Abstract
Significant knowledge about the pathophysiology of Alzheimer’s disease (AD) has been gained in the last century; however, the understanding of its causes of onset remains limited. Late-onset AD is observed in about 95% of patients, and APOE4-encoding apolipoprotein E4 (ApoE4) is strongly associated with these cases. As an apolipoprotein, the function of ApoE in brain cholesterol transport has been extensively studied and widely appreciated. Development of new technologies such as human-induced pluripotent stem cells (hiPSCs) and CRISPR-Cas9 genome editing tools have enabled us to develop human brain model systems in vitro and readily manipulate genomic information. In the context of these advances, recent studies provide strong evidence that abnormal cholesterol metabolism by ApoE4 could be linked to AD-associated pathology. In this review, we discuss novel discoveries in brain cholesterol dysregulation by ApoE4. We further elaborate cell type-specific roles in cholesterol regulation of four major brain cell types, neurons, astrocytes, microglia, and oligodendrocytes, and how its dysregulation can be linked to AD pathology.
Keywords: Aβ, Alzheimer’s disease, ApoE4, apolipoprotein, cholesterol
INTRODUCTION
Alzheimer’s disease (AD), a progressive neurodegenerative disease, is one of the most common forms of irreversible dementia. Patients with AD suffer from cognitive impairment including language problems, mood changes, as well as memory loss. AD brains display amyloid-beta (Aβ) plaques, tau tangles, synaptic degradation, and neuronal loss. Among these neurobiological changes, Aβ plaque is considered to be one of the major hallmarks of AD because of its early appearance during the progression of the disease. Therefore, a major focus of ongoing research has been identifying the mechanisms of Aβ plaque formation and aggregation to target Aβ metabolism (O’Brien and Wong, 2011; Selkoe and Hardy, 2016). Although many studies and clinical trials for candidate drugs are currently underway, it is not yet clear how AD progresses, and no effective treatment for the disease exists. Currently-available drugs such as cholinesterase inhibitors only delay the onset of symptoms.
While early-onset AD pathology has been shown to be associated with genetic mutations in coding regions for proteins involved in Aβ metabolism (e.g., amyloid precursor protein; APP, presenilin 1 and 2; PSEN1 and PSEN2), such mutations were not consistently observed in late-onset AD (LOAD), which comprises about 95% of patients; thus these cases have long been believed to be sporadic. However, recent genome-wide association studies revealed that compared to cognitively normal controls, several genetic variants are frequently observed in patients with LOAD, suggesting a strong association of these variants with late-onset cases (Kunkle et al., 2019; Lambert et al., 2013). Genes that contain these genetic variants or are closely located to them are not those typically associated with Aβ generation or cleavage, rather these are genes that are associated with various cellular functions related to immune responses, endocytosis, and cholesterol metabolism (Karch and Goate, 2015). Among identified genetic risk factors for LOAD, the most influential factor, for which the odds ratio is above 10 for homozygotes and found in about 40% of patients, is located on the APOE gene encoding apolipoprotein E (Corder et al., 1993).
ApoE is an apolipoprotein that plays a major role in cholesterol transport. In the brain, ApoE was previously believed to be mainly produced by astrocytes (Zhang et al., 2014; 2016). However, recent transcriptome analysis from human brain samples and hiPSC-derived brain cell types showed considerable expression of ApoE transcripts in microglia as well, and its expression is upregulated even in neurons in AD brains (Keren-Shaul et al., 2017; Lin et al., 2018; Mathys et al., 2019; Xu et al., 2006). There are three isoforms of ApoE; ApoE2, ApoE3, and ApoE4; only one or two amino acid variations differentiate these isoforms. ApoE3, the most common isoform present in about 78% of the population, has cysteine and arginine residues Cys112 and Arg158, whereas ApoE2 contains cysteine residues Cys112 and Cys158, and ApoE4 carries two arginine residues, Arg112 and Arg158. Although the difference is very subtle, it results in drastically differential effects on the risk for AD development. The allele frequency of ApoE2 is about 8%, and it is known to be neuroprotective. However, ApoE4 is one of the strongest risk factors for AD with a prevalence of about 14% (Liu et al., 2013; Strittmatter et al., 1993). Since its strong association with AD was first reported about twenty-five years ago (Corder et al., 1993; Strittmatter et al., 1993), many studies have been dedicated to determining how only one amino acid alteration in ApoE4 compared to ApoE3 could drastically increase the risk of AD (Liu et al., 2013).
In terms of the role of ApoE protein on Aβ metabolism, contradictory data have been reported in different studies. Some studies have reported a decrease in Aβ plaque in familial AD (fAD) mouse models with ApoE deficiency, while others showed that ApoE is required for Aβ reduction, including astrocyte-mediated Aβ clearance, and deletion of ApoE accelerated the accumulation of fibrillar amyloid in the brains of fAD mice (Bales et al., 1997; Holtzman et al., 1999; 2000; Kim et al., 2011; Koistinaho et al., 2004; Tai et al., 2011; Ulrich et al., 2018). Regarding the role of ApoE isoforms in AD pathogenesis, most studies agree that ApoE4 induces the highest Aβ accumulation and gliosis compared with other isoforms (Lin et al., 2018; Liu et al., 2013; 2017). Hyperphosphorylation of tau and related pathology (tauopathy), another hallmark of AD, has also been observed to be accelerated in the presence of the ApoE4 isoform (Shi et al., 2017). However, the mechanisms of ApoE4-induced AD pathogenesis still remain unclear.
