Abstract
Purpose
The aim of this study was to investigate the influence of epithelial-mesenchymal transition (EMT) occurring in gastric carcinoma cells and the involvement of programmed death ligand 1 (PD-L1) expression in tumor cells that undergo EMT. The mechanisms underlying PD-L1 expression during EMT in gastric carcinoma cells were also explored.
Methods
The capacities of migration and invasion were tested by cell scratch-wound assay and transwell chamber assay. PD-L1 expression by SGC7901 cell line and related mechanism were measured by Western blot and QRT-PCR.
Results
Treating with TGF-β1 promotes the motility of SGC7901 and PD-L1 expression in vitro, while activating the NF-κB signal pathway.
Conclusion
EMT increases the capacities of migration and invasion in gastric cancer cells, which resulted in up-regulation of PD-L1 expression via a mechanism that is dependent on NF-κB activation.
Keywords: EMT, PD-L1, migration and invasion, gastric carcinoma
Introduction
Gastric cancer is one of the most common malignancies and a leading cause of cancer death in China, with about 680,000 new cases and nearly 500,000 deaths every year; both morbidity and mortality rank only second to lung cancer.1 Despite great progress in surgical operation treatment, effective methods for early diagnosis, monitoring metastasis and prognosis are currently unavailable for gastric cancer.2 In addition, there are difficulties for surgeon in local relapse and distant metastasis.3 Targeted therapy and immunotherapy for cancer metastasis are now in full swing, but with little progress and few new drugs for gastric cancer due to its heterogeneity of gene expression,4 only trastuzumab for rare HER-2 positive patients5 and nivolumab/pembrolizumab for third-line treatment.6,7
Metastasis often means more difficult to treat and worse survival among patients with gastric cancer,8 which includes detachment of tumor cells from the primary tumor mass, microinvasion of tumor cells into stromal tissues, intravasation of tumor cells into the lymphatic or blood vessels and extravasation and growth of tumor cells in secondary sites.9,10 To achieve the control of cancer metastasis, the earlier the treatment begins, the better effect is.
One of the key factors in treatment is finding metastasis as early as possible. Epithelial-Mesenchymal Transition (EMT) refers to the loss of polarity and tight junction and adhesion of epithelial cells under the inducement of different factors, acquiring the ability of infiltration and migration, the change of epithelial cells into cells with morphological and characteristic of interstitial cells.11 Tumor cells, which undergo EMT, are able to invade normal tissues and induce metastases. The other crucial step of metastases is the acquisition by tumor cells of the ability to evade the immune system. Programmed death ligand-1 (PD-L1) is a member of the B7 superfamily of co-stimulating molecules expressed by antigen-presenting cells and tumor cells that functions as a T lymphocyte inhibitory molecule. PD-L1 has been shown to induce T lymphocyte anergy and/or apoptosis through binding to its receptor PD-1, which may be one reason for immune escape of tumor cells that induces the development and metastasis of tumor.12 Up-regulation of PD-L1 is associated with tumorigenesis and poor prognosis of gastric cancer,13 melanoma, head and neck or lung cancer.14–16 We therefore hypothesized that the expression of PD-L1 might be specifically regulated during EMT to allow invasion and immune escape. Indeed, the role of ZEB-1 and miR200 on PD-L1 in EMT was recently shown in breast cancer models.17 However, the association between EMT and PD-L1 expression in gastric cancer was unknown.
In the current study, we selected an EMT model based on TGF-β1 treatment of gastric cancer cell line SGC7901. We show that treating with TGF-β1 promotes the motility of SGC7901 and PD-L1 expression in vitro; the expression of PD-L1 might partly be regulated by NF-κB during EMT signaling in gastric carcinoma.
Materials And Methods
Cell Preparation
Human gastric cancer SGC7901 cells were purchased from the Cell Bank of the Chinese Academy of Sciences, Shanghai, China.
