conditions. Given that pathogen interactions with host cells under flow conditions are largely affected by shear forces and that the interaction in vivo is different from in vitro (30), it is of importance to address this gap.
Considering the fact that bacterial pathogens can directly activate complement (29) and can directly bind CRIg via lipoteichoic acid recognition (11), it is difficult to distinguish whether CRIg on KCs functions as a complement receptor or as a pattern recognition receptor to capture the circulating bacterial pathogens. Thus, infection of mice with bacterial pathogens may not be an ideal model to address the function of CRIg as a complement receptor for recognition of bloodborne pathogens in vivo under flow conditions. In contrast to bacterial pathogens, our results demonstrate that CRIg cannot function as a pattern recognition receptor for KCs to directly bind to circulating African trypanosomes. In addition, antibody-activated complement is essential for CRIg-mediated capture of the circulating parasites by KCs in vivo. Thus, infection of mice with African trypanosomes provides a unique model to study the interaction of CRIg expressed on KCs with complement deposited on microbes circulating in the bloodstream. Our study not only identifies the essential role of CRIg in intravascular clearance of bloodborne parasites, thereby preventing the early mortality of infected mice, but more importantly, reveals that CRIg, functions as a complement receptor and not a pattern recognition receptor, mediating the capture of circulating pathogens by interacting with complement deposited on pathogens in vivo under flow conditions.
It remains unknown as to whether CRIg plays a role in other parasitic diseases. One of the most devastating parasitic diseases is malaria caused by Plasmodium parasites. In vitro studies have shown that KCs purified from mice and rats can ingest and destroy Plasmodium sporozoites coated with antibodies (31, 32). In addition, sporozoites were also observed undergoing cytolysis within phagolysosomes of KCs in rats infected with Plasmodium berghei (33). However, it was also reported that Plasmodium sporozoites can traverse KCs to reach hepatocytes during liver infection (34, 35). CD68 was identified as a receptor expressed on KCs for sporozoite invasion of KCs (36). It is likely that KCs internalize opsonized sporozoites and destroy them within phagolysosomes in immune animals. However, in nonimmune animals, KCs may be targeted by unopsonized sporozoites as a gateway out of the liver sinusoids. The role of CRIg expressed on KCs during infections with Plasmodium parasites deserves further investigation.
It should be pointed out that the mechanism of intravascular clearance of the parasites was dissected using a mouse model system in the current study. It is presently unknown whether humans use the same mechanism to clear the parasites. Nevertheless, the mouse has many similarities to humans in terms of immune responses to African trypanosomes. Of note, IgM antibodies are the first predominant class of anti-trypanosomal antibodies and high levels of IgM antibodies are detected in both mice and humans during trypanosomal infections (37–39). In addition, KCs represent about 35% of the liver nonparenchyma cells in mice (2) and about 30% in humans (40). Importantly, CRIg, the receptor identified for intravascular clearance of the parasites, is not only expressed on mouse KCs but also highly expressed on human KCs (7). Thus, it is likely that CRIg also plays a prominent role in intravascular clearance of the parasites in human infections.
In summary, we demonstrate that antibody-induced activation of complement mediates the capture of the bloodborne parasite African trypanosomes by KCs, resulting in parasitemia control. We further reveal that CRIg functions as a complement receptor in vivo, but not as a pattern recognition receptor, to capture bloodborne parasites by interacting with complement deposited on the parasites, preventing the early mortality of infected mice. Thus, CRIg plays an essential role in intravascular clearance of bloodborne parasites through interactions with complement and may represent a target for treatment.
Materials and Methods
Mice.
Eight- to 12-week-old C57BL/6 mice and 5- to 6-week-old outbred Swiss white mice (CD1) were purchased from the National Cancer Institute (Frederick, MD). C3−/− (Stock No. 003641), CR3−/− (Stock No. 003991), and CRIg−/− (Stock No. 024408) mice in C57BL/6 background were purchased from the Jackson Laboratory and bred in-house. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and Institutional Bio-safety Committee of the University of Maryland, College Park.
Parasites.
T. congolense, Trans Mara strain, variant antigenic type TC13 was provided by Dr. Jude Uzonna (University of Manitoba, Canada). The passages of the parasites were made every third day by infecting CD1 mice, which were immunosuppressed with cyclophosphamide as described previously (41). The parasites were purified from the blood of infected CD1 mice by DEAE-cellulose chromatography (41) or gradient centrifugation with 60% Percoll at 700xg for 8 min with the brake off. A monomorphic strain of T. b. brucei Tb221 labeled by dTomato was also used in this study. The Tb221 strain was routinely cultured at 37 °C, 5% CO2 in HMI-9 (Axenia Biologix) supplemented with 10% heat-inactivated FBS, penicillin-G (100 units/mL), and streptomycin sulfate (100 μg/mL).
Live Cell Imaging.