Recent evidence has emerged showing abnormal cholesterol metabolism by ApoE4 could mediate AD-associated pathology. Here, we review recent research, providing some insights on how ApoE4-mediated cholesterol dysregulation could affect functions of different brain cell types in the context of AD.
NEURONS
Neurons are the most vulnerable to the toxicity of Aβ accumulation, and they are also the major cell type responsible for generating Aβ in the brain (Sinha and Lieberburg, 1999; Zhang et al., 2016). Various pathological features are induced by Aβ accumulation in neurons, such as synaptic degradation, altered synaptic and neuronal circuit activity, and cell death, which have been extensively reviewed elsewhere (Canter et al., 2016; De Strooper and Karran, 2016; Palop and Mucke, 2010; Selkoe and Hardy, 2016). In this review, we focus on how ApoE4-induced cholesterol metabolic abnormalities could affect Aβ generation and alter the synaptic function in neurons.
Neuronal lipid rafts provide the platform for APP to encounter its cleaving enzymes, resulting in Aβ generation. Cholesterol is one of the major components of lipid rafts, and its dysregulation affects Aβ production in neurons (Fassbender et al., 2001; Simons et al., 1998). Tethering of cholesterol to methyl-β-cyclodextrin (MβCD) enables its delivery to the plasma membrane, and neurons treated with MβCD-cholesterol complex show increased lipid raft formation and generation of Aβ (Cossec et al., 2010; Marquer et al., 2014). However, it remains unknown whether cholesterol-dependent facilitation of Aβ cleavage is through the regulation of β-secretase activity or/and increased formation of APP/β-secretase clusters (Kalvodova et al., 2005; Marquer et al., 2011). Some genetic variants associated with the LOAD are found on or near genes related to cholesterol transport such as APOE and ATP-binding cassette transporter 7 (ABCA7), which implies that Aβ generation could be altered by these variants through cholesterol dysregulation and altered lipid raft formation (Karch and Goate, 2015).
In the brain, cholesterol is mainly transmitted from astrocytes to neurons via lipoprotein particles, of which ApoE is one of the major components (Liu et al., 2013; Vance, 2012). ApoE, mainly produced by astrocytes, forms the lipoprotein complex with cholesterol and is secreted through the function of ABCAs. Among isoforms, ApoE4 displays lower transport affinity and binding capacity for lipids (Hatters et al., 2006; Vance, 2012), which could reduce cholesterol transport from astrocytes to neurons. As a result, the formation of neuronal lipid rafts and Aβ production might be affected by ApoE4 in the brain. Two recent studies with astrocytes generated from hiPSCs addressed this point, and both showed higher accumulation of cholesterol in ApoE4-expressing astrocytes than in isogenic ApoE3 astrocytes (Lin et al., 2018; TCW et al., 2019). However, regarding the secretion of cholesterol from these ApoE4 astrocytes, Lin et al. (2018) showed increased extracellular cholesterol levels by directly measuring secreted cholesterol in cultured media, while TCW et al. (2019) speculated that ApoE4 astrocytes might secrete less cholesterol due to a reduction of ApoE and Abca1, which mediate the efflux of cholesterol. Further studies are required to directly examine cholesterol transport to neurons from astrocytes with different ApoE isoforms.
Although cholesterol synthesis in neurons is lower than in astrocytes, particularly in adults (Nieweg et al., 2009; Pfrieger and Ungerer, 2011), ApoE isoform-dependent changes in cholesterol metabolism in neurons could also be driven in a cell-autonomous manner. For example, impaired efflux of cholesterol in ApoE4 neurons could contribute to its intracellular accumulation, leading to AD-related pathology, as observed in mice with cholesterol 24-hydroxylase-dependent cholesterol efflux suppressed in hippocampal neurons (Djelti et al., 2015). It is also important to investigate whether ApoE4 neurons have altered the expression and/or function of ApoE receptors such as those belonging to the low-density lipoprotein receptor family that can directly affect the uptake of extracellular cholesterol as well as Aβ clearance (Holtzman et al., 2012).
Cholesterol plays critical roles in synaptic function. It helps maintain adequate curvature of membrane to facilitate soluble NSF attachment protein receptor (SNARE)-mediated membrane fusion. Depleting cholesterol significantly reduces synaptic transmission, which can be reversed by reloading cholesterol (Linetti et al., 2010; Tong et al., 2009). Lack of cholesterol in neurons leads to impaired synaptic plasticity, evidenced by changes in paired-pulse facilitation and long-term potentiation (Koudinov and Koudinova, 2002). Hippocampal synapses in mice with decreased astrocytic cholesterol secretion also showed fewer synaptic vesicles, more immature synapses, and reduced expression of presynaptic synaptosomal nerve-associated protein 25 (SNAP-25) required for the exocytic release of neurotransmitters (van Deijk et al., 2017).
