Significance
This longitudinal study demonstrates within-individual pubertal recalibration of the HPA axis in humans. Findings provide empirical support for an adolescent window of plasticity during which the brain resamples the environment and alters HPA functioning if the current caregiving environment is sufficiently different from the early caregiving environment in which the system was originally organized. This suggests that intervention efforts to improve outcomes for children who have experienced early life adversity should include a focus on the prepubertal and peripubertal period in order to maximize their impact on recalibrating systems like the HPA axis.
Keywords: HPA axis, puberty, recalibration, early life stress, institutional care
Abstract
Nonhuman animal models reveal that the hypothalamic–pituitary–adrenocortical (HPA) axis calibrates to the harshness of the environment during a sensitive period in infancy. Humans exposed to depriving institutional care in infancy show reduced HPA axis responsivity, even years after they are placed in supportive, well-resourced families. This study examined whether puberty opens a window of opportunity to recalibrate the HPA axis toward more typical reactivity when children shift from harsh deprived conditions in infancy into supportive conditions in childhood and adolescence. Participants (n = 129 postinstitutionalized, 68.2% female; n = 170 comparison, 52.4% female) completed 3 annual sessions beginning at ages 7 to 15 (M = 11.28, SD = 2.31). Each session assessed pubertal stage via nurse examination and cortisol reactivity to the Trier social stress test for children. The linear mixed-effects model controlling for sex and between-individual differences in pubertal stage showed a significant group by pubertal stage interaction: within-individual increases in pubertal stage were associated with increases in cortisol stress reactivity for postinstitutionalized youth but not nonadopted comparison youth. This study indicates that pubertal development reopens a window of opportunity for the HPA axis to recalibrate based on significant improvements in the supportiveness of the environment relative to that in infancy. The peripubertal period may be an important time in development where the caregiving environment has a substantial impact on the HPA axis and, perhaps, other stress-mediating systems. Future research is needed to examine the mechanisms of recalibration and whether HPA recalibration impacts physical and psychological health.
Institutional care during infancy impairs the responsiveness of the HPA axis to psychosocial stressors. It also is associated with reductions in risk-taking and sensation-seeking (1, 2), increased amygdala responses to threat stimuli (3, 4), and increases in anxiety symptoms (5) that are associated with alterations in brain connectivity (6) and white matter pathways (7). Taken together, these effects suggest that the defensive system was calibrated to the harsh, unsupportive environment of the institution (orphanage) and that even years after adoption into supportive, well-resourced homes, the defensive system maintains its original calibration. Studies of the adrenocortical response to stressors have shown that early institutional deprivation results in a blunted cortisol response to stressors (8–10). This is seen within the first months after removal from institutional care (9) and lasts at least until middle childhood (8). The effect is causal, as shown by the Bucharest Early Intervention Study. They found that the cortisol and autonomic reactivity were both blunted in response to the Trier social stress test in children randomly assigned to institutional care as usual and those randomly assigned to removal from institutional care. However, this was only true for those removed from the institution after 2 y of age but not for those removed before age 2 (10). The causal nature of this blunting of the HPA axis is also demonstrated in studies of nonhuman primates randomly assigned to nursery rearing instead of maternal rearing (11). Glucocorticoids have an inverted U shape relation to cognitive and behavioral functioning due to differential activation of mineralocorticoid and glucocorticoid receptors in the hippocampus and amygdala, among other areas (12). Thus, underactivity of the HPA axis has been associated with various physical and mental health problems (13–15). The question is, can this effect be reversed?
Simply removing children from deprivation and placing them in supportive, well-resourced homes does not appear to be sufficient to allow the HPA axis to recalibrate. This is the case even when the parents in those homes score very high on observational measures of parenting quality (16) and children report high levels of perceived support (8). However, development may allow windows of opportunity for recalibration. In particular, for the HPA axis, puberty may reopen the system and functionally create a second sensitive period. Two cross-sectional studies suggest this may be the case. Both involved the cortisol awakening response (CAR) and thus were not actually studies of the stress response (17, 18). Both found a blunted CAR for early life stress children at earlier stages of pubertal development but an increased CAR for those at later stages. The increased CAR was more similar to that shown by children and adolescents who had not experienced adverse care.