Cell Scratch-Wound Assay
When cells reached 90% confluence, a single wound was created and phase-contrast images were digitally photographed immediately and at 24 hrs after incubation. The original opening distances of the wound were set as 100%. The opening distance after 24 hrs were measured from three areas randomly selected per well, and the distances in three wells of each group were quantified and normalized by the original opening distance. The experiment was performed three times in triplicate, and the percentage of the migration rate was calculated by measuring the length of cell migration and expressed as percentage compared to the control group. Migration rate = (treatment group cell migration distance/control group migration distance) * 100%.18
Invasion And Migration Assays
Cells (2×104 cells in 200 μL medium without FBS) were plated onto a 24-well cell culture insert coated with or without Matrigel with 8 μm cores (Costar, USA). A medium containing 5% FBS (500μL) was added to the lower wells. After incubation for 24 hrs, penetrated cells on the lower surface of the membrane were fixed with 100% methanol, stained with 0.1% crystal violet and quantified in five areas randomly selected per insert. The infiltrated cells in three inserts were quantified in each experiment. The experiment was performed three times in triplicate, and the percentage of the migration rate and invasion rate was calculated by measuring the number of cell migration and invasion as percentage compared to the control group. Migration rate = (treatment group migrated cell count/control group migrated cell count) * 100%. Invasion rate = (treatment group invaded cell count/control group invaded cell count) * 100%.19
Quantitative Real-Time Reversed Transcription-PCR
PD-L1, IL8, BIRC3 mRNA were normalized to GAPDH mRNA. Primer sequences were: PD-L1, forward: TGCGGACTACAAGCGAATCA and reverse: GATCCACGGAAATTCTCTGGTT;20 IL8, forward: ATGACTTCCAAGCTGGCCGTGCT and reverse: TCTCAGCCCTCTTCAAAAACTTCTC;21 BIRC3, forward: AAGCTACCTCTCAGCCTACTTT and reverse: CCACTGTTTTCTGTACCCGGA;22 GAPDH, forward: CAGGGCTGCTTTTAACTC and reverse: GGAAGATGGTGATGGGAT. Results were calculated using 2−△△CT method and are expressed as means ± SD.23
SDS-PAGE And Western Blot Analysis
Western blotting was conducted as described previously. In brief, cell lysates were centrifuged at 12,000 rpm for 30 mins at 4°C. Protein concentrations of cell lysates were determined with the BCA assay (Pierce) and 40 μg of proteins was loaded onto SDS polyacrylamide gels (7.5%–15%) Following separation by SDS-PAGE, the proteins were transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The membranes were blocked in blocking buffer (5% non-fat dried milk/1% Tween 20 in PBS) for 2 hrs at room temperature and then incubated with appropriate primary antibodies (1:1000) in blocking buffer overnight at 4°C. The blot was incubated with appropriate alkaline phosphatase (AP)-conjugated secondary antibodies. Immunoreactivity was detected by the addition of 5 mL AP buffer containing 16.5 mL BCIP and 33 mL of NBT at room temperature for 10 to 20 mins. Blots were then photographed. Tubulin was used as a loading control.24
Antibodies And Reagents
E-cadherin (Signalway Antibody 48801), N-cadherin (Signalway Antibody 48779), Vimentin (Signalway Antibody 38104), Tubulin (Signalway Antibody 48885), Galectine 9 (Abcam ab153673), CEACAM1 (CD66) (Abcam ab108397), PD-L2 (Abcam ab256386), Vista (Abcam ab230950), IκBα (pS32) (Cell Signaling #2859), IκBα (Cell Signaling #9242), RelA(pS536) (Cell Signaling #3033), TGFβ (Abcam ab92486), TGFβ inhibitor (LY-364947, MCE, USA), NFκB inhibitor (BAY11, MCE, USA). PD-L1 was measured using the Human PD-L1 ELISA kit (Abcam ab214565).