The role of ApoE isoforms on the formation of neuronal synapses and their function is not yet clear (Kim et al., 2014). A recent study with ApoE2, ApoE3 and ApoE4 knock-in (KI) mice in which the murine APOE gene was replaced with the human APOE2, APOE3 or APOE4 gene showed that ApoE4 KI mice displayed reduced phagocytic capacity in astrocytes and increased senescence synapses compared to mice carrying other ApoE isoforms (Chung et al., 2016). This result seems to be consistent with a study that demonstrated an increased number of synapses in hiPSC-derived neurons carrying APOE4 alleles compared to ApoE3 neurons (Lin et al., 2018). Nonetheless, further studies are necessary to understand the role of ApoE4-dependent cholesterol dysregulation in synaptic dysfunction and whether restoration of cholesterol metabolism in ApoE4 brain would be sufficient to recover from synaptopathy.
ASTROCYTES
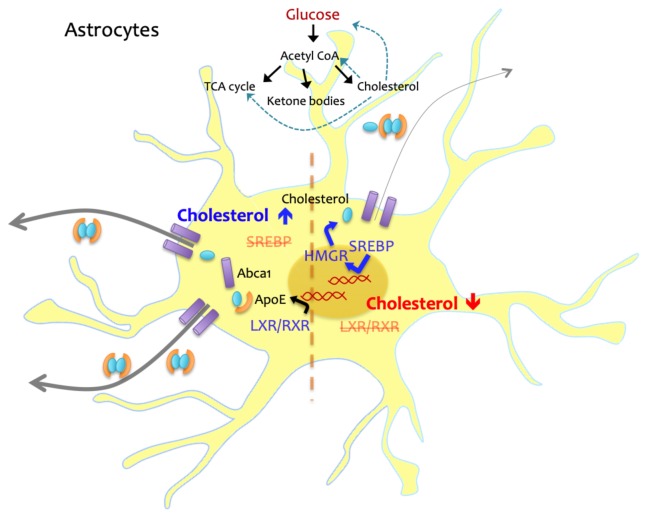
Lipoprotein-bound cholesterol from circulating plasma is prevented from entering the brain due to the presence of the blood-brain barrier (BBB), and astrocytes, which account for up to 40% of all brain cells in humans, are mainly responsible for producing cholesterol in the brain (Nieweg et al., 2009; Zhang et al., 2016). Cholesterol synthesis in astrocytes is tightly regulated by an internal feedback loop; if the intracellular level of cholesterol is low, cholesterol synthesis is induced by increasing proteolytic processes of sterol regulatory element-binding proteins (SREBPs) and reducing levels of proteins that mediate cholesterol efflux (Benarroch, 2008). When the intracellular cholesterol level is high, liver X receptor/retinoid X receptor (LXR/RXR)-mediated transcription for cholesterol transport proteins is increased, and efflux of lipoprotein complex is facilitated (Fig. 1). Cholesterol accumulation in ApoE4 astrocytes may not be due, solely, to impaired efflux resulting from reduced ApoE levels. Transcriptome analysis in hiPSC-derived astrocytes suggests the expression of genes involved in lipid metabolism is dysregulated in ApoE4 astrocytes, with an increase in the expression levels of cholesterol biosynthesis-related genes. Lysosomal cholesterol degradation processes also seem to be impaired in these cells (Lin et al., 2018; TCW et al., 2019). Further study is required to precisely elucidate the mechanisms underlying ApoE4-induced cholesterol accumulation in astrocytes, and how it can be targeted to resolve the abnormality.
Fig. 1. Cholesterol homeostasis in astrocytes.
Intracellular cholesterol level is tightly regulated and maintained in astrocytes, major producers of cholesterol in the brain. When cholesterol level is low, SREBP-mediated cholesterol synthesis is triggered. If the level of cholesterol is increased, SREBP is suppressed and LXR/RXR-induced transcription for cholesterol transport proteins such as ApoE and Abca1 is facilitated. Cholesterol is also synthesized from glucose-derived Acetyl CoA, and the level of cholesterol can affect glucose metabolism.
Because cholesterol is a multifunctional metabolite, abnormal cholesterol metabolism by ApoE4 would lead not only altered cholesterol transport to other cell types but also functional deficits in astrocytes as observed in neurons (Fukui et al., 2015; Martín-Segura et al., 2019) and microglia (Churchward and Todd, 2014; Račková, 2013). However, there have not been many studies investigating the effects of intracellular cholesterol on cellular functions or formation of organelles in astrocytes. Therefore, investigating whether/how ApoE4 induces cholesterol-related cellular alterations in astrocytes are a priority. Cholesterol levels in astrocytes and glucose metabolism are closely linked (Ferris et al., 2017), A recent study showed ApoE isoform-dependent changes of brain glucose metabolism in mice (Wu et al., 2018), and it suggests that cholesterol abnormalities caused by ApoE4 could alter glucose metabolism (Fig. 1). Since astrocytes provide not only structural but metabolic supports for neurons, further investigations of astrocyte-specific changes in glucose metabolism in humanized ApoE KI mouse models or single-cell type culture systems with different ApoE isoforms, and its association with cholesterol dysregulation are needed.