The possibility that puberty may open a window of plasticity allowing the HPA axis to recalibrate is supported by studies in nonhuman animal models. Romeo (19, 20) has argued that the peripubertal period is another sensitive period for programming of the HPA axis. He has shown that stressors during that period have longer-term impacts on the axis than the same stressors imposed on adult animals. It is also the case that positive experiences in adolescent nonhuman animals functionally reverse the effects of prenatal stress (21) and postnatal repeated maternal separations (22). These studies used environmental enrichment to reverse the hyperresponsiveness of the axis induced by prenatal and postnatal stress.
While studies using the CAR suggest that there may be a recalibration of the HPA axis with puberty, the CAR is not a measure of stress reactivity. To answer the question of whether the HPA axis stress response is recalibrated with puberty, it is necessary to assess the cortisol response during a stressor task. We used the Trier social stress test for children (TSST-C), a social evaluative stressor (23, 24) and one of the most reliable stressor tasks in the literature, to examine whether youth adopted as infants from institutions would become increasingly more stress responsive and would respond more similarly to low-risk, comparison youth with increasing pubertal stage. An accelerated longitudinal design was used (25) in which children began the first session at 7 to 15 y old and then were assessed annually over 2 y, totaling up to 3 sessions per participant. See SI Appendix, SI Materials and Methods, for details of our method for maintaining responses across repeated trials of the TSST. We previously reported preliminary evidence of recalibration using a cross-sectional examination of the data from the first session (26). The present analysis focused on the cortisol response to the TSST-C as a function of within individual increases in pubertal stage. We hypothesized that youth exposed to institutional care in infancy would exhibit increasing cortisol reactivity with increasing pubertal stage, gradually becoming more similar to the cortisol responses seen in typically developing youth, who would show no or comparatively little change in cortisol reactivity across puberty. Importantly, this framework begins to tease apart the role of age- and puberty-related change, as within-individual increases in pubertal stage do not differ if, for example, an individual moves from pubertal stage 1 to 2 or stage 4 to 5. To compare biological and psychological responses to the TSST-C across puberty, see SI Appendix, Pubertal Changes in Self-Reported Stress, for additional analyses examining whether increasing pubertal stage predicts within-individual changes in self-reported subjective stress during the TSST-C.
Results
Salivary cortisol reactivity and pubertal status were characterized in 299 children and adolescents across 3 annual sessions beginning when participants were 7 to 15 y old. Participants were either previously institutionalized as infants and toddlers (postinstitutionalized [PI]) or born and raised in their natal families (nonadopted [NA]). At each session, saliva samples were collected across 2 h, capturing cortisol reactivity and recovery in response to a modified version of the TSST-C (see SI Appendix, Fig. S1 and SI Materials and Methods, for detail on sampling protocol). A linear mixed-effects model was fit to examine whether within-individual changes in pubertal status would predict changes in salivary cortisol reactivity, moderated by group. Data were modeled hierarchically with cortisol sample nested within session, nested within participant. Between-individual differences in pubertal stage were modeled by calculating each individual’s mean pubertal stage across all sessions (hereon referred to as “mean pubertal stage”). The focal predictor, within-individual changes in pubertal stage, was calculated as the difference from an individual’s mean at each separate session (hereon “pubertal change”). Because puberty may also increase differences in HPA axis functioning between males and females (27), we also explored the possibility that sex would modify the pubertal stress recalibration effect. Participant information is described in Table 1. Descriptive statistics and correlations between focal variables are included in SI Appendix, Table S1.
Table 1.
