Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a severe and dose-limiting side effect of cancer treatment that affects millions of cancer survivors throughout the world and current treatment options are extremely limited by their side effects. Cannabinoids are highly effective in suppressing pain symptoms of chemotherapy-induced and other peripheral neuropathies but their widespread use is limited by central nervous system (CNS)-mediated side effects. Here, we tested one compound from a series of recently developed synthetic peripherally restricted cannabinoids (PRCBs) in a rat model of cisplatin-induced peripheral neuropathy. Results show that local or systemic administration of 4-{2-[-(1E)-1[(4-propylnaphthalen-1-yl)methylidene]-1H-inden-3-yl]ethyl}morpholine (PrNMI) dose-dependently suppressed CIPN mechanical and cold allodynia. Orally administered PrNMI also dose-dependently suppressed CIPN allodynia symptoms in both male and female rats without any CNS side effects. Co-administration with selective cannabinoid receptor subtype blockers revealed that PrNMI’s anti-allodynic effects are mediated by CB1 receptor (CB1R) activation. Expression of CB2Rs was reduced in dorsal root ganglia from CIPN rats, whereas expression of CB1Rs and various endocannabinoid synthesizing and metabolizing enzymes was unaffected. Daily PrNMI treatment of CIPN rats for two weeks showed a lack of appreciable tolerance to PrNMI’s anti-allodynic effects. In an operant task which reflects cerebral processing of pain, PrNMI also dose-dependently suppressed CIPN pain behaviors. Our results demonstrate that PRCBs exemplified by PrNMI may represent a viable option for the treatment of CIPN pain symptoms.
Keywords: Chemotherapy neuropathy, Allodynia, CB1 receptor, CB2 receptor, Endocannabinoid enzymes, Operant behavior, Tolerance
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a severe and dose limiting side-effect of cancer treatment by antineoplastic agents, causing significant disability among cancer survivors (Argyriou et al., 2014). Neuropathy starts distally with a characteristic stocking glove distribution presenting signs of numbness, paresthesia, dysesthesia and pain aggravated by physical activity. Symptoms are observed within a few days after initiation of the therapy and continue to progress for several months, especially in the case of platinum based compounds (van der Hoop et al., 1990). Medications recommended for other neuropathy types, although used for CIPN, show limited efficacy (Hammack et al., 2002; Majithia et al., 2016; Rao et al., 2007).
Mechanisms underlying CIPN development vary depending on the cytostatic agent used (Areti et al., 2014). However, lack of protection by the blood-nerve barrier make neurons within dorsal root ganglia (DRG) highly susceptible to the actions of anticancer agents (Abram et al., 2006). Thus, damage to mitochondria of long axons and somata of DRG neurons is considered the primary reason for neuropathy (Bennett et al., 2014; Sisignano et al., 2014). In addition to mitotoxicity, direct activation of ligand or voltage gated ion channels and intracellular signaling pathways of DRG neurons by cytostatic agents alter peripheral nerve excitability resulting in dysesthesia and pain (Bennett et al., 2014; Park et al., 2009; Sisignano et al., 2014).
Cannabinoids suppress allodynia and hyperalgesia associated with chronic inflammatory and neuropathic pain states in animal and human studies (Berman et al., 2004; Herzberg et al., 1997). In fact, activation of both cannabinoid 1 and cannabinoid 2 receptor (CB1R and CB2R) induces potent anti-allodynic effects in rodent models of CIPN (Deng et al., 2012; Vera et al., 2013). While the primary site of CB2R-mediated anti-allodynic effects is still unclear (Atwood and Mackie, 2010), both tissue-selective knockout and site-specific drug administration studies demonstrate that activation of CB1Rs on primary sensory neurons contributes to the majority of anti-allodynic effects of cannabinoids observed in rodent models of chronic pain (Agarwal et al., 2007; Fox et al., 2001), which eventually led to the development of several peripherally restricted cannabinoid receptor agonists (PRCBs) to harness the potential of CB1R-mediated peripheral analgesic effects and circumvent CNS side effects (Adam et al., 2012; Dziadulewicz et al., 2007; Seltzman et al., 2016; Yu et al., 2010). Despite being known for their potent anti-allodynic effects in models of peripheral nerve injury, and most recently in a model of cancer-induced bone pain (Zhang et al., 2018), the efficacy of PRCBs in alleviating CIPN symptoms is yet to be determined. Here, we use both conventional withdrawal reflex and operant task-based behavioral assays in a rat model of CIPN to examine the anti-allodynic efficacy of 4-{2-[-(1E)-1[(4-propylnaphthalen-1-yl)methylidene]-1H-inden-3-yl]ethyl} morpholine (PrNMI), one of the compounds from our recently developed indene series of PRCBs which we showed to be full agonists at CB1Rs and partial agonists at CB2Rs (Seltzman et al., 2016). We also examine the effect of CIPN on expression of CB1Rs and CB2Rs, as well as the various endocannabinoid synthesizing and metabolizing enzymes. Furthermore, we address cannabinoid receptor selectivity, sex differences, and development of tolerance to repeated PrNMI administration.
2. Materials and methods
2.1. Subjects
Adult male and female Sprague–Dawley rats (Envigo, Placentia, CA) weighing 200–225 g are used throughout the study. Rats are housed in the vivarium under a 12 h light/dark cycle (lights on at 6AM) and have ad libitum access to food and water during the entire experiment. All experimental procedures are carried out in accordance with the National Institute of Health guidelines for the handling and use of laboratory animals and with approval from the Animal Research Committee of the University of California, Los Angeles.
2.2. Cisplatin induced peripheral neuropathy
Cisplatin (Sigma-Aldrich, St. Louis, MO) is dissolved in sterile bacteriostatic 0.9% NaCl solution (Hospira, Lake Forest, IL) at a concentration of 1 mg/mL and administered by intraperitoneal (i.p.) injection (27½ gauge needle) once a week at a dose of 3 mg/kg for 4 weeks. Control rats are injected similarly with equal amounts of saline. To minimize nephrotoxicity, all cisplatin-treated rats are pre-injected subcutaneously with 2 mL of 4% NaHCO3 in sterile saline (Hospira).
2.3. Behavioral testing
All behavioral experiments are performed between 9 a.m. and 6 p.m. Investigators involved in behavioral testing and data analysis are blinded to the nature and dose of the experimental drugs administered.
2.3.1. Mechanical sensitivity testing
Rats are placed in a plastic-walled cage (10 × 20 × 13 cm) with a metal mesh floor (0.6 × 0.6 cm holes) and allowed to acclimate for 15 min. The hindpaw withdrawal thresholds are determined in response to pressure from a digital von Frey anesthesiometer (Model 1601C, IITC Instruments, Woodland Hills, CA). The amount of pressure (g) needed to produce a paw-withdrawal response is measured two times on each paw separated by 5 min intervals to prevent sensitization/desensitization of responses. The results of the tests are averaged across both hindpaws. For intraplantar administration and testing, 3 trials are averaged for each paw. Allodynia suppression is calculated using the formula:
2.3.2. Cold sensitivity testing
The method to evaluate withdrawal responses to cold stimulation is adapted from Guindon et al. (2013). Briefly, rats are placed in an individual plastic cages on an elevated metal mesh floor similar to that described in mechanical hypersensitivity testing and allowed to acclimate for 15 min. A 1 mL syringe without the needle is loaded with acetone at room temperature and a single drop (~20 μL) is applied through the mesh platform by gently touching the plantar surface of the hindpaw. Care is taken to avoid mechanical stimulation by the syringe tip. A flick or brisk withdrawal is considered a positive response and the percent response is obtained by averaging a total of six trials (3 from each hindpaw). Each trial is performed at least 5 min apart. Data are expressed as percent withdrawal response or % allodynia suppression (calculated as described above).
2.3.3. Rotarod
Rats are tested for motor function and the ataxic effects of drugs as described previously (Seltzman et al., 2016). Rats are trained 72 h before the test (3 sessions 24 h apart) to remain for at least 180 s on a rotarod revolving at an acceleration of 4–40 revs over 5 min. Rats are tested 1 h before vehicle or drug injections and tested again at 2, 6, 24, and 48 h after drug administration. The time for which the rats are able to remain on the rotarod is recorded up to a cutoff of 3 min.
2.3.4. Hypothermia
Rats are acclimated to a plastic restrainer apparatus (Model RTV-180 Braintree Scientific Inc., Braintree, MA) on the day of testing by placing them in the restrainer twice for 5 min separated by 20 min. Core temperature measurements are obtained by inserting rectally (1 inch depth) a sterile lubricating jelly (First Priority, Elgin, IL)-coated thermometer probe (model RET-2, Physitemp Instruments, Clifton, NJ) and recording the digital temperature readout (model BAT-12, Physitemp Instruments) at 1 min after probe insertion. Baseline core temperature is taken before treatment, and again at 2, 6, 24 and 48 h after drug administration.
2.3.5. Catalepsy (ring) test
Catalepsy is determined with a ring immobility test modified for rats (Fox et al., 2001). Rats are placed with their forepaws on a horizontal metal ring (12 cm diameter) at a height that allowed their hindpaws to just touch the bench surface. Immobility is recorded as the time for the rat to move off the ring with a 100s cutoff. Rats are tested before drug administration and again at 2, 6, 24, and 48 h after administration.