MICROGLIA
The role of microglia in AD has been extensively investigated; nonetheless, their precise contribution to the development of AD pathology remains unclear. A recent development in single-cell transcriptome analysis technology revealed the spectrum of microglia phenotypes during AD pathogenesis and the species difference in transcriptomic information of disease-associated microglia between mice and humans (Keren-Shaul et al., 2017; Mathys et al., 2017; 2019). Although this topic is of great interest, it is beyond the scope of this review. Nevertheless, it is important to note that in both mouse and human cells, ApoE, along with triggering receptor expressed on myeloid cells 2 (TREM2), is shown to be upregulated and mediates microglial phenotypic changes and recruitment to amyloid plaques during the progression of AD (Cheng-Hathaway et al., 2018; Keren-Shaul et al., 2017; Parhizkar et al., 2019; Ulrich et al., 2018). Transition of microglia from resting (homeostatic) to reactive (disease-associated) phenotypes is generally mediated by transcriptional regulation (Deczkowska et al., 2018; Holtman et al., 2017; Krasemann et al., 2017). Although ApoE isoform-dependent transcriptional regulation was recently suggested by multiple studies (Huang et al., 2017; Lin et al., 2018; TCW et al., 2019), its precise mechanism is unclear.
Because excessive inflammatory response is associated with AD, the relationship between the level of cholesterol and immune responses in microglia, the brain resident macrophages, is of great interest. Interaction of cholesterol and inflammatory response in macrophages was suggested (Ricote et al., 2004). In microglia, however, regulation of inflammatory responses by peroxisome proliferator-activated receptor γ (PPARγ), LXR/RXR-mediated cholesterol synthesis, and phagocytic activity by PPARγ and LXR/RXR were reported in separate studies (Bernardo and Minghetti, 2006; Courtney and Landreth, 2016; Saijo et al., 2013; Savage et al., 2015). Direct interaction between cholesterol and inflammatory responses in this cell type has not been assessed.
The effect of microglial cholesterol on AD pathogenesis has been previously investigated using primary cultured cells. In primary microglia, accumulation of intracellular cholesterol induced by either MβCD-mediated loading or Niemann–Pick disease, type C1 (NPC1) inhibition (thus blocking cholesterol export from the lysosome) also increases intracellular Aβ. Furthermore, a negative correlation between cholesterol accumulation-induced intracellular Aβ and ApoE levels in these cells was observed. These data suggest that ApoE contributes to intracellular Aβ clearance through the regulation of cholesterol levels in microglia (Lee et al., 2012).
Recently developed protocols to generate microglia-like cells from hiPSC have enabled researchers to investigate functions and underlying mechanisms of ApoE4 variants in human microglia (Abud et al., 2017; Muffat et al., 2016; Pandya et al., 2017). Microglia-like cells from hiPSC carrying the APOE4 allele have fewer processes compared to isogenic cells with the APOE3 allele, and live-imaging of Aβ-uptake assays and transcriptome analysis suggest impaired phagocytic activity as well as upregulation of immune responses in microglia by ApoE4 (Lin et al., 2018). Interestingly, a recent study showed that glycolysis in microglia is important for acute response against Aβ (Baik et al., 2019). The next several years will likely produce more exciting and important data elucidating the contribution of microglial cholesterol dysregulation by ApoE4 to the pathogenesis of AD.
OLIGODENDROCYTES
Oligodendrocytes have a unique structure called myelin that ensheathes axons, facilitating conduction of action potentials and protecting neurons from other possible extracellular insults (Domingues et al., 2016). Lipids are the major components of myelin membranes, consisting of at least 70% of their dry weight, whereas membranes in other cell types are generally composed of about 30% lipids and 70% proteins (Chrast et al., 2011; Ingólfsson et al., 2017). This suggests that altered lipid composition by abnormal metabolic processes would affect not only intracellular compartments generally seen in other cell types but also the formation and function of myelin, which is critical for neuronal function.
Among lipid metabolites in myelin membranes, cholesterol has been shown to have a significant role in myelin formation. Inhibition of cholesterol-synthesizing ability in oligodendrocytes by deleting squalene synthases leads to a significant reduction of myelination in oligodendrocytes, inducing behavioral abnormalities such as ataxia and tremors in mice (Saher et al., 2005). Increased levels of gene expression associated with cholesterol biosynthesis were also observed in oligodendrocyte lineage cells during the remyelination phase following axonal damage (Voskuhl et al., 2019). These data demonstrate the major contribution of oligodendrocyte-derived cholesterol for myelination in the brain. A recent study also reported the role of astrocytic cholesterol in the formation of myelin following axonal injury (Camargo et al., 2017). In this study, cell type-specific deletion of SREBP cleavage-activating protein (SCAP), which mediates cholesterol synthesis, either in oligodendrocytes or astrocytes, showed only partial impairment of myelination in mice. When SCAP is ablated in both cell types, however, mice showed an almost complete absence of myelination in the brain. These results suggest that altered levels of extracellular and intracellular cholesterol for oligodendrocytes could regulate myelination in the brain.
As a major cholesterol transporter, it would not be surprising that ApoE is involved in myelination processes. Indeed, a recent study showed that ApoE is required for cholesterol clearance in demyelinating lesions to prevent lysosomal accumulation of myelin debris (Cantuti-Castelvetri et al., 2018). Studies have found an association between amyloid pathology with myelin alteration in AD animal models and preclinical studies, including a reduction of myelination associated with brain atrophy (Dean et al., 2017; Wu et al., 2017). Further work is needed to determine the differential role of ApoE isoforms in oligodendrocytes and whether and how ApoE4-associated cholesterol dysregulation in these cells drive AD-associated pathology.
