Abstract
Glaucoma is an age-dependent disease closely related to oxidative stress and is regarded as the second leading cause of irreversible blindness worldwide. In recent years, many studies have shown that morphological and functional abnormalities of the trabecular meshwork (TM) are closely related to glaucoma, especially with respect to oxidative stress. In this review, the mechanisms of oxidative stress in the TM and treatment strategies for this condition, including strategies involving antioxidants, noncoding RNAs and exogenous compounds, are discussed. Although many questions remain to be answered, the reviewed findings provide insights for further research on oxidative stress alleviation in glaucoma and suggest new targets for glaucoma prevention.
Keywords: Trabecular meshwork, Oxidative stress, Antioxidants, POAG
Introduction
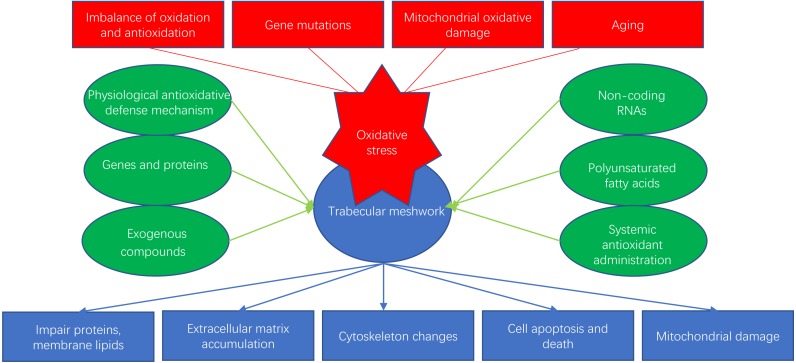
Glaucoma is an age-dependent disease closely related to oxidative stress and is considered to be the second leading cause of irreversible human blindness worldwide, especially in the elderly population (Quigley & Broman, 2006). Oxidative stress can happen in many ocular cells, such as corneal epithelial cells (CECs), trabecular meshwork (TM) cells (TMCs), retinal pigment epithelial cells (RPEs) and retinal ganglion cells (RGCs). In particular, oxidative stress-induced dysfunction of TMCs can obstruct the outflow of the aqueous humor, leading to pathologically high intraocular pressure (IOP) and contributing to glaucoma. Several studies have suggested that the progression of primary open-angle glaucoma (POAG) may be related to reductions in the antioxidant capacity of the TM (Ammar, Hamweyah & Kahook, 2012a). In this review, we discuss the mechanisms of oxidative stress and recent research on antioxidative strategies for the TM (Fig. 1).
Figure 1. Oxidative stress and antioxidants of trabecular meshwork.
Survey Methodology
This review focuses on hot topics in glaucoma research: oxidative stress and antioxidants. All references were retrieved using search engines such as PubMed and Web of Science using keywords including “trabecular meshwork cells”, “oxidative stress”, “antioxidants” and “glaucoma.”
ROS and oxidative stress
Free radicals are substances with unpaired electrons that are regularly produced through normal metabolic processes. Free radicals can be divided into oxygen and nonoxygen radicals, although oxygen free radicals account for 95% of all free radicals (Zhao et al., 2016). Oxygen radicals include oxygen and highly reactive oxygen molecules, such as hydrogen peroxide (H2O2), hydroxyl radicals (OH•), peroxide hydroxyl radicals, alkoxy radicals, superoxide and anionic radicals (O2-), which are collectively referred to as reactive oxygen species (ROS). The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family is an enzyme family whose main function is to produce ROS upon stimulation by different growth factors and cytokines in various cell types (Wulf, 2002; Yun et al., 2011). Important exogenous stimulants of free radical production include electromagnetic radiation (visible, ultraviolet (UV), and infrared radiation) and known environmental pollutants such as tobacco smoke. Endogenous sources of free radicals include mitochondria, which form superoxide through the respiratory chain, and polynuclear cells in the inflammatory environment, which perform important functions during the physiological response to injury.
Oxidative stress is usually caused by imbalance between ROS production and elimination as a result of biological defense mechanisms, mitochondrial dysfunction, impaired antioxidant systems or a combination of these factors. Oxidative stress increases the production of ROS, creating a vicious cycle. Abnormal ROS accumulation can cause oxidative damage to deoxyribonucleic acid (DNA), proteins, and lipids. DNA damage can induce apoptosis, autophagy, and mutation, which are associated with cataracts, age-related macular degeneration (AMD), retinopathies, and glaucoma.
TM oxidative stress and glaucoma
Patients with POAG are susceptible to oxidative damage because their total reactive antioxidant capacity is 60%–70% lower than that of healthy individuals (Ferreira et al., 2004; Tanito et al., 2015). POAG patients’ serum samples always exhibit low levels of circulating glutathione (Doina et al., 2005), total antioxidant capacity (TAC), advanced oxidation protein products (AOPPs), superoxide dismutase (SOD), glutathione peroxidase (Gpx) (Engin et al., 2010) and catalase (CAT) (Majstereka et al., 2011) but high levels of malondialdehyde (MDA). Interestingly, results obtained from serum samples are consistent with those obtained from aqueous humor samples (Nucci et al., 2013; Sorkhabi et al., 2011), indicating that systemic antioxidant capacity can reflect local ocular redox status. Various studies have shown that TMCs are some of the most ROS-sensitive cells in the anterior chamber (Alberto et al., 2009) and serve as regulators of aqueous humor outflow. The TM structure can sustain oxidative stress due to the effects of UV-based oxidative byproducts of aqueous, corneal and crystalline epithelial cells (Stamer & Clark, 2017). ROS-mediated damage to the TM has been shown to impair the structural and functional components of mtDNA in TMCs and to damage proteins and membrane lipids (Abu-Amero, Jose & Bosley, 2006), increasing aqueous humor outflow resistance (Izzotti et al., 2003). Furthermore, elevations in IOP may accelerate oxidative adduct formation, which is greatest near neuronal cell bodies, resulting in a positive feedback loop (Weinreb & Tee, 2004). In vitro studies have shown that oxidative stress is often induced by hydrogen peroxide at different concentrations (Ammar, Hamweyah & Kahook, 2012b; Liu & Zhang, 2019; Lu & Wang, 2017; Zhao et al., 2019a) or by homocysteine (You et al., 2018) and rotenone (He et al., 2019). In addition, oxygen free radical generation in TMCs may increase with age, leading to gradual increases in oxidative damage, extracellular matrix (ECM) accumulation, cytoskeletal changes, apoptosis and changes in the structures and functions of plasmids and lysosomes (Gabelt & Kaufman, 2005).
(1) Imbalance between oxidation and antioxidation in the anterior chamber
Imbalance between oxidants and antioxidants or excessive ROS accumulation can cause oxidative stress (Aydin Yaz et al., 2019). Under conditions of oxidative stress, TMCs express a variety of reductases, such as SOD, glutathione S-transferase (GS-T), and GPx, that neutralize the active substances, and total antioxidant status (TAS) (Abu-Amero et al., 2011), CAT, vitamin C (Ferreira & Lerner, 2008), paraoxonase, and arylesterase can be measured as antioxidant markers. Furthermore, total oxidative stress (TOS) (Dursun et al., 2015), MDA (D’Azy et al., 2016) (16, 17), 8-hydroxydeoxyguanosine (8-OHdG) (Sorkhabi et al., 2011), 4-hydroxynonenal (4-HNE), protein carbonyl (PC) (Mesut et al., 2011), and nitric oxide (NO) have been measured as pro-oxidant markers in various studies. Other inflammatory markers, such as interleukin-1 α (IL-1 α) and endothelial leukocyte adhesion molecule (ELAM)-1, have been evaluated in animals and TMCs (Avotri, Eatman & Russell-Randall, 2019). Imbalance between oxidants and antioxidants can lead to ROS accumulation, TMC structural remodeling, TM enlargement or TM collapse. In addition, oxidative stress stimulates the migration of human TMCs in vitro, resulting in thickening, enlargement and fusion of the TM (Hogg et al., 2000)
(2) Genes and mutations
CyP1B1
Cytochrome P450 family 1 subfamily B member 1 (CYP1B1) is part of the CYP450 family, whose main function is to catalyze reactions of exogenous and endogenous molecules through NADPH (Savas et al., 1994). Mutations in CYP1B1 have been found in patients with congenital glaucoma. Appropriate expression of periostin (Postn) helps to maintain the structural integrity of TM tissue, and the expression of this molecule is influenced by Cyp1b1 (Yun et al., 2013)
LTBP2 (https://www.ncbi.nlm.nih.gov/gene/4053)
The latent transforming growth factor (TGF)- β binding protein (LTBP) 2 gene encodes the protein LTBP2, which is closely connected with ECM molecules including fibrillin proteins and other LTBPs (Rifkin, 2005). Knockdown of LTBP2 affects not only the ECM but also TMC apoptosis through a mechanism that may be mediated by the TGF β and BMP signaling pathways; these effects are similar to those induced by oxidative stress (Suri, Yazdani & Elahi, 2018).
MYOC
Myocilin (MYOC) is the first gene whose mutations were demonstrated to cause familial forms of glaucoma (Stone et al., 1997). One mutation in MYOC activates the IL-1/NF- κB pathway, significantly stimulating IL1A and IL1B expression, which may be associated with POAG (Itakura, Peters & Fini, 2015).
8-OHdG
8-OHdG, a product of oxidative damage to DNA, is produced by reaction of hydroxyl radicals with deoxyguanosine, which causes c-8-hydroxylation (Sun, 2016). As an endogenous mutagenic agent, 8-OHdG can cause a G:C → T:A mutation. One study using 8-OHdG as a marker of oxidative stress revealed that oxidative DNA damage is significantly elevated in TMCs of patients with POAG compared to TMCs of healthy individuals (Sacca et al., 2005). Further analysis revealed a significant positive correlation of 8-OHdG levels in the TM with visual field defects and increased IOP (Sergio Claudio et al., 2005).
TXNRD2
The thioredoxin reductase 2 (TXNRD2) gene encodes a mitochondrial protein of the same name that belongs to the pyridine nucleotide-disulfide oxidoreductase family and is a member of the Trx system. This protein is necessary for reducing damaging ROS generated by oxidative phosphorylation (OXPHOS) and other mitochondrial functions (Chen, Cai & Jones, 2006). A genome-wide association analysis reported that TXNRD2 loci are significantly associated with POAG (Shiga et al., 2018). Additionally, Bailey et al revealed that TXNRD2 loci are significantly associated with IOP in another genome-wide association study (Bailey et al., 2016).
(3) Humor outflow impairment and the ECM
Excessive accumulation of ECM proteins (e.g., collagen, fibronectin (FN), and laminin) in the TM may induce elevations in IOP. In vitro induction of oxidative stress in TMCs leads to typical POAG-like changes (ECM accumulation, cell death, cytoskeletal disorders, inflammatory marker release, etc.), which can be significantly reduced by pretreatment with antioxidants and vasopressors (prostaglandin analogs and carbonic anhydride inhibitors) (Welge-Lussen & Birke, 2010). The levels of FN, an ECM component, are significantly increased in the context of POAG. Increased FN concentrations can not only cause TMC dysfunction but also reduce the numbers of TMCs, thus affecting normal aqueous filtration (Hogg et al., 2000). FN can also change the structures of TMCs, causing dysfunction (Padma et al., 2012). In addition, FN can change other ECM characteristics, increasing the outflow resistance of the aqueous humor. With regard to DNA damage, continuous oxidative stress decreases the function of miR-29b, which negatively regulates the expression of ECM-related genes, thereby promoting the deposition of ECM in the TM and impeding the flow of water out of the chamber (Luna et al., 2009).
(4) Mitochondrial oxidative damage in TMCs
Mitochondria are important sites of intracellular aerobic respiration that play vital roles in maintaining cell homeostasis by regulating processes including oxidative energy metabolism, intracellular calcium balance, neuronal excitability and synaptic transmission, and apoptosis (Chan, 2006). Mitochondrial dysfunction can decrease intracellular ATP synthesis and inhibit mitochondrial OXPHOS, inducing excessive ROS production. Excessive accumulation of ROS leads to mitochondrial DNA damage, which further damages mitochondrial structure and function and in turn generates additional ROS. In recent years, increasing evidence has shown that mitochondrial injury and oxidative stress are involved in TMC damage in glaucoma (Zhao et al., 2016). Mitochondrial complex I defects have been reported to be associated with the degradation of TMCs in POAG patients (Yuan et al., 2008). In addition, patients with POAG are more likely to have a maternal family history than a paternal family history, suggesting a role for mitochondrial inheritance (Paul et al., 2002). Abu-Amero et al. (2011) found 27 nonsynonymous mtDNA mutations in POAG patients, 22 of which were potentially pathogenic, while no such mutations were found in a healthy control group. Mean mitochondrial respiratory activity was decreased in 24 cases, further indicating that oxidative stress and mitochondrial dysfunction contribute significantly to POAG. Chen, Cai & Jones (2006) found that the redox status of mitochondrial thioredoxin (mtTrx) underlies the vulnerability of mitochondria to oxidative injury. These findings indicate that glaucoma is a mitochondrial neurodegenerative disease and thus may suggest new options for glaucoma treatment.
(5) Inflammatory response to oxidative stress
Previous results (Li et al., 2007a) have revealed that the pathological changes induced by oxidative stress include cell death, intracellular ROS production, proinflammatory factor induction, senescence marker activation, PC accumulation, proteasome activity promotion, and apoptosis promotion, all of which are hallmarks of glaucoma. Inflammatory cells release active substances at inflammatory sites, leading to excessive oxidative stress (Li et al., 2007b). Reactive oxygen and nitrogen species (RONS) can activate the expression of proinflammatory genes through intracellular signaling cascades (Yang et al., 2012). For example, ROS can activate the NF-κB pathway, whose downstream target genes include components of mitogen-activated protein kinase (MAPK) signaling pathways, phosphoinositide 3-kinase (PI3K)-Akt, extracellular signal-regulated kinase (ERK) and p38 (Li et al., 2007b), which may alter TM mobility and cause contractile dysfunction. Additionally, oxidative stress can increase the expression of some inflammatory mediators, including IL-1α, IL-6, IL-8 and ELAM-1, not only in glaucomatous TMCs but also in vivo (Tourtas et al., 2012). This effect is further exacerbated by upregulation of the expression of ELAMs due to oxidative stress and activation of the inflammatory cytokine IL-1. Sirtuin 1 (SIRT1) is a member of the sirtuin family of nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases; this protein helps to regulate lifespan in several organisms and may provide protection against diseases related to oxidative stress-induced ocular damage. In the case of glaucoma, such protection is likely to occur through the interaction of SIRT1 with endothelial nitric oxide synthase (eNOS) (Thomas et al., 2002), which regulates inflow and outflow pathways of TMCs.
(6) Aging and oxidative stress
Aging refers to the gradual loss of tissue and organ functions over time (Losordo & Henry, 2016). Aging, in which oxidative stress plays a major role, is a risk factor frequently associated with various degenerative diseases. Age-related structural damage and functional loss are due to the accumulation of oxidative damage in macromolecules (lipids, DNA and proteins) mediated by electrons (Beckman & Ames, 1998). The TM shows striking morphological decay during aging; its cellularity diminishes in a linear manner with age. The exact mechanism by which oxidative stress induces senescence is unclear, but increased RONS levels are known to cause cellular senescence. Autophagy plays a critical role in the removal of aged or damaged intracellular organelles and in the delivery of damaged organelles to lysosomes for degradation (Cuervo et al., 2005). Aging promotes TM senescence due to increased oxidative stress, and this process is paralleled by increased autophagy (Pulliero et al., 2014). Furthermore, production of advanced glycation end products (AGEs) is induced by nonenzymatic reactions between sugars and proteins under conditions of abnormally increased glucose concentrations, especially in aged patients or in patients with diabetes mellitus (Bucala, Tracey & Cerami, 1991); AGEs can enhance TMC senescence and increase oxidative stress (Park & Kim, 2012).
Antioxidative strategies
(1) Physiological antioxidative defense mechanisms
Physiological antioxidative defense mechanisms involve a number of enzymes, such as SOD, CAT, GPx, GS-T, and the thioredoxin (TRX) system (Rokicki et al., 2016). Nonenzymatic antioxidants include endogenously produced GSH and dietary compounds, such as vitamins C and E (Zanon-Moreno et al., 2013); vitamin-like antioxidant compounds, including polyphenols and oligoelements; and certain metalloreductases. The function of these antioxidants is to capture free radicals by accepting and transferring unpaired electrons or through UV light absorption. In addition to the antioxidants described above, TMCs have been shown to be able to synthesize β-crystalline as a molecular chaperone to prevent oxidative damage (Pinazo-Durãn et al., 2017).
(2) Genes and proteins
FOXC1
Forkhead box C1 (FOXC1) is a member of the Forkhead Box or FOX class of transcription factors. The FOX class regulates cellular functions, the development of many organ systems, energy homeostasis and oncogenesis (Carlsson & Mahlapuu, 2002; Lehmann et al., 2003). FOXC1 is essential for the survival of TMCs under conditions of oxidative stress (Berry et al., 2008).
Prdx6
Peroxiredoxin 6 (Prdx6), a protective protein together with GPx and acidic calcium-independent phospholipase A2, acts as a rheostat to regulate cellular physiology by clearing ROS (Singh et al., 2016). ROS accumulation and pathobiological changes in aging or glaucomatous TMCs are partly due to the loss of Prdx6 (Chhunchha et al., 2017) and are correlated with increases in senescence markers and reductions in telomerase activity.
HES1
Hairy and enhancer of split 1 (HES1), which belongs to the basic helix-loop-helix family of transcription factors, is a transcriptional repressor. HES1 regulates the development of cells in the nervous and digestive systems by functioning downstream of the Notch signaling pathway (Kageyama, Ohtsuka & Kobayashi, 2007). Xu et al. found that HES1 promotes ECM expression and inhibits TMC proliferation and migration under oxidative stress (Xu et al., 2017). More importantly, HES1 short hairpin RNA (shRNA) has been shown to attenuate ECM protein upregulation and functional defects caused by oxidative stress.
TGF-β2
TGF-β2 in the aqueous humor may cause molecular changes and increase outflow resistance in POAG (Inatani et al., 2001; Junglas et al., 2009). The effect of connective tissue growth factor (CTGF) in oxidative stress is associated with ECM synthesis and increased contractility of the TM, contributing to a decrease in aqueous humor outflow facility and an increase in IOP (Sabrina et al., 2015). A recent study showed that mitochondrial-targeted antioxidants (XJB-5-131 and MitoQ) can attenuate TGF- β2/Smad signaling in TMCs through processes including reductions in CTGF and collagen isoform gene and protein expression (Rao et al., 2019).
NRF2
Nuclear factor (erythroid-derived 2)-like 2 (NRF2) plays a key role in regulating cellular oxidation reactions through oxidative stress defense mechanisms (Sachdeva, Cano & Handa, 2014). After exposure to ROS, Kelch-like ECH-associated protein 1 (Keap1) undergoes conformational changes, translocating NRF2 into the nucleus, binding to the antioxidant response element (ARE) region, and initiating the transcription of targets, including heme oxygenase-1 (HO-1) (Batliwala et al., 2017; Suzuki & Yamamoto, 2015) and NAD (P)H:quinone oxidoreductase1 (NQO1). Recently, many NRF2 activators, including the antioxidants sulforaphane (SFN), quercetin, and resveratrol (RSV), have been intensively studied and show great potential for protection against oxidative stress; these findings may offer new strategies for glaucoma treatment.
Rho kinase family members and their inhibitors
The Rho family kinases (Pinazo-Durãn et al., 2017) and their inhibitors (AMA0076, AR-13324, K-115, PG324, Y-39983, RKI-983, H-1152 recoverin and Y-27632) (Fujimoto et al., 2017) modulate signal transduction pathways; actin cytoskeleton function; and TMC, canal of Schlemm and ciliary muscle cell motility. In vivo, inhibition of p38 MAPK phosphorylation decreases tert-butyl hydroperoxide-induced apoptosis in TMCs.
(3) Noncoding RNAs
MicroRNAs are a class of small noncoding RNAs (19–25 nucleotides in length) that regulate a wide range of cellular processes by repressing the transcription or translation of their target genes (Van Rooij, 2011). MiRNAs are abundantly present in biological fluids and are reliable diagnostic and predictive biomarkers (Weber et al., 2010). Long noncoding RNAs are >200 nucleotide-long RNA molecules that lack or have limited protein-coding potential but can regulate miRNAs or protein formation through several different mechanisms (Wawrzyniak et al., 2018). Recently, noncoding RNAs have become popular subjects of glaucoma research (Table 1), providing attractive opportunities to defend against oxidative stress and to identify novel biomarkers for the diagnosis and prognosis of glaucoma.
Table 1. Role of antioxidative stress of miRNAs and lncRNAs.
| Name | Functions and Mechanisms | References |
|---|---|---|
| miR-1 | Regulates TMCs under oxidative stress by targeting FN expression. | Guo et al. (2019) |
| miR-29b | Downregulated by TGF-β2 and oxidative stress. Negatively regulates the expression of multiple genes involved in the synthesis and deposition of ECM proteins, including SPARC (secreted protein, acidic, and rich in cysteine), FBN1, laminin, collagens, BMP1, ADAM12, NKIRAS2, and SP1. | Guadalupe et al. (2011), Li et al. (2009), Luna et al. (2009), Srikumar et al. (2008), Zhaoyong et al. (2009) |
| miR-21 | Increases the production of the ECM by silencing its target gene PTEN and by regulating TGF-β2 expression. | Dang (2017) |
| miR-181a | Inhibits the TMCs apoptosis induced by H2O2 through the suppression of the NF-κB and JNK pathways. | Wang et al. (2018) |
| miR-1298 | Protects TMCs against the damage caused by chronic oxidative stress (COS) via inhibiting the TGF- β2/Smad4 pathway and activating the canonical Wnt pathway. | Ruibin et al. (2018) |
| miR-483-3p | Inhibits the ECM after oxidative stress by targeting Smad4. | Shen et al. (2015) |
| miR-24 | Regulates TGF β1 during cyclic mechanical stress by targeting FURIN. | Coralia et al. (2011) |
| miR-200c | Inhibits the expression of genes (ZEB1, ZEB2, FHOD1, LPAR1/EDG2, ETAR, and RHOA) related to the contraction of TMCs. | Luna et al. (2012) |
| miR-146a | Modulates inflammatory markers. | Guorong et al. (2010) |
| miR-204 | Affects the sensitivity of TMCs to apoptosis and the number of cells. Acts as a direct target of AP1S2, Bcl2l2, BIRC2, EDEM1, EZR, FZD1, M6PR, RAB22A, RAB40B, SERP1, TCF12, TCF4, CLOCK, PLEKHG5, and ITGB1 MEIS2 and as a potential target of FOXC1. | Guorong et al. (2011), Matthew et al. (2012), Paylakhi et al. (2013), Redis et al. (2012) |
| miR-155 | Regulates the ECM though interacting with the TGF β pathway. | Bjoern et al. (2006), Johannes et al. (2004) |
| miR-184 | Regulates the growth, apoptosis and cytotoxicity by inhibiting HIF-l α. | Wang et al. (2017) |
| miR-93 | Inhibits the viability and induces the apoptosis via the suppression of NRF2. | Wang, Li & Wang (2016) |
| miR-175p | MiR-17-5p was downregulated in TMCs under oxidative conditions, and may regulate the apoptosis of TMCs by targeting PTEN | Wang et al. (2019) |
| miR-27a | Regulates Nrf2 expression at the posttranscriptional level. Salidroside (Sal) mitigates hydrogen peroxide-induced injury by activating the PI3K/AKT and Wnt/b-catenin pathways by increasing miR-27a. | Zhao et al. (2019a) |
| miR-199-5p | Targets the 3’-UTR of TGF β2. The increase in TGF β2expression induced by oxidative stress may be related to the downregulation of mir-199-5p expression. | Feng (2014) |
| miR-182 | MiR-182 expression is upregulated in primary TMCs with stress-induced premature senescence. The overexpression of miR-182 contributes to the phenotypic alterations of senescent cells. | Liu et al. (2016) |
| miR-183 | Decreases the expression of laminin, gel, and type I collagen by targeting ITG β1 without a 3’-UTR. | Li et al. (2010) |
| miR-450 | Influences the shrinkage of TMCs by targeting the MyoD family of proteins. | Sun et al. (2014) |
| miR-107 | Regulates Nestin expression and counteracts the apoptosis of TMCs. | Xue et al. (2006) |
| miR-144-3p | The over-expression of miR-144-3p promotes the proliferation and invasion of TMCs by inhibiting the expression of FN-1 in oxidative stress TMCs | Yin & Chen (2019) |
| LncRNA-RP11-820 | Promotes ECM production via regulating miR-3178/MYOD1 | Shen et al. (2019) |
| LncRNA antisense noncoding RNA in the INK4 locus (ANRIL) | Down-regulates microRNA-7 to protect TMCs in an experimental model for glaucoma | Zhao et al. (2019b) |
(4) PUFAs
Polyunsaturated fatty acids (PUFAs) have numerous anti-inflammatory and antioxidant properties (Sacca et al., 2018) that can influence mitochondrial energy production; improve mitochondrial function (Putti et al., 2015); influence cellular energy metabolism, neuronal plasticity, and membrane homeostasis (Dyall, 2017); and improve synaptic function. Omega-3 and omega-6 fatty acids exert preventative effects against oxidative stress in TMCs by abolishing the stimulation of NF-κB and IL-6. Therefore, the physiological basis of the PUFA-mediated protection of TMCs from oxidative stress has been revealed, which may provide new targets for antioxidation treatment (Tourtas et al., 2012).
(5) Exogenous compounds
Due to the association between oxidative stress and age-related disease, many types of phytochemicals, including polyphenols and terpenoids, which have anti-inflammatory and antioxidant properties, have been reported to be potential preventative treatments for ocular diseases. Additionally, other compounds, including rapamycin (He et al., 2019), ethyl pyruvate (Famili, Ammar & Kahook, 2013), and 1α,25-dihydroxyvitamin D3 (Lv et al., 2019), exert protective effects against oxidative stress through different pathways. The functions and mechanisms of these compounds are shown in Table 2. Studies investigating exogenous compounds have revealed new treatment options for oxidative stress.
Table 2. Role of antioxidative exogenous compounds.
| Name | Functions and Mechanisms | References |
|---|---|---|
| Resveratrol | Increases mitochondrial mass and mitochondrial DNA. Activates SIRT1 and upregulates NO and eNOS. Activates Nrf2 pathways. | Avotri, Eatman & Russell-Randall (2019), Coralia et al. (2011) |
| Lycium barbarum polysaccharides (LBP) | Activates the PI3K/AKT and ERK signaling pathways by upregulating miR-4295. | Liu & Zhang (2019) |
| Curcumin | Inhibits proinflammatory factors, including IL-6, ELAM-1, IL-1α, and IL-8, decreases the activities of the senescence marker SA-β-gal, and lowers the levels of carbonylated proteins and the number of apoptotic cells. | Lin, Ciolino & Pasquale (2017) |
| Baicalin | Increases cell survival and decreases iROS production. Inhibits the production of IL-1α and ELAM-1, decreases the activity of senescence-associated SA-β-gal, and lowers the level of carbonylated proteins. | Gong & Zhu (2018) |
| Sulforaphane | Attenuates H2O2-induced oxidative stress via PI3K/AKT-mediated NRF2 signaling activation. | Liu & Zhang (2019) |
| Quercetin | Upregulates antioxidant peroxiredoxins through the activation of the NRF2/NRF1 transcription pathway and protects against oxidative stress-induced ocular disease. | Naoya et al. (2011) |
| Procyanidins | Decreases the apoptotic rate of TMCs under oxidative stress and reduces the release of cytochrome C. | Shi & Wang (2017) |
| Salidroside | Protects TMCs against H2O2-induced oxidative damage by activating the PI3K/AKT and Wnt/β-catenin pathways by increasing miR-27a. | Zhao et al. (2019a), Zhao et al. (2019b) |
| Polyphenols (derived from red wine, tea and dark chocolate) | Targets eNOS and induces the accumulation of NRF2. | Mann et al. (2007), Upadhyay & Dixit (2015) |
| Rapamycin | Protects TM-1 cells from COS by inhibiting mTOR and inducing autophagy. In addition, removes damaged mitochondria. | He et al. (2019) |
| Ethyl pyruvate | Able to nonenzymatically reduce hydrogen peroxide and scavenge hydroxyl radicals. | Dobsak et al. (1999), Famili, Ammar & Kahook (2013) |
| 1α,25-dihydroxyvitamin D3 | Attenuates OS-induced damage in TMCs by inhibiting TGFβ-SMAD3-VDR pathway | Lv et al. (2019) |
(6) Systemic antioxidant administration for glaucoma treatment
As described in the section “TM oxidative stress and glaucoma”, systemic antioxidant capacity can reflect local ocular redox status. Some researchers have hypothesized and verified that increases in systemic antioxidant levels due to long-term antioxidant intake can increase local antioxidant levels, but the evidence is limited. Intake of vitamins C, A, and E is not significantly associated with the risk of POAG (Kang et al., 2003; Wang, Singh & Lin, 2013). Notably, in the case of glaucoma, systemic drugs have greater difficulty crossing the blood-retinal barrier than local drugs (Lin, Ciolino & Pasquale, 2017); in addition, systemic drugs have more systemic side effects and lower bioavailability than local drugs. These differences remain challenges to be solved. Many new drug delivery systems (such as in situ gels, liposomes, niosomes, hydrogels, dendrimers, nanoparticles, and solid lipid nanoparticles) are in clinical trials. The goal of related research is to improve drug delivery in appropriate recipients, which may improve efficacy and compliance and reduce side effects (Yadav, Rajpurohit & Sharma, 2019).
Conclusion
Various studies on humans and laboratory animals have demonstrated that a variety of antioxidants, particularly noncoding RNAs and exogenous compounds, help to regulate IOP and protect TMCs from oxidative stress. Based on these studies, it is believed that new methods with broad applicability and promise for the treatment of oxidative stress and glaucoma will be developed in the near future.
Acknowledgments
We would like to thank Dr. Yajuan Zheng in the production of this review. Her perspective and advice have been invaluable and have helped us a lot in completing this review.
Funding Statement
This study was supported by the National Natural Science Foundation of China (no. 81271002). There was no additional external funding received for this study The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Mingxuan Wang conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Yajuan Zheng conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review.
References
- Abu-Amero, Jose & Bosley (2006).Abu-Amero KK, Jose M, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Investigative Ophthalmology & Visual Science. 2006;47:2533–2541. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- Abu-Amero et al. (2011).Abu-Amero KK, Kondkar AA, Mousa A, Osman EA, Al-Obeidan SA. Decreased total antioxidants status in the plasma of patients with pseudoexfoliation glaucoma. Molecular Vision. 2011;17:2769–2775. [PMC free article] [PubMed] [Google Scholar]
- Alberto et al. (2009).Alberto I, Saccà SC, Mariagrazia L, Cristina C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Investigative Ophthalmology & Visual Science. 2009;50:5251–5258. doi: 10.1167/iovs.09-3871. [DOI] [PubMed] [Google Scholar]
- Ammar, Hamweyah & Kahook (2012a).Ammar DA, Hamweyah KM, Kahook MY. Antioxidants protect trabecular meshwork cells from hydrogen peroxide-induced cell death. Translational Vision Science & Technology. 2012a;1 doi: 10.1167/tvst.1.1.4. Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar, Hamweyah & Kahook (2012b).Ammar DA, Hamweyah KM, Kahook MY. Antioxidants protect trabecular meshwork cells from hydrogen peroxide-induced cell death. Transl Vis Sci Technol. 2012b;1 doi: 10.1167/tvst.1.1.4. Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avotri, Eatman & Russell-Randall (2019).Avotri S, Eatman D, Russell-Randall K. Effects of resveratrol on inflammatory biomarkers in glaucomatous human trabecular meshwork cells. Nutrients. 2019;11(5) doi: 10.3390/nu11050984. Article 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin Yaz et al. (2019).Aydin Yaz Y, Yildirim N, Yaz Y, Tekin N, Inal M, Sahin FM. Role of oxidative stress in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Turkish Journal of Ophthalmology. 2019;49:61–67. doi: 10.4274/tjo.galenos.2018.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey et al. (2016).Bailey JN, Loomis SJ, Kang JH, Allingham RR, Gharahkhani P, Khor CC, Burdon KP, Aschard H, Chasman DI, Jr IR. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nature Genetics. 2016;48:189–194. doi: 10.1038/ng.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batliwala et al. (2017).Batliwala S, Xavier C, Liu Y, Wu H, Pang IH. Involvement of Nrf2 in ocular diseases. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/1703810. Article 1703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman & Ames (1998).Beckman KB, Ames BN. The free radical theory of aging matures. Physiological Reviews. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Berry et al. (2008).Berry FB, Skarie JM, Farideh M, Yannick F, Hudson TJ, Vincent R, Link BA, Walter MA. FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Human Molecular Genetics. 2008;17:490–505. doi: 10.1093/hmg/ddm326. [DOI] [PubMed] [Google Scholar]
- Bjoern et al. (2006).Bjoern B, Marco B, Daniel K, Michael E, Elke LD. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Investigative Ophthalmology and Visual Science. 2006;47:2011–2020. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- Bucala, Tracey & Cerami (1991).Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. Journal of Clinical Investigation. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson & Mahlapuu (2002).Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Developmental Biology. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- Chan (2006).Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen, Cai & Jones (2006).Chen Y, Cai J, Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Letters. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhunchha et al. (2017).Chhunchha B, Singh P, Stamer WD, Singh DP. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discovery. 2017;3:17060. doi: 10.1038/cddiscovery.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coralia et al. (2011).Coralia L, Guorong L, Jianming Q, Epstein DL, Pedro G. MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. Journal of Cellular Physiology. 2011;226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo et al. (2005).Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining clean cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Dang (2017).Dang X. Effects of miR-21 on protein expression of extracellular matrix in human trabecular meshwork cells under oxidative stress. Recent Advances in Ophthalmology. 2017;37:30–34. [Google Scholar]
- D’Azy et al. (2016).D’Azy CB, Pereira B, Chiambaretta F, Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: a systematic review and meta-analysis. PLOS ONE. 2016;11:e0166915. doi: 10.1371/journal.pone.0166915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobsak et al. (1999).Dobsak P, Courderot MC, Zeller M, Vergely C, Laubriet A, Assem M, Eicher J, Teyssier J, Wolf J, Rochette L. Antioxidative properties of pyruvate and protection of the ischemic rat heart during cardioplegia. Journal of Cardiovascular Pharmacology. 1999;34:651–659. doi: 10.1097/00005344-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Doina et al. (2005).Doina G, Helen Rosemary G, Emma Jane H, Ian Andrew C, Sarah Louise H. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Investigative Ophthalmology and Visual Science. 2005;46:877–883. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- Dursun et al. (2015).Dursun F, Ozec AV, Aydin H, Topalkara A, Dursun A, Toker MI, Erdogan H, Arici MK. Total oxidative stress, paraoxonase and arylesterase levels at patients with pseudoexfoliation syndrome and pseudoexfoliative glaucoma. International Journal of Ophthalmology. 2015;8:985. doi: 10.3980/j.issn.2222-3959.2015.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall (2017).Dyall SC. Interplay between n-3 and n-6 long-chain polyunsaturated fatty acids and the endocannabinoid system in brain protection and repair. Lipids. 2017;52:885–900. doi: 10.1007/s11745-017-4292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin et al. (2010).Engin KN, Yemişci B, Yiğit U, Ağaçhan A, Coşkun C. Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Molecular Vision. 2010;16:1260–1271. [PMC free article] [PubMed] [Google Scholar]
- Famili, Ammar & Kahook (2013).Famili A, Ammar DA, Kahook MY. Ethyl pyruvate treatment mitigates oxidative stress damage in cultured trabecular meshwork cells. Molecular Vision. 2013;19:1304–1309. [PMC free article] [PubMed] [Google Scholar]
- Feng (2014).Feng X. D Thesis. 2014. The mechanism of miRNA-199b-5p regulating TGF-β2 in human trabecular meshwork cells under oxidative stress. [Google Scholar]
- Ferreira & Lerner (2008).Ferreira SM, Lerner SR. Antioxidant status in the aqueous humour of patients with glaucoma associated with exfoliation syndrome. Eye. 2008;23:1691–1697. doi: 10.1038/eye.2008.352. [DOI] [PubMed] [Google Scholar]
- Ferreira et al. (2004).Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients ✩. American Journal of Ophthalmology. 2004;137:62–69. doi: 10.1016/S0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Fujimoto et al. (2017).Fujimoto T, Inoue T, Ohira S, Awai-Kasaoka N, Kameda T, Inoue-Mochita M, Tanihara H. Inhibition of Rho kinase induces antioxidative molecules and suppresses reactive oxidative species in trabecular meshwork cells. Journal of Ophthalmology. 2017;2017 doi: 10.1155/2017/7598140. Article 7598140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelt & Kaufman (2005).Gabelt BAT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Progress in Retinal & Eye Research. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gong & Zhu (2018).Gong L, Zhu J. Baicalin alleviates oxidative stress damage in trabecular meshwork cells in vitro. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2018;391:51–58. doi: 10.1007/s00210-017-1433-9. [DOI] [PubMed] [Google Scholar]
- Guadalupe et al. (2011).Guadalupe V, Dong-Jin O, Hyung KM, Rhee DJ. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Investigative Ophthalmology & Visual Science. 2011;52:3391–3397. doi: 10.1167/iovs.10-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2019).Guo JH, Su C, Jiang SY, Wang F, Feng X, Wang JT. MicroRNA-1 regulates fibronectin expression in human trabecular meshwork cells under oxidative stress. Zhonghua Yan Ke Za Zhi. 2019;55:355–360. doi: 10.3760/cma.j.issn.0412-4081.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Guorong et al. (2010).Guorong L, Coralia L, Jianming Q, Epstein DL, Pedro G. Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Investigative Ophthalmology & Visual Science. 2010;51:2976–2985. doi: 10.1167/iovs.09-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guorong et al. (2011).Guorong L, Coralia L, Jianming Q, Epstein DL, Pedro G. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Investigative Ophthalmology & Visual Science. 2011;52:2999–3007. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2019).He JN, Zhang SD, Qu Y, Wang HL, Tham CC, Pang CP, Chu WK. Rapamycin removes damaged mitochondria and protects human trabecular meshwork (TM-1) cells from chronic oxidative stress. Molecular Neurobiology. 2019;56(9):6586–6593. doi: 10.1007/s12035-019-1559-5. [DOI] [PubMed] [Google Scholar]
- Hogg et al. (2000).Hogg P, Calthorpe M, Batterbury M, Grierson I. Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Investigative Ophthalmology & Visual Science. 2000;41:1091–1098. [PubMed] [Google Scholar]
- Inatani et al. (2001).Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-β2 levels in aqueous humor of glaucomatous eyes. Graefes Archive for Clinical & Experimental Ophthalmology. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Itakura, Peters & Fini (2015).Itakura T, Peters DM, Fini ME. Glaucomatous MYOC mutations activate the IL-1/NF-kappaB inflammatory stress response and the glaucoma marker SELE in trabecular meshwork cells. Molecular Vision. 2015;21:1071–1084. [PMC free article] [PubMed] [Google Scholar]
- Izzotti et al. (2003).Izzotti A, Cartiglia C, De Flora S, Sacca S. Methodology for evaluating oxidative DNA damage and metabolic genotypes in human trabecular meshwork. Toxicology Mechanisms and Methods. 2003;13(3):161–168. doi: 10.1080/15376510309830. [DOI] [PubMed] [Google Scholar]
- Johannes et al. (2004).Johannes G, Darren C, Michael E, Elke LD, Ross EC. Effects of TGF-beta2 in perfused human eyes. Investigative Ophthalmology and Visual Science. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Junglas et al. (2009).Junglas B, Yu AHL, Welge-Lüssen U, Tamm ER, Fuchshofera R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Experimental Eye Research. 2009;88:1065–1075. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Kageyama, Ohtsuka & Kobayashi (2007).Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- Kang et al. (2003).Kang JH, Pasquale LR, Walter W, Bernard R, Egan KM, Nicholaus F, Hankinson SE. Antioxidant intake and primary open-angle glaucoma: a prospective study. American Journal of Epidemiology. 2003;158:337–346. doi: 10.1093/aje/kwg167. [DOI] [PubMed] [Google Scholar]
- Lehmann et al. (2003).Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. Fox’s in development and disease. Trends in Genetics. 2003;19:339–344. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Li et al. (2007a).Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Molecular Vision. 2007a;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2007b).Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Molecular Vision. 2007b;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2009).Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence ✩. Mechanisms of Ageing & Development. 2009;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2010).Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Targeting of integrin Î21 and Kinesin 2Î ± by MicroRNA 183. Journal of Biological Chemistry. 2010;285:2976–2985. doi: 10.1074/jbc.M109.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Ciolino & Pasquale (2017).Lin MM, Ciolino JB, Pasquale LR. Novel glaucoma drug delivery devices. International Ophthalmology Clinics. 2017;57:57–71. doi: 10.1097/IIO.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2016).Liu Y, Bailey JC, Helwa I, Dismuke WM, Cai J, Drewry M, Brilliant MH, Budenz DL, Christen WG, Chasman DI. A common variant inMIR182Is associated with primary open-angle glaucoma in the NEIGHBORHOOD Consortium. Investigative Ophthalmology & Visual Science. 2016;57:4528–4535. doi: 10.1167/iovs.16-19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu & Zhang (2019).Liu Y, Zhang Y. Lycium barbarum polysaccharides alleviate hydrogen peroxide-induced injury by up-regulation of miR-4295 in human trabecular meshwork cells. Experimental and Molecular Pathology. 2019;106:109–115. doi: 10.1016/j.yexmp.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Losordo & Henry (2016).Losordo DW, Henry TD. New definition of aging? Circulation Research. 2016;119:774–775. doi: 10.1161/CIRCRESAHA.116.309622. [DOI] [PubMed] [Google Scholar]
- Lu & Wang (2017).Lu S, Wang CY. Protective effects of procyanidins on human trabecular meshwork cells against H_2O_2 induced oxidative stress. Recent Advances in Ophthalmology. 2017;37(2):121–124. [Google Scholar]
- Luna et al. (2012).Luna C, Li G, Huang J, Qiu J, Wu J, Yuan F, Epstein DL, Gonzalez P. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLOS ONE. 2012;7:e51688. doi: 10.1371/journal.pone.0051688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna et al. (2009).Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Molecular Vision. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- Lv et al. (2019).Lv Y, Han X, Yao Q, Zhang K, Zheng L, Hong W, Xing X. 1apha, 25- dihydroxyvitamin D3 attenuates oxidative stress-induced damage in human trabecular meshwork cells by inhibiting TGFbeta-SMAD3-VDR pathway. Biochemical and Biophysical Research Communications. 2019;516:75–81. doi: 10.1016/j.bbrc.2019.06.027. [DOI] [PubMed] [Google Scholar]
- Majstereka et al. (2011).Majstereka I, Stanczyk M, Kowalski M, Blaszczyk J, Kurowska AK, Kaminska A, Szaflik J, Szaflik JP. Evaluation of oxidative stress markers in pathogenesis of primary open-angle glaucoma. Experimental & Molecular Pathology. 2011;90:231–237. doi: 10.1016/j.yexmp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Mann et al. (2007).Mann GE, Rowlands DJ, Li FYL, Patricia DW, Siow RCM. Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovascular Research. 2007;75:261–274. doi: 10.1016/j.cardiores.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Matthew et al. (2012).Matthew W, Xing-Hua G, Li Z, Qing-Sheng M. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. Rna Biology. 2012;9:850–859. doi: 10.4161/rna.20378. [DOI] [PubMed] [Google Scholar]
- Mesut et al. (2011).Mesut E, Ramazan YC, Ömer A, Remzi K, Ali A, Hepşen IF. Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Current Eye Research. 2011;36:713–718. doi: 10.3109/02713683.2011.584370. [DOI] [PubMed] [Google Scholar]
- Naoya et al. (2011).Naoya M, Hiroto I, Rie M, Hiroyuki K, Akihiko T, Yasuyuki S, Kimitoshi K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Investigative Ophthalmology & Visual Science. 2011;52:1055. doi: 10.1167/iovs.10-5777. [DOI] [PubMed] [Google Scholar]
- Nucci et al. (2013).Nucci C, Pierro DD, Varesi C, Ciuffoletti E, Russo R, Gentile R, Cedrone C, Duran MDP, Coletta M, Mancino R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Molecular Vision. 2013;19:1841–1846. [PMC free article] [PubMed] [Google Scholar]
- Padma et al. (2012).Padma I, Rupalatha M, Pattabiraman PP, Ponugoti Vasantha R. Connective tissue growth factor-mediated upregulation of neuromedin U expression in trabecular meshwork cells and its role in homeostasis of aqueous humor outflow. Investigative Ophthalmology & Visual Science. 2012;53:4952–4962. doi: 10.1167/iovs.12-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park & Kim (2012).Park CH, Kim JW. Effect of advanced glycation end products on oxidative stress and senescence of trabecular meshwork cells. Korean Journal of Ophthalmology Kjo. 2012;26:123–131. doi: 10.3341/kjo.2012.26.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul et al. (2002).Paul M, Elena R, Lee AJ, Jin WJ. Bias in self-reported family history and relationship to glaucoma: the Blue Mountains eye study. Ophthalmic Epidemiology. 2002;9:333–345. doi: 10.1076/opep.9.5.333.10335. [DOI] [PubMed] [Google Scholar]
- Paylakhi et al. (2013).Paylakhi SH, Moazzeni H, Yazdani S, Rassouli P, Arefian E, Jaberi E, Arash EH, Gilani AS, Fan JB, April C. FOXC1 in human trabecular meshwork cells is involved in regulatory pathway that includes miR-204, MEIS2, and ITGβ1. Experimental Eye Research. 2013;111:112–121. doi: 10.1016/j.exer.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Pinazo-Durãn et al. (2017).Pinazo-DurÃn MD, Shoaie-Nia K, Zanã3N-Moreno V, Sanz-GonzÃlez SM, Del Castillo JB, Garcã”-A-Medina JJ. Strategies to reduce oxidative stress in glaucoma patients. Current Neuropharmacology. 2017;15:903–918. doi: 10.2174/1570159X15666170705101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliero et al. (2014).Pulliero A, Seydel A, Camoirano A, Saccà SC, Sandri M, Izzotti A. Oxidative damage and autophagy in the human trabecular meshwork as related with ageing. PLOS ONE. 2014;9:e98106. doi: 10.1371/journal.pone.0098106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putti et al. (2015).Putti R, Sica R, Migliaccio V, Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Frontiers in Physiology. 2015;6 doi: 10.3389/fphys.2015.00109. Article 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley & Broman (2006).Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao et al. (2019).Rao VR, Lautz JD, Kaja S, Foecking EM, Lukacs E, Stubbs Jr EB. Mitochondrial-targeted antioxidants attenuate TGF-beta2 signaling in human trabecular meshwork cells. Investigative Ophthalmology and Visual Science. 2019;60:3613–3624. doi: 10.1167/iovs.19-27542. [DOI] [PubMed] [Google Scholar]
- Redis et al. (2012).Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacology & Therapeutics. 2012;136:169–174. doi: 10.1016/j.pharmthera.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin (2005).Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. Journal of Biological Chemistry. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Rokicki et al. (2016).Rokicki W, Zalejska-Fiolka J, Pojda-Wilczek D, Kabiesz A, Majewski W. Oxidative stress in the red blood cells of patients with primary open-angle glaucoma. Clinical Hemorheology and Microcirculation. 2016;62:369–378. doi: 10.3233/CH-152029. [DOI] [PubMed] [Google Scholar]
- Ruibin et al. (2018).Ruibin W, Zheng X, Chen J, Zhang X, Yang X, Lin Y. Micro RNA-1298 opposes the effects of chronic oxidative stress on human trabecular meshwork cells via targeting on EIF4E3. Biomedicine & Pharmacotherapy. 2018;100:349–357. doi: 10.1016/j.biopha.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Sabrina et al. (2015).Sabrina K, Benjamin J, Braunger BM, Tamm ER, Rudolf F. The regulation of connective tissue growth factor expression influences the viability of human trabecular meshwork cells. Journal of Cellular & Molecular Medicine. 2015;19:1010–1020. doi: 10.1111/jcmm.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca et al. (2018).Sacca SC, Cutolo CA, Ferrari D, Corazza P, Traverso CE. The eye, oxidative damage and polyunsaturated fatty acids. Nutrients. 2018;10(6) doi: 10.3390/nu10060668. Article 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca et al. (2005).Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Archives of Ophthalmology. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- Sachdeva, Cano & Handa (2014).Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Experimental Eye Research. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas et al. (1994).Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. Journal of Biological Chemistry. 1994;269:14905–14911. [PubMed] [Google Scholar]
- Sergio Claudio et al. (2005).Sergio Claudio S, Antonio P, Paola C, Paolo C, Alberto I. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Archives of Ophthalmology. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2015).Shen W, Han Y, Huang B, Qi Y, Xu L, Guo R, Wang X, Wang J. MicroRNA-483-3p inhibits extracellular matrix production by targeting Smad4 in human trabecular meshwork cells. Investigative Ophthalmology & Visual Science. 2015;56:8419–8427. doi: 10.1167/iovs.15-18036. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2019).Shen W, Huang B, He Y, Shi L, Yang J. Long non-coding RNA RP11-820 promotes extracellular matrix production via regulating miR-3178/MYOD1 in human trabecular meshwork cells. The FEBS Journal. 2019 doi: 10.1111/febs.15058. Epub ahead of print Sep 08 2019. [DOI] [PubMed] [Google Scholar]
- Shi & Wang (2017).Shi L, Wang C. Effects of procyanidins on cell apoptosis and the release of cytochrome C in human trabecular meshwork cells under oxidative stress. Recent Advances in Ophthalmology. 2017;37:931–934. [Google Scholar]
- Shiga et al. (2018).Shiga Y, Akiyama M, Nishiguchi KM, Sato K, Shimozawa N, Takahashi A, Momozawa Y, Hirata M, Koichi M, Yamaji T. Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Human Molecular Genetics. 2018;27(8):1486–1496. doi: 10.1093/hmg/ddy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh et al. (2016).Singh SP, Chhunchha B, Fatma N, Kubo E, Singh SP, Singh DP. Delivery of a protein transduction domain-mediated Prdx6 protein ameliorates oxidative stress-induced injury in human and mouse neuronal cells. American Journal of Physiology. Cell Physiology. 2016;90:ajpcell.0022902015. doi: 10.1152/ajpcell.00229.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkhabi et al. (2011).Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Molecular Vision. 2011;17:41–46. [PMC free article] [PubMed] [Google Scholar]
- Srikumar et al. (2008).Srikumar S, Den Boon JA, Chen IH, Newton MA, Stanhope SA, Chen Y-J, Chen C-J, Allan H, Bill S, Paul A. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer & Clark (2017).Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Experimental Eye Research. 2017;158:112–123. doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone et al. (1997).Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2014).Sun MM, Li JF, Guo LL, Xiao HT, Dong L, Wang F, Huang FB, Cao D, Qin T, Yin XH, Li JM, Wang SL. TGF-beta1 suppression of microRNA-450b-5p expression: a novel mechanism for blocking myogenic differentiation of rhabdomyosarcoma. Oncogene. 2014;33:2075–2086. doi: 10.1038/onc.2013.165. [DOI] [PubMed] [Google Scholar]
- Sun (2016).Sun R. Oxidative injury on trabecular meshwork. Chinese Journal of Experimental Ophthalmology. 2016;34:375–379. [Google Scholar]
- Suri, Yazdani & Elahi (2018).Suri F, Yazdani S, Elahi E. LTBP2 knockdown and oxidative stress affect glaucoma features including TGFβ pathways, ECM genes expression and apoptosis in trabecular meshwork cells. Gene. 2018;88 doi: 10.1016/j.gene.2018.06.038. S0378111918306899- [DOI] [PubMed] [Google Scholar]
- Suzuki & Yamamoto (2015).Suzuki T, Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radical Biology and Medicine. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Tanito et al. (2015).Tanito M, Kaidzu S, Takai Y, Ohira A. Correlation between systemic oxidative stress and intraocular pressure level. PLOS ONE. 2015;10:e0133582. doi: 10.1371/journal.pone.0133582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas et al. (2002).Thomas W, Göran D, Thomas T, Henrik A, Huige L, Klaus W, Ulrich FR. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.CIR.0000029925.18593.5C. [DOI] [PubMed] [Google Scholar]
- Tourtas et al. (2012).Tourtas T, Birke MT, Kruse FE, Welge-Lussen UC, Birke K. Preventive effects of omega-3 and omega-6 Fatty acids on peroxide mediated oxidative stress responses in primary human trabecular meshwork cells. PLOS ONE. 2012;7:e31340. doi: 10.1371/journal.pone.0031340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay & Dixit (2015).Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxidative Medicine & Cellular Longevity. 2015;2015:504253. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij (2011).Van Rooij E. The art of microRNA research. Circulation Research. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang D, Wang H, Wu X, Wang H, Wang S, Liu C. MiR-184 prevents chronic oxidative stress induced human trabecular meshwork cells apoptosis and cytotoxicity in vitro by targeting hypoxia-inducible factor 1α. International Journal of Clinical and Experimental Pathology. 2017;10:2754–2763. [Google Scholar]
- Wang, Singh & Lin (2013).Wang SY, Singh K, Lin SC. Glaucoma and vitamins A, C, and E supplement intake and serum levels in a population-based sample of the United States. Eye. 2013;27:487–494. doi: 10.1038/eye.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019).Wang X, Li Z, Bai J, Song W, Zhang F. miR175p regulates the proliferation and apoptosis of human trabecular meshwork cells by targeting phosphatase and tensin homolog. Molecular Medicine Reports. 2019;19:3132–3138. doi: 10.3892/mmr.2019.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Li & Wang (2016).Wang Y, Li F, Wang S. MicroRNA93 is overexpressed and induces apoptosis in glaucoma trabecular meshwork cells. Molecular Medicine Reports. 2016;14:5746–5750. doi: 10.3892/mmr.2016.5938. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang Y, Zhou H, Liu X, Han Y, Pan S, Wang Y. MiR-181a inhibits human trabecular meshwork cell apoptosis induced by HâOâ through the suppression of NF-ÎoB and JNK pathways. Advances in Clinical & Experimental Medicine. 2018;27(5):577–582. doi: 10.17219/acem/69135. [DOI] [PubMed] [Google Scholar]
- Wawrzyniak et al. (2018).Wawrzyniak O, Zarebska Z, Rolle K, Gotz-Wieckowska A. Circular and long non-coding RNAs and their role in ophthalmologic diseases. Acta Biochimica Polonica. 2018;65:497–508. doi: 10.18388/abp.2018_2639. [DOI] [PubMed] [Google Scholar]
- Weber et al. (2010).Weber JA, Baxter DH, Shile Z, Huang DY, How HKuo, Jen LM, Galas DJ, Kai W. The microRNA spectrum in 12 body fluids. Clinical Chemistry. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb & Tee (2004).Weinreb RN, Tee KP. Primary open-angle glaucoma. New England Journal of Medicine. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen & Birke (2010).Welge-Lussen U, Birke K. Oxidative stress in the trabecular meshwork of POAG. Klinische Monatsblätter für Augenheilkunde. 2010;227:99–107. doi: 10.1055/s-0029-1245171. [DOI] [PubMed] [Google Scholar]
- Wulf (2002).Wulf DG. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu L, Zhang Y, Guo R, Shen W, Qi Y, Wang Q, Guo Z, Qi C, Yin H, Wang J. HES1 promotes extracellular matrix protein expression and inhibits proliferation and migration in human trabecular meshwork cells under oxidative stress. Oncotarget. 2017;8:21818–21833. doi: 10.18632/oncotarget.15631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al. (2006).Xue LP, Lu J, Cao Q, Hu S, Ding P, Ling EA. Müller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 2006;139:723–732. doi: 10.1016/j.neuroscience.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Yadav, Rajpurohit & Sharma (2019).Yadav KS, Rajpurohit R, Sharma S. Glaucoma: current treatment and impact of advanced drug delivery systems. Life Sciences. 2019;221:362–376. doi: 10.1016/j.lfs.2019.02.029. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2012).Yang Y, Xing L, Huang J, Zhong Y, Zhen M, Hui X, Mei L, Zhuo Y. Inhibition of p38 mitogen-activated protein kinase phosphorylation decrease tert-butyl hydroperoxide-induced apoptosis in human trabecular meshwork cells. Molecular Vision. 2012;18:2127–2136. [PMC free article] [PubMed] [Google Scholar]
- Yin & Chen (2019).Yin R, Chen X. Regulatory effect of miR-144-3p on the function of human trabecular meshwork cells and fibronectin-1. Experimental and Therapeutic Medicine. 2019;18:647–653. doi: 10.3892/etm.2019.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You et al. (2018).You ZP, Zhang YZ, Zhang YL, Shi L, Shi K. Homocysteine induces oxidative stress to damage trabecular meshwork cells. Experimental & Therapeutic Medicine. 2018;15:4379–4385. doi: 10.3892/etm.2018.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2008).Yuan H, Kar Wah L, Yue-Hong Z, Shan D, Xiu-Feng Z, Ru-Zhang J, Zhan P, Joyce TT, Jian G. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Investigative Ophthalmology & Visual Science. 2008;49:1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- Yun et al. (2011).Yun SB, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Molecules & Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun et al. (2013).Yun Z, Shoujian W, Sorenson CM, Leandro T, Dubielzig RR, Peters DM, Conway SJ, Jefcoate CR, Nader S. Cyp1b1 mediates periostin regulation of trabecular meshwork development by suppression of oxidative stress. Molecular & Cellular Biology. 2013;33:4225–4240. doi: 10.1128/MCB.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon-Moreno et al. (2013).Zanon-Moreno V, Asensio-Marquez EM, Ciancotti-Oliver L, Garcia-Medina JJ, Sanz P, Ortega-Azorin C, Pinazo-Duran MD, Ordovás JM, Corella D. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Molecular Vision. 2013;19:231–242. [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2019a).Zhao J, Du X, Wang M, Yang P, Zhang J. Salidroside mitigates hydrogen peroxide-induced injury by enhancement of microRNA-27a in human trabecular meshwork cells. Artif Cells Nanomed Biotechnol. 2019a;47:1758–1765. doi: 10.1080/21691401.2019.1608222. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2019b).Zhao J, Sun H, Zhang JM, Wang M, Du XJ, Zhang JL. Long non-coding RNA ANRIL down-regulates microRNA-7 to protect human trabecular meshwork cells in an experimental model for glaucoma. European Review for Medical and Pharmacological Sciences. 2019b;23(8):3173–3182. doi: 10.26355/eurrev_201904_17675. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2016).Zhao J, Wang S, Zhong W, Yang B, Sun L, Zheng Y. Oxidative stress in the trabecular meshwork (Review) International Journal of Molecular Medicine. 2016;38:995. doi: 10.3892/ijmm.2016.2714. [DOI] [PubMed] [Google Scholar]
- Zhaoyong et al. (2009).Zhaoyong L, Hassan MQ, Mohammed J, Aqeilan RI, Ramiro G, Croce CM, Van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. Journal of Biological Chemistry. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review.