Statistical Analysis
Results are represented as means ± SD. Statistical analyses were one way ANOVA. Result was considered statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Treatment With TGFβ1 Effectively Induced EMT In SGC7901
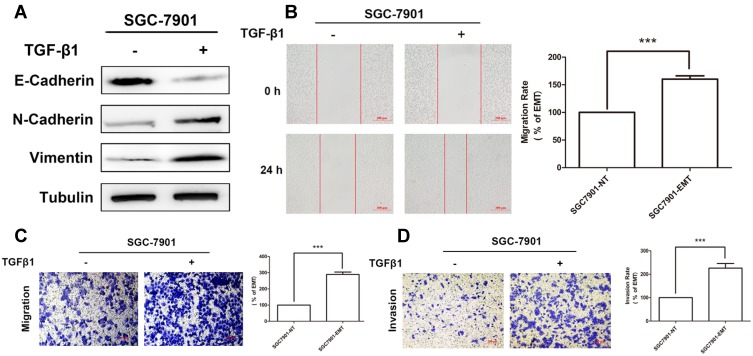
Cytokine TGFβ1 has been shown to induce a EMT model in NSCLC A549 cell line.25,26 To confirm this method is available in our study, GC cell line SGC7901 was exposed to TGFβ1 for 5 days to establish an EMT model. Samples were collected from untreated (0 days) and cytokine-treated cultures (5 days), Epithelical (E-cadherin) and mesenchyme (N-cadherin, Vimentin) makers were measured by Western blotting to confirm EMT status of SGC7901. Treatment with TGFβ1 resulted in a loss of E-cadherin expression with an increase in N-cadherin and Vimentin (Figure 1A).
Figure 1.
Treatment with TGFβ1 effectively induced EMT in SGC7901. (A) Expression of epithelial marker E-Cadherin and mesenchymal marker N-Cadherin and Vimentin in SGC7901 after treatment with TGF-β1 were measured by Western-Blotting. (B) Wound-healing assay showing the migration ability of SGC7901 after treatment with TGF-β1. Scale bar, 200 μm. Transwell assay showing the migration (C) and invasion (D) ability of SGC7901 after treatment with TGF-β1. Scale bar, 200 μm.
Enhanced migration and invasion is one of the hallmarks of tumor cell undergoing EMT. Cell scratch-wound and transwell chamber assay were performed to further confirm EMT status. As a result, the wound of SGC7901 (treated for 5 days) was obviously narrower than that of the untreated cells (0 days) (Figure 1B). Transwell assays showed improved migratory ability of SGC7901, as indicated by the increasing number of migrated (Figure 1C) and invaded cells (Figure 1D).
In conclusion, stimulation with TGFβ1 for five days effectively induced EMT in SGC7901 in vitro.
PD-L1 Expression Was Up-Regulated In Cytokine-Induced EMT Model
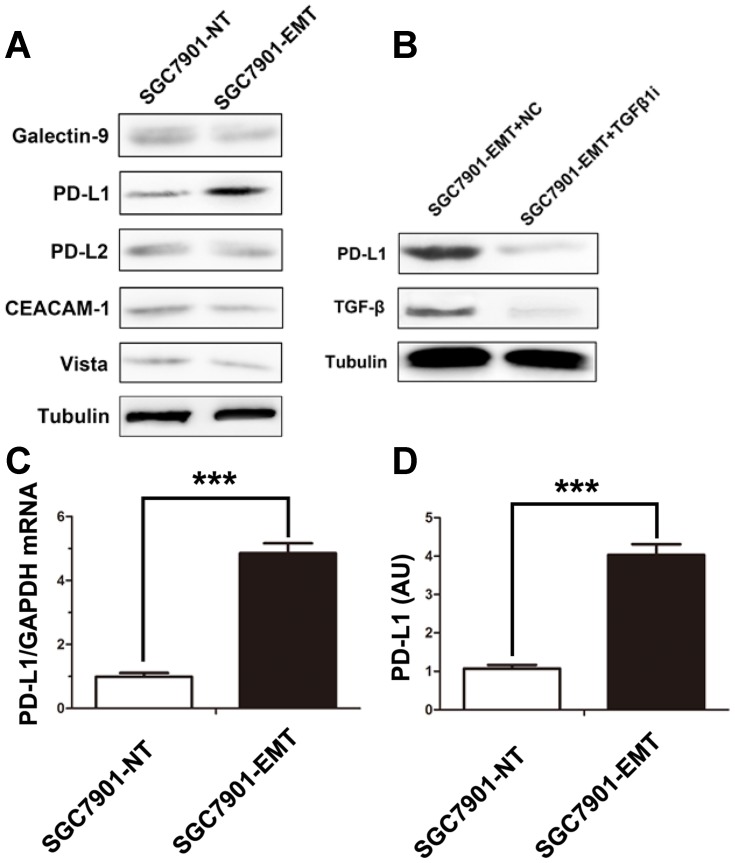
PD-L1 expression is associated with EMT in head and neck squamous cell carcinoma,27 lung carcinoma28 and renal cell carcinoma.29 To investigate the association between EMT and PD-L1 expression in gastric cancer, we next tested the expression of immune checkpoint ligands in SGC7901 according to EMT status. Western blotting results showed that EMT induction led to the up-regulation of PD-L1 but not TIM3 ligands Galectine 9, CEACAM1 (CD66), PD-L2 or Vista in this model (Figure 2A). Moreover, TGF-β1 receptor inhibition (used as a specific inhibitor of TGF-β receptor to block TGFβ1 induced EMT) blocked PD-L1 expression (Figure 2B). These results indicated that cytokine-mediated EMT promoted the expression of PD-L1. The up-regulation of PDL1 by exposition to TGF-β1 was also confirmed by QRT-PCR (Figure 2C) and ELISA (Figure 2D).