CONCLUSION
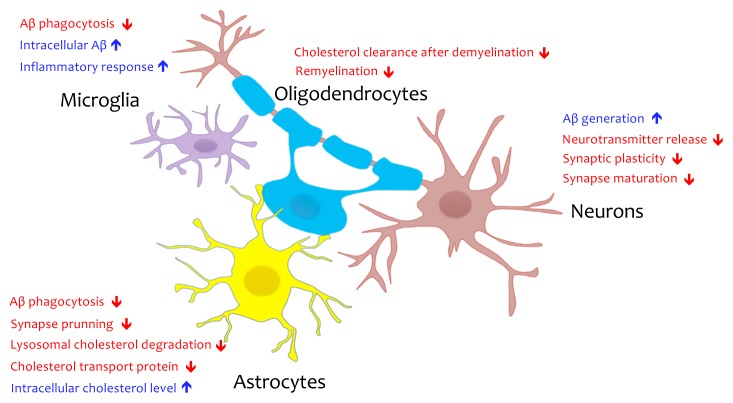
Investigating pathological features of whole AD brain samples would be of interest. However, it is also necessary to tease out cell type-specific alterations and their contributions to AD pathogenesis. Recent investigations of cell type-specific functions of ApoE4 from various brain cell types isolated from ApoE KI mice or derived from hiPSC showed impaired cholesterol metabolism by ApoE4, specifically in glial cells that were not evident from previous studies that relied on bulk brain samples composed of mixed cell populations (Lin et al., 2018; Nuriel et al., 2017; TCW et al., 2019). As we reviewed, cholesterol metabolism is closely linked to various functions in different types of cells in the brain, and its dysregulation can lead to AD-associated pathological phenotypes in each cell type, including Aβ upregulation in neurons, abnormal glucose metabolism in astrocytes, inflammatory responses in microglia, and myelination defects in oligodendrocytes (Fig. 2).
Fig. 2. Potential pathological role of cholesterol dysregulation by ApoE4 in different brain cell types.
Cholesterol dysregulation by ApoE4 could lead to cell type-specific functional abnormalities in the brain such as Aβ upregulation and impaired synaptic function in neurons, reduced synapse prunning activity in astrocytes, impaired remyelination in oligodendrocytes, and Aβ accumulation and inflammatory response in microglia.
In addition to these cell types, the BBB, which is composed of various cell types, including pericytes and endothelial cells, was also shown to be influenced by ApoE4. Multiple studies suggest that ApoE4 leads to BBB leakage through the degeneration of pericytes and the disruption of the integrity of tight junctions (Casey et al., 2015; Nishitsuji et al., 2011), which leads to the accumulation of serum proteins in the brain (Bell et al., 2012). This can result in further elevation of brain cholesterol. It is also possible that increased cholesterol in the brain or plasma by ApoE4 affects BBB integrity and may lead to leakage (Jiang et al., 2012; Kalayci et al., 2009). While there is a close coupling between neurovascular dysfunction and AD, the cell types mentioned above are understudied in the context of AD. Exploring detailed mechanisms in BBB pathogenesis in ApoE4 brain may provide insights into a new therapeutic avenue.
While we largely discuss ApoE4 as an AD risk factor in terms of cholesterol metabolism in the brain, it is also important to mention that in several studies it has been suggested that plasma cholesterol level and AD risk/pathology are linked (Pappolla et al., 2003; Wingo et al., 2019). Epidemiological studies show an increased concentration of plasma cholesterol in AD patients (Wood et al., 2014), and diet-induced hypercholesterolemia in AD mouse models leads to increased Aβ and impaired memory (Park et al., 2013). Unlike physiological conditions in which the translocation of peripheral cholesterol to the brain is limited, it is facilitated when leakage of the BBB occurs under pathological conditions. Although more studies are necessary to identify plasma cholesterol as a causative factor, it is important to understand the dynamic relationship between brain cholesterol and plasma cholesterol in terms of AD pathogenesis.
Further studies are also required to determine whether other AD genetic risk factors associated with cholesterol homeostasis, including Clustrin (apolipoprotein J) and ABCA7, share pathological mechanisms with APOE4. In this regard, brain cholesterol may be targeted for therapeutic treatments in LOAD. Statins are β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors reducing the levels of cholesterol by blocking mevalonate synthesis. Statins including simvastatin which across the BBB have been tested in multiple studies and have demonstrated beneficial effects in various AD model systems (Chu et al., 2018; Li et al., 2018). However inconsistent data regarding the effect of statins in clinical trials exist (Di Paolo and Kim, 2011; Wood et al., 2014). Further clinical investigation with larger cohorts would provide more definitive insights into the effects of statins in AD. Alternatively, a cell type-specific approach or one that targets the signaling pathway that is affected by cholesterol dysregulation might be promising approaches for the development of novel therapeutics for AD.
ACKNOWLEDGMENTS
This work was supported by DGIST MIREBraiN program and the National Research Foundation of Korea (NRF) grants (2018M3C7A1056275). We thank Joonho Cho for careful reading of the manuscript and helpful comments.