To examine the dynamics of uptake of dTomato-expressing T. b. brucei Tb221 by macrophages in vitro, J774 cells (American Type Culture Center) were routinely grown at 37 °C, 5% CO2 in DMEM supplemented with 10% heat-inactivated FBS, penicillin (100 units/mL), and streptomycin (100 μg/mL). Cultures were performed in 35 mm glass bottom culture dishes (MatTEK) at a cell density of ∼5 × 105 cells/mL. Cells were stained with 40 µM CSFE at 37 °C for 10 min. After washing, cells were pretreated with 150 ng/mL PMA (Acros Organics) for 1 h, and phagocytosis of trypanosomes was performed in the presence of 5 μg/mL anti-Tb221 IgM mAb (clone 13A8) and 30% mouse serum with a constant macrophage: trypanosome ratio of 1:10. The culture system was subjected to confocal live-cell imaging (Zeiss LSM 510) at 37 °C, 5% CO2. Images were taken at 1s interval with 63× oil lens.
Infections and Treatments.
Mice were i.p. infected with 1 × 103 T. congolense TC13 or 1 × 103 dTomato-expressing T. b. brucei Tb221. For i.v. infection, purified 5 × 107 T. congolense TC13 were injected into mice via the tail vein. In some experiments, T. congolense TC13 parasites were labeled by TRITC in vitro prior to i.v. infection. In brief, the parasites were stained with TRITC (ThemoFisher Scientific) at 10 µg/mL in PBS for 10 min at 37 °C, 5% CO2, and extensively washed in Tris⋅HCl (pH 7.8). In some experiments, mice were i.v. injected with 1 mg IgM (clone 6C1) or IgG (clone 1D11) mAb specific to T. congolense TC13 (42) in 100 µL PBS following i.v. infection with the parasites. To deplete KCs, mice were injected with 200 μL clodronate liposomes or control PBS liposomes (Encapsula Nano Sciences) via the tail vein 24 h prior to infection.
Estimation of Parasitemia and Survival Time.
To quantify parasitemia, 1 μL of blood was collected via tail vein bleeding and diluted in 99 μL sterile PBS. Parasitemia was counted at 40× magnification by phase-contrast microscopy. The survival time was defined as the number of days after infection that the infected mice remained alive.
Leukocyte Isolation and Flow Cytometry.
Leukocytes were isolated and flow cytometry was performed as described previously (41). For more details, see SI Appendix.
Intravital Microscopy (IVM).
IVM was performed as previously described (43). For more details, see SI Appendix.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (19). For more details, see SI Appendix.
C3b/iC3b Deposition Assay.
In vivo deposition of C3b/iC3b on parasites was determined by cell imaging and flow cytometry. To prevent internalization of the parasites by KCs, WT mice were i.v. injected with clodronate liposomes to deplete KCs and 24 h later i.v. infected with 5 × 107 T. congolense TC13 parasites labeled by TRITC in vitro. The infected mice were i.v. injected with or without 1 mg anti-TC13 IgM mAb (clone 6C1) 10 min after infection. Parasites were purified from the blood of infected mice 20 min after infection and washed with 5% glucose in PBS (pH 7.8). Parasites were then stained with APC anti-mouse C3b/iC3b mAb (clone 11H9, Novus) for 20 min at 4 °C followed by a final wash and resuspension in 200 μL 5% glucose/PBS. Stained parasites were imaged by microscopy or analyzed by flow cytometry immediately without fixation.
ELISAs for Trypanosome-Specific Antibodies.
The serum titers of anti-TC13 IgM and IgG antibodies were quantified by ELISA as previously described (44). For more details, see SI Appendix.
Statistical Analysis.
Data are represented as the mean ± SEM. Significance of differences was determined by 2-tailed Student’s t test, ANOVA or a log-rank test using the GraphPad Prism 8.0 software (GraphPad). Values of P < 0.05 are considered statistically significant.
All data discussed in the paper are available to readers in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Banchao Shu for establishing the in vitro phagocytosis experiment. We also thank Drs. Chunyan Wu and Yuchen Nan for helpful discussion. The Chinese Scholarship Council (CSC) provided fellowship to G.L. and Y.F., the U.S.-Egypt Science and Technology Joint Fund (STDF) provided fellowship to M.Y., and the University of Maryland provided startup funding to M.S.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913443116/-/DCSupplemental.