2.3.6. Tail-flick test
(model 390, IITC Instr.) is also used to measure tail-flick latency (TFL). Radiant heat is directed to a point 3 cm from the tail tip and the TFL observed and timed with a photo cell counter. The intensity of the radiant heat is adjusted for a baseline TFL of approximately 5–7 s for naïve rats, with a 25 s cutoff set to avoid tissue damage.
2.3.7. Operant behavior analysis
A mechanical conflict avoidance system (Stoelting, Wood Dale, IL) based on Harte-Morrow method is used to assess the operant behavior of CIPN and control rats (Harte et al., 2016). The apparatus is made of bright and dark compartments separated by 15-inch (38 cm) long middle compartment arranged with an array of height-adjustable probes (0.5–4.0 mm). Before baseline measurements, 4 training sessions are carried out over 4 days. Rats are allowed to explore all the compartments without nociceptive probes for 5 min and the bright compartment is illuminated with a low heat generating light-emitting diode bulb delivering an average of 544 lm/ft2 at floor level. On the day of baseline recordings, rats are tested for escape latencies with nociceptive probes set at variable heights (0.5, 1.0, 2.0 and 3.0 mm). For each trial, rats are placed individually into the light compartment with the light turned off and an escape door closed. Following 10 s of dark acclimatization, compartment light is turned on for the rest of the trial. The escape door is released 20 s after the light is turned on. The animal is considered inside the dark compartment when all four paws are placed completely inside that compartment. For rats that initiated walking on the nail bed but failed to reach the dark compartment during the 3-min testing period, a cutoff latency of 120 s is used. All trials are video recorded for offline analysis.
2.4. Quantitative PCR
L4-L5 DRG are harvested from CIPN and control rats ~1 week after the administration of the 4th cisplatin or saline injection. Total RNA is extracted with RNEasy mini kit (Qiagen, Valencia, CA) and concentration is determined by Nanophotometer (Implen, Munich, Germany). 1 μg of total RNA is used for cDNA synthesis (Superscript III, Life Technologies, Carlsbad, CA) and qPCR reaction in triplicates is carried out with Taqman gene expression master mix (Life Technologies) on a 7900 HT real time fast PCR system. Primers and probes (Life Technologies, and Integrated DNA Technologies, San Diego, CA) used for qPCR reaction are shown in Table 1. The mRNA levels of cannabinoid receptors and endocannabinoid metabolizing enzymes are normalized to the internal reference gene Gapdh. Fold change in mRNA is calculated using the 2−ddCt method (Livak and Schmittgen, 2001).
Table 1.
Primers and probes used for qPCR.
| Cnr1 | Rn00562880_m1 |
|---|---|
| Cnr2 | Primer 1: 5′-TCA TAG GGT TGA ACT CCA AGC-3′ |
| Primer 2: 5′-CAT CTA TGC TGA CTC TGA ATG GG-3′ | |
| Probe: 5'-/56-FAM/ACT CCA GCT/ZEN/CCC GGC ATC C/3IABkFQ/-3′ | |
| Napepld | Primer 1: 5′-ACG GAT CTC TTG GCT TGA AC-3′ |
| Primer 2: 5′-TTT GAC CTC GCG GCT ATT C-3′ | |
| Probe: 5'-/56-FAM/TGT GAA TCC/ZEN/TTA CAG CAT CCT CTG GG/3IABkFQ/-3′ | |
| Dagla | Primer 1: 5′-AGA GGA ACA CTT TTA GAC GGC-3′ |
| Primer 2: 5′-AAC CTG CGG ACT TAC AAC C-3′ | |
| Probe: 5'-/56-FAM/CAT CGG TTA/ZEN/GAG GAG GGT CAG GC/3IABkFQ/-3′ | |
| Mgll | Primer 1: 5′-GGC AGG ACA AAA TTG AGC AG-3′ |
| Primer 2: 5′-AGA GAC CAA CCC ACT TTT CTG-3′ | |
| Probe: 5'-/56-FAM/CCG GAT TGG/ZEN/CAA GGA TCA GAG GT/3IABkFQ/-3′ | |
| Faah | Primer 1: 5′-TCA CAG TCG GTC AGA TAG GAG-3′ |
| Primer 2: 5′-CAG AAG TTA CAG AGT GGA GAG C-3′ | |
| Probe: 5'-/56-FAM/CCT GGG AAG/ZEN/TGA ACA AAG GGA CCA A/3IABkFQ/-3′ | |
| Gapdh | Primer 1: 5′-GTA ACC AGG CGT CCG ATA C-3′ |
| Primer 2: 5′- TCT CTG CTC CTC CCT GTT C-3′ | |
| Probe: 5'-/56-FAM/CAC ACC GAC/ZEN/CTT CAC CAT CTT GTC T/3IABkFQ/-3′ | |
2.5. Western blotting
L4-L5 DRG samples are isolated from the same rats used in qPCR experiments. Tissue samples are homogenized with Dounce homogenizer in a RIPA lysis buffer (Sigma-Aldrich) with Halt protease inhibitor cocktail (Invitrogen, Carlsbad, CA) and total protein concentration is determined by DC protein assay (Biorad, Hercules, CA). Samples (25 μg) are mixed with a loading dye in a 1:1 ratio and heated to 65 °C for 10 min prior to running on 4–15% polyacrylamide stain free gels (Mini-protean TGX Stain free gels, Biorad). Proteins are segregated under denatured running conditions and transferred to 0.2 μ PVDF (Immun-blot, Biorad) using a wet transfer method. Blots are incubated with anti-L15 CB1R 1° antibody in 2% ECL prime blocking reagent (1:1000) overnight (16 h) at 4° C followed by an HRP-conjugated 2° antibody (2.5% NFDM, 1:2000, Millipore, Temecula, CA) at room temperature for 1 h. The CB1R 1° antibody has been extensively characterized in numerous publications (Coutts et al., 2002; Hajos et al., 2000). Stain-free images are taken prior to application of substrate (ECL Prime detection reagent, GE) on the blot using ChemiDoc MP imaging system (Biorad). CB1R bands are visualized by chemiluminescence detection by ChemiDoc MP imager (Biorad). Acquired images are then analyzed by ImageLab software (BioRad). Band volume intensity of each lane is normalized to the total lane protein.
2.6. Drugs
PrNMI is synthesized by Dr. Seltzman at Research Triangle Institute (RTI, Research Triangle Park, NC). SR141716 and SR144528 are also synthesized at RTI as part of the National Institute of Drug Abuse (NIDA) drug supply program. For intraperitoneal (i.p.) and intraplantar injections, PrNMI is first dissolved in a 50/50% mixture of pure DMSO and Tween 80 (Fisher Scientific), then appropriately diluted in sterile saline (1.5 mL/kg for i.p.) and administered using 29½ gauge sterile needles and 1mL syringes equipped with a 0.22 μm filter. For oral administration, PrNMI, selective CB1R antagonist, SR141716, and selective CB2R antagonist, SR144528, are dissolved in pure DMSO, appropriately diluted in 20% sweet condensed milk (16 mL/kg), and delivered intragastrically (i.g.) by oral intubation with the aid of a ball-tipped gavage needle and a 5-mL syringe. For vehicle administration, vehicles had the same composition but the active drug was omitted. In all rat cohorts, experimental drugs are administered 2–7 days after the last cisplatin injection and a minimum of 3-day interval is maintained for all the subsequent drug administrations except for tolerance studies.
2.7. Data analysis
Data are expressed as mean ± standard error of mean (S.E.M). Two group comparisons are made with unpaired t-test. Analysis of variance with appropriate post-hoc method is used for within and between group comparisons. Least squares non-linear regression is used to plot the dose-response curves and estimate ED50 values. Between group dose-response curves are compared by multiple sum of squares F test. A p value of <0.05 is considered statistically significant. All statistical analyses are performed using Prism 7 software (GraphPad Software Inc., La Jolla, CA).
3. Results
3.1. Cisplatin induced peripheral neuropathy
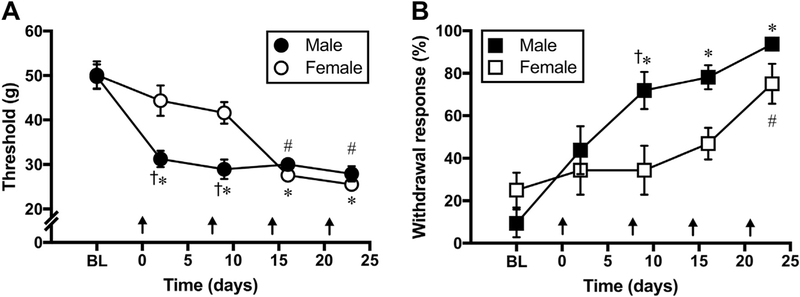
Before the start of cisplatin injections, the average mechanical withdrawal thresholds of male and female rats are 49.73 ± 2.76 g and 50.14 ± 3.06 g, respectively. Withdrawal responses to acetone application are 9.37 ± 6.57% for males and 25 ± 8.18% for females. Repeated injections of cisplatin (3 mg/kg, i.p., once weekly) result in significantly decreased withdrawal thresholds in male [Ftreatment (2.68, 18.78) = 25.62, p < 0.0001 vs baseline] and female rats [Ftreatment (2.71, 19.02) = 20.58, p < 0.0001 vs baseline], indicating mechanical allodynia (Fig. 1A–B). Similarly, withdrawal responses to acetone application become exaggerated after cisplatin injections in both cohorts [Male: Ftreatment (1.55, 10.9) = 24.15, p < 0.0001 vs baseline; Female: Ftreatment (2.48, 17.42) = 4.14; p < 0.05 vs baseline], indicating cold allodynia (Fig. 1C–D). However, a significant delay in the development of mechanical [Fgender (1, 70) = 9.23, p < 0.05] and cold allodynia [Fgender (1, 70) = 8.62, p < 0.05] is observed in female vs male cisplatin-treated rats.