Figure 2.
PD-L1 expression was up-regulated in cytokine-induced EMT model. (A) Expression of immune-checkpoint inhibitors as Galectine 9, CEACAM1, PD-L1, PD-L2, Vista before and after induction of EMT on SGC7901 cells. (B) The changes in the expression of PD-L1 with TGFβ receptor inhibitor treatment in SGC7901-EMT cells. (D) Expression of PD-L1 mRNA and protein were measured following TGFβ1 treatment by QRT-PCR (C) or ELISA (D). Data are expressed as means ± S.D of three independent experiments, each performed in triplicate. ***P < 0.001.
NF-κB Pathway Is Constitutively Active And Is Required For Induction Of EMT
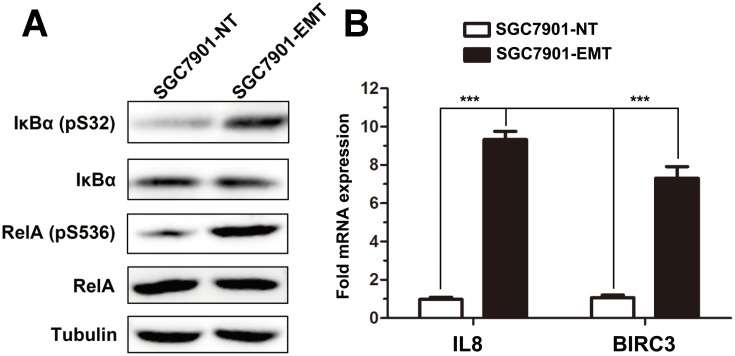
As the increased transcription of PD-L1 during cytokine-induced EMT, we first analyzed the transcription factors correlated with PD-L1 promoter. NF-κB has been shown to regulate the EMT master-switch transcription factors in multiple model systems30–33 and present a binding site in the PD-L1 promoter. Interestingly, a strong increase of NF-κB activity was observed in this EMT model (Figure 3A); mesenchymal SGC7901 cells displayed constitutive NF-κB activated pathways, as determined using phosphor-specific antibodies to IKBα and RelA. Moreover, QRT-PCR experiments demonstrated increased expression of NF-kB-regulated genes IL8 and BIRC3/cIAP2 (Figure 3B).
Figure 3.
NF-κB pathway is constitutively active and is required for induction of EMT. (A) Activity of NF-κB was measured in SGC7901 cells treated or not with TGFβ1. (B) QRT-PCR analysis of the expression of IL-8, BIRC3 in SGC7901 cells following TGFβ1 treatment. ***P < 0.001.
These data suggested that TGF-β1 treatment of SGC7901 results in the increased phosphorylation of IKK-regulated substrates and constitutive NF-kB transcriptional activation.