Demographic information for each group
| Postinstitutionalized (n = 129) | Nonadopted (n = 170) | Test of difference | |
| Session 1 age in years, M (SD) | 11.38 (2.39) | 11.20 (2.24) | t(279) = 0.63, P = 0.53 |
| Sex, n (%) female | 88 (68.22%) | 89 (52.35%) | χ2(1, n = 299) = 4.84, P = 0.03 |
| Child race, n (%) | χ2(4, n = 276) = 103.36, P < 0.001 | ||
| American Indian/Alaskan Native | 14 (11.29%) | 1 (0.62%) | |
| Asian | 48 (38.71%) | 2 (1.23%) | |
| Black/African American/African | 7 (5.65%) | 4 (2.47%) | |
| White | 50 (40.32%) | 142 (87.65%) | |
| Multiracial | 5 (4.03%) | 13 (8.02%) | |
| Child region of origin, n (%) | — | ||
| Russia/Eastern Europe | 63 (48.84%) | — | |
| China/Southeast Asia | 45 (34.89%) | — | |
| Latin America | 16 (12.40%) | — | |
| Africa/Haiti | 5 (3.88%) | — | |
| Session 1 puberty, n (%)* | t(86) = −1.15, P = 0.25 | ||
| Stage 1 | 41 (33.61%) | 60 (37.97%) | |
| Stage 2 | 16 (13.11%) | 39 (24.68%) | |
| Stage 3 | 28 (22.95%) | 14 (8.86%) | |
| Stage 4 | 19 (15.57%) | 14 (8.86%) | |
| Stage 5 | 18 (14.75%) | 31 (19.62%) | |
| Session 1 medication index, M (% 0)† | 2.76 (74.24%) | 2.26 (86.71%) | t(279) = 3.69, P < 0.001 |
| Annual family income, n (%) | χ2(8, n = 276) = 9.73, P = 0.28 | ||
| <$40,000 | — | 7 (4.46%) | |
| $40,001 to 70,000 | 19 (15.97%) | 18 (11.46%) | |
| $70,001 to 100,000 | 25 (21.01%) | 40 (25.48%) | |
| $100,001 to 150,000 | 40 (33.61%) | 42 (26.75%) | |
| $150,001 to 200,000 | 20 (16.81%) | 28 (17.83%) | |
| > $200,000 | 15 (12.61%) | 22 (14.01%) | |
| Primary caregiver education, n (%) | χ2(9, n = 280) = 7.39, P = 0.60 | ||
| High school degree/GED or less | 2 (1.64%) | 3 (1.90%) | |
| Some college | 16 (13.11%) | 25 (15.82%) | |
| Undergraduate degree | 42 (34.43%) | 68 (43.04%) | |
| Graduate/professional degree | 62 (50.82%) | 62 (39.24%) |
Significant group differences are indicated in bold.
Statistic tests group difference in age at pubertal stage 3 (central puberty) in order to examine group differences in pubertal timing.
Mean calculated excluding zeroes.
Pubertal Recalibration of Salivary Cortisol Reactivity.
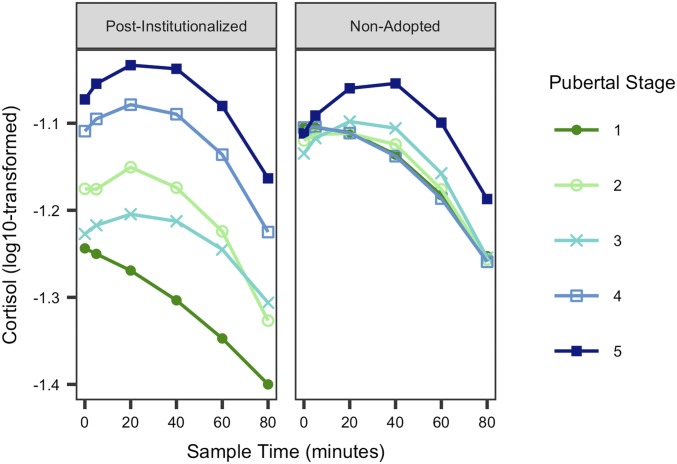
Full model results are displayed in SI Appendix, Table S2. The pubertal change by group interaction was significant (t = 2.74, P = 0.01), but the mean pubertal stage by group interaction was not (t = −0.77, P = 0.44), suggesting that exposure to early institutional care moderated the association between within-individual changes in pubertal stage and cortisol reactivity but not between-individual differences in pubertal stage and cortisol reactivity (Fig. 1). Least-squares mean contrasts indicated that cortisol reactivity at pubertal stages 1 and 5 were significantly different for postinstitutionalized youth (95% CI [−1.40, −1.15] and [−1.14, −0.92], respectively). There were no significant differences in cortisol reactivity for nonadopted youth at any pubertal stage. There was no evidence that sex moderated the recalibration effect.