References
- 1.Jenne C. N., Kubes P., Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Bilzer M., Roggel F., Gerbes A. L., Role of Kupffer cells in host defense and liver disease. Liver Int. 26, 1175–1186 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Walport M. J., Complement. Second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Walport M. J., Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Pangburn M. K., Müller-Eberhard H. J., Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J. Exp. Med. 152, 1102–1114 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holers V. M., Kinoshita T., Molina H., The evolution of mouse and human complement C3-binding proteins: Divergence of form but conservation of function. Immunol. Today 13, 231–236 (1992). [DOI] [PubMed] [Google Scholar]
- 7.He J. Q., Wiesmann C., van Lookeren Campagne M., A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol. Immunol. 45, 4041–4047 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Helmy K. Y., et al. , CRIg: A macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Vogt L., et al. , VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J. Clin. Invest. 116, 2817–2826 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broadley S. P., et al. , Dual-Track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe 20, 36–48 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Zeng Z., et al. , CRIg Functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe 20, 99–106 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Gregory S. H., et al. , Complementary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J. Immunol. 168, 308–315 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Beschin A., Van Den Abbeele J., De Baetselier P., Pays E., African trypanosome control in the insect vector and mammalian host. Trends Parasitol. 30, 538–547 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Stijlemans B., Radwanska M., De Trez C., Magez S., African trypanosomes undermine humoral responses and vaccine development: Link with Inflammatory Responses? Front. Immunol. 8, 582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simarro P. P., et al. , Estimating and mapping the population at risk of sleeping sickness. PLoS Negl. Trop. Dis. 6, e1859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steverding D., The history of African trypanosomiasis. Parasit. Vectors 1, 3 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempsey W. L., Mansfield J. M., Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J. Immunol. 130, 405–411 (1983). [PubMed] [Google Scholar]
- 18.Macaskill J. A., et al. , Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology 40, 629–635 (1980). [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M., Wei G., Pan W., Tabel H., Trypanosoma congolense infections: Antibody-mediated phagocytosis by Kupffer cells. J. Leukoc. Biol. 76, 399–405 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Tabel H., Wei G., Shi M., T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol. Rev. 225, 128–139 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Serbina N. V., Pamer E. G., Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Hanna R. N., et al. , The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol. 12, 778–785 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misharin A. V., et al. , Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 9, 591–604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devine D. V., Falk R. J., Balber A. E., Restriction of the alternative pathway of human complement by intact Trypanosoma brucei subsp. gambiense. Infect. Immun. 52, 223–229 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engstler M., et al. , Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 131, 505–515 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Stijlemans B., et al. , Immune evasion strategies of Trypanosoma brucei within the mammalian host: Progression to pathogenicity. Front. Immunol. 7, 233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magez S., et al. , Interferon-gamma and nitric oxide in combination with antibodies are key protective host immune factors during trypanosoma congolense Tc13 Infections. J. Infect. Dis. 193, 1575–1583 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Aderem A., Underhill D. M., Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, 593–623 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Flannagan R. S., Jaumouillé V., Grinstein S., The cell biology of phagocytosis. Annu. Rev. Pathol. 7, 61–98 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Isberg R. R., Barnes P., Dancing with the host; flow-dependent bacterial adhesion. Cell 110, 1–4 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Danforth H. D., Aikawa M., Cochrane A. H., Nussenzweig R. S., Sporozoites of mammalian malaria: Attachment to, interiorization and fate within macrophages. J. Protozool. 27, 193–202 (1980). [DOI] [PubMed] [Google Scholar]
- 32.Seguin M. C., Ballou W. R., Nacy C. A., Interactions of Plasmodium berghei sporozoites and murine Kupffer cells in vitro. J. Immunol. 143, 1716–1722 (1989). [PubMed] [Google Scholar]
- 33.Shin S. C., Vanderberg J. P., Terzakis J. A., Direct infection of hepatocytes by sporozoites of Plasmodium berghei. J. Protozool. 29, 448–454 (1982). [DOI] [PubMed] [Google Scholar]
- 34.Frevert U., et al. , Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3, e192 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavares J., et al. , Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 210, 905–915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha S. J., et al. , CD68 acts as a major gateway for malaria sporozoite liver infection. J. Exp. Med. 212, 1391–1403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binz G., Timperman G., Hutchinson M. P., Estimation of serum immunoglobulin M as a screening technique for trypanosomiasis. A field trial in the Democratic Republic of the Congo. Bull W. H. O. 38, 523–545 (1968). [PMC free article] [PubMed] [Google Scholar]
- 38.Greenwood B. M., Whittle H. C., Cerebrospinal-fluid IgM in patients with sleeping-sickness. Lancet 2, 525–527 (1973). [DOI] [PubMed] [Google Scholar]
- 39.Hudson K. M., Byner C., Freeman J., Terry R. J., Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature 264, 256–258 (1976). [DOI] [PubMed] [Google Scholar]
- 40.Malarkey D. E., Johnson K., Ryan L., Boorman G., Maronpot R. R., New insights into functional aspects of liver morphology. Toxicol. Pathol. 33, 27–34 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Liu G., et al. , IL-27 signaling is crucial for survival of mice infected with African trypanosomes via preventing lethal effects of CD4+ T Cells and IFN-gamma. PLoS Pathog. 11, e1005065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei G., Qualtiere L., Tabel H., Trypanosoma congolense: Complement independent immobilization by a monoclonal antibody. Exp. Parasitol. 70, 483–485 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Marques P. E., et al. , Imaging liver biology in vivo using conventional confocal microscopy. Nat. Protoc. 10, 258–268 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Liu G., et al. , Distinct contributions of CD4+ and CD8+ T cells to pathogenesis of Trypanosoma brucei infection in the context of gamma interferon and interleukin-10. Infect. Immun. 83, 2785–2795 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.