Fig. 1. Cisplatin-induced mechanical and cold allodynia in male and female rats.
(A-B) Time course of the development of cisplatin induced mechanical (A) and cold allodynia (B) after weekly injections of cisplatin (3 mg/kg, i.p.) in male and female rats. One-way analysis of variance shows that cisplatin treatments significantly reduce mechanical withdrawal thresholds and increase acetone withdrawal responses (%) in male (*, p < 0.05 vs baseline) and female rats (#, p < 0.05 vs baseline). However, a slight delay in the development of allodynia symptoms is noticed in female rats (†, p < 0.05 vs female; two-way analysis of variance, n = 8 rats/group). Arrows indicate timing of cisplatin injections.
3.2. PrNMI suppression of CIPN symptoms is more effective after local rather than systemic administration
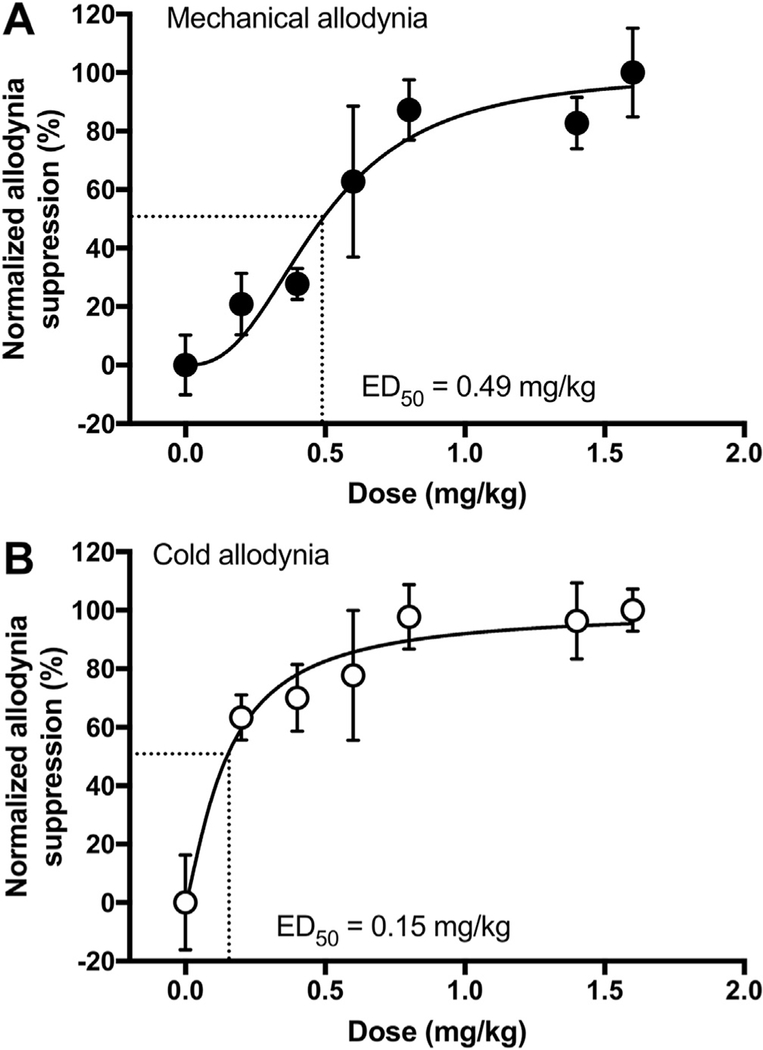
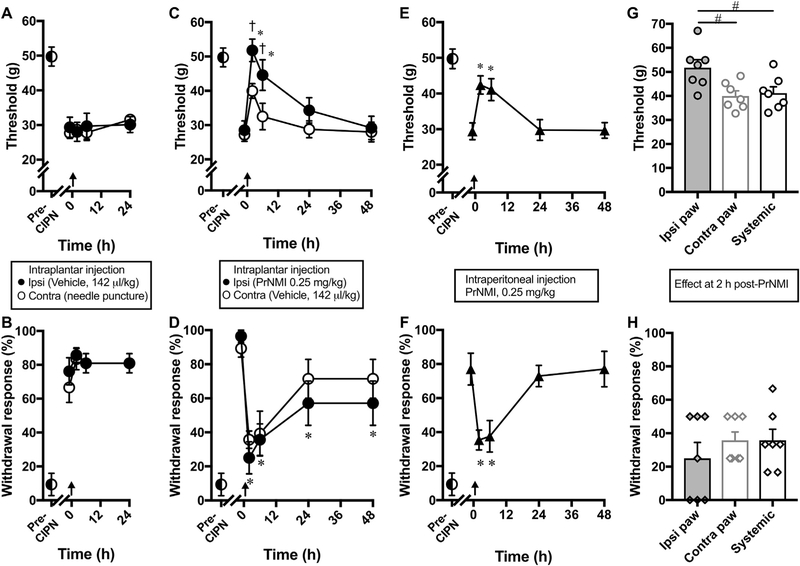
Dose dependent anti-allodynic effects of PrNMI in male rats measured two hours after intraperitoneal administration are shown in Fig. 2. The 2-h time period is chosen based on our previous pharmacokinetic data from rats (Seltzman et al., 2016). Dose-response curves yielded an estimated ED50 of 0.49 ± 0.06 mg/kg for mechanical allodynia suppression and 0.15 ± 0.07 mg/kg for cold allodynia suppression (Fig. 2A–B). We also compared the effectiveness of local PrNMI administration at the site of allodynia testing with its systemic administration. Intraplantar administration of a vehicle or needle puncture alone has no significant effect on withdrawal thresholds to mechanical [Ftreatment (1.91, 11.48) = 0.27, p = 0.758 for vehicle; Ftreatment (1.92, 11.55) = 1.8, p = 0.209 for puncture] and cold stimuli [Ftreatment (1.35, 8.15) = 1.28, p = 0.308 for vehicle; Ftreatment (1.19, 7.16) = 1.32, p = 0.29 for puncture] (Fig. 3A–B). At 2 h post-intraplantar injection, PrNMI (0.25 mg/kg) completely suppresses cisplatin-induced mechanical and cold allodynia symptoms ipsilateral to injection [Ftreatment (2.05,12.32) = 21.95, p < 0.0001 for mechanical; Ftreatment (2.67,16.04) = 11.75, p < 0.001 for cold] with only a partial effect at the contralateral hindpaw [Ftreatment (2.1, 12.66) = 9.86, p = 0.002 for mechanical; Ftreatment (2.24, 13.46) = 10.7, p = 0.0013 for cold] (Fig. 3C–D). In contrast, systemic administration of the same dose produces only partial suppression of neuropathy symptoms at its peak effect [Ftreatment (2.23, 15.61) = 11.02, p = 0.0008 for mechanical; Ftreatment (2.49, 17.45) = 10.54, p = 0.0006 for cold] (Fig. 3E–F). One-way analysis of variance revealed that at 2 h post-PrNMI, mechanical but not cold allodynia suppression is significantly greater on the ipsilateral paw compared to contralateral paw or systemic administration [Ftreatment (2, 18) = 5.7, p = 0.012] (Fig. 3G–H).
Fig. 2. PrNMI dose-dependently suppresses cisplatin-induced mechanical (A) and cold allodynia (B) in male rats.
PrNMI is administered by intraperitoneal injection. Data points are fit by least squares non-linear regression to estimate ED50 values (n = 6–7 rats).
Fig. 3. Suppression of CIPN-induced allodynia symptoms after local compared to systemic administration of PrNMI.
(A–B) Pre-CIPN (◐) responses to mechanical and cold stimuli become exaggerated after cisplatin treatment, indicative of allodynia development. Intraplantar ipsilateral vehicle (●) administration and contralateral puncture with a 29½ gauge needle (○) does not affect cisplatin-induced symptoms of mechanical (A) or cold (B) allodynia. (C–D) Intraplantar ipsilateral PrNMI (0.25 mg/kg) administration (●) and contralateral vehicle (○) administration results in complete suppression (at peak effect) of mechanical (C) and cold (D) allodynia symptoms on the ipsilateral side and a smaller effect on the contralateral side. (E–F) Systemic PrNMI (0.25 mg/kg, i.p.) administration (▲) also produces a significant but sub-maximal suppression of mechanical (E) and cold (F) allodynia symptoms. (G–H) Comparison of site-specific effects revealed that local PrNMI is more effective on the ipsi paw compared to contra paw or systemic dose at 2 h post-injection. Each data point represents mean ± SEM of 7 rats. Arrows indicate timing of puncture, or injection of vehicle or PrNMI. *, p < 0.05 vs. pre-drug values (one-way repeated measures analysis of variance). †, p < 0.05 vs. contralateral values (two-way analysis of variance). #, p < 0.05 (one-way analysis of variance).