Fig. 1.
Line graph illustrating model-implied results of a linear mixed-effects model examining the association between pubertal stage and cortisol stress reactivity by group (postinstitutionalized, Left; nonadopted, Right). Saliva samples were collected at time 0, +5, +20, +40, +60, and +80 min shown on the x axis above, with time 0 indicating beginning of TSST-C prep. For clarity, the data shown are collapsed across multiple data points per participant; however, model results in SI Appendix, Table S2, indicate that cortisol reactivity is associated with within-individual increases in pubertal stage, not just between-individual differences across puberty (see SI Appendix, Table S2, for model results and SI Appendix, Fig. S2, for 95% confidence envelopes).
Discussion
Research suggests that there is an initial sensitive period in infancy during which the HPA axis calibrates to the harshness of the environmental conditions which, for humans, tends to result in reduced HPA responsivity (9, 10). This study sought to determine whether puberty provides a second window of plasticity during which the human HPA axis could recalibrate if conditions are sufficiently different from those in infancy. In the case of the present study, conditions were harsh and unsupportive in infancy, and once adopted, the children entered well-resourced and generally supportive homes. Thus, our prediction was that HPA axis reactivity, with advancing pubertal stage, would increasingly resemble the typical responsiveness of youth reared in well-resourced, supportive homes. If postinstitutionalized children’s HPA activity can be restored to moderate, but not prolonged, levels of responding to environmental challenges, this may signal other downstream improvements in physical and mental health as well (13, 15). Because glucocorticoids are known to be critical regulators of plasticity (14), recalibration of the system with puberty may help support the plasticity of this period of brain development.
Indeed, the results of this study provide within-individual evidence of pubertal recalibration of the HPA axis in humans. Using the first year of data collection in this study, we previously reported that cross-sectional differences in puberty predict greater cortisol reactivity. The present analysis allows us to confirm that this was, in fact, a developmental process occurring within individuals. For postinstitutionalized youth, within-individual increases in pubertal stage significantly predicted increased (i.e., more typical) cortisol stress reactivity. Nonadopted comparison youth who did not experience institutional deprivation in infancy did not show a similar pattern of pubertal recalibration. These findings corroborate nonhuman animal models (19, 22) and extend cross-sectional human research (17, 18, 28) which demonstrated between-individual differences in HPA functioning across the peripubertal period, particularly for those who experienced early life adversity.
Importantly, this change in cortisol reactivity does not occur immediately upon removal from the depriving environment. The majority of the youth in this study were adopted by age 2, and yet even in the earlier stages of pubertal development (at least 5 to 7 y postadoption) the axis still appears relatively blunted. It appears to take the physiological changes associated with pubertal development to open the axis for recalibration. From a life history perspective (29), the axis is recalibrating prior to the onset of the reproductive period to match the conditions likely to be present during that life stage. However, the mechanisms of this recalibration are still unclear. There was no particular pubertal stage that was associated with changes in cortisol reactivity in this study. For this reason, it is unlikely that the activity of any individual hormone is responsible for driving recalibration. Instead, it may be that more global changes in neural plasticity and the associated enhanced processing of environmental stimuli occurring during puberty (30) allow the caregiving environment to have a disproportionately large influence on HPA axis functioning during this period.
Further, this study measured cortisol, which is a distal end product of the HPA axis that involves complex neural inputs and influences. It is unclear whether the change in HPA reactivity occurred at the level of the adrenal, pituitary gland, hypothalamus, or limbic inputs to the hypothalamus. This knowledge could have functional implications for our understanding of pubertal development and the recalibration of the HPA axis following substantial changes in environmental conditions. Future studies are needed to determine the mechanisms through which pubertal recalibration of the stress response takes place in humans. It will also be important to examine the extent to which variations in current environmental conditions impact the magnitude of pubertal recalibration and whether recalibration is associated with physical and psychological functioning in adolescence into adulthood.