PDL1 Expression Is Regulated Via NF-κB Signaling During EMT
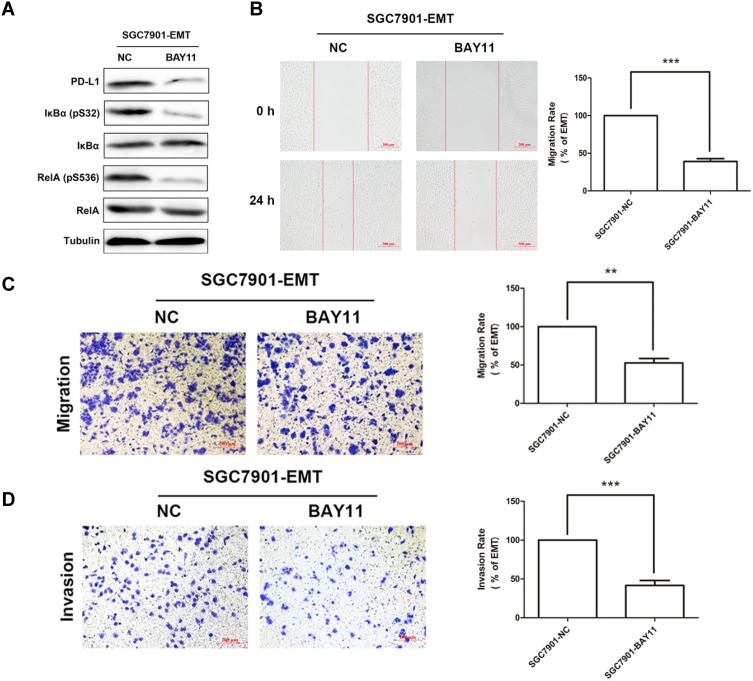
According to the above result that the PD-L1 expression is up-regulated in this cytokine-induced EMT model, we questioned whether inhibiting NF-κB activity would dampen PD-L1 expression. Indeed, we used a specific inhibitor (BAY11) to inhibition of NF-κB activity resulting in a significantly reduced PD-L1 expression (Figure 4A). Meanwhile, inhibition of NF-κB activity suppressed the migratory ability of SGC7901 according to EMT status in wound-healing (Figure 4B) and transwell assay (Figure 4C).
Figure 4.
PDL1 expression is regulated via NF-κB signaling during EMT. (A) The activity of NF-κB measured by Western Blotting after treatment with NF-κB inhibitor. (B) Wound-healing assay showing the migration ability of SGC7901-EMT after treatment with NF-κB inhibitor. Scale bar, 200 μm. Transwell assay showing the migration (C) and invasion (D) ability of SGC7901-EMT after treatment with NF-κB inhibitor. Scale bar, 200 μm.
In summary, all our data contribute to show for the first time that PD-L1 expression is at least partly regulated by NF-κB signaling during the cytokine TGF-β1-induced EMT.
Discussion
EMT has become a focus of research due to its vital role in tumor progress throughout the body and complexity in a variety of immune processes.34–36 In this study, we tried to explore the influence of EMT in immune-related process through the study of PD-L1 expression regulation in gastric cancer.
We mimic the invasion phase with a cytokine-induced EMT model. After treated with TGF-β1 for five days, SGC7901 cells were actually driven to EMT.37,38 In this model, E-cadherin expression was suppressed while N-cadherin and Vimentin were overexpressed in SGC7901 following TGF-β1 treatment. In the present study, we showed that EMT phenotype conferred by TGF-β1 to SGC7901 was associated with the up-regulation of PD-L1. Meanwhile, the capacities of migration and invasion were also verified with cell scratch-wound assay and trans-well chamber assay. We found that the wound of SGC7901 (treated with TGFβ1 for 5 days) was obviously narrower than that of the untreated cells (0 days) (Figure 1B) and transwell assays showed improved migratory ability of SGC7901 indicated by the increasing number of migrated (Figure 1C) and invaded cells (Figure 1D).
The mechanism underlying the inducible expression of PD-L1 is not clear. Several studies figure out TNF-α stimulates NF-κB in cancer cells like A549 and increases the ability of TGF-β1 to induce EMT.28 Here, we found that inducible expression of PD-L1 in SGC7901 cells was TGF-β1-dependent manner (Figure 2A and B). We suspected that cumulative effects leading to the transition of SGC7901 cells occurred during the stimulation process.
Figure 3 shows that the PD-L1 expression is related to NF-κB signals. Thus, we suspected that TGFβ1 might up-regulate ICOSL by activation of the NF-κB signaling pathway.39,40 Unfortunately, a direct connection between NF-κB signaling activation and PD-L1 expression could not be investigated due to the restrictions of time and conditions. Further studies are required to confirm TGFβ receptor expression on SGC7901 cells for clarification of the mechanism underlying TGFβ1-mediated stimulation of gastric cancer cells, as well as the potential expression of other mediators that might connect NF-κB activation with PD-L1 expression in the TGFβ1 culture system.
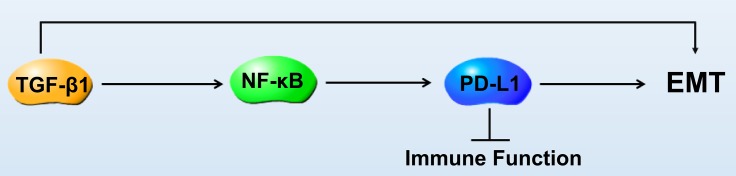
In summary, although the exact mechanism of TGFβ1-dependent PD-L1 expression on SGC7901 cells was not fully elucidated, we found the influence of EMT during the transition of gastric cancer cells (Figure 5). These findings provide insights into the molecules and signaling pathways involved in the interaction of EMT with other immune processes, which is hoped to promote the development of different therapeutic strategies aimed at boosting or suppressing specific EMT functions, depending on the pathological context.
Figure 5.
PD-L1 expression is regulated by NF-κB during EMT signaling in gastric carcinoma.
Acknowledgments
We would like to thank Prof. Ping Gong for technical assistance and insightful discussions. Financial support of this work was provided by the school-level subject of the First Affiliated Hospital of Shihezi University School of Medicine (Grant YL2016-R004), The 8th Shihezi City Science and Technology Plan Project (Grant 2017H220).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Wang L, Yang Y, Gong L, Xiao B, Liu X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem Biophys Res Commun. 2017;493:1322. doi: 10.1016/j.bbrc.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 3.Shi X, Huang Y, Wang H, Zheng W, Chen S. MFSD2A expression predicts better prognosis in gastric cancer. Biochem Biophys Res Commun. 2018;505(3):699–704. doi: 10.1016/j.bbrc.2018.09.156 [DOI] [PubMed] [Google Scholar]
- 4.Strand MS, Lockhart AC, Fields RC. Genetics of gastric cancer. Surg Clin North Am. 2017;97:345–370. doi: 10.1016/j.suc.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van CE, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 6.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 7.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 8.Yamamichi K, Uehara Y, Kitamura N, Nakane Y, Hioki K. Increased expression of CD44v6 mRNA significantly correlates with distant metastasis and poor prognosis in gastric cancer. Int J Cancer. 1998;79:256–262. doi: 10.1002/(ISSN)1097-0215 [DOI] [PubMed] [Google Scholar]
- 9.Pepper MS. Lymphangiogenesis and tumor metastasis myth or reality? Clin Cancer Res. 2001;7:462–468. [PubMed] [Google Scholar]
- 10.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Z H W, Lin C, Liu CC, et al. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res Commun. 2018;501(4):1068–1073. [DOI] [PubMed] [Google Scholar]
- 12.Amarnath S, Mangus CW, Wang JCM, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111ra120–111ra120. doi: 10.1126/scitranslmed.3003130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qing Y, Li Q, Ren T, et al. Upregulation of PD-LI and APEI is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. 2015;9:901–909. doi: 10.2147/DDDT.S75152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ascierto PA, Mcarthur GA. Checkpoint inhibitors in melanoma and early phase development in solid tumors: what’s the future? J Transl Med. 2017;15(1):173. doi: 10.1186/s12967-017-1278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. doi: 10.1177/030089161209800612 [DOI] [PubMed] [Google Scholar]
- 16.Prat A, Navarro A, Parã© L, et al. Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 2017;77:3540–3550. doi: 10.1158/0008-5472.CAN-16-3556 [DOI] [PubMed] [Google Scholar]
- 17.Noman MZ, Janji B, Abdou A, et al. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. OncoImmunology. 2017;6(1):e1263412. doi: 10.1080/2162402X.2016.1263412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai A, Landman K, Hughes BD. Multi-scale modeling of a wound-healing cell migration assay. J Theor Biol. 2007;245:576–594. doi: 10.1016/j.jtbi.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 19.Kramer N, Walzl A, Unger C, et al. In vitro cell migration and invasion assays. Mutat Res-Rev Mutat Res. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 20.Eppihimer MJ, Gunn J, Freeman GJGreenfield EA, Chernova T, Erickson J, Leonard JP. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2015;9:133–145. doi: 10.1080/713774061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maria P, Anna N, Laura S, et al. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol. 2014;109:279–287. doi: 10.1038/ajg.2013.403 [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Berglund A, Kenchappa RS, Forsyth PA, Mulé JJ, Etame AB. BIRC3 is a novel driver of therapeutic resistance in Glioblastoma. Sci Rep. 2016;6:21710. doi: 10.1038/srep21710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan-Malek R, Wang Y. Statistical analysis of quantitative RT-PCR results. Methods Mol Biol. 2011;691:227–241. [DOI] [PubMed] [Google Scholar]
- 24.Liang X, Shi H, Yang L, et al. Inhibition of polypyrimidine tract-binding protein 3 induces apoptosis and cell cycle arrest, and enhances the cytotoxicity of 5-fluorouracil in gastric cancer cells. Br J Cancer. 2017;116(7):903–911. doi: 10.1038/bjc.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasuhiro Y, Tadashi K, Hajime T, et al. Tumor necrosis factor-alpha enhances both epithelial-mesenchymal transition and cell contraction induced in A549 human alveolar epithelial cells by transforming growth factor-beta1. Exp Lung Res. 2010;36:12–24. doi: 10.3109/01902140903042589 [DOI] [PubMed] [Google Scholar]
- 26.Akira S, Suzuki HI, Masafumi H, et al. An integrated expression profiling reveals target genes of TGF-β and TNF-α possibly mediated by microRNAs in lung cancer cells. PLoS One. 2013;8(2):e56587. doi: 10.1371/journal.pone.0056587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ock CY, Kim S, Keam B, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016;7(13):15901–15914. doi: 10.18632/oncotarget.7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asgarova A, Asgarov K, Godet Y, et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. OncoImmunology. 2018;7(6):e1423170. doi: 10.1080/2162402X.2017.1423170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wang H, Zhao Q, et al. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol. 2015;32(8):212. doi: 10.1007/s12032-015-0655-2 [DOI] [PubMed] [Google Scholar]
- 30.Barberà MJ, Puig I, Domínguez D, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990 [DOI] [PubMed] [Google Scholar]
- 31.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808 [DOI] [PubMed] [Google Scholar]
- 32.Julien S, Puig I, Caretti E, et al. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26(53):7445–7456. doi: 10.1038/sj.onc.1210546 [DOI] [PubMed] [Google Scholar]
- 33.Pham CG, Bubici C, Zazzeroni F, et al. Upregulation of twist-1 by NF-κB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27:3920–3935. doi: 10.1128/MCB.01219-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricciardi M, Zanotto M, Malpeli G, et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112:1067–1075. doi: 10.1038/bjc.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terry S, Savagner P, Ortiz-Cuaran S, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11(7):824–846. doi: 10.1002/mol2.2017.11.issue-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biddle A. Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev. 2012;31:285–293. doi: 10.1007/s10555-012-9345-0 [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka J, Yashiro M, Doi Y, et al. Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFβ signaling. PLoS One. 2013;8:e62310. doi: 10.1371/journal.pone.0062310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Long J, Du R, Ge C, Guo K, Xu Y. miR-204 regulates the EMT by targeting snai1 to suppress the invasion and migration of gastric cancer. Tumor Biol. 2016;37:8327–8335. doi: 10.1007/s13277-015-4627-0 [DOI] [PubMed] [Google Scholar]
- 39.Oh H, Ghosh S. NF‐κB: roles and regulation in different CD4+ T‐cell subsets. Immunol Rev. 2013;252:41–51. doi: 10.1111/imr.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen C, Hupin C, Froidure A, Detry B, Pilette C. Impaired ICOSL in human myeloid dendritic cells promotes Th2 responses in patients with allergic rhinitis and asthma. Clin Exp Allergy. 2014;44:831–841. doi: 10.1111/cea.12308 [DOI] [PubMed] [Google Scholar]