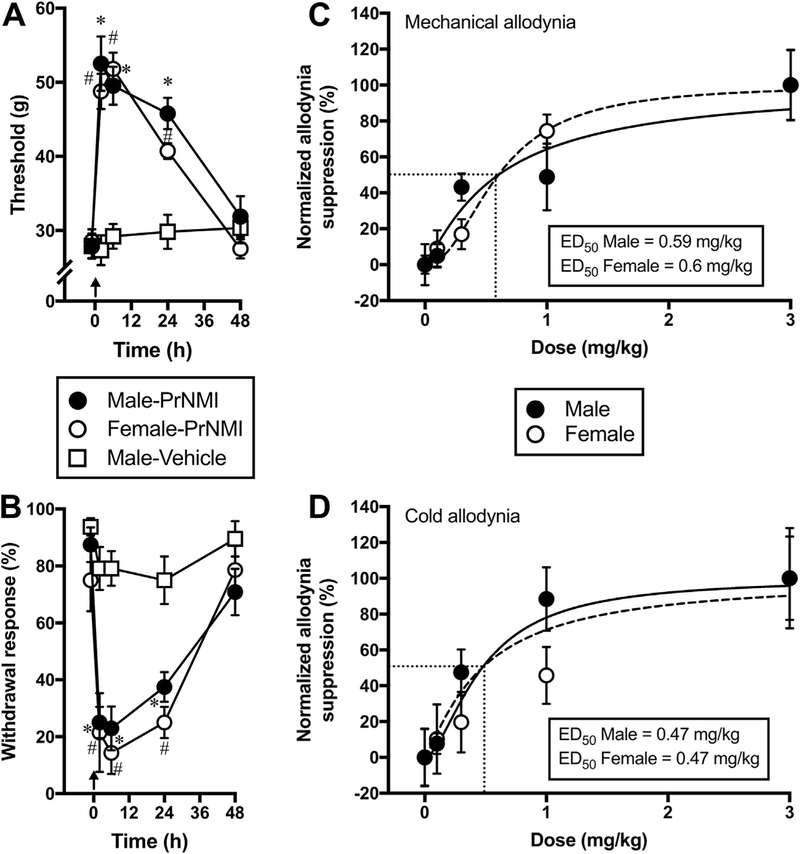
3.3. PrNMI is equally effective in male and female rats after oral administration
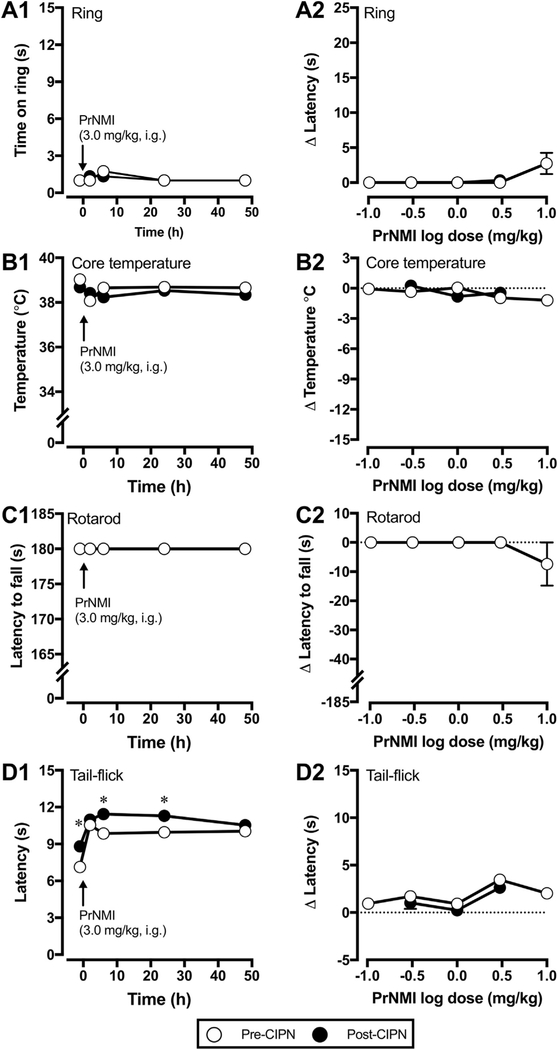
Previous studies demonstrated sex differences in rodent responses to administration of centrally active cannabinoids (Cooper and Craft, 2018; Cooper and Haney, 2016; Craft et al., 2013b; Tseng and Craft, 2001 ). To address possible sex differences, we compared the dose response curves of PrNMI after oral administration. PrNMI at 3.0 mg/kg completely suppresses mechanical [p < 0.0001 vs pre-drug] and cold allodynia symptoms [p < 0.0001 vs pre-drug] in male and female rats with significant effects lasting >24 h after administration (Fig. 4A–B). Furthermore, oral PrNMI dose-dependently reduces CIPN symptoms in both male and female rats with no significant difference among their ED50 values (Fig. 4C–D). The estimated ED50 values in male and female rats after oral administration are 0.59 and 0.60 mg/kg for mechanical allodynia suppression and 0.47 mg/kg each for cold allodynia suppression. In addition, oral PrNMI does not elicit any CNS mediated side-effects in naïve female rats at doses 0.1, 0.3,1.0, 3.0 & 10.0 mg/kg (Fig. 5), similarly to our previously reported results in male rats (Seltzman et al., 2016). Tetrad testing of the same female rats after cisplatin treatments also shows no CNS side effects at 0.3, 1.0 & 3.0 mg/kg, i.g. PrNMI (Fig. 5), suggesting that the restriction of PrNMI to the periphery is unaffected by cisplatin treatments. However, tail flick latencies of these rats are significantly increased post-chemotherapy (p < 0.05 vs pre-CIPN) indicating cisplatin-induced heat hypoalgesia (Fig. 5D1 and D2) (Han et al., 2014; Hopkins et al., 2016; Seto et al., 2016). Since PrNMI is equally effective in male and female CIPN rats, we continued all subsequent experiments with male rats.
Fig. 4. Oral PrNMI dose-dependently suppresses CIPN symptoms in both male and female rats.
(A-B) Oral PrNMI at 3.0 mg/kg significantly reduces cisplatin induced mechanical (A) and cold (B) allodynia in male (●) and female (○) rats at 2, 6 and 24 h post-drug. No effect of vehicle (□) is observed in male CIPN rats. Arrows indicate timing of vehicle or PrNMI administrations. *(male), #(female), p < 0.05 vs pre-drug (one-way repeated measures analysis of variance; Šídák’s multiple comparison test, n = 8 rats). (C-D) Cisplatin-induced mechanical (C) and cold (D) allodynia symptoms are dose-dependently reduced in male (●) and female rats (○) 2 h after oral administration of PrNMI at 0.1, 0.3,1.0 or 3.0 mg/kg. Data points are fit by least squares non-linear regression to estimate ED50 values (n = 7–8 rats/group).
Fig. 5. Tetrad assays of oral PrNMI effects in female rats before and after CIPN.
(A1-D1) Comparison of time-dependent effects of PrNMI at 3.0 mg/kg, i.g. before (○) and after (●) induction of CIPN in ring, core temperature, rotarod and tail-flick assays. Cisplatin treatments do not affect peripheral restriction of PrNMI as observed by the lack of significant effects in ring, core temperature and rotarod assays. However, tail-flick latencies are significantly increased after CIPN when tested at 1 h before and 6 & 24 h after PrNMI administration, indicative of cisplatin induced heat hypoalgesia (*,p < 0.05 vs. pre-CIPN, paired t-test; Bonferroni-Šídák correction method, n = 6 rats). (A2-D2) At peak effect (2 h), after oral administration of different doses of PrNMI, tetrad assays are compared before and after CIPN induction. Note the lack of significant effects at all doses tested.
3.4. Anti-allodynic effects of PrNMI in CIPN are mediated mainly by CB1Rs
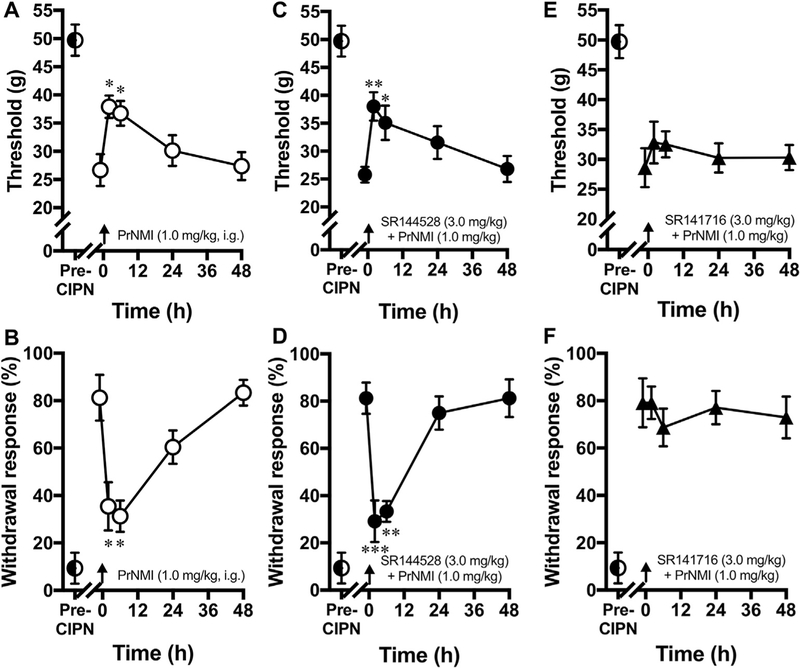
PrNMI is a full agonist at CB1R and only a partial agonist at CB2R (Seltzman et al., 2016). To determine the relative contribution of CBR subtypes to the alleviation of CIPN symptoms, we co-administered PrNMI with either CB1R or CB2R antagonists. Oral administration of PrNMI at 1.0 mg/kg produces submaximal suppression of CIPN symptoms (Fig. 6A–B) which are blocked, albeit incompletely, by the selective CB1R antagonist SR141716 (Fig. 6E–F), but not the selective CB2R antagonist SR144528 (Fig. 6C–D), indicating that the anti-allodynic effects of PrNMI in CIPN are mediated mainly by CB1Rs.
Fig. 6. Anti-allodynic effects of PrNMI in the CIPN are mediated mainly by CB1Rs.
(A–B) Suppression of cisplatin-induced mechanical (A) and cold (B) allodynia by PrNMI (1.0 mg/kg, i.g., ○). (C–D) In the same rats, co-administration of the selective CB2R blocker SR144528 (3 mg/kg, i.g., ●) does not affect the response to PrNMI. (E–F) However, PrNMI co-administration with the selective CB1R blocker SR141716 (3 mg/kg, i.g., ▲) blocks the response to PrNMI. Arrows indicate timing of drug administrations. *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. pre-drug (one-way repeated measures analysis of variance, Šídák’s multiple comparison method, n = 8/group).
3.5. Lack of appreciable tolerance after repeated PrNMI administration
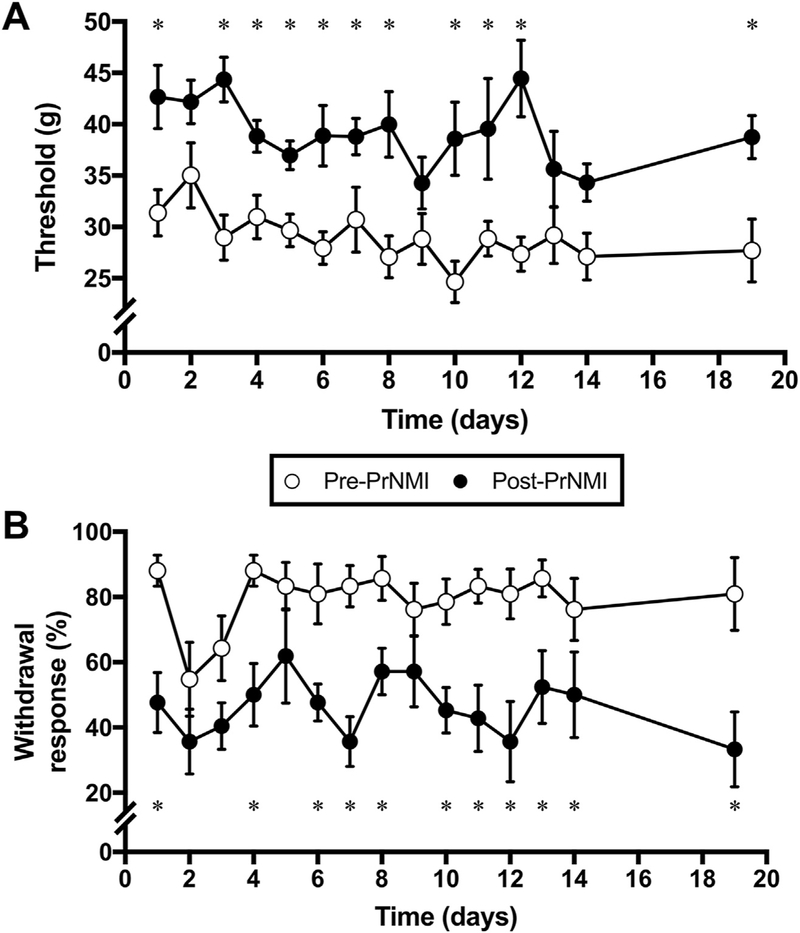
Development of tolerance and dependence are of major concern with the repeated use of synthetic and naturally occurring cannabinoids. Tolerance to antinociceptive effects has been observed after 3–7 daily administrations in uninjured animals (Bass and Martin, 2000; Gonzalez et al., 2005). By contrast, no appreciable tolerance to the anti-allodynic effects of cannabinoids have been observed in clinical trials (Burstein, 2005; Russo, 2008) or to continuous delivery of cannabinoids using osmotic mini-pumps in a rodent CIPN model (Rahn et al., 2014). To address the potential development of tolerance to anti-allodynic effects of PrNMI in the CIPN model, we administered PrNMI daily (1 mg/kg, i.g.) for two weeks and compared the post-treatment responses to their pre-treatment baselines. Results show no significant tolerance to suppression of both mechanical (significant effect of treatment [Ftreatment (1,12) = 30.56, p = 0.0001] and no interaction [Ftreatment × time (14,168) = 1.1, p = 0.354]) and cold allodynia (significant effect of treatment [Ftreatment (1,12) = 32.07, p = 0.0001] and no interaction [Ftreatment × time (14,168) = 0.71, p = 0.761]) during the two-week testing period (Fig. 7A–B).
Fig. 7. Daily oral administration of PrNMI (1 mg/kg) for 2 weeks suppresses mechanical (A) and cold (B) allodynia CIPN symptoms.
*, p < 0.05 (two-way repeated measures analysis of variance with Fisher’s least significant difference test, n = 8).
3.6. PrNMI suppresses CIPN symptoms in an operant-based mechanical conflict system
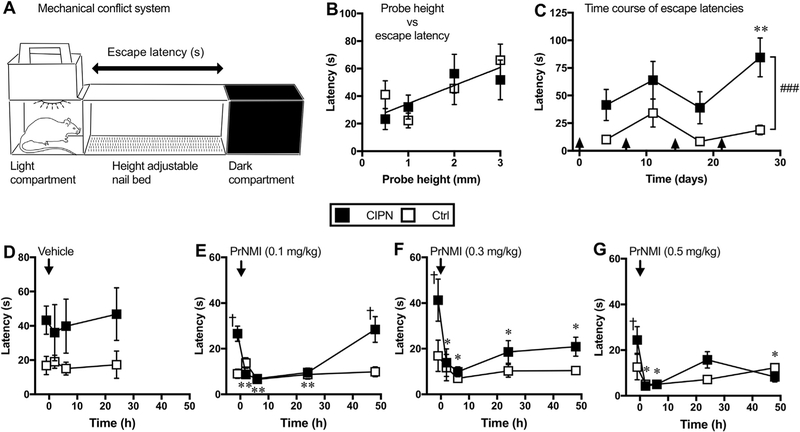
A significant portion of cancer survivors experience CIPN-induced functional disability often characterized by slow walking speed and increased risk of falls (Hile et al., 2010; Lichtman et al., 2012; Winters-Stone et al., 2017). To date there have been no studies that examined the efficacy of cannabinoids in improving CIPN-induced functional disability in rodent models. Mechanical conflict system is an operant task in which rats are given a choice to remain in an aversive bright light compartment or to escape to a dark compartment by crossing an array of nociceptive probes (Fig. 8A). Prior to saline/cisplatin treatment, the average escape latencies measured in control and CIPN groups combined at probe heights of 0.5, 1, 2 and 3 mm are 32.13 ± 6.54, 27.16 ± 5.07, 50.91 ± 8.96 and 58.94 ± 9.15 s respectively (Fig. 8B). These baseline escape latencies show a positive correlation between probe height and latency (r = 0.93, Pearson correlation; p = 0.03, one-tailed t-test; n =16 rats) suggesting that elevated probes are relatively more nociceptive. However, in subsequent tests the escape latencies measured at 3 mm probe height decreased significantly in control rats, most likely due to improved performance in navigating through the nail bed after repeated testing. In contrast, the mean escape latencies measured at the same probe height have remained significantly higher in cisplatin-treated rats indicating that CIPN impairs rat’s ability to cross the nail bed [Ftreatment (1, 56) = 20.77, p < 0.001 vs control; Ftime (3, 56) = 2.96, p = 0.039; Ftreatment × time (3, 56) = 1.04, p = 0.37]. (Fig. 8C). Administration of PrNMI (0.1 mg/kg, i.p.), but not vehicle, in CIPN rats significantly reduces escape latencies at 2, 6 and 24 h post-injection (Fig. 8D–E). However, higher doses of PrNMI at 0.3 and 0.5 mg/kg decrease escape latencies for up to 48 h post-injection indicating longer-lasting suppression of CIPN-induced pain behaviors (Fig. 8F–G). These results demonstrate that PrNMI can effectively improve performance of CIPN rats in a mechanical conflict system-based operant assay.
Fig. 8. CIPN behaviors are potently suppressed by PrNMI in the mechanical conflict system-based operant assay.
(A) Schematic view of the mechanical conflict system equipped with light and dark compartments separated by a 15-inch (38-cm) long middle compartment with a height adjustable nail bed. (B) XY scatter plot of baseline measurements showing a positive correlation between the probe height (mm) and escape latency (s). A single correlation coefficient is calculated for both CIPN (■) and control (□) groups. (r = 0.93, Pearson correlation; p = 0.03, one-tailed t-test; n = 8 rats/group). Each data point represents an average of three baseline measurements recorded before the start of cisplatin or saline injections. (C) Time course of escape latencies of CIPN and control rats measured with 3 mm probe height after weekly injections of cisplatin or saline. Arrows indicate time points of intraperitoneal injections. Analysis revealed significant treatment effect between the groups (###, p < 0.001, two-way analysis of variance). Tukey post hoc analysis revealed significant increase of escape latency in CIPN vs. control rats: **, p < 0.01. (D-G) Intraperitoneal administration of PrNMI at 0.1, 0.3 & 0.5 mg/kg, but not vehicle, significantly reduces escape latencies post-injection. *, p < 0.05; **, p < 0.01 vs. pre-injection (one-way repeated measures analysis of variance with Dunnett’s post hoc method, n = 7 rats/group); †, p < 0.05 vs. control (two-way analysis of variance). Similar administration of PrNMI or vehicle in control rats has no significant effect on their escape latencies at all post-injection time points.
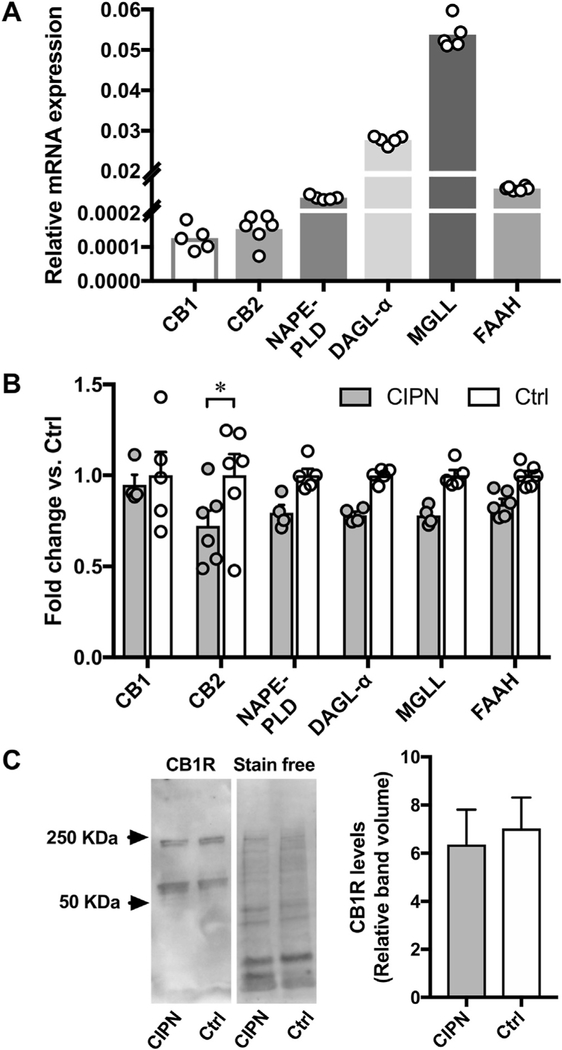
3.7. CB2R mRNA is downregulated in L4-L5 DRG of CIPN rats
The relative transcript levels of cannabinoid receptors and endocannabinoid metabolizing enzymes of control rats are shown in Fig. 9 (Fig. 9A). Compared to saline, cisplatin treatments have no significant effect on the relative mRNA and protein levels of CB1Rs in L4-L5 DRG (Fig. 9B–C). In addition, mRNAs of endocannabinoid synthesizing and degrading enzymes also remain statistically unaffected in individual comparisons although an overall treatment effect is observed on mRNA expression [Ftreatment (1,48) = 22.68, p < 0.0001 vs control]. By contrast, CB2R mRNA expression is significantly decreased in the DRG of cisplatin-treated rats (Fig. 9B).
Fig. 9. Cisplatin treatments decrease CB2R mRNA without affecting CB1R mRNA, protein, and mRNAs of endocannabinoid metabolizing enzymes in L4-L5 DRG.
(A) Relative mRNA levels of cannabinoid receptors and endocannabinoid metabolizing enzymes after normalizing to the internal control GAPDH from the L4-L5 DRG of control rats (n = 5–6/assay). (B) CB2R mRNA levels are downregulated in the L4-L5 DRG of CIPN rats (*, p < 0.05; two-way analysis of variance with Šídák’s multiple comparison test, n = 4–6/group). (C) Western blot analysis reveals no significant change in CB1R levels in L4-L5 DRG of CIPN rats (p = 0.739 vs. control group; unpaired t-test, n = 6/group). Note. Additional bands at −220 KDa indicative of CB1R oligomers are also included in the quantitative analysis. NAPE-PLD, N-acyl phosphatidylethanolamine-specific phospholipase D; DAGL-α, diacyl glycerol lipase - alpha; MGLL, monoacylglycerol lipase; FAAH, fatty acid amide hydrolase.
4. Discussion
The current study demonstrates that PrNMI, one of our recently developed indene series of PRCBs (Seltzman et al., 2016), potently suppresses painful CIPN symptoms after local, systemic and oral administration. The anti-allodynic effects of PrNMI are demonstrated to be primarily mediated by CB1R and not CB2R activation, with minimal centrally-mediated side effects. Moreover, we demonstrate a lack of appreciable tolerance to repeated PrNMI administration, similar anti-allodynic potency and efficacy in males and females, and prolonged suppression of pain behaviors in the operant task.
Using intraplantar administration of PrNMI, we show a complete suppression of CIPN symptoms ipsilateral to injection with a partial effect on the contralateral paw, suggestive of drug entry into systemic circulation to reach the contralateral paw. Anti-allodynic efficacy of cannabinoids after local administration has been demonstrated previously in several models of peripheral nerve injury and inflammation (Agarwal et al., 2007; Amaya et al., 2006; Fox et al., 2001 ). Our results are consistent with previous findings that showed complete suppression of CIPN symptoms by a peripherally restricted fatty acid amide hydrolase inhibitor, URB937, in a rat model of cisplatin-induced neuropathy (Guindon et al., 2013). In contrast, intraplantar administration of WIN 55,212–2, a non-selective cannabinoid agonist, failed to produce anti-allodynic effects in rat models of paclitaxel and vincristine induced neuropathy (Pascual et al., 2005; Rahn et al., 2007). Thus, it remains to be investigated if PRCBs will be effective in other CIPN models.
The dose-dependent anti-allodynic effects of PrNMI revealed an ED50 of 0.49 and 0.15 mg/kg for inhibition of mechanical and cold hypersensitivity, respectively. To our knowledge, this is the first study to demonstrate differential effectiveness of peripheral CB1R activation on mechanical and cold allodynia suppression in a CIPN model. However, suppression of cold allodynia by WIN 55,212–2 at doses without significant effects on mechanical allodynia has been shown previously in a rat model of spinal nerve ligation neuropathy (Bridges et al., 2001). Although the underlying mechanisms are still unclear, studies suggest that cannabinoids may antagonize the actions of a menthol and icilin sensitive transient receptor potential M8 (TRPM8) ion channel (De Petrocellis et al., 2007, 2008, 2011), which is activated by cold and is mostly expressed in a nociceptive subpopulation of DRG neurons (Dhaka et al., 2007, 2008). The role of TRPM8 in the development of cold hypersensitivity has been demonstrated previously in animal and human CIPN studies (Gauchan et al., 2009; Kono et al., 2012; Ta et al., 2010). A recent study showed that global CB1R deletion exaggerated the responses to hindpaw cold stimulation in naive and neuropathic mice suggesting that CB1R activation may protect against the development of cold allodynia (Sideris et al., 2016). In contrast, other studies have demonstrated a lack of such effects of CB1R deletion on cold sensitivity before and after induction of neuropathy (Castane et al., 2006; Deng et al., 2015). Future studies using tissue selective knockout mice should clarify the role of peripheral CB1Rs in cold allodynia development.
We show that orally administered PrNMI is equipotent in male and female rats as determined by their ED50 values. Previous studies of non-selective brain-permeant cannabinoids have demonstrated greater potency and efficacy of their analgesic effects in females than in males (Cooper and Craft, 2018). For example, peripheral or systemic administration of tetrahydrocannabinol was more effective in females than males in a rat model of persistent hindpaw inflammation and the anti-allodynic/anti-hyperalgesic effects of peripherally administered tetrahydrocannabinol were mediated by both CB1R and CB2Rin females, contrary to CB1R only in males (Craft et al., 2013a). In another study, selective activation of peripheral CB1Rs in a rat model of persistent orofacial inflammation showed robust anti-hyperalgesic effects in males and only a moderate effect in females (Niu et al., 2012) suggesting that peripheral CB1R activation mediates most of the analgesic effects of cannabinoids in male rats. Even though PrNMI is equipotent in male and female CIPN rats, we acknowledge that it will be important to determine the type(s) of cannabinoid receptor(s) involved in the anti-allodynic actions of PrNMI in female CIPN rats.
We also show that PrNMI inhibits CIPN behaviors in an operant task with greater efficacy and longer duration of action than in reflex withdrawal assays. The increased duration of action cannot be due to drug accumulation because the estimated plasma half-life of PrNMI after i.p. administration in rats is 7.2 h (Seltzman et al., 2016) and test doses are administered randomly at 3-day intervals (vehicle-0.3–0.1–0.5 mg/kg). Unlike operant assays, reflex measures may not mirror clinical states of pain as they don’t necessarily address cerebral processing in animals (Vierck et al., 2008). In the mechanical conflict system operant assay, an animal’s choice to escape aversive bright light by crossing an array of nociceptive probes measures a behavior that reflects affective and motivational components of pain (Harte et al., 2016). The linear relationship observed between the probe height and escape latency suggests that an escape pathway with elevated and relatively more painful probes increases the latency to reach the dark compartment. Moreover, longer escape latencies in CIPN rats as compared to control rats also suggests that neuropathy may amplify pain intensity or increase aversiveness to probes in CIPN rats. Impaired mobility as a causative factor for increased latencies is unlikely as previous studies (Cata et al., 2008; Garcia et al., 2008) and our rotarod data indicates no significant motor effects of cisplatin at doses administered in this study. Greater efficacy and longer duration of action of PrNMI in the operant task versus reflex assays also indicates an involvement of affective and motivational components of pain. Previously, peripherally and spinally administered analgesics have been shown to induce motivated behavior in rats (LaBuda and Fuchs, 2001; Martin et al., 2006) and in humans (Duse et al., 2009) in chronic pain states. After PrNMI administration, CIPN rats may perceive nociceptive probes as less painful while escaping from the light compartment and exhibit greater motivational drive to escape resulting in shorter latencies. In contrast, we do not observe any significant effect of PrNMI treatment in control rats, unlike morphine which significantly increases escape latencies in the mechanical conflict system assay (Harte et al., 2016), presumably due to an anxiolytic effect and reduced aversiveness to bright light by morphine. Lack of significant anxiolytic effects by PrNMI in control rats underscores its peripherally mediated effects on CIPN behaviors.
Our qPCR data show that CB2R mRNA levels decrease in the DRG of CIPN rats. Previous studies in CIPN models reported either no change or were unable to detect CB2R transcript expression in the DRG (Guindon et al., 2013; Khasabova et al., 2014). It has been shown that mRNA of CB2R undergoes alternative splicing with differential expression of splice variants in various tissues (Zhang et al., 2015). In the current study, qPCR primers are designed to target exons 1–2 of rat cnr2 transcript variant NM_001164142, which is highly expressed in pure neuronal cultures of rat DRG as determined by high throughput RNA sequencing in our previous study (Hirai et al., 2017). This isoform has also been shown previously to be expressed in CNS neurons (Van Sickle et al., 2005; Zhang et al., 2017). Reduced levels of CB2R protein in the hindpaw skin has been reported in a murine model of cisplatin induced neuropathy (Khasabova et al., 2014). Despite decreases in peripheral CB2R expression, CB2R agonists effectively suppress allodynia in CIPN models, suggesting that these actions may be centrally mediated (Rahn et al., 2007, 2014). We also show lack of significant changes in CB1R mRNA and protein levels in CIPN which is in agreement with previous studies that reported lack of significant changes in CB1R mRNA in DRG (Guindon et al., 2013) and spinal cord (Guindon et al., 2013; Rahn et al., 2014) in CIPN models indicating that chemotherapy does not affect CB1R expression. By contrast, other peripheral neuropathy models do exhibit site-specific changes in CB1R expression. For example, increased CB1R expression is seen in rat L4-L5 DRG ipsilateral to L5 spinal nerve ligation (Mitrirattanakul et al., 2006). In the rat diabetic neuropathy model decreased CB1R expression is seen in the DRG (Zhang et al., 2007). In the rat sciatic nerve chronic constriction injury, CB1R expression is predominantly increased in the ipsilateral spinal dorsal horn (Lim et al., 2003). Interestingly, in the same model, there is a decrease in CB1R expression in the ventrolateral periaqueductal grey, consistent with decreased analgesic effectiveness of WIN 55,212–2 microinjections in this area in the injured rats (Palazzo et al., 2012). These studies underscore the site-specificity of changes in cannabinoid receptor expression in different neuropathy models.
Daily oral administration of PrNMI did not result in significant tolerance to either mechanical or cold allodynia suppression during the two-week testing period. In contrast, tolerance to anti-allodynic effects has been observed with a centrally acting cannabinoid in a mouse CIPN model (Deng et al., 2015), suggesting that the site of CB1R activation might play a role in the development of tolerance to cannabinoids. Other factors such as receptor density, potency of the CB1R agonist, agonist bias for intracellular pathways activated, and disease-induced alterations in membrane trafficking of CB1Rs could also affect the development of tolerance to cannabinoids (Breivogel et al., 2003; Lichtman and Martin, 2005; Martin et al., 2004; Tappe-Theodor et al., 2007). Further studies would be needed to assess the possible development of tolerance to continuous administration of PRCBs in CIPN models.
In conclusion, the current study demonstrates effective suppression of CIPN pain symptoms by PrNMI without appreciable CNS side effects or tolerance to repetitive administration. Thus, PCRBs may represent a viable option for the treatment of CIPN pain symptoms.
Supplementary Material
Acknowledgments
We thank the NIDA drug supply program for the gift of SR141716 and SR144528. This work was made possible with support by: UC Center for Accelerated Innovation grant U54HL119893 (I.S.), DA021696 and DA009158 (K.M.) and CA196263 (B.L.S., I.S.). The authors declare no conflicts of interests.
Footnotes
Appendix A. Supplementary video
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuropharm.2018.07.002.
Appendix B. Western blot images
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuropharm.2018.07.002.
Portions of the data in this manuscript have been presented in abstract form at the following scientific meetings: 15thWorld Congress on Pain, Buenos Aires, 2014; Society for Neuroscience, Washington, DC, 2014; Society for Neuroscience, Chicago, IL, 2015; Society for Neuroscience, San Diego, 2016; Society for Neuroscience, Washington, DC, 2017.
References
- Abram SE, Yi J, Fuchs A, Hogan QH, 2006. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology 105,146–153. [DOI] [PubMed] [Google Scholar]
- Adam JM, Clark JK, Davies K, Everett K, Fields R, Francis S, Jeremiah F, Kiyoi T, Maidment M, Morrison A, Ratcliffe P, Prosser A, Schulz J, Wishart G, Baker J, Boyce S, Campbell R, Cottney JE, Deehan M, Martin I, 2012. Low brain penetrant CB1 receptor agonists for the treatment of neuropathic pain. Bioorg. Med. Chem. Lett 22, 2932–2937. [DOI] [PubMed] [Google Scholar]
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R, 2007. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 10, 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M, 2006. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124,175–183. [DOI] [PubMed] [Google Scholar]
- Areti A, Yerra VG, Naidu V, Kumar A, 2014. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP, 2014. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Canc. Manag. Res. 6,135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K, 2010. CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Martin BR, 2000. Time course for the induction and maintenance of tolerance to Delta(9)-tetrahydrocannabinol in mice. Drug Alcohol Depend. 60, 113–119. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Doyle T, Salvemini D, 2014. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 10, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JS, Symonds C, Birch R, 2004. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 112, 299–306. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR, 2003. The effects of delta9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur. J. Pharmacol. 459,139–150. [DOI] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice AS, 2001. The synthetic cannabinoid WIN55,212–2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 133, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S, 2005. Ajulemic acid (IP-751): synthesis, proof of principle, toxicity studies, and clinical trials. AAPS J. 7, E143–E148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castane A, Celerier E, Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O, 2006. Development and expression of neuropathic pain in CB1 knockout mice. Neuropharmacology 50,111–122. [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Dougherty PM, 2008. Behavioral and electrophysiological studies in rats with cisplatin-induced chemoneuropathy. Brain Res. 1230, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM, 2018. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 43, 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2016. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend. 167, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S, 2002. Localisation of cannabinoid CB(1) receptor immunoreactivity in the Guinea pig and rat myenteric plexus. J. Comp. Neurol. 448, 410–422. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM, 2013a. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Delta(9)-tetrahydrocannabinol in the rat. Pain 154, 1709–1717. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL, 2013b. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 92, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello A.s, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V, 2011. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163,1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V, 2007. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 313, 1911–1920. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V, 2008. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Therapeut. 325, 1007–1015. [DOI] [PubMed] [Google Scholar]
- Deng L, Cornett BL, Mackie K, Hohmann AG, 2015. CB1 knockout mice unveil sustained CB2-mediated antiallodynic effects of the mixed CB1/CB2 agonist CP55,940 in a mouse model of paclitaxel-induced neuropathic pain. Mol. Pharmacol. 88, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, Hohmann AG, 2012. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB(2) receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol. Pain 8, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A, 2008. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 28, 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A, 2007. TRPM8 is required for cold sensation in mice. Neuron 54, 371–378. [DOI] [PubMed] [Google Scholar]
- Duse G, Davia G, White PF, 2009. Improvement in psychosocial outcomes in chronic pain patients receiving intrathecal morphine infusions. Anesth. Analg. 109, 1981–1986. [DOI] [PubMed] [Google Scholar]
- Dziadulewicz EK, Bevan SJ, Brain CT, Coote PR, Culshaw AJ, Davis AJ, Edwards LJ, Fisher AJ, Fox AJ, Gentry C, Groarke A, Hart TW, Huber W, James IF, Kesingland A, La Vecchia L, Loong Y, Lyothier I, McNair K, O’Farrell C, Peacock M, Portmann R, Schopfer U, Yaqoob M, Zadrobilek J, 2007. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J. Med. Chem. 50, 3851–3856. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I, 2001. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 92, 91–100. [DOI] [PubMed] [Google Scholar]
- Garcia JM, Cata JP, Dougherty PM, Smith RG, 2008. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology 149, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchan P, Andoh T, Kato A, Kuraishi Y, 2009. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 458, 93–95. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J, 2005. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol. Biochem. Behav. 81, 300–318. [DOI] [PubMed] [Google Scholar]
- Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG, 2013. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 67, 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF, 2000. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 12, 3239–3249. [DOI] [PubMed] [Google Scholar]
- Hammack JE, Michalak JC, Loprinzi CL, Sloan JA, Novotny PJ, Soori GS, Tirona MT, Rowland KM Jr., Stella PJ, Johnson JA, 2002. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 98, 195–203. [DOI] [PubMed] [Google Scholar]
- Han FY, Wyse BD, Smith MT, 2014. Optimization and pharmacological characterization of a refined cisplatin-induced rat model of peripheral neuropathic pain. Behav. Pharmacol. 25, 732–740. [DOI] [PubMed] [Google Scholar]
- Harte SE, Meyers JB, Donahue RR, Taylor BK, Morrow TJ, 2016. Mechanical conflict system: a novel operant method for the assessment of nociceptive behavior. PLoS One 11, e0150164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ, 1997. The analgesic effects of R(+)- WIN 55,212–2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci. Lett. 221, 157–160. [DOI] [PubMed] [Google Scholar]
- Hile ES, Fitzgerald GK, Studenski SA, 2010. Persistent mobility disability after neurotoxic chemotherapy. Phys. Ther. 90,1649–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Mulpuri Y, Cheng Y, Xia Z, Li W, Ruangsri S, Spigelman I, Nishimura I, 2017. Aberrant plasticity of peripheral sensory axons in a painful neuropathy. Sci. Rep. 7, 3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins HL, Duggett NA, Flatters SJL, 2016. Chemotherapy-induced painful neuropathy: pain-like behaviours in rodent models and their response to commonly used analgesics. Curr. Opin. Support. Palliat. Care 10, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Yao X, Paz J, Lewandowski CT, Lindberg AE, Coicou L, Burlakova N, Simone DA, Seybold VS, 2014. JZL184 is anti-hyperalgesic in a murine model of cisplatin-induced peripheral neuropathy. Pharmacol. Res. 90, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T, Satomi M, Suno M, Kimura N, Yamazaki H, Furukawa H, Matsubara K, 2012. Oxaliplatin-induced neurotoxicity involves TRPM8 in the mechanism of acute hypersensitivity to cold sensation. Brain Behav. 2, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN, 2001. Low dose aspirin attenuates escape/avoidance behavior, but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain. Neurosci. Lett. 304, 137–140. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR, 2005. Cannabinoid tolerance and dependence. Handb. Exp. Pharmacol. 691–717. [DOI] [PubMed] [Google Scholar]
- Lichtman SM, Hurria A, Cirrincione CT, Seidman AD, Winer E, Hudis C, Cohen HJ, Muss HB, Cancer and Leukemia Group B, 2012. Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: combined analysis of CALGB 9342 and 9840. Ann. Oncol. 23, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Sung B, Ji RR, Mao J, 2003. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212–2 on neuropathic pain behaviors in rats. Pain 105, 275–283. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Majithia N, Loprinzi CL, Smith TJ, 2016. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology (Williston Park) 30, 1020–1029. [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE, 2004. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol. Sci. 25, 325–330. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Eisenach JC, 2006. Clonidine maintains intrathecal self-administration in rats following spinal nerve ligation. Pain 125, 257–263. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, Mackie K, Faull KF, Spigelman I, 2006. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain 126, 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu KY, Zhang Y, Ro JY, 2012. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain 153, 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo E, Luongo L, Bellini G, Guida F, Marabese I, Boccella S, Rossi F, Maione S, de Novellis V, 2012. Changes in cannabinoid receptor subtype 1 activity and interaction with metabotropic glutamate subtype 5 receptors in the periaqueductal gray-rostral ventromedial medulla pathway in a rodent neuropathic pain model. CNS Neurol. Disord. - Drug Targets 11, 148–161. [DOI] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC, 2009. Oxaliplatin-induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain 132, 2712–2723. [DOI] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Suardiaz M, Martin MI, 2005. A cannabinoid agonist, WIN 55,212–2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 118, 23–34. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Deng L, Thakur GA, Vemuri K, Zvonok AM, Lai YY, Makriyannis A, Hohmann AG, 2014. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol. Pain 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Makriyannis A, Hohmann AG, 2007. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 152, 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY, North Central Cancer Treatment G, 2007. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110, 2110–2118. [DOI] [PubMed] [Google Scholar]
- Russo EB, 2008. Cannabinoids in the management of difficult to treat pain. Therapeut. Clin. Risk Manag. 4, 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzman HH, Shiner C, Hirt EE, Gilliam AF, Thomas BF, Maitra R, Snyder R, Black SL, Patel PR, Mulpuri Y, Spigelman I, 2016. Peripherally selective cannabinoid 1 receptor (CB1R) agonists for the treatment of neuropathic pain. J. Med. Chem. 59, 7525–7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y, Okazaki F, Horikawa K, Zhang J, Sasaki H, To H, 2016. Influence of dosing times on cisplatin-induced peripheral neuropathy in rats. BMC Canc. 16, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideris A, Piskoun B, Russo L, Norcini M, Blanck T, Recio-Pinto E, 2016. Cannabinoid 1 receptor knockout mice display cold allodynia, but enhanced recovery from spared-nerve injury-induced mechanical hypersensitivity. Mol. Pain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisignano M, Baron R, Scholich K, Geisslinger G, 2014. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 10, 694–707. [DOI] [PubMed] [Google Scholar]
- Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ, 2010. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol. Pain 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R, 2007. A molecular basis of analgesic tolerance to cannabinoids. J. Neurosci. 27, 4165–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Craft RM, 2001. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur. J. Pharmacol. 430, 41–47. [DOI] [PubMed] [Google Scholar]
- van der Hoop RG, van der Burg ME, ten Bokkel Huinink WW, van Houwelingen C, Neijt JP, 1990. Incidence of neuropathy in 395 patients with ovarian cancer treated with or without cisplatin. Cancer 66, 1697–1702. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA, 2005. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. [DOI] [PubMed] [Google Scholar]
- Vera G, Cabezos PA, Martin MI, Abalo R, 2013. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol. Biochem. Behav. 105, 205–212. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP, 2008. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain 135, 7–10. [DOI] [PubMed] [Google Scholar]
- Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, Faithfull S, 2017. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J. Clin. Oncol. 35, 2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XH, Cao CQ, Martino G, Puma C, Morinville A, St-Onge S, Lessard E, Perkins MN, Laird JM, 2010. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain 151, 337–344. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hong S, Stone V, Smith PJ, 2007. Expression of cannabinoid CB1 receptors in models of diabetic neuropathy. J. Pharmacol. Exp. Therapeut. 323, 508–515. [DOI] [PubMed] [Google Scholar]
- Zhang H, Lund DM, Ciccone HA, Staatz WD, Ibrahim MM, Largent-Milnes TM, Seltzman HH, Spigelman I, Vanderah TW, 2018. A Peripherally Restricted Cannabinoid 1 Receptor Agonist as a Novel Analgesic in Cancer-induced Bone Pain. Pain Articles in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES, Gardner EL, Xi ZX, Liu QR, 2015. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 40, 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Shen H, Bi GH, Yang HJ, Liu QR, Wu J, Gardner EL, Bonci A, Xi ZX, 2017. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biol. 22, 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.