Repeated administration of the same stressor, as was done in this study, may change the individual’s psychological interpretation and/or physiological response to the stressor. Indeed, participants reported lower self-reported subjective stress across puberty (SI Appendix, Table S3). However, this would typically be associated with a reduced cortisol stress response over time. The fact that this study found increasing cortisol reactivity despite reduced novelty of the stressor and decreasing subjective stress further strengthens the evidence for pubertal recalibration. To test the pubertal recalibration hypothesis, one could hypothetically use equivalent but different stressors at each time point in order to maintain novelty of the stressor. Unfortunately, there is not enough evidence in the literature that any 2 laboratory stressors are comparable enough to be confidently treated as equivalent. In any case, repeated exposure to the TSST-C is ecologically valid because youth commonly encounter multiple experiences of speaking in front of the class or engaging in other public social evaluations. Thus, this study provides the best approximation of an adolescent’s cortisol response to multiple exposures of social evaluative stress.
Of particular note, we did not find that age of adoption altered the impact of puberty on recalibration of the cortisol response. We had a wide range of adoption age (5.5 to 59 mo, M = 19.43) and so would have expected to have identified an impact of adoption age if it was there. This means that even those adopted later than age 2 were exhibiting changes in reactivity, consistent with the idea that the increased plasticity of the peripubertal period allowed them to recalibrate. We might have expected less recalibration among those adopted younger because previous work would suggest that they would have a less blunted response (10); however, we did not see evidence of this. It should also be noted that other studies have found persistently reduced cortisol reactivity into adulthood in individuals who experienced both early life stress and repeated, ongoing adversity (31, 32), suggesting that puberty alone does not increase axis reactivity following early adversity in humans. Indeed, it seems likely that conditions need to change markedly for recalibration to be evident. One study examining postinstitutionalized children who were adopted prior to adolescence found continued HPA axis dysregulation in adulthood (33), although this was measured via the CAR and not reactivity to a stressor. It is yet unclear whether evidence of recalibration will generalize beyond situations of early institutional deprivation followed by adoption into well-resourced homes. It will be important to determine whether other conditions of adversity (e.g., child abuse and neglect) that remit prior to the peripubertal period also exhibit evidence of recalibration of the HPA axis.
In conclusion, pubertal development seems to open a window of opportunity for changes in the HPA axis of postinstitutionalized children that tend not to be seen prior to puberty when they are adopted out of institutions into well-resourced homes. There is also a steep increase in the incidence of mental health disorders during the pubertal period (34). The ability to recalibrate the HPA axis toward more typical functioning during puberty could promote resilience in high-risk adolescents who experienced early life adversity, protecting against the emergence of mental health symptoms, although this remains to be tested and should be a focus of future research. To take advantage of the apparent heightened plasticity seen during pubertal development, the present findings argue that interventions to improve the supportiveness of children’s environments should include a focus on the peripubertal period to maximize their effectiveness for children’s stress-mediating systems like the HPA axis.
Materials and Methods
Participants and Study Design.
Participants included 299 youths aged 7 to 15 y at the start of the study (M = 11.28, SD = 2.31). Of those participants, 129 (88 female) were previously institutionalized as infants and toddlers (PI) and 170 (89 female) were born and raised in their natal families (NA). PI youth were selected to have been adopted prior to age 5 (M = 19.43 mo, SD = 12.64) and spent at least 50% of their preadoptive life in the institution (M = 95.19%, SD = 9.54). PI and NA families were roughly matched on socioeconomic status; see Table 1 for demographic information and analyses of group differences in demographics (see SI Appendix, SI Materials and Methods, for additional information on identification of study participants). We assessed participants’ cortisol stress reactivity and pubertal stage at 3 consecutive, annual sessions. We used an accelerated longitudinal design (25), with multiple age cohorts (7 to 15 y) that we followed once per year for 3 assessment periods. This design thus captured a wider developmental period (∼7 to 17 y of age, from the youngest participants at session 1 to the oldest participants at session 3) in a shorter time span than a traditional longitudinal design. Consent was received from 1 parent for each participant, and written and verbal assent were received from all participants. This study was approved by the University of Minnesota Institutional Review Board.
Pubertal Staging.
Trained nurses conducted pubertal staging, scored according to Marshall and Tanner criteria (35, 36). Breast and genital scores, for girls and boys, respectively, were used as a measure of central puberty ranging from 1, indicating pubertal development not yet begun, to 5, indicating pubertal development is complete (see ref. 37 and SI Appendix, SI Materials and Methods, for additional details on pubertal assessment procedures, quality control analyses, and data processing).
Salivary Cortisol Reactivity.
At every annual session, participants completed a modified version of the TSST-C (see refs. 8, 24, and 26 for additional details on this procedure) while being filmed by an obvious camera in front of a 2-way mirror. Seven whole, unstimulated saliva samples were collected throughout the 2-h laboratory visit, as shown in SI Appendix, Fig. S1 (26), to later be assayed for cortisol concentration. The samples were stored in a laboratory freezer at −20 °C until being shipped to the University of Trier, Germany. All samples were assayed in duplicate using a time-resolved fluorescence immunoassay (DELFIA). Additional details on laboratory measures, procedures, quality control analyses, and data processing are provided in SI Appendix, SI Materials and Methods.
Data Analysis.
A hierarchical linear mixed-effects model (38) was used to examine change in cortisol reactivity over time. Time since start of the TSST-C preparatory period and the quadratic effect of time were used as predictors to model cortisol reactivity and recovery, with random intercepts and random effects of time and quadratic time nested within session and within participant. The quadratic effect of time captures the expected inverted U pattern of cortisol production across the TSST-C: an initial increase (reactivity) that eventually peaks and starts to decline again (recovery). To account for the accelerated longitudinal design and disentangle between- and within-person effects of pubertal stage, and capture both an individual’s change in puberty over time as well as an individual’s mean pubertal score compared with other individuals in the study, within- and between-individual pubertal change scores were calculated. Within-individual change in puberty, or the longitudinal change in pubertal score for each individual (pubertal change), was modeled using pubertal stage centered around each participant’s mean across all sessions. Between-individual change in puberty was modeled as each participant’s mean pubertal stage across all sessions (mean pubertal stage). Each pubertal stage variable was then moderated by group (PI vs. NA) to examine whether changes in pubertal stage predicted changes in cortisol reactivity differently for the 2 groups. Thus, the focal predictor was the quadratic time by pubertal change by group moderation term, representing a change in cortisol reactivity and recovery associated with within-individual increases in puberty that differs by group. All models adjusted for sex, as well as sex by group and sex by pubertal stage interactions (additional details on model construction are provided in SI Appendix, SI Materials and Methods).
Data Availability.
The data from this manuscript are available on GitHub (39).
Supplementary Material
Acknowledgments
This study was funded by National Institute of Child Health and Human Development Grant R01 HD075349 (to M.R.G.), National Institute of Mental Health Grant T32 MH015755 (to C.E.D.), and NSF Graduate Research Fellowship 00039202 (to B.M.R.). The content is solely the responsibility of the authors and does not represent the views of the National Institutes of Health or the National Science Foundation. The authors wish to thank the families who participated, the International Adoption Project, and the Center for Neurobehavioral Development at the University of Minnesota. We also thank Tori Simenec, Bao Moua, Lea Neumann, Heather Taylor, and Dr. Chris Desjardins for their assistance with the study; our nurses Janet Goodwalt, Terri Jones, and Melissa Stoll for Tanner staging; and Drs. Brad Miller and Lorah Dorn for providing training in pubertal assessment.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. I.H.G. is a guest editor invited by the Editorial Board.
Data deposition: The data from this manuscript are available at https://github.com/cdepasquale/Gunnar-DePasquale-Reid-Donzella-2019-PNAS.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909699116/-/DCSupplemental.
References
- 1.Loman M. M., Johnson A. E., Quevedo K., Lafavor T. L., Gunnar M. R., Risk-taking and sensation-seeking propensity in postinstitutionalized early adolescents. J. Child Psychol. Psychiatry 55, 1145–1152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopetz C., et al. , Early psychosocial deprivation and adolescent risk-taking: The role of motivation and executive control. J. Exp. Psychol. Gen. 148, 388–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maheu F. S., et al. , A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn. Affect. Behav. Neurosci. 10, 34–49 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tottenham N., et al. , Elevated amygdala response to faces following early deprivation. Dev. Sci. 14, 190–204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiik K. L., et al. , Behavioral and emotional symptoms of post-institutionalized children in middle childhood. J. Child Psychol. Psychiatry 52, 56–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvers J. A., et al. , Vigilance, the amygdala, and anxiety in youths with a history of institutional care. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 493–501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bick J., Fox N., Zeanah C., Nelson C. A., Early deprivation, atypical brain development, and internalizing symptoms in late childhood. Neuroscience 342, 140–153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hostinar C. E., Johnson A. E., Gunnar M. R., Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study. Dev. Psychol. 51, 1597–1608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koss K. J., Mliner S. B., Donzella B., Gunnar M. R., Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology 66, 31–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin K. A., et al. , Causal effects of the early caregiving environment on development of stress response systems in children. Proc. Natl. Acad. Sci. U.S.A. 112, 5637–5642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capitanio J. P., Mendoza S. P., Mason W. A., Maninger N., Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta). Dev. Psychobiol. 46, 318–330 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Herbert J., et al. , Do corticosteroids damage the brain? J. Neuroendocrinol. 18, 393–411 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Fries E., Hesse J., Hellhammer J., Hellhammer D. H., A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Liston C., Gan W. B., Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. U.S.A. 108, 16074–16079 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen B. S., What is the confusion with cortisol? Chronic Stress (Thousand Oaks) 3, 1–3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawler J. M., Koss K. J., Gunnar M. R., Bidirectional effects of parenting and child behavior in internationally adopting families. J. Fam. Psychol. 31, 563–573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King L. S., et al. , The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology 77, 68–74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quevedo K., Johnson A., Loman M., Lafavor T., Gunnar M. R., The confluence of early deprivation and puberty on the cortisol awakening response: A study of post-institutionalized children. Int. J. Behav. Dev. 36, 19–28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romeo R. D., Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol. 31, 232–240 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Romeo R. D., The metamorphosis of adolescent hormonal stress reactivity: A focus on animal models. Front. Neuroendocrinol. 49, 43–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morley-Fletcher S., Rea M., Maccari S., Laviola G., Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur. J. Neurosci. 18, 3367–3374 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Francis D. D., Diorio J., Plotsky P. M., Meaney M. J., Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 22, 7840–7843 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen A. P., et al. , The Trier Social Stress Test: Principles and practice. Neurobiol. Stress 6, 113–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yim I. S., Quas J. A., Cahill L., Hayakawa C. M., Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology 35, 241–248 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Galbraith S., Bowden J., Mander A., Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Stat. Methods Med. Res. 26, 374–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DePasquale C. E., Donzella B., Gunnar M. R., Pubertal recalibration of cortisol reactivity following early life stress: A cross-sectional analysis. J. Child Psychol. Psychiatry 60, 566–575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroud L. R., Papandonatos G. D., Williamson D. E., Dahl R. E., Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology 36, 1226–1238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumenthal H., Leen-Feldner E. W., Badour C. L., Trainor C. D., Babson K. A., Pubertal maturation and cortisol level in response to a novel social environment among female adolescents. J. Adolesc. 37, 893–900 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Del Giudice M., Ellis B. J., Shirtcliff E. A., The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 35, 1562–1592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoki C., Romeo R. D., Smith S. S., Adolescence as a critical period for developmental plasticity. Brain Res. 1654, 85–86 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Goldman-Mellor S., Hamer M., Steptoe A., Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology 37, 1755–1768 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Young E. S., et al. , The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: A prospective study. Psychol. Sci. 30, 739–747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumsta R., et al. , HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology 86, 196–202 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Ormel J., et al. , Mental health in Dutch adolescents: A TRAILS report on prevalence, severity, age of onset, continuity and co-morbidity of DSM disorders. Psychol. Med. 45, 345–360 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Marshall W. A., Tanner J. M., Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall W. A., Tanner J. M., Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid B. M., et al. , Early growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatr. Res. 82, 278–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinheiro J., Bates D., Mixed-Effects Models in S and S-PLUS (Springer Science & Business Media, 2006). [Google Scholar]
- 39.Gunnar M. R., Gunnar, DePasquale, Reid, & Donzella 2019 PNAS. GitHub. https://github.com/cdepasquale/Gunnar-DePasquale-Reid-Donzella-2019-PNAS. Deposited 8 October 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this manuscript are available on GitHub (39).