Footnotes
Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C., et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik S.H., Kang S., Lee W., Choi H., Chung S., Kim J.I., Mook-Jung I. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 2019;30:493–507.e6. doi: 10.1016/j.cmet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Bales K.R., Verina T., Dodel R.C., Du Y., Altstiel L., Bender M., Hyslop P., Johnstone E.M., Little S.P., Cummins D.J., et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Brain cholesterol metabolism and neurologic disease. Neurology. 2008;71:1368–1373. doi: 10.1212/01.wnl.0000333215.93440.36. [DOI] [PubMed] [Google Scholar]
- Bernardo A., Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- Camargo N., Goudriaan A., van Deijk A.L.F., Otte W.M., Brouwers J.F., Lodder H., Gutmann D.H., Nave K.A., Dijkhuizen R.M., Mansvelder H.D., et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017;15:e1002605. doi: 10.1371/journal.pbio.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Fitzner D., Bosch-Queralt M., Weil M.T., Su M., Sen P., Ruhwedel T., Mitkovski M., Trendelenburg G., Lütjohann D., et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684–688. doi: 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- Casey C.S., Atagi Y., Yamazaki Y., Shinohara M., Tachibana M., Fu Y., Bu G., Kanekiyo T. Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA protein-mediated pathway. J Biol Chem. 2015;290:14208–14217. doi: 10.1074/jbc.M114.625251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Hathaway P.J., Reed-Geaghan E.G., Jay T.R., Casali B.T., Bemiller S.M., Puntambekar S.S., von Saucken V.E., Williams R.Y., Karlo J.C., Moutinho M., et al. The Trem2 R47H variant confers loss-of-function-like phenotypes in Alzheimer’s disease. Mol Neurodegener. 2018;13:29. doi: 10.1186/s13024-018-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrast R., Saher G., Nave K.A., Verheijen M.H.G. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52:419–434. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.S., Tseng P.T., Stubbs B., Chen T.Y., Tang C.H., Li D.J., Yang W.C., Chen Y.W., Wu C.K., Veronese N., et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2018;8:5804. doi: 10.1038/s41598-018-24248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.S., Verghese P.B., Chakraborty C., Joung J., Hyman B.T., Ulrich J.D., Holtzman D.M., Barres B.A. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A. 2016;113:10186–10191. doi: 10.1073/pnas.1609896113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward M.A., Todd K.G. Statin treatment affects cytokine release and phagocytic activity in primary cultured microglia through two separable mechanisms. Mol Brain. 2014;7:85. doi: 10.1186/s13041-014-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cossec J.C., Simon A., Marquer C., Moldrich R.X., Leterrier C., Rossier J., Duyckaerts C., Lenkei Z., Potier M.C. Clathrin-dependent APP endocytosis and Aβ secretion are highly sensitive to the level of plasma membrane cholesterol. Biochim Biophys Acta. 2010;1801:846–852. doi: 10.1016/j.bbalip.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Courtney R., Landreth G.E. LXR regulation of brain cholesterol: from development to disease. Trends Endocrinol Metab. 2016;27:404–414. doi: 10.1016/j.tem.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Dean D.C., Hurley S.A., Kecskemeti S.R., O’Grady J.P., Canda C., Davenport-Sis N.J., Carlsson C.M., Zetterberg H., Blennow K., Asthana S., et al. Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurol. 2017;74:41–49. doi: 10.1001/jamaneurol.2016.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deczkowska A., Keren-Shaul H., Weiner A., Colonna M., Schwartz M., Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173:1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Di Paolo G., Kim T.W. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djelti F., Braudeau J., Hudry E., Dhenain M., Varin J., Bièche I., Marquer C., Chali F., Ayciriex S., Auzeil N., et al. CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain. 2015;138:2383–2398. doi: 10.1093/brain/awv166. [DOI] [PubMed] [Google Scholar]
- Domingues H.S., Portugal C.C., Socodato R., Relvas J.B. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol. 2016;4:71. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K., Simons M., Bergmann C., Stroick M., Lutjohann D., Keller P., Runz H., Kuhl S., Bertsch T., von Bergmann K., et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris H.A., Perry R.J., Moreira G.V., Shulman G.I., Horton J.D., Kahn C.R. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc Natl Acad Sci U S A. 2017;114:1189–1194. doi: 10.1073/pnas.1620506114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Ferris H.A., Kahn C.R. Effect of cholesterol reduction on receptor signaling in neurons. J Biol Chem. 2015;290:26383–26392. doi: 10.1074/jbc.M115.664367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatters D.M., Peters-Libeu C.A., Weisgraber K.H. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Holtman I.R., Skola D., Glass C.K. Transcriptional control of microglia phenotypes in health and disease. J Clin Invest. 2017;127:3220–3229. doi: 10.1172/JCI90604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D.M., Bales K.R., Wu S., Bhat P., Parsadanian M., Fagan A.M., Chang L.K., Sun Y., Paul S.M. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D.M., Fagan A.M., Mackey B., Tenkova T., Sartorius L., Paul S.M., Bales K., Ashe K.H., Irizarry M.C., Hyman B.T. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–747. doi: 10.1002/1531-8249(200006)47:6<739::AID-ANA6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Holtzman D.M., Herz J., Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W.A., Zhou B., Wernig M., Südhof T.C. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell. 2017;168:427–441.e21. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H.I., Carpenter T.S., Bhatia H., Bremer P.T., Marrink S.J., Lightstone F.C. Computational lipidomics of the neuronal plasma membrane. Biophys J. 2017;113:2271–2280. doi: 10.1016/j.bpj.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Guo M., Su J., Lu B., Ma D., Zhang R., Yang L., Wang Q., Ma Y., Fan Y. Simvastatin blocks blood-brain barrier disruptions induced by elevated cholesterol both in vivo and in vitro. Int J Alzheimers Dis. 2012;2012:109324–109327. doi: 10.1155/2012/109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayci R., Kaya M., Uzun H., Bilgic B., Ahishali B., Arican N., Elmas I., Küçük M. Influence of hypercholesterolemia and hypertension on the integrity of the blood-brain barrier in rats. Int J Neurosci. 2009;119:1881–1904. doi: 10.1080/14647270802336650. [DOI] [PubMed] [Google Scholar]
- Kalvodova L., Kahya N., Schwille P., Ehehalt R., Verkade P., Drechsel D., Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kim J., Jiang H., Park S., Eltorai A.E.M., Stewart F.R., Yoon H., Basak J.M., Finn M.B., Holtzman D.M. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-β amyloidosis. J Neurosci. 2011;31:18007–18012. doi: 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Yoon H., Basak J., Kim J. Apolipoprotein E in synaptic plasticity and Alzheimer’s disease: potential cellular and molecular mechanisms. Mol Cells. 2014;37:767–776. doi: 10.14348/molcells.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K.R., et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Koudinov A.R., Koudinova N.V. Cholesterol’s role in synapse formation. Science. 2002;295:2213. doi: 10.1126/science.295.5563.2213a. [DOI] [PubMed] [Google Scholar]
- Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O’Loughlin E., Xu Y., Fanek Z., et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581.e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J.C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., DeStafano A.L., Bis J.C., Beecham G.W., Grenier-Boley B., et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y.D., Tse W., Smith J.D., Landreth G.E. Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem. 2012;287:2032–2044. doi: 10.1074/jbc.M111.295451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu Q., Sun J., Wang J., Liu X., Gao J. Mitochondrial protective mechanism of simvastatin protects against amyloid β peptide-induced injury in SH-SY5Y cells. Int J Mol Med. 2018;41:2997–3005. doi: 10.3892/ijmm.2018.3456. [DOI] [PubMed] [Google Scholar]
- Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetti A., Fratangeli A., Taverna E., Valnegri P., Francolini M., Cappello V., Matteoli M., Passafaro M., Rosa P. Cholesterol reduction impairs exocytosis of synaptic vesicles. J Cell Sci. 2010;123:595–605. doi: 10.1242/jcs.060681. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Zhao N., Fu Y., Wang N., Linares C., Tsai C.W., Bu G. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017;96:1024–1032.e3. doi: 10.1016/j.neuron.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquer C., Devauges V., Cossec J.C., Liot G., Lécart S., Saudou F., Duyckaerts C., Lévêque-Fort S., Potier M.C. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 2011;25:1295–1305. doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- Marquer C., Laine J., Dauphinot L., Hanbouch L., Lemercier-Neuillet C., Pierrot N., Bossers K., Le M., Corlier F., Benstaali C., et al. Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer’s disease early phenotypes. Mol Neurodegener. 2014;9:60. doi: 10.1186/1750-1326-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Segura A., Ahmed T., Casadomé-Perales Á, Palomares-Perez I., Palomer E., Kerstens A., Munck S., Balschun D., Dotti C.G. Age-associated cholesterol reduction triggers brain insulin resistance by facilitating ligand-independent receptor activation and pathway desensitization. Aging Cell. 2019;18:e12932. doi: 10.1111/acel.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H., Adaikkan C., Gao F., Young J.Z., Manet E., Hemberg M., De Jager P.L., Ransohoff R.M., Regev A., Tsai L.H. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 2017;21:366–380. doi: 10.1016/j.celrep.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H., Davila-Velderrain J., Peng Z., Gao F., Mohammadi S., Young J.Z., Menon M., He L., Abdurrob F., Jiang X., et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J., Li Y., Yuan B., Mitalipova M., Omer A., Corcoran S., Bakiasi G., Tsai L.H., Aubourg P., Ransohoff R.M., et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016;22:1358–1367. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieweg K., Schaller H., Pfrieger F.W. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem. 2009;109:125–134. doi: 10.1111/j.1471-4159.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K., Hosono T., Nakamura T., Bu G., Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel T., Angulo S.L., Khan U., Ashok A., Chen Q., Figueroa H.Y., Emrani S., Liu L., Herman M., Barrett G., et al. Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nat Commun. 2017;8:1464. doi: 10.1038/s41467-017-01444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya H., Shen M.J., Ichikawa D.M., Sedlock A.B., Choi Y., Johnson K.R., Kim G., Brown M.A., Elkahloun A.G., Maric D., et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat Neurosci. 2017;20:753–759. doi: 10.1038/nn.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappolla M.A., Bryant-Thomas T.K., Herbert D., Pacheco J., Fabra Garcia M., Manjon M., Girones X., Henry T.L., Matsubara E., Zambon D., et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.WNL.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- Parhizkar S., Arzberger T., Brendel M., Kleinberger G., Deussing M., Focke C., Nuscher B., Xiong M., Ghasemigharagoz A., Katzmarski N., et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci. 2019;22:191–204. doi: 10.1038/s41593-018-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Kim J.H., Choi K.H., Jang Y.J., Bae S.S., Choi B.T., Shin H.K. Hypercholesterolemia accelerates amyloid β-induced cognitive deficits. Int J Mol Med. 2013;31:577–582. doi: 10.3892/ijmm.2013.1233. [DOI] [PubMed] [Google Scholar]
- Pfrieger F.W., Ungerer N. Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Račková L. Cholesterol load of microglia: contribution of membrane architecture changes to neurotoxic power? Arch Biochem Biophys. 2013;537:91–103. doi: 10.1016/j.abb.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Ricote M., Valledor A.F., Glass C.K. Decoding transcriptional programs regulated by PPARs and LXRs in the macrophage: effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:230–239. doi: 10.1161/01.ATV.0000103951.67680.B1. [DOI] [PubMed] [Google Scholar]
- Saher G., Brügger B., Lappe-Siefke C., Möbius W., Tozawa R.I., Wehr M.C., Wieland F., Ishibashi S., Nave K.A. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Saijo K., Crotti A., Glass C.K. Regulation of microglia activation and deactivation by nuclear receptors. Glia. 2013;61:104–111. doi: 10.1002/glia.22423. [DOI] [PubMed] [Google Scholar]
- Savage J.C., Jay T., Goduni E., Quigley C., Mariani M.M., Malm T., Ransohoff R.M., Lamb B.T., Landreth G.E. Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer’s disease. J Neurosci. 2015;35:6532–6543. doi: 10.1523/JNEUROSCI.4586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W., Tsai R.M., Spina S., Grinberg L.T., Rojas J.C., et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Keller P., De Strooper B., Beyreuther K., Dotti C.G., Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Lieberburg I. Cellular mechanisms of beta-amyloid production and secretion. Proc Natl Acad Sci U S A. 1999;96:11049–11053. doi: 10.1073/pnas.96.20.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S., Roses A.D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai L.M., Youmans K.L., Jungbauer L., Yu C., Ladu M.J. Introducing human APOE into Aβ transgenic mouse models. Int J Alzheimers Dis. 2011;2011 doi: 10.4061/2011/810981. 810981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCW J., Liang S.A., Qian L., Pipalia N.H., Chao M.J., Shi Y., Bertelsen S.E., Kapoor M., Marcora E., Sikora E., et al. Cholesterol and matrisome pathways dysregulated in human APOE ε4 glia. bioRxiv. 2019;99:713362. doi: 10.1016/j.cell.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J., Borbat P.P., Freed J.H., Shin Y.K. A scissors mechanism for stimulation of SNARE-mediated lipid mixing by cholesterol. Proc Natl Acad Sci U S A. 2009;106:5141–5146. doi: 10.1073/pnas.0813138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J.D., Ulland T.K., Mahan T.E., Nyström S., Nilsson K.P., Song W.M., Zhou Y., Reinartz M., Choi S., Jiang H., et al. ApoE facilitates the microglial response to amyloid plaque pathology. J Exp Med. 2018;215:1047–1058. doi: 10.1084/jem.20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deijk A.F., Camargo N., Timmerman J., Heistek T., Brouwers J.F., Mogavero F., Mansvelder H.D., Smit A.B., Verheijen M.H. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia. 2017;65:670–682. doi: 10.1002/glia.23120. [DOI] [PubMed] [Google Scholar]
- Vance J.E. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl R.R., Itoh N., Tassoni A., Matsukawa M.A., Ren E., Tse V., Jang E., Suen T.T., Itoh Y. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116:10130–10139. doi: 10.1073/pnas.1821306116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo T.S., Cutler D.J., Wingo A.P., Le N.A., Rabinovici G.D., Miller B.L., Lah J.J., Levey A.I. Association of early-onset Alzheimer disease with elevated low-density lipoprotein cholesterol levels and rare genetic coding variants of APOB. JAMA Neurol. 2019;76:809–817. doi: 10.1001/jamaneurol.2019.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W.G., Li L., Müller W.E., Eckert G.P. Cholesterol as a causative factor in Alzheimer’s disease: a debatable hypothesis. J Neurochem. 2014;129:559–572. doi: 10.1111/jnc.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhang X., Zhao L. Human ApoE isoforms differentially modulate brain glucose and ketone body metabolism: implications for Alzheimer’s disease risk reduction and early intervention. J Neurosci. 2018;38:6665–6681. doi: 10.1523/JNEUROSCI.2262-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Ma Y., Liu Z., Geng Q., Chen Z., Zhang Y. Alterations of myelin morphology and oligodendrocyte development in early stage of Alzheimer’s disease mouse model. Neurosci Lett. 2017;642:102–106. doi: 10.1016/j.neulet.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Xu Q., Bernardo A., Walker D., Kanegawa T., Mahley R.W., Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S.B., Li